Dear Editor,
Congenital Factor VII deficiency is a rare autosomal recessive coagulation disorder affecting approximately 1 in 500,000 live births [1]. Factor VII is one of the key coagulation factors in the classical extrinsic coagulation pathway. Factor VII is coded by the gene on band 13q34, closely located to the gene for factor X (F10). It has the shortest half-life among all procoagulant factors: 3–6 h. Alexander et al. [2] first described about congenital factor VII deficiency in 1951. Pediatric cardiac surgery in Factor VII deficiency disease has rarely been performed and till date only few case reports have been found in literature. For the first time in India, we are reporting a case of congenital Factor VII deficiency with Ventricular Septal Defect (VSD), who successfully underwent transcatheter VSD closure with Amplatzer Duct Occluder II device using recombinant Factor VII activated (rFVIIa).
A 9 year old male child weighing 20 kg, presented to us with complaints of shortness of breath, since the age of 6 months with occasional waxing and waning. Echocardiography showed 4 mm perimembranous VSD, partially restricted by septal leaflet aneurysm of Tricuspid Valve, left to right shunt gradient 72 mm Hg, dilated left atrium (LA), left ventricle (LV) with no pulmonary arterial hypertension. During preoperative evaluation, it was found that his Prothombin Time (PT) was 74.6 s (control 12 s, INR 6.8), Activated Partial Thromboplastine Time (APTT) was 33 s (control 28 s) and platelets were 4.97 × 109/L. The complete coagulation work up revealed isolated prolongation of PT with normal APTT and Thrombin Time (TT).The correction study was performed with normal human plasma which normalized the prolonged PT, suggesting a coagulation factor deficiency involving extrinsic pathway. The liver function test and ultrasonographic study of abdomen were normal. Further investigations revealed deficiency of FVII with 5.7% activity (laboratory normal range: 60–150%). There were no spontaneous bleeding episodes except frequent episodes of gum bleeding, which used to cease with pressure. Patient had never been evaluated or diagnosed for any bleeding disorder before. There was no history of consanguinity or bleeding disorders in family.
After discussion with parents and taking proper consent, he was planned for VSD device closure with Factor VII replacement. On the day before procedure, repeat INR was 5.4. Inhibitor screening was performed to rule out presence of any immediate or delayed type of inhibitors. Human rFVIIa (NovoSeven® RT, Novo Nordisk HealthCare AG) bolus injection was given intravenously at a dose of 20 μg/kg, fifteen minutes prior to the cardiac intervention. Post transfusion PT and INR came down to 11 s and 0.9 respectively before starting of cardiac intervention.
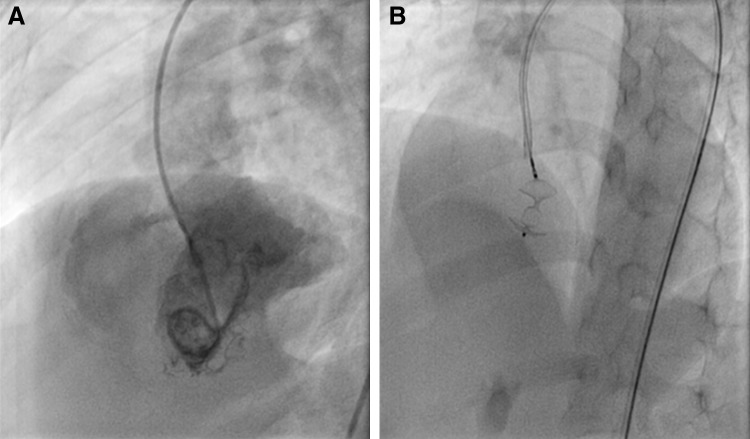
Accesses were obtained in right femoral vein and artery. The VSD was crossed with 5F 3.5 mm Judkin’s Right catheter and 0.025 × 260 cm J tip Terumo wire from LV. The wire was placed in the left pulmonary artery. A 5F Launcher catheter (Medtronic, Inc, MN USA) passed over the Terumo wire and was placed in right ventricle. The 5 × 6 mm Amplatzer Duct Occlude II (AGA medical corporation, MN USA) device was deployed under fluoroscopic and transthoracic echocardiographic guidance. Post procedure echocardiography showed VSD device in situ (Fig. 1), no residual shunt, no aortic incompetence, good biventricular function. Hemostasis was achieved after removal of sheath.
Fig. 1.
The angiographic and fluoroscopic image of patient. a Pre procedure LV angiogram showing perimemranous VSD with left to right shunt. b Post procedure fluoroscopic image showing VSD occluder deice in situ
The post procedure INR, done three hours after first dose of factor infusion, was 1.78. The patient was monitored by PT 12 hourly for next 3 days (Table 1). As there was no significant hemodynamic complication and no bleeding, further dose of recombinant Factor VII was omitted. Post procedure 6 months follow up period showed device in situ, no residual shunt, no Aortic regurgitation and patient was stable with sinus rhythm.
Table 1.
Serial monitoring of PT/INR
| Serial monitoring of PT/INR Control PT (12 s) |
P time(s) Test |
INR |
|---|---|---|
| Before rFVIIa infusion | 68 | 5.40 |
| 15 min after rFVIIa infusion | 11 | 0.90 |
| 3 h post infusion | 21 | 1.78 |
| 12 h post infusion | 28 | 2.33 |
| 24 h post infusion | 38 | 3.38 |
| 36 h post infusion | 50 | 4.28 |
| 48 h post infusion | 44 | 3.8 |
| 72 h post infusion | 56 | 4.8 |
The treatment of FVII deficiency disorders requires replacement with Fresh Frozen Plasma or Prothombin Complex Concentrate. But given the short half life of FVII, multiple transfusions are required, which may lead to volume overload especially in pediatric cases. Also other non deficient factor concentrations are highly increased during these transfusions leading to hypercoaguability [3]. The rFVIIa is therefore preferable as replacement therapy in FVII deficiency [4]. It forms complex with tissue factor, activates coagulation Factor X to Factor Xa. Factor Xa, in complex with other factors, then converts prothrombin to thrombin, which leads to the formation of a hemostatic plug by converting fibrinogen to fibrin and thereby inducing local hemostasis. Despite the strong recommendations on use of rFVIIa in FVII deficiency, the published data on dosage and treatment schedules in surgical setting are scarce [5].
There were few cases of successful cardiac surgery performed in such rare coagulation disorder, that have been reported previously [6, 7]. They used a high dose of 40 μg/kg 2 h before surgery. On contrary Ozan Emiroglu et al. [8] used low dose FVII of 20 μg/kg fifteen minutes before surgery for ASD closure. Till now, no literature is found about catheter based cardiac intervention, performed in patients with FVII deficiency. So optimal replacement dosing in such clinical scenario is not known. The UK guidelines on the management of rare bleeding disorders recommended target trough FVII: C level of 10–15% in FVII-deficient patients undergoing major surgery [5]. Factor VII levels of 15% (0.075 micrograms per mL) are generally sufficient to achieve normal hemostasis [9]. So, a 20 kg child with FVII deficiency (plasma volume of approximately 900 mL) would thus require 3.4 micrograms per kg of rFVIIa to secure hemostasis, assuming 100% recovery. Since the mean plasma recovery for rFVIIa is 20% for FVII-deficient patients, a dose of 17 micrograms per kg rFVIIa would be required to achieve sufficient FVII plasma levels for hemostasis, which is consistent with the recommended dose range and the dose (20 μg/kg) we have used in our case.
In conclusion, using a low-dose single infusion substitution strategy with rFVIIa concentrate, VSD repair can be performed successfully with transcatheter device closure technique in presence of congenital FVII deficiency. During surgery with rFVIIa concentrate, both bleeding and thrombotic complications need to be monitored. Also, optimal dosing and timing of administration of rFVIIa in cardiac surgeries needs to be validated with more such studies.
Acknowledgements
We acknowledge the contributions of late Dr. Bhanu Kumar Bansal, pediatric cardiac interventionist, in this case.
Compliance with Ethical Standards
Funding
The study did not involve any funding.
Disclosure of Potential Conflicts of Interest
No financial or other relationships, which might lead to a conflict of interest, are related to this article.
Informed Consent
Informed consent was taken from the patient/guardian to publish his photograph and/or clinical documents in medical journals.
References
- 1.Perry DJ. Factor VII deficiency. Br J Haematol. 2002;118(3):689–700. doi: 10.1046/j.1365-2141.2002.03545.x. [DOI] [PubMed] [Google Scholar]
- 2.Alexander B, Goldstein R, Landwehr G, CooK CD, et al. Congenital SPCA deficiency: a hitherto unrecognized coagulation defect with hemorrhage rectified by serum and serum fractions. J Clin Invest. 1951;30(6):596–608. doi: 10.1172/JCI102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariani G, Herrmann FH, Schulman S, et al. Thrombosis in inherited factor VII deficiency. J Thromb Haemost. 2003;1(10):2153–2158. doi: 10.1046/j.1538-7836.2003.00395.x. [DOI] [PubMed] [Google Scholar]
- 4.Mariani G, Konkle BA, Ingerslev J. Congenital factor VII deficiency: therapy with recombinant activated factor VII: a critical appraisal. Haemophilia. 2006;12(1):19–27. doi: 10.1111/j.1365-2516.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 5.Bolton-Maggs PH, Perry DJ, Chalmers EA, et al. The rare coagulation disorders: review with guidelines for management from the United Kingdom Haemophilia Centre Doctors’ Organisation. Haemophilia. 2004;10(5):593–628. doi: 10.1111/j.1365-2516.2004.00944.x. [DOI] [PubMed] [Google Scholar]
- 6.Tokunaga C, Hiramatsu Y, Horigome H, et al. Palliative open heart surgery in an infant with factor VII deficiency. Ann Thorac Surg. 2003;76(6):2093–2094. doi: 10.1016/S0003-4975(03)01038-5. [DOI] [PubMed] [Google Scholar]
- 7.Ferster A, Capouet V, Deville A, et al. Cardiac surgery with extracorporeal circulation in severe factor VII deficiency. Haemostasis. 1993;23(1):65–68. doi: 10.1159/000216855. [DOI] [PubMed] [Google Scholar]
- 8.Emiroglu Ozan, Eyileten Zeynep, Uçar Tayfun, et al. Pediatric cardiac surgery under cardiopulmonary bypass in factor VII deficiency. Turk J Pediatr. 2010;52(1):101–103. [PubMed] [Google Scholar]
- 9.Bauer KA. Treatment of factor VII deficiency with recombinant factor VIIa. Haemostasis. 1996;26(suppl 1):155–158. doi: 10.1159/000217259. [DOI] [PubMed] [Google Scholar]