Abstract
Multiple myeloma (MM) is a neoplastic disorder, which accounts for 13% of all hematological malignancies globally. While, conventional chemotherapy used to be the mainstay treatment for the disease, the landscape of treatment witnessed a paradigm shift with the introduction of high-dose chemotherapy and autologous stem cell transplant (ASCT). In this paper, we present a cost analysis of various services provided to multiple myeloma patients, using either of the two modalities of treatments i.e. conventional chemotherapy or ASCT. Bottom-up costing methodology was used to collect data on all health system resources, i.e. capital or recurrent, which were used to provide various services to MM patients. Capital costs were annualized for their useful life using a discount rate of 5%. Out of pocket expenditure on treatment was also ascertained. Cost was assessed for various services, including outpatient consultation, bed day hospitalization in general ward, high dependency unit intensive care setting and bone marrow transplant unit. Unit costs were calculated from both health system and patient perspective. The overall cost per patient for ASCT (including high dose chemotherapy) and conventional chemotherapy from societal perspective was INR 395,527 (USD 6085) and INR 62,785 (USD 966) respectively. Estimates on cost from our study could be used for planning health services, and evaluating cost effectiveness of different modalities of care for multiple myeloma.
Electronic supplementary material
The online version of this article (doi:10.1007/s12288-017-0843-7) contains supplementary material, which is available to authorized users.
Keywords: Cost analysis, Autologous stem cell transplant, Conventional chemotherapy, Multiple myeloma
Introduction
As per global burden of disease, the number of incident cases of multiple myeloma (MM) globally increased from 62,738 in 1990 to 116,947 in 2013 [1]. In India, the estimated incident cases of disease in 2015 were 12,605 males and 5329 females, which are projected to increase by the year 2020 [2]. Multiple myeloma accounts for 13% of all hematological malignancies globally [3]. It constitutes 2.5% of all hematological malignancies in India [4]. Moreover, a considerable variation is reported in demographic features like age at which MM is diagnosed in Asian and Western countries [5]. Although the disease remains incurable, there have been advancements on the therapeutic front concurrent with an improved understanding of disease dynamics [6]. Advent of high dose therapy supported with autologous stem cell transplantation (ASCT) as a treatment modality in MM has been reported to have promising results [7–15].
However, advanced treatment comes with a higher cost, be it direct in terms of out of pocket (OOP) expenditure spent by the patient, or cost incurred by the health system. This has implications in terms of choosing cost effective interventions so that the best value for money spent can be gained. The present study was undertaken as part of an overall cost effectiveness analysis for the two alternative modalities of treatment of MM-autologous stem cell transplant and conventional chemotherapy [16]. The objective of this paper included a detailed cost analysis for providing various services to multiple myeloma patients. Specifically, we estimate the unit cost per outpatient consultation (OP) and hospitalization costs are estimated as per bed day cost in bone marrow transplant unit (BMT) and high dependency unit (HDU). Using these estimates, we compute the unit cost per treating a multiple myeloma patient using ASCT and conventional chemotherapy.
Methods
Study Setting
This study was conducted at a 1950-bedded tertiary care, government funded hospital in Chandigarh [17]. The study hospital provides out-patient consultation, special clinic, in-patient hospitalization, intensive care, bone marrow transplant (BMT) and other support services. Patients of MM from a wide geographic catchment area like Jammu and Kashmir, Uttar Pradesh, Himachal Pradesh, Punjab and Haryana seek treatment in this hospital. The conventional chemotherapy that is used at our centre is combination chemotherapy and generic drugs are used. The combination of drugs are based on financial affordability of patient and various induction regimens used are melphalan/prednisolone/bortezomib (MPV), bortezomib/cyclophosphamide/dexamethasone (VCD) or cyclophosphamide/thalidomide/dexamethasone (CTD). Patients of multiple myeloma are categorized as transplant eligible and transplant ineligible. Transplant eligible patients are given 4–6 cycles of chemotherapy consisting of bortezomib, dexamethasone with either cyclophosphamide/thalidomide/lenalidomide. After 4–6 cycles of chemotherapy, mobilization of stem cells is done with granulocyte colony stimulating factors (G CSF). The harvested stem cells are stored at 4 °C. Patients are given conditioning with high dose melphalan 200 mg/m2 and autologous stem cells are re-infused 24-h later. All transplant are carried out in patient because of multiple reasons; most important being risk of life threatening infections and inadequate supportive care on out-patient basis. Patients ineligible for ASCT are also treated with triplet regimen consisting of novel agents with melphalan for 6–9 cycles. Patients who are younger than 65-years are eligible for ASCT.
Study Design
A primary costing survey was undertaken to collect data on resources spent for treatment of MM for both the modalities i.e. ASCT and conventional chemotherapy for the period March 2015–April 2016. In general, there are two types of approaches commonly used in the costing studies. The first approach is the ‘top down’ while the second is referred to as the ‘bottom-up approach’. The top down approach employs the use of financial records of expenditure done in the past to assess the utilization of resources. On the other hand, bottom up costing approach exercises a more comprehensive method of specifying the type and measuring the quantity of inputs being involved in the treatment [18]. In our study, we used bottom up approach to determine the resources used as per adaptation of standard methods of economic costing [18–20]. Further, the costing survey was carried as per these standard methods which have also previously been employed in costing studies elsewhere in India [21–24]. Detailed assessment of out of pocket payments borne by the patients was also undertaken.
Firstly, an initial assessment of the various service centres was done in order to allocate the costs of treatment in both the arms. A service centre was considered primary in case it was directly involved in delivery of health services to the patient. Out-patient department, in-patient department, bone marrow transplant centre (where in-patient ASCT delivered) and high dependency unit (where patients with severe hematological illness are admitted) were taken as direct primary cost centres. While the secondary service centres included electricity, water etc. which were not directly involved in delivery of services to the patient.
Secondly, an output of each service centre was identified and measured for the last 1 year (March 2015–April 2016) using hospital records. Thirdly, the resources used to produce these outputs were outlined and measured. Broadly, these input resources are classified as capital and recurrent resource based on their time of usage. For example, buildings, medical equipment, non-medical equipment like beds, furniture which are expected to last for more than a year are considered as capital resources. Salaries of staff, consumables, drugs, overheads like electricity, water etc. comprised of recurrent resources. Enlisting of staff involved in provision of treatment modalities under consideration either full time or part-time, whether contractual or permanent was done.
Data Collection
Various sources of information like hospital records, stock registers, prescription slips, and relevant records were reviewed to elicit the number of services under each service centre like OP, BMT, HDU, IPD (general) rendered as well as annual number of patients who sought treatment during the study period. Stock registers were reviewed to gather information on input resources like drugs and consumables, medical and non-medical equipment, etc. for service centres. Utility of each room and use of capital resources like equipment, building, furniture etc. was assessed. The data on floor area of the rooms, waiting areas and any other space being used were also obtained from records of engineering department.
Information on salaries were deduced from salary slips of staff involved in delivery of services like doctors, nurses, other medical and non-medical personnel. Several staff members were engaged in more than one activity. Using standard schedules [23, 24] detailed time allocation data with respect to different services for last 1 week was ascertained by interviewing, which was supplemented with actual observation. Data on life of capital items, for example equipment, was assessed through expert consultations. Price of each of the items whether capital or recurrent along with information on year of purchase from the hospital procurement department. In case the price was not available, market prices were used.
Data on out of pocket payments (OOP) incurred by the patients for the specific services such as ASCT, chemotherapy at study hospital was collected by interviewing—31 MM patients who had accessed treatment (26 patients for conventional chemotherapy and 5 ASCT patients) during last year. Demographic and socio-economic details like age, gender, address, income, monthly consumption expenditure were also elicited. OOP expenditures included any hospital user charges, expenditure on medication/drugs, consumables, diagnostic tests, travel, boarding, lodging or food related expenditures [17, 25–27].
Data Analysis
Unit health system cost of various services used by multiple myeloma patients was computed. These included cost per out-patient consultation, per patient who underwent ASCT, high dependency unit intensive care unit or per patient hospitalization in general ward. For capital resources, the costs were annualized. By annualization, we mean spreading out the costs of capital goods over the useful life with discounting to estimate its present equivalent monetary value. All the costs of equipment were discounted at 5% [28] and standard assumptions for their useful life were made. The economic value of space used for treatment was calculated by multiplying the floor area being used under that service centre with the market rental prices of similar space. In case of space jointly utilized for more than one activity, it was suitably apportioned by the proportion of time it was used for that activity. Overhead costs like water, electricity were apportioned as per proportional floor area [29].
For recurrent items, the quantities consumed were multiplied with the unit price to obtain overall cost. For staff costs, an apportioning statistic based on the proportion of time devoted to treatment services related to both the treatment arms was computed. Further, this apportioning statistic was then multiplied with the gross salary of that staff in order to assess the cost of human resource for that particular service centre. Subsequently, all the costs were summed up for each service centre to estimate an annual cost in Indian National Rupee (INR). For out of pocket payments on chemotherapy and ASCT mean expenditures (95% CI) were computed in SPSS version 17. A conversion rate of 1 USD equivalent to 65 INR in 2015 was used for all the costs reported [30].
Sensitivity Analysis
A univariate sensitivity analysis was performed to see the effect of variation in inputs over the annual costs. The base values of salaries, capital, medical and non-medical equipment were varied by 25% on both sides. Assuming a wide variation in prices in consumables and drugs, the prices of the latter were varied by 50% on either side of base value.
Ethical Approval
This study was approved by the Institute Ethics Committee of the Post Graduate Institute of Medical Education and Research, Chandigarh. Written informed consent was obtained from all study participants.
Results
Health System Costs
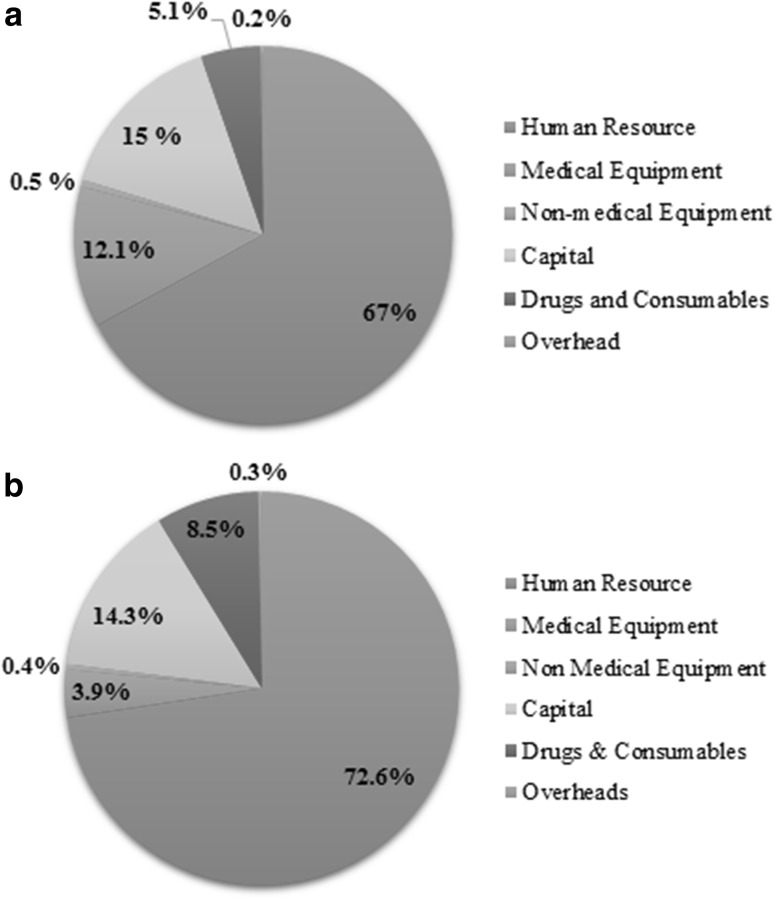
The average health system cost per patient and per bed day in bone marrow transplant centre was INR 160,027 (USD 2462) and INR 11,617 (USD 179) respectively. The average health system cost per patient and per bed day in intensive care setting (high dependency unit) was INR 62,565 (USD 963) and INR 8683 (USD 134) respectively. Average length of stay in BMT and HDU was 14 and 7.2 days, respectively. Health system incurred a cost of INR 510 (USD 7.84) for per outpatient visit and INR 10,107 (USD 155) per hospitalization in general ward as shown in Table 1. Cost on human resource was the major component of health system costs in all the services as shown in Fig. 1a, b.
Table 1.
Unit costs for treatment of multiple myeloma in a public sector tertiary care hospital
| Cost centre | Unit cost estimation | Health system unit cost in INR (USD) |
|---|---|---|
| Bone marrow transplant unit | Per patient | 160,027 (USD 2462) |
| High dependency unit (intensive care setting) | Per patient | 62,565 (USD 963) |
| Hospitalization in general ward | Per patient | 10,107 (USD 155) |
| Outpatient visit | Per OPD visit | 510 (USD 7.84) |
Fig. 1.
a Distribution of annual health system costs in bone marrow transplant unit at public sector tertiary care hospital in India. b Distribution of annual health system costs in high dependency unit ICU at public sector tertiary care hospital in India
Out of Pocket Expenditure
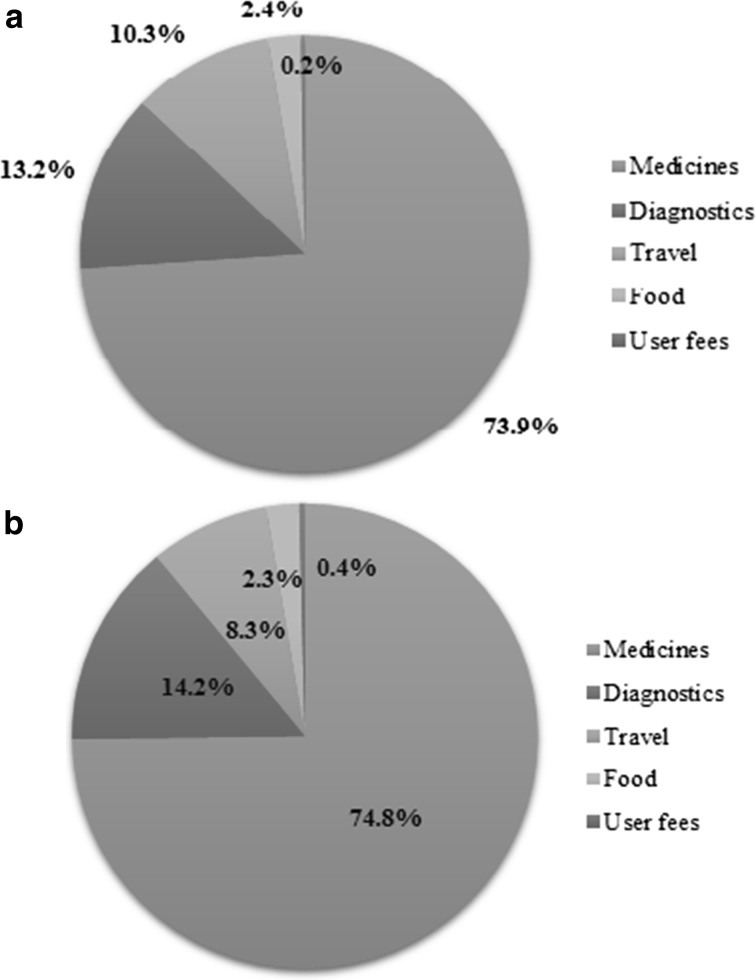
More than half (60%) of recipients of ASCT in last year were males. A patient who underwent ASCT reported a mean OOP expenditure (including prior conventional chemotherapy) of INR 235,500 (USD 3623; 95% CI INR 225,000–247,400). Majority of chemotherapy patients were males (73.1%) with median age as 62 years and median monthly income of INR 10,000 (USD 154). The mean OOP expenditure of these patients with an average six cycles of chemotherapy was found to be INR 62,275 (USD 958; 95% CI INR 50,962–73,960). Expenditure on medicines was the major component of OOP followed by diagnostics and travel in all services ASCT and chemotherapy used by patient as shown in Fig. 2a, b.
Fig. 2.
a Components of out of pocket payments on chemotherapy for multiple myeloma treatment at public sector tertiary care hospital in India. b Components of out of pocket payments on ASCT for multiple myeloma treatment at public sector tertiary care hospital in India
Overall societal cost (inclusive of health system and patient perspective) of per ASCT and conventional chemotherapy was estimated to be INR 395,527 (USD 6085) and INR 62,785 (USD 966) respectively.
Sensitivity Analysis
Annual BMT health system costs were most sensitive to variation in salaries (90.4%) followed by capital (4.5%); while HDU costs were most sensitive to salaries (91.2%) followed by drugs and consumables (5%) and capital (3.5%). Similarly, salaries followed by capital were significant factors determining variation in annual costs for out-patient consultation and hospitalization (general ward).
Discussion
Overall, we estimated the cost of treatment of multiple myeloma for two treatment modalities-ASCT and conventional chemotherapy. A societal perspective, generally wider and realistic, to account for all the costs was considered. The overall costs of provision of ASCT per patient (including high dose chemotherapy) was estimated to be INR 395,527 (USD 6085) while for a chemotherapy patient it was INR 62,785 (USD 966).
In context of developed nations like Norway, a multi-centric study reported the mean cost of HDT followed by hospitalization as USD 25,616 (USD 13,978–43,277) from health system perspective. Similar to findings from our study, it concluded that personnel costs constituted the largest share of cost followed by medications [31]. Sharma et al. [32] reported that the median cost of autologous transplant was USD 12,500 (USD 10,331–39,367) in a private sector super-specialty hospital in India, which is almost double the cost in public sector as reported in our study. Similar to costs of ASCT reported in our study, a non-profit organization running transplant centre in Eastern India also reported autologous transplant cost of around INR 3–4 lakhs [33]. Nevertheless as reported in number of studies on out of pocket payments, expenditure on medicines was the chief component of OOP in our study as well [34–36].
Strengths
Evidently, there is dearth of literature on costing of tertiary care health services. A number of surveys are undertaken, which primarily assess the OOP expenditure, but there are very limited studies which assess the health system cost. Our study is first of its kind in the country to give comprehensive cost estimates for plausible service centres that may be involved in the provision of care for multiple myeloma. We undertook a primary costing survey to assess the unit health system costs and OOP for various services. Accordingly, costs were reported from both health system and patient perspective. In addition, we carried out a detailed sensitivity analysis for health system costs major service centres in order to account for the various uncertainties related to assumptions as well as to see its effect of any variation on final cost estimations.
Limitations
We would also like to acknowledge few data limitations in our study. Firstly, our cost estimates are based on a single public sector tertiary care hospital. We would like to mention that there could be significant variations in cost estimates between various public sector hospitals. In addition, costs in private sector can be very different. Secondly, we did not account for cost of cryopreservation as the same is not routinely used during ASCT in Indian setting. Thirdly, we did not undertake further analysis based on varying length of stay for different disease conditions. Fourthly, there is a possibility of recall bias in reporting of OOP by patients. However, it is not likely to result in a systematic bias. Further these out of pocket payments were assessed from five ASCT cases while for conventional chemotherapy they were assessed from the 26 patients. The regimen of treatment for patients of multiple myeloma and drugs on which they chiefly incur out of pocket expenditures is standardized for all patients using either ASCT or conventional chemotherapy. The average OOP expenditure incurred by patients undergoing ASCT during the study period is same for the other ASCT patients. Hence these out of pocket expenditures represent average levels of resource utilization for these treatments. Nonetheless it is recommended to undertake studies with a larger sample to assess any variation in costs. Fifthly we did not estimate indirect costs or any productivity losses due to the disease which could have an impact on the costs involved. Lastly, in the context of full economic evaluation measuring the extent of occurrence of adverse events following both the treatment regimens for multiple myeloma and their respective costs should be accounted. However this was beyond the scope of the present study which focusses solely on the cost of the intervention for multiple myeloma. Any full cost effectiveness analysis for different treatment options in case of multiple myeloma should however include such a cost assessment.
Policy Implications and Research
Costing studies provide an invaluable insight to resources being used and its financial implications. Be it with an objective of an assessment of resources, cost containment or further delving into the means of cost-reduction, costing studies offers multitude of benefits to clinicians, health care providers, policy makers and researchers. Further, it is an indispensable input to cost effectiveness analysis carried out to ascertain best value for money for particular health service, program, treatment, drugs or diagnostic under consideration [18]. While ASCT in general is not cost effective to treat multiple myeloma, its use could be targeted to those detected and treated early where there was some value for money spent [16]. The cost analysis in the present paper included both the health system as well as the patient perspective which is important for planning health care services for the provision of treatment of multiple myeloma. Further detailed breakdown of costs as reported in the paper are relevant for policy makers in order to establish infrastructure for providing the treatment to patients of multiple myeloma. Evidence on costs is also important from payers’ perspective in order to generate reimbursement rates/provider payments. Our estimates should also be used in general while purchasing care under various publicly financed insurance schemes. Moreover, there is a concomitant need to undertake more costing and cost-effectiveness studies of various health services at tertiary level for informed decision-making especially in light of Universal Health Care.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Conflict of interest
None.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12288-017-0843-7) contains supplementary material, which is available to authorized users.
References
- 1.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre M, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takiar R, Nadayil D, Nandakumar A. Projections of number of cancer cases in India (2010–2020) by cancer groups. Asian Pac J Cancer Prev. 2010;11:1045–1049. [PubMed] [Google Scholar]
- 3.Raab M, Podar K, Breitkreutz I, Richardson P, Anderson K. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 4.Ghalaut P, Chaudhuri S, Singh R. Recent advances in diagnosis and management of multiple myeloma: an update. India: The Association of Physicians of India, Medicine Update; 2013. [Google Scholar]
- 5.Lu J, Hou J, Liu KY, Parmar S, De La Fuente A, Andersson B, et al. Asia-Pacific Hematology Consortium Report on approach to multiple myeloma. Survey results from the 6th international hematologic malignancies conference: bridging the gap 2015, Beijing, China. Leuk Lymphoma. 2016;57(7):1534–1538. doi: 10.3109/10428194.2015.1135434. [DOI] [PubMed] [Google Scholar]
- 6.Yanamandra U, Khattry N, Kumar S, Raje N, Jain A, Jagannath S, et al. Consensus in the management of multiple myeloma in India at myeloma state of the art 2016 conference. Indian J Hematol Blood transfus. 2017;33(1):15–21. doi: 10.1007/s12288-016-0773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlogie B, Hall R, Zander A, Dicke K, Alexanian R. High-dose melphalan with autologous bone marrow transplantation for multiple myeloma. Blood. 1986;67(5):1298–1301. [PubMed] [Google Scholar]
- 8.Fermand JP, Chevret S, Ravaud P, Divine M, Leblond V, Dreyfus F, et al. High-dose chemoradiotherapy and autologous blood stem cell transplantation in multiple myeloma: results of a phase II trial involving 63 patients. Blood. 1993;82(7):2005–2009. [PubMed] [Google Scholar]
- 9.Cunningham D, Paz-Ares L, Milan S, Powles R, Nicolson M, Hickish T, et al. High-dose melphalan and autologous bone marrow transplantation as consolidation in previously untreated myeloma. J Clin Oncol. 1994;12(4):759–763. doi: 10.1200/JCO.1994.12.4.759. [DOI] [PubMed] [Google Scholar]
- 10.Harousseau JL, Attal M, Divine M, Marit G, Leblond V, Stoppa AM, et al. Autologous stem cell transplantation after first remission induction treatment in multiple myeloma: a report of the French Registry on autologous transplantation in multiple myeloma. Blood. 1995;85(11):3077–3085. [PubMed] [Google Scholar]
- 11.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais Du Myelome. N Engl J Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 12.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 13.Kumar L, Raju GM, Ganessan K, Shawgi S, Menon H, Wadhwa J, et al. High dose chemotherapy followed by autologous haemopoietic stem cell transplant in multiple myeloma. Natl Med J India. 2003;16(1):16–21. [PubMed] [Google Scholar]
- 14.Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13(2):183–196. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Kumar L, Ghosh J, Ganessan P, Gupta A, Hariprasad R, Kochupillai V. High-dose chemotherapy with autologous stem cell transplantation for multiple myeloma: what predicts the outcome? Experience from a developing country. Bone Marrow Transplant. 2009;43(6):481–489. doi: 10.1038/bmt.2008.343. [DOI] [PubMed] [Google Scholar]
- 16.Prinja S, Kaur G, Malhotra P, Jyani G, Ramachandran R, Bahuguna P, et al. Cost-effectiveness of autologous stem cell treatment as compared to conventional chemotherapy for treatment of multiple myeloma in India. Indian J Hematol Blood Transfus. 2017 doi: 10.1007/s12288-017-0776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prinja S, Jagnoor J, Chauhan AS, Aggarwal S, Nguyen H, Ivers R. Economic burden of hospitalization due to injuries in North India: a cohort study. Int J Environ Res Public Health. 2016 doi: 10.3390/ijerph13070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriks ME, Kundu P, Boers AC, Bolarinwa OA, Te Pas MJ, Akande TM, et al. Step-by-step guideline for disease-specific costing studies in low- and middle-income countries: a mixed methodology. Global Health Action. 2014;7:23573. doi: 10.3402/gha.v7.23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck EJ, Harling G, Gerbase S, DeLay P. The cost of treatment and care for people living with HIV infection: implications of published studies, 1999–2008. Curr Opin HIV AIDS. 2010;5(3):215–224. doi: 10.1097/COH.0b013e32833860e9. [DOI] [PubMed] [Google Scholar]
- 20.Adam T, Bishai D, Khan M, Evans D. Methods for the costing component of multi-country evaluation of IMNCI. Geneva: World Health Organization; 2004. [Google Scholar]
- 21.Prinja S, Jeet G, Verma R, Kumar D, Bahuguna P, Kaur M, et al. Economic analysis of delivering primary health care services through community health workers in 3 North Indian states. PLoS ONE. 2014;9(3):e91781. doi: 10.1371/journal.pone.0091781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prinja S, Mazumder S, Taneja S, Bahuguna P, Bhandari N, Mohan P, et al. Cost of delivering child health care through community level health workers: how much extra does IMNCI program cost? J Trop Pediatr. 2013;59(6):489–495. doi: 10.1093/tropej/fmt057. [DOI] [PubMed] [Google Scholar]
- 23.Prinja S, Manchanda N, Mohan P, Gupta G, Sethy G, Sen A, et al. Cost of neonatal intensive care delivered through district level public hospitals in India. Indian Pediatr. 2013;50(9):839–846. doi: 10.1007/s13312-013-0234-6. [DOI] [PubMed] [Google Scholar]
- 24.Prinja S, Gupta A, Verma R, Bahuguna P, Kumar D, Kaur M, et al. Cost of delivering health care services in public sector primary and community health centres in North India. PLoS ONE. 2016;11(8):e0160986. doi: 10.1371/journal.pone.0160986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prinja S, Bahuguna P, Gupta R, Sharma A, Rana SK, Kumar R. Coverage and financial risk protection for institutional delivery: how universal is provision of maternal health care in India? PLoS ONE. 2015;10(9):e0137315. doi: 10.1371/journal.pone.0137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta I, Chowdhury S, Prinja S, Trivedi M. Out-of-pocket spending on out-patient care in India: assessment and options based on results from a district level Survey. PLoS ONE. 2016;11(11):e0166775. doi: 10.1371/journal.pone.0166775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prinja S, Gupta R, Bahuguna P, Sharma A, Kumar Aggarwal A, Phogat A, et al. A composite indicator to measure universal health care coverage in India: way forward for post-2015 health system performance monitoring framework. Health Policy Plan. 2017;32(1):43–56. doi: 10.1093/heapol/czw097. [DOI] [PubMed] [Google Scholar]
- 28.ISPOR (2016) Pharmacoeconomics and outcomes research guidelines for India PEOR Guidelines 2016. https://www.ispor.org/consortiums/asia/PEGuidelines_India_March2016.pdf
- 29.Sangwan A, Prinja S, Aggarwal S, Jagnoor SJ, Bahuguna P, Ivers R, et al. Cost of trauma care in secondary and tertiary care public sector hospitals in north India. Appl Health Eco Health Policy. 2017 doi: 10.1007/s40258-017-0329-7. [DOI] [PubMed] [Google Scholar]
- 30.The Economic Times (2015) Forex rates 2015. http://economictimes.indiatimes.com/markets/forex
- 31.Mishra V, Andresen S, Brinch L, Kvaloy S, Ernst P, Lonset MK, et al. Cost of autologous peripheral blood stem cell transplantation: the Norwegian experience from a multicenter cost study. Bone Marrow Transplant. 2005;35(12):1149–1153. doi: 10.1038/sj.bmt.1704988. [DOI] [PubMed] [Google Scholar]
- 32.Sharma SK, Choudhary D, Gupta N, Dhamija M, Khandelwal V, Kharya G, et al. Cost of hematopoietic stem cell transplantation in India. Mediterr J Hematol Infect Dis. 2014;6(1):e2014046. doi: 10.4084/mjhid.2014.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhopadhyay A, Gupta P, Basak J, Chakraborty A, Bhattacharyya D, Mukhopadhyay S, et al. Stem cell transplant: an experience from eastern India. Indian J Med Paediatr Oncol. 2012;33(4):203–209. doi: 10.4103/0971-5851.107078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garg CC, Karan AK. Reducing out-of-pocket expenditures to reduce poverty: a disaggregated analysis at rural–urban and state level in India. Health Policy Plan. 2009;24(2):116–128. doi: 10.1093/heapol/czn046. [DOI] [PubMed] [Google Scholar]
- 35.Bhojani U, Thriveni B, Devadasan R, Munegowda C, Devadasan N, Kolsteren P, et al. Out-of-pocket healthcare payments on chronic conditions impoverish urban poor in Bangalore, India. BMC Public Health. 2012;12:990. doi: 10.1186/1471-2458-12-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NSSO (2015) Key indicators of social consumption in India Health NSS 71st Round (January–June 2014). Ministry of Statistical and Programme Implementation, New Delhi
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.