ABSTRACT
Pseudouridine (Ψ) is present at conserved, functionally important regions in the ribosomal RNAs (rRNAs) from all three domains of life. Little, however, is known about the functions of Ψ modifications in bacterial ribosomes. An Escherichia coli strain has been constructed in which all seven rRNA Ψ synthases have been inactivated and whose ribosomes are devoid of all Ψs. Surprisingly, this strain displays only minor defects in ribosome biogenesis and function, and cell growth is only modestly affected. This is in contrast to a strong requirement for Ψ in eukaryotic ribosomes and suggests divergent roles for rRNA Ψ modifications in these two domains.
IMPORTANCE Pseudouridine (Ψ) is the most abundant posttranscriptional modification in RNAs. In the ribosome, Ψ modifications are typically located at conserved, critical regions, suggesting they play an important functional role. In eukarya and archaea, rRNAs are modified by a single pseudouridine synthase (PUS) enzyme, targeted to rRNA via a snoRNA-dependent mechanism, while bacteria use multiple stand-alone PUS enzymes. Disruption of Ψ modification of rRNA in eukarya seriously impairs ribosome function and cell growth. We have constructed an E. coli multiple deletion strain lacking all Ψ modifications in rRNA. In contrast to the equivalent eukaryotic mutants, the E. coli strain is only modestly affected in growth, decoding, and ribosome biogenesis, indicating a differential requirement for Ψ modifications in these two domains.
KEYWORDS: pseudouridine, rRNA modification, ribosome assembly, antibiotic resistance, decoding accuracy
INTRODUCTION
Pseudouridine (Ψ) is the most common posttranscriptional modification found in RNA. This uridine isomer is found in tRNAs, rRNAs, and several classes of stable, noncoding RNAs, including snRNAs (reviewed in reference 1). More recently, transcriptome-wide approaches have identified Ψ modifications in mRNAs from a variety of eukaryotes (2–4), and the alterations in mRNA pseudouridylation in response to various environmental stresses suggest that this mRNA modification may play a regulatory role (4). Ψ is formed by replacing the N1-C1′ glycosidic bond of uridine with a C5-C1′ linkage. Chemically, Ψ modifications enhance the rigidity of the phosphodiester backbone, and Ψ-A base pairs have greater thermodynamic stability than U-A pairs, perhaps accounting for its widespread distribution in all classes of RNAs (5).
Ribosomes from all three domains of life contain various amounts of Ψ. There is a single Ψ in mitochondrial ribosomes (6, 7), and there are 11 Ψs in Escherichia coli rRNA (Fig. 1). The number varies between 3 and 9 in the archaea that have been analyzed (8–10), while eukaryotic cytoplasmic ribosomes have many more, ranging from 44 in yeast to 91 in humans (11). Ψ modifications are clustered in functionally important centers of the ribosome (7, 12). In recent, high-resolution structures of bacterial ribosomes, Ψ (and other) modifications can be seen to surround and “cradle” the A- and P-site tRNAs (13–15) (Fig. 1). Moreover, despite the wide variation in Ψ number in ribosomes from different organisms, certain Ψs are almost invariant; these include those in the functionally critical helix H69 and the peptidyltransferase and peptide tunnel regions of the large ribosomal subunit (16). Pseudouridylation of rRNA is achieved via fundamentally different mechanisms in bacteria and archaea/eukarya. Bacteria encode a set of “stand-alone” protein pseudouridine synthases (PUSs) that both recognize their RNA substrates and carry out the isomerization of U to Ψ. While the archaea and eukarya have some stand-alone PUSs, Ψ modification of rRNA in these organisms is achieved by a set of RNA protein complexes, the box H/ACA snoRNPs. These RNPs contain a catalytic subunit, Cbf5 (dyskerin in mammals), and small RNAs that target the complex to the rRNA substrate via complementary base pairing. In addition to their enzymatic activity, at least one PUS has recently been shown to act as an RNA chaperone (17).
FIG 1.
Modified nucleosides in the E. coli ribosome (PDB 5AFI). Pseudouridines (cyan) and methylations (magenta) are clustered around the decoding site (DC), peptidyltransferase center (PTC), and tRNA binding sites (P-site tRNA in green). The mRNA is depicted in yellow. The Ψ modifications, with the relevant PUS responsible for the isomerization(s) in parentheses, are as follows: 16S rRNA, Ψ516 (RsuA); 23S rRNA, Ψ746 (RluA), Ψ2605 (RluB), Ψ955, Ψ2504, Ψ2580 (RluC), Ψ1911, Ψ1915, Ψ1917 (RluD), Ψ2557 (RluE), and Ψ2604 (RluF).
The distribution of Ψ in bacterial, archaeal, and eukaryotic rRNAs and their conserved locations in discrete, functionally important regions argues for important functions for these Ψ modifications. This conclusion is consistent with the ribosomal and other phenotypes of yeast cells expressing a catalytically inactive Cbf5p and mouse cells expressing a partially active dyskerin protein or human cell lines in which dyskerin levels have been reduced (18). The Ψ-lacking ribosomes in these cells showed defects in internal ribosome entry site (IRES)-mediated initiation, tRNA binding, and frame maintenance (18). Moreover, the phenotypes of deletions of individual yeast snoRNA genes responsible for discrete Ψ modifications (19, 20) all support the conclusion that in eukaryotic cytoplasmic ribosomes, Ψ is important for ribosome biogenesis and function. In contrast to the eukaryotic results, the archaeal Cbf5 gene in Haloferax volcanii is surprisingly not essential. Deletion of the H. volcanii Cbf5 leads to a complete loss of Ψ from rRNA but has little effect on growth (21). Loss of the single mitochondrial Ψ in the large subunit (LSU) rRNA (at the position equivalent to 2580 in E. coli 23S rRNA) has diverse phenotypic effects, depending on the organism. Deletion of PUS5 from yeast leads to loss of Ψ in 21S rRNA but does not affect growth on fermentable carbon sources (6). However, in human cell lines, depletion of the essential enzyme RPUSD4, responsible for the single Ψ in the LSU rRNA, inhibited mitochondrial ribosome assembly and protein synthesis (22). Thus, while Ψ rRNA modifications are critical in eukaryotic cytoplasmic and mammalian mitochondrial ribosomes, in yeast mitochondria and at least one archaeon, Ψ is not required for ribosome function.
Studies of Ψ functions in bacterial ribosomes have been carried out largely in K-12 strains of E. coli. Seven different PUS enzymes (RsuA and RluA, -B, -C, -D, -E, and -F) are responsible for the 11 Ψ modifications in rRNA (Fig. 1), and only a limited number of phenotypes have been reported for the corresponding deletion mutants. In K-12 strains, deletions of rsuA, rluA, rluB, rluC, rluE, and rluF are well tolerated with few phenotypic consequences. The sole exception is deletion of rluD, responsible for Ψ1911, Ψ1915, and Ψ1917 in helix H69 of 23S rRNA. Loss of RluD caused a severe slow-growth phenotype and affected ribosome biogenesis profoundly. Helix H69 is a universally conserved element of large subunit rRNAs and forms part of bridge B2a, one of the intersubunit bridges connecting the large and small ribosomal subunits. Moreover, RluD is one of the few rRNA modification enzymes found even in bacteria with drastically reduced genomes (16). However, work from this and other laboratories showed that the severe rluD deletion phenotype is essentially an artifact of K-12 strains of E. coli (23–25). This strain of E. coli carries a partially defective allele of the termination factor RF2, with a Thr at position 246, in contrast to the Ala or Ser found at this position in all other class 1 RFs, even in the RFs of other E. coli strains. Replacement of the partially defective Thr246 RF2 with the fully active Ala246 RF2 allele allowed the deletion of rluD with little or no phenotypic effect. Similar results were obtained with RF3, the termination factor that recycles RF1 and RF2 off posttermination ribosomes (23). Deletion of the prfC gene encoding RF3 in K-12 strains of E. coli produces distinct cold-sensitive growth, antibiotic hypersensitivity, and stop codon readthrough phenotypes (23). These phenotypes were largely eliminated upon replacement of the K-12 Thr246 RF2 with the Ala246 allele in the prfC deletion strains (23). Loss of H69 Ψs also affected RF2 activity in vitro, and reintroduction of pseudouridines into H69 by treatment with purified RluD restored the activity of RF2 in peptide release (26). These studies uncovered a genetic (synthetic-sick) interaction between RF2 and H69 Ψs. The cell can tolerate single mutations affecting either RF2 or H69, but double mutant combinations hamper this interaction, to the extent that termination is now very inefficient and growth is compromised. This genetic result is completely consistent with the known interaction of RF1 and RF2 with the ribosome (27, 28).
Even less is known about the physiological roles of other PUS enzymes: RluC is responsible for modifications at positions 955, 2504, and 2580 in 23S rRNA. Ψ2504 contributes to the ribosomal response to a range of peptidyltransferase-targeting antibiotics (29), while inactivation of rluC suppresses the cold sensitivity associated with mutants lacking the translational GTPase BipA (30). RsuA is responsible for Ψ516 in 16S rRNA and has been linked to cell survival after induction of the MazF endoribonuclease toxin (31). Interestingly, isomerization of uridines at nonspecific positions inhibits progression of ribosome assembly, supporting the idea of Ψ stabilizing the RNA backbone structure (32).
To explore the different requirements for rRNA Ψ in E. coli and eukaryotes and to determine if loss of multiple Ψ modifications in bacteria produces deleterious phenotypes that are not evident in single-gene deletions, we have constructed an E. coli strain lacking all Ψ modifications in rRNA. Surprisingly, lack of Ψ modifications has only modest effects on the phenotypes assayed. The Ψ-free ribosomes support cell growth at levels close to wild type under a variety of medium types and growth temperatures.
RESULTS
Generation of the ΔΨ7 strain lacking all rRNA pseudouridine synthases.
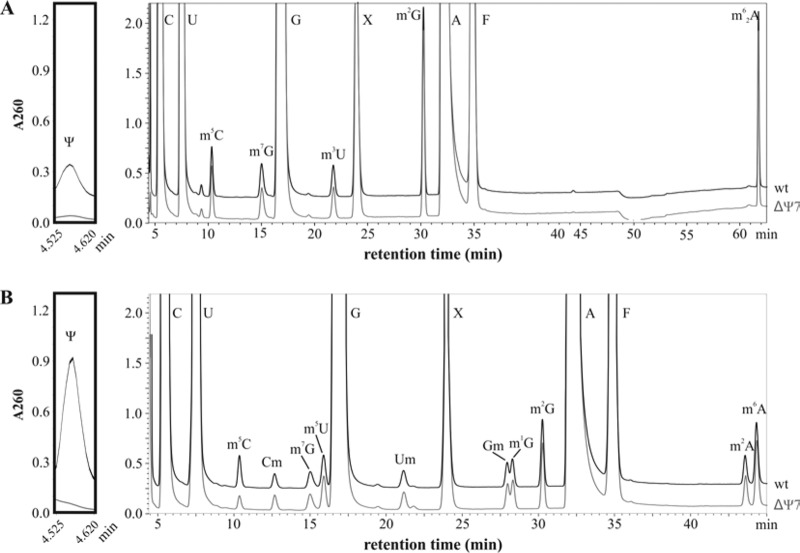
Since pseudouridylation of bacterial ribosomes involves several stand-alone PUS enzymes, the generation of bacterial strains equivalent to the Cbf5 mutant strains of yeast or H. volcanii lacking all Ψs in rRNA requires the deletion of multiple PUS genes. The rluD studies described above together with previous work showed that each of the 7 E. coli rRNA PUS genes could be individually deleted with little phenotypic consequence. Starting with MC415 expressing the fully active (Ala246) RF2, deletions in each of the 7 rRNA PUS genes were introduced into this strain to generate the ΔΨ7 strain, MC452. To demonstrate that Ψ was totally lacking in ribosomes from the ΔΨ7 strain, ribosomes were isolated from wild-type and ΔΨ7 strains, purified, and dissociated into subunits on sucrose gradients. This step avoids Ψ contamination derived from residual tRNAs bound to 70S ribosomes. RNAs were then extracted from isolated ribosomal subunits, digested, and analyzed by high-performance liquid chromatography (HPLC) (Fig. 2). These experiments confirmed the absence of Ψ in ribosomes from the ΔΨ7 strain.
FIG 2.
HPLC analysis of rRNA nucleosides. 16S rRNA (A) and 23S rRNA (B) were isolated from mature ribosome subunits obtained by dissociation of 70S ribosomes from wild-type E. coli MC415 (black trace) and the ΔΨ7 strain, MC452 (gray trace). Peaks corresponding to four standard nucleosides (C, U, G, and A) and Ψ, m5C, Cm, m7G, m5U, m3U, m4Cm, Um, Gm, m1G, m2G, m2A, m6A, and m62A are indicated. X corresponds to unknown compounds, and F corresponds to phenol. The inset to the left of both panels shows an enlargement of the Ψ region (retention time, 4.5 to 4.6 min) of the chromatogram. The peak corresponding to m5C of the 23S rRNA also contains m3Ψ and is significantly smaller for the ΔΨ7 strain.
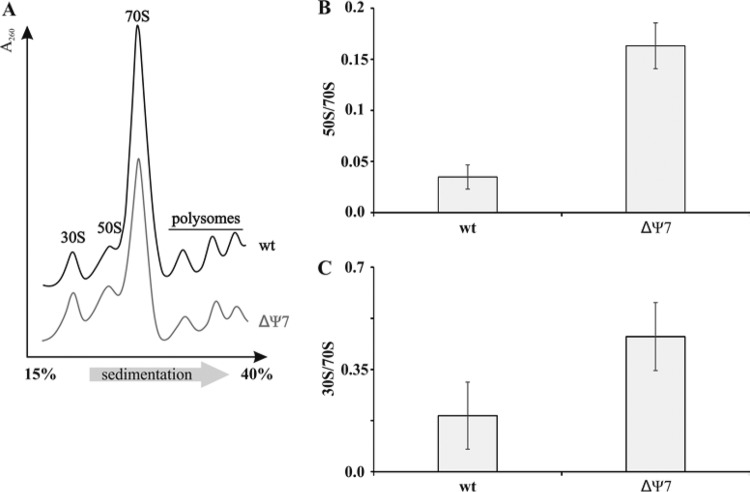
When lysates from wild-type and ΔΨ7 strains were examined on sucrose gradients, a small increase in the amount of free 30S and 50S subunits was seen in the ΔΨ7 strain (Fig. 3). In principle, the increased subunit level can be due to either effects on subunit joining or effects on the kinetics of subunit assembly. A majority of the Ψ modifications are introduced at the early and intermediate stages of assembly, with only RluD acting at late assembly stages (33). The increased subunit levels may thus reflect multiple delays in assembly steps. Nonetheless, the 70S levels in the ΔΨ7 strain remained high, the 70S/polysome ratios were the same in both strains, and no additional peaks, corresponding to accumulated assembly intermediates, were observed.
FIG 3.
Sucrose gradient analysis of ribosomal particles. (A) Ribosome profiles of the wild-type (wt [MC415]) and ΔΨ7 (MC452) strains. The direction of sedimentation is from left to right. (B) Molar ratio of free 50S subunits and 70S ribosomes on the gradient profile. (C) Molar ratio of free 30S subunits and 70S ribosomes on the gradient profile.
Effects of Ψ loss on growth, antibiotic interactions, and bacterial physiology.
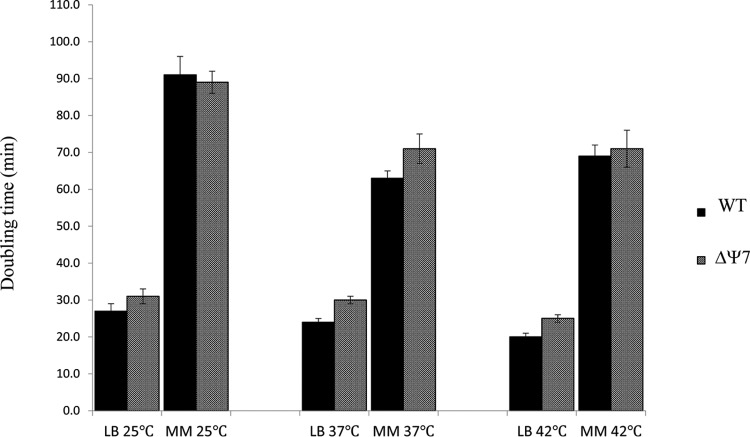
Successful construction of the ΔΨ7 strain suggested that loss of Ψ modifications from rRNA did not have a major effect on growth on solid, rich medium at 37°C. Determination of doubling times of isogenic wild-type and ΔΨ7 strains in both rich and minimal media, at temperatures ranging from 25 to 42°C (Fig. 4), indicated that lack of Ψ modifications had a small (15%) but detectable effect on growth in rich medium at all temperatures. In minimal medium, which supports slower growth of all strains, growth of the strain pairs was identical (at 25 and 42°C) or very similar (at 37°C). Thus, under rich medium growth conditions, where the demand for ribosome function is greatest, growth is modestly affected by loss of Ψ modifications from rRNA. Small growth effects due to loss of highly conserved posttranscriptional modifications have also been noted for certain tRNA modifications, and such mutants with seemingly minimal growth defects are nonetheless readily outcompeted in coculture by a wild-type strain (34). Thus, the 15% slower growth of the Ψ-free strain may well be sufficient to explain the conservation of Ψ modifications in bacterial rRNAs. However, the effects of Ψ loss from bacteria are clearly distinct and far less deleterious than the severe phenotypes resulting from their loss in eukarya.
FIG 4.
Effects of Ψ modifications on cell growth in rich and minimal media. Doubling times of wild-type (WT) MC415 and ΔΨ7 (MC452) strains were determined in LB or minimal E medium (MM) (47) supplemented with glucose and thiamine (48) at the indicated temperatures. Each bar represents the mean from at least three independent growth determinations ± standard deviation (SD).
The response of Ψ-free ribosomes to a variety of antibiotics was assessed by determining the MICs of wild-type and ΔΨ7 strains (see Table S1 in the supplemental material). The ΔΨ7 strain shows decreased resistance to linezolid, clindamycin, and tiamulin. The decreased resistances to the latter 3 antibiotics were not unexpected, since previous work had shown that the RluC-dependent Ψ modification at position 2504 in E. coli 23S rRNA rendered the ribosomes hypersensitive to the same antibiotics (29). Moreover, the MIC for linezolid was restored to wild-type levels in ΔΨ6 C+, a strain carrying deletions in all rRNA pseudouridine synthase genes except rluC (not shown). Thus, at least for the range of compounds tested, only rluC-dependent Ψ modifications appear to affect ribosome-antibiotic interactions.
To explore the effects of loss of rRNA Ψ modifications on bacterial physiology, wild-type and ΔΨ7 strains were subjected to phenotypic profiling (carried out by Biolog, Hayward, CA). By monitoring growth on various carbon, nitrogen, and phosphate sources, as well as in the presence of a multitude of inhibitors, this approach screens simultaneously for approximately 2,000 phenotypes. Only a limited number of differences were reproducibly detected. In addition to the previously noted sensitivity to peptidyltransferase-targeting antibiotics, the ΔΨ7 strain displayed increased osmotic sensitivity for sodium chloride, potassium chloride, and urea, as well as hypersensitivity to two ribonucleotide diphosphate reductase inhibitors, two aminoacyl-tRNA synthetase-inhibiting hydroxamates, and several antibiotics that inhibit cell wall biosynthesis. The ΔΨ7 strains also showed an increased ability to use adenosine 3′-5′-cyclic monophosphate as a phosphorus source. These phenotypes do not have immediately obvious links to ribosome function. Two of the genes deleted in the ΔΨ7 strain, rluA and rluF, encode dual-specificity enzymes responsible for Ψ modifications in certain tRNAs as well as in rRNA (35, 36). Thus, some of the phenotypes of the ΔΨ7 strain, including, perhaps, hypersensitivity to hydroxamates, may be due to altered tRNA functionality rather than to ribosome hypomodification. The altered osmotic sensitivity was reproducible on solid LB medium containing 1 M NaCl (see Fig. S1 in the supplemental material) and is of potential relevance, since Hase et al. (37) have shown that inactivation of ribosome maturation factors or loss of ribosomal protein bS6 conferred increased salt tolerance on the mutant E. coli strains. However, using precisely the same liquid growth assays described by Hase et al. (37), our wild-type and ΔΨ7 strains did not show any difference in growth cessation and subsequent resumption after the addition of 0.9 M NaCl to growing cultures (data not shown). The connection between the few altered phenotypes and Ψ loss thus remains unclear.
Decoding by Ψ-free ribosomes.
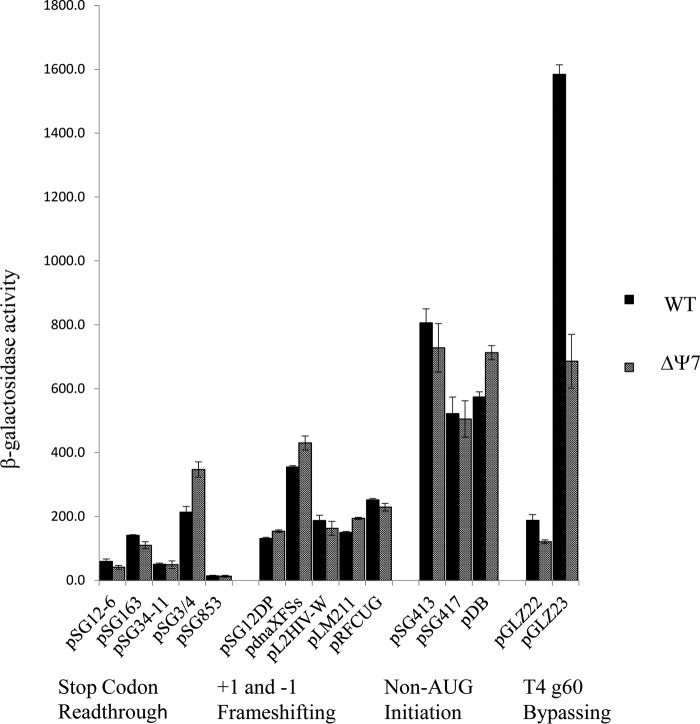
Previous analyses of Ψ function in eukaryotic cells showed that the mutant ribosomes were defective in (IRES-mediated) initiation and frame maintenance. These steps of translation were assessed here using a range of lacZ constructs that report on non-AUG initiation, stop codon readthrough, +1 and −1 frameshifting, and other recoding events. Loss of Ψ led to modestly increased levels (1.6-fold) of UGA readthrough in one codon context (pSG3/4 UGA) but not in others (pSG34-11) (Fig. 5). In most cases, β-galactosidase levels measured in the ΔΨ7 strain were close (1.1- to 1.4-fold differences) to those measured in the wild-type strain, suggesting that the fidelity of initiation, elongation, and termination is not greatly affected by Ψ in rRNA. A notable exception is the decreased bypassing of the coding gap in gene 60 of phage T4 (38) observed in the ΔΨ7 strain. Phage T4's gene 60 encodes a topoisomerase, and the gene 60 mRNA contains a 50-nucleotide (nt) coding gap that is bypassed at high frequency during translation in the E. coli host (38). Bypassing was assessed using two reporter plasmids, pGLZ22 and pGLZ23 (39). Plasmid pGLZ22 carries a T4 gene 60-lacZ fusion, and bypassing is required for β-galactosidase production. A derivative, pGLZ23, carries a precise deletion of the 50-nt coding gap, so no bypassing is required for pGLZ23-directed β-galactosidase synthesis. The levels of β-galactosidase supported by pGLZ22 and pGLZ23 measured here were both decreased in the ΔΨ7 strain (1.5- and 2.4-fold, respectively). Decreased expression from both T4 gene 60 templates appears to be due to loss of RluC-dependent Ψ modifications, since a similar decrease in pGLZ23-directed β-galactosidase activity was observed in a strain with deletion only for rluC (data not shown). Moreover, high-level expression of β-galactosidase from pGLZ23 was restored in a strain (ΔΨ6 C+ [data not shown]) with deletion for 6 Ψ synthases but carrying an intact rluC gene. The 2.4-fold decrease in β-galactosidase levels seen with pGLZ23 in the ΔΨ7 and ΔrluC strains is noteworthy: while bypassing is not required for pGLZ23-directed β-galactosidase synthesis, this plasmid does carry the cis-acting mRNA and nascent peptide signals that normally promote bypassing, which suggests that one or more of the RluC-dependent Ψ residues in rRNA are sensitive to the presence of these bypassing signals The interactions of the nascent peptide with the ribosome at the onset of bypassing have recently been uncovered, and while none involves RluC-dependent Ψ residues directly, several Ψ-adjacent 23S rRNA residues (U2586 and U2506) contact the Tyr33-Thr40 regions of the nascent peptide. Our current hypothesis is that the loss of Ψ modifications in the peptidyltransferase and peptide exit tunnel regions of the ribosome affects T4 gene 60 nascent peptide-ribosome interactions at one or more stages during bypassing.
FIG 5.
Effects of Ψ loss on stop codon readthrough, +1 and −1 frameshifting, initiation on non-AUG codons and leaderless mRNA, and T4 g60 bypassing in lacZ reporter constructs. Assay results are presented as Miller units of β-galactosidase activity. Each bar represents the mean of measurements from at least three independent cultures ± SD. An engineered, wild-type lacZ plasmid, pSG25, supported 22,246 ± 884 and 27,318 ± 2,353 Miller units of activity in the wild-type and ΔΨ7 strains, respectively.
DISCUSSION
The major finding of this work is that loss of Ψ modifications from a bacterial ribosome has surprisingly little effect on cell growth or ribosome biogenesis and function. This is unexpected, given the high degree of conservation of Ψ modifications in functionally critical regions of the ribosome. The result is also in contrast to what is observed in eukaryotic systems, where loss of the modifications greatly impairs ribosome assembly and function. However, the E. coli results presented here are strikingly similar to what has been found in the model archaeon, H. volcanii (21). These results raise the question as to why Ψ is dispensable in the rRNAs of model bacteria and archaea but required in eukaryotes (18). One major difference between bacterial/archaeal rRNAs and their eukaryotic counterparts is the far greater number of Ψ modifications in the latter (9, 11, 40). Potentially, during their evolutionary diversification, eukaryotic ribosomes have become much more dependent on the stabilizing properties of Ψ and their ribosomes cannot tolerate the loss of 40 to 90 such modifications resulting from loss of the Cbf5 PUS.
Posttranscriptional modifications in both tRNAs and rRNAs have been proposed to “fine tune” the functions of the respective RNAs (41). Our previous findings that RluD-dependent modifications of H69 in 23S rRNA were critical only when termination was compromised suggest the additional possibility that Ψ modifications participate in functionally redundant RNA-RNA and RNA-protein interactions, both within the ribosome and with ribosomal ligands. Both models are compatible with Ψ being normally dispensable but critical for function under particular physiological conditions or when translation is compromised by mutation or other insults.
There are two important caveats to our studies with E. coli: the first is that while we have examined the effects of Ψ loss under various growth conditions (Fig. 4 and phenotypic profiling data), there may well exist other conditions that E. coli encounters routinely in its natural habitats where Ψ modifications play important roles in protein synthesis. A second caveat, which also applies to the studies of Blaby et al. (21) on H. volcanii, is that the dispensability of Ψ modifications in these model organisms may not necessarily be extendable to all the bacteria and archaea, respectively. E. coli's mesophilic lifestyle may render Ψ dispensable for growth, while thermophiles, psychrophiles, or other extremophilic bacteria may have a greater requirement for these modifications. Experiments to test this hypothesis are in progress.
MATERIALS AND METHODS
Bacterial strains and plasmids.
MC415 [F− Δ(araD-araB)567 ΔlacZ4787::rrnB-3 λ− rph-1 Δ(rhaD-rhaB)568 hsdR514 prfB(E. coli B)] is a modified E. coli K-12 strain carrying the fully active (Ala246) RF2 allele (23). The rluD gene was readily deleted in this strain without substantial effects on growth (23) and kanamycin-marked deletions in each of the other rRNA PUS genes (rsuA and rluA, -B, -C, -E, and -F deletions, all obtained from the Keio collection [42]) were introduced sequentially by P1-mediated transduction. At each step, the kanamycin resistance cassette was removed by transient expression of the FLP recombinase prior to introduction of the next deletion (43). Initially, three series of strains carrying different combinations of deletions (ΔrluDBE, ΔrluDAC, and ΔrluDFE) were constructed in parallel to determine if particular combinations of PUS gene deletions might be deleterious. Since all the triple deletion mutants grew at identical rates on agar plates, only the ΔrluDBE strain was pursued further. For unknown reasons, the ΔrluC753 deletion mutant obtained from the Keio collection generated an unexpected PCR fragment when transduced into new strain backgrounds. An equivalent rluC deletion (ΔrluC100) mutant was reconstructed using standard recombineering techniques and was used successfully in subsequent manipulations. The eventual kanamycin-sensitive strain MC452, carrying deletions in all 7 rRNA PUS genes, was designated “ΔΨ7′.” The genotype of MC452 is F− Δ(araD-araB)567 ΔlacZ4787::rrnB-3 λ− rph-1 Δ(rhaD-rhaB)568 hsdR514 prfB(E. coli B) ΔrsuA740::frt ΔrluA768::frt ΔrluB777::frt ΔrluC100::frt ΔrluD::frt ΔrluE723::frt ΔrluF789::frt. Pairs of primers ∼100 bp upstream and downstream of each PUS gene were used to amplify the rsuA and rluA-F regions from the wild-type and ΔΨ7 strains. The ΔΨ7 strain generated the expected ∼300-bp fragments for each deleted gene, while the wild-type strain generated the larger fragments expected for intact PUS genes (not shown).
Stop codon readthrough, frameshifting, and initiations from non-AUG codons were assessed using a series of previously described lacZ reporter plasmids (44, 45). Plasmids pSG12-6 and pSG163 carry in-frame UAG stop codons, pSG3/4 and pSG34-11 carry UGA codons, and pSG853 carries a UAA codon. Plasmids pSG12-DP, pdnaXFSs, and pL2HIV-W report on −1 frameshifting events, while pLM211 and pRFCUG report on +1 frameshifting. In plasmids pSG413 and pSG417, translation initiates on CUG and AUU codons, respectively. pDB is a leaderless lacZ construct (46), with the 5′ coding region derived from phage λ's cI gene. Plasmids pGLZ22 and pGLZ23 are ampicillin-resistant T4 gene 60-lacZ fusion plasmids (39). Bypassing of an untranslated 50-nt region is required for synthesis of β-galactosidase encoded by pGLZ22. Plasmid pGLZ23 is an in-frame control plasmid carrying a precise deletion of the bypassed region but still containing the upstream and downstream elements that promote bypassing. An engineered wild-type lacZ plasmid, pSG25, was used as the lacZ control.
Growth rate and antibiotic resistance determinations and β-galactosidase measurements.
Growth rates were determined at various temperatures in LB or minimal E medium (47) supplemented with glucose and thiamine. Overnight cultures were diluted into fresh medium, and the increases in turbidity were monitored using a Klett-Summerson colorimeter. MICs of antibiotics were determined using 2-fold dilutions of antibiotics in microtiter dishes. Cultures for β-galactosidase assays were grown to mid-logarithmic phase in minimal E medium supplemented with glucose, thiamine, Casamino Acids, isopropyl-β-d-1-thiogalactopyranoside (IPTG; 1 mM) and appropriate antibiotics (ampicillin at 100 ml/liter or tetracycline at 12.5 mg/liter). For each strain-plasmid combination, assays of at least 3 independent cultures were carried out as described previously (44, 48) and activities were calculated in Miller units (48).
Analyses of ribosomes and nucleosides.
For ribosome preparation, cells were collected by low-speed centrifugation, resuspended in LLP buffer (10 mM Tris-HCl [pH 8.0], 60 mM KCl, 60 mM NH4Cl, 12 mM magnesium acetate [MgOAc], 6 mM 2-mercaptoethanol), and lysed with a Precellys 24 homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France), according to the manufacturers' protocol. Up to 100 optical density (OD) units of the lysate were layered onto a 15% to 40% (wt/vol) sucrose gradient (in LLP buffer) and centrifuged in a Beckman SW28 rotor at 22,000 rpm for 16 h (ω2t = 3.0 × 1011) at 4°C. Sucrose gradient fractions were collected by displacement with heavy sucrose and ribosomal profiles were continuously monitored at 260 nm with a UV absorption monitor. Fractions containing 70S ribosomes were collected and pelleted by ultracentrifugation in 45Ti rotor at 41,000 rpm for 15 h (ω2t = 1.0 × 1012). The 70S pellet was dissolved in dissociation buffer (10 mM Tris-HCl [pH 8.0], 60 mM KCl, 60 mM, NH4Cl, 1 mM MgOAc, 6 mM 2-mercaptoethanol), layered onto a 15 to 30% sucrose gradient in dissociation buffer, and centrifuged in a SW28 rotor at 22,000 rpm for 18 h (ω2t = 3.5 × 1011) at 4°C. 30S and 50S subunits were collected, and rRNA was extracted by phenol-chloroform treatment. ImageJ software was used to calculate the peak areas in the sucrose density gradient profiles and then estimate the difference in molar ratios of ribosomal subunits and 70S ribosomes.
For analysis of the nucleoside composition in rRNAs extracted from 30S or 50S subunits, 2 A260 units of (23S plus 5S rRNA) or 1 A260 unit of 16S rRNA was digested with nuclease P1 (Sigma, St. Louis, MO) and treated with bacterial alkaline phosphatase (Thermo Scientific) according to the method of Gehrke and Kuo (49). Nucleoside composition was determined by reverse phase (RP)-HPLC on a Supelcosil LC-18-S HPLC column (25 cm by 4.6 mm, 5 μm), equipped with a precolumn (4.6 mm by 20 mm) at 30°C on a Shimadzu Prominence HPLC system. The following buffers were used: buffer A (10 mM NH4H2PO4 plus 2.5% methanol at pH 5.3), buffer B (10 mM NH4H2PO4 plus 20% methanol at pH 5.1), and buffer C (10 mM NH4H2PO4 plus 35% acetonitrile at pH 4.9). RP-HPLC analysis was performed using the following previously described gradient conditions (49): flow rate of 1.0 ml/min held at 0% buffer B for 12 min, to 10% buffer B over 8 min, to 25% buffer B over 5 min, to 60% buffer B over 8 min, to 64% buffer B over 4 min, to 100% buffer B over 9 min, from 0 to 100% buffer C over 35 min, held at 100% buffer C for 10 min, and equilibrated with 100% buffer A for 30 min.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from the School of Biological Sciences, University of Missouri—Kansas City and by Institutional Research Funding Projects of the Estonian Ministry of Education and Research (IUT20-21).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00540-17.
REFERENCES
- 1.Karijolich J, Yi C, Yu YT. 2015. Transcriptome-wide dynamics of RNA pseudouridylation. Nat Rev Mol Cell Biol 16:581–585. doi: 10.1038/nrm4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, Leon-Ricardo BX, Engreitz JM, Guttman M, Satija R, Lander ES, Fink G, Regev A. 2014. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovejoy AF, Riordan DP, Brown PO. 2014. Transcriptome-wide mapping of pseudouridines: pseudouridine synthases modify specific mRNAs in S. cerevisiae. PLoS One 9:e110799. doi: 10.1371/journal.pone.0110799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV. 2014. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515:143–146. doi: 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charette M, Gray MW. 2000. Pseudouridine in RNA: what, where, how, and why. IUBMB Life 49:341–351. doi: 10.1080/152165400410182. [DOI] [PubMed] [Google Scholar]
- 6.Ansmant I, Massenet S, Grosjean H, Motorin Y, Branlant C. 2000. Identification of the Saccharomyces cerevisiae RNA:pseudouridine synthase responsible for formation of psi(2819) in 21S mitochondrial ribosomal RNA. Nucleic Acids Res 28:1941–1946. doi: 10.1093/nar/28.9.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakin A, Lane BG, Ofengand J. 1994. Clustering of pseudouridine residues around the peptidyltransferase center of yeast cytoplasmic and mitochondrial ribosomes. Biochemistry 33:13475–13483. doi: 10.1021/bi00249a036. [DOI] [PubMed] [Google Scholar]
- 8.Noon KR, Bruenger E, McCloskey JA. 1998. Posttranscriptional modifications in 16S and 23S rRNAs of the archaeal hyperthermophile Sulfolobus solfataricus. J Bacteriol 180:2883–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Campo M, Recinos C, Yanez G, Pomerantz SC, Guymon R, Crain PF, McCloskey JA, Ofengand J. 2005. Number, position, and significance of the pseudouridines in the large subunit ribosomal RNA of Haloarcula marismortui and Deinococcus radiodurans. RNA 11:210–219. doi: 10.1261/rna.7209905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massenet S, Ansmant I, Motorin Y, Branlant C. 1999. The first determination of pseudouridine residues in 23S ribosomal RNA from hyperthermophilic Archaea Sulfolobus acidocaldarius. FEBS Lett 462:94–100. doi: 10.1016/S0014-5793(99)01524-0. [DOI] [PubMed] [Google Scholar]
- 11.Ofengand J, Malhotra A, Remme J, Gutgsell NS, Del Campo M, Jean-Charles S, Peil L, Kaya Y. 2001. Pseudouridines and pseudouridine synthases of the ribosome. Cold Spring Harbor Symp Quant Biol 66:147–159. doi: 10.1101/sqb.2001.66.147. [DOI] [PubMed] [Google Scholar]
- 12.Ofengand J. 2002. Ribosomal RNA pseudouridines and pseudouridine synthases. FEBS Lett 514:17–25. doi: 10.1016/S0014-5793(02)02305-0. [DOI] [PubMed] [Google Scholar]
- 13.Polikanov YS, Melnikov SV, Soll D, Steitz TA. 2015. Structural insights into the role of rRNA modifications in protein synthesis and ribosome assembly. Nat Struct Mol Biol 22:342–344. doi: 10.1038/nsmb.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noeske J, Wasserman MR, Terry DS, Altman RB, Blanchard SC, Cate JH. 2015. High-resolution structure of the Escherichia coli ribosome. Nat Struct Mol Biol 22:336–341. doi: 10.1038/nsmb.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer N, Neumann P, Konevega AL, Bock LV, Ficner R, Rodnina MV, Stark H. 2015. Structure of the E. coli ribosome-EF-Tu complex at <3 A resolution by Cs-corrected cryo-EM. Nature 520:567–570. doi: 10.1038/nature14275. [DOI] [PubMed] [Google Scholar]
- 16.Grosjean H, Breton M, Sirand-Pugnet P, Tardy F, Thiaucourt F, Citti C, Barre A, Yoshizawa S, Fourmy D, de Crecy-Lagard V, Blanchard A. 2014. Predicting the minimal translation apparatus: lessons from the reductive evolution of mollicutes. PLoS Genet 10:e1004363. doi: 10.1371/journal.pgen.1004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keffer-Wilkes LC, Veerareddygari GR, Kothe U. 2016. RNA modification enzyme TruB is a tRNA chaperone. Proc Natl Acad Sci U S A 113:14306–14311. doi: 10.1073/pnas.1607512113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack K, Bellodi C, Landry DM, Niederer RO, Meskauskas A, Musalgaonkar S, Kopmar N, Krasnykh O, Dean AM, Thompson SR, Ruggero D, Dinman JD. 2011. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol Cell 44:660–666. doi: 10.1016/j.molcel.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badis G, Fromont-Racine M, Jacquier A. 2003. A snoRNA that guides the two most conserved pseudouridine modifications within rRNA confers a growth advantage in yeast. RNA 9:771–779. doi: 10.1261/rna.5240503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang XH, Liu Q, Fournier MJ. 2007. rRNA modifications in an intersubunit bridge of the ribosome strongly affect both ribosome biogenesis and activity. Mol Cell 28:965–977. doi: 10.1016/j.molcel.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Blaby IK, Majumder M, Chatterjee K, Jana S, Grosjean H, de Crecy-Lagard V, Gupta R. 2011. Pseudouridine formation in archaeal RNAs: the case of Haloferax volcanii. RNA 17:1367–1380. doi: 10.1261/rna.2712811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonicka H, Choquet K, Lin ZY, Gingras AC, Kleinman CL, Shoubridge EA. 2017. A pseudouridine synthase module is essential for mitochondrial protein synthesis and cell viability. EMBO Rep 18:28–38. doi: 10.15252/embr.201643391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Connor M. 2015. Interactions of release factor RF3 with the translation machinery. Mol Genet Genomics 290:1335–1344. doi: 10.1007/s00438-015-0994-x. [DOI] [PubMed] [Google Scholar]
- 24.Schaub RE, Hayes CS. 2011. Deletion of the RluD pseudouridine synthase promotes SsrA peptide tagging of ribosomal protein S7. Mol Microbiol 79:331–341. doi: 10.1111/j.1365-2958.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baggett NE, Zhang Y, Gross CA. 2017. Global analysis of translation termination in E. coli. PLoS Genet 13:e1006676. doi: 10.1371/journal.pgen.1006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kipper K, Sild S, Hetenyi C, Remme J, Liiv A. 2011. Pseudouridylation of 23S rRNA helix 69 promotes peptide release by release factor RF2 but not by release factor RF1. Biochimie 93:834–844. doi: 10.1016/j.biochi.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Korostelev A, Zhu J, Asahara H, Noller HF. 2010. Recognition of the amber UAG stop codon by release factor RF1. EMBO J 29:2577–2585. doi: 10.1038/emboj.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weixlbaumer A, Jin H, Neubauer C, Voorhees RM, Petry S, Kelley AC, Ramakrishnan V. 2008. Insights into translational termination from the structure of RF2 bound to the ribosome. Science 322:953–956. doi: 10.1126/science.1164840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toh SM, Mankin AS. 2008. An indigenous posttranscriptional modification in the ribosomal peptidyl transferase center confers resistance to an array of protein synthesis inhibitors. J Mol Biol 380:593–597. doi: 10.1016/j.jmb.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan K, Flower AM. 2008. Suppression of DeltabipA phenotypes in Escherichia coli by abolishment of pseudouridylation at specific sites on the 23S rRNA. J Bacteriol 190:7675–7683. doi: 10.1128/JB.00835-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amitai S, Kolodkin-Gal I, Hananya-Meltabashi M, Sacher A, Engelberg-Kulka H. 2009. Escherichia coli MazF leads to the simultaneous selective synthesis of both “death proteins” and “survival proteins.” PLoS Genet 5:e1000390. doi: 10.1371/journal.pgen.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leppik M, Liiv A, Remme J. 2017. Random pseuoduridylation in vivo reveals critical region of Escherichia coli 23S rRNA for ribosome assembly. Nucleic Acids Res 45:6098–6108. doi: 10.1093/nar/gkx160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siibak T, Remme J. 2010. Subribosomal particle analysis reveals the stages of bacterial ribosome assembly at which rRNA nucleotides are modified. RNA 16:2023–2032. doi: 10.1261/rna.2160010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjork GR, Neidhardt FC. 1975. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J Bacteriol 124:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raychaudhuri S, Niu L, Conrad J, Lane BG, Ofengand J. 1999. Functional effect of deletion and mutation of the Escherichia coli ribosomal RNA and tRNA pseudouridine synthase RluA. J Biol Chem 274:18880–18886. doi: 10.1074/jbc.274.27.18880. [DOI] [PubMed] [Google Scholar]
- 36.Addepalli B, Limbach PA. 2016. Pseudouridine in the anticodon of Escherichia coli tRNATyr(QPsiA) is catalyzed by the dual specificity enzyme RluF. J Biol Chem 291:22327–22337. doi: 10.1074/jbc.M116.747865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hase Y, Yokoyama S, Muto A, Himeno H. 2009. Removal of a ribosome small subunit-dependent GTPase confers salt resistance on Escherichia coli cells. RNA 15:1766–1774. doi: 10.1261/rna.1687309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herr AJ, Atkins JF, Gesteland RF. 2000. Coupling of open reading frames by translational bypassing. Annu Rev Biochem 69:343–372. doi: 10.1146/annurev.biochem.69.1.343. [DOI] [PubMed] [Google Scholar]
- 39.Maldonado R, Herr AJ. 1998. Efficiency of T4 gene 60 translational bypassing. J Bacteriol 180:1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ofengand J, Bakin A. 1997. Mapping to nucleotide resolution of pseudouridine residues in large subunit ribosomal RNAs from representative eukaryotes, prokaryotes, archaebacteria, mitochondria and chloroplasts. J Mol Biol 266:246–268. doi: 10.1006/jmbi.1996.0737. [DOI] [PubMed] [Google Scholar]
- 41.Kimura S, Suzuki T. 2010. Fine-tuning of the ribosomal decoding center by conserved methyl-modifications in the Escherichia coli 16S rRNA. Nucleic Acids Res 38:1341–1352. doi: 10.1093/nar/gkp1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006–2008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Connor M, Thomas CL, Zimmermann RA, Dahlberg AE. 1997. Decoding fidelity at the ribosomal A and P sites: influence of mutations in three different regions of the decoding domain in 16S rRNA. Nucleic Acids Res 25:1185–1193. doi: 10.1093/nar/25.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connor M, Gregory ST, Dahlberg AE. 2004. Multiple defects in translation associated with altered ribosomal protein L4. Nucleic Acids Res 32:5750–5756. doi: 10.1093/nar/gkh913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shean CS, Gottesman ME. 1992. Translation of the prophage lambda cl transcript. Cell 70:513–522. doi: 10.1016/0092-8674(92)90175-C. [DOI] [PubMed] [Google Scholar]
- 47.Vogel HJ, Bonner DM. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97–106. [PubMed] [Google Scholar]
- 48.Miller JH. 1993. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, New York, NY. [Google Scholar]
- 49.Gehrke CW, Kuo KC. 1989. Ribonucleoside analysis by reversed-phase high-performance liquid chromatography. J Chromatogr 471:3–36. doi: 10.1016/S0021-9673(00)94152-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.