ABSTRACT
Little is known about the sensitivity of the BinaxNOW pneumococcal urinary antigen (PUA) test for adult pneumococcal pneumonia caused by different serotypes. In this study, we aimed to analyze the trends in the sensitivity of the PUA test over a 15-year period (2001 to 2015) and to analyze its sensitivity for pneumococcal pneumonia caused by different serotypes. In total, we analyzed 1,096 pneumococcal isolates from adults with pneumococcal pneumonia who had a PUA test performed at the onset of the episode. Three periods were analyzed: 2001 to 2005 (early use of the seven-valent pneumococcal conjugate vaccine [early PCV7]), 2006 to 2010 (late PCV7), and 2011 to 2015 (early PCV13). The sensitivity of the PUA test varied from 76.4% (95% confidence interval [CI], 70.5% to 82.4%) in the period from 2001 to 2005 to 77.9% in 2006 to 2010 (95% CI, 74.4% to 81.4%) and decreased to 60.5% (95% CI, 55.4% to 65.6%) in 2011 to 2015. This decrease was observed in 560 proven (83.2% in 2001 to 2005, 86.5% in 2006 to 2010, and 78.1%) and 536 probable (70.0% in 2001 to 2005, 68.7% in 2006 to 2010, and 41.5% in 2011 to 2015) episodes of pneumococcal pneumonia. Differences were observed in the sensitivity of the PUA test for diagnosing pneumonia caused by certain serotypes, being highest for the 9V (90.6%), 14 (86.8%), 18C (100%), and 20 (100%) serotypes and lowest for the 8 (55.2%), 9L/N (39.1%), 11A (48.8%), 23B (33.3%), and nontypeable (47.8%) serotypes. Comparing 2001 to 2005, 2006 to 2010, and 2011 to 2015, the prevalence of serotypes 9V (3.1%, 3.7%, and 1.7%, respectively) and 14 (7.2%, 5.1%, and 3.1%, respectively) decreased, while the prevalence of serotypes 23B (0%, 0.7%, and 1.4%, respectively), 9L/N (1.0%, 1.6%, and 3.4%, respectively), 11A (2.6%, 4.2%, and 3.7%, respectively), and 8 (1.5%, 1.5%, and 5.1%, respectively) increased. The PUA test sensitivity varied by pneumococcal pneumonia serotype, and these differences and the changes in serotype distribution were associated with an overall decrease in the sensitivity of the PUA test.
KEYWORDS: Streptococcus pneumoniae, pneumococcal conjugate vaccine, pneumococcal urinary antigen test, community-acquired pneumonia, invasive pneumococcal disease
INTRODUCTION
Community-acquired pneumonia (CAP) is an important cause of morbidity and mortality worldwide. The early diagnosis of the etiology of CAP helps to establish accurate therapy and adequate antibiotic de-escalation. Streptococcus pneumoniae (pneumococcus) is the most important cause of potentially life-threatening CAP; therefore, its detection early in the course of infection is important for prognosis and medical cost (1, 2).
The pneumococcal urinary antigen (PUA) test is useful for diagnosing pneumococcal infections in adults (3). It works by detecting the pneumococcal C-polysaccharide antigen, which is the teichoic acid of the bacterial cell wall, in a patient's urine by immunochromatographic membrane assay. Although the BinaxNOW PUA test is a rapid, simple, and useful test in adults, it is not useful in children because of higher rates of pneumococcal colonization (3). A recent meta-analysis reported an overall PUA test sensitivity of 74% (95% confidence Interval [CI], 66.6% to 82.3%) and a specificity of 97% (95% CI, 92.7% to 99.8%) when diagnosing adult pneumococcal pneumonia (4). However, this analysis reported a broad range of PUA test sensitivities that could be due not only to different target populations but also to different serotype distributions. To date, there is only limited information regarding the sensitivity of the PUA test for the diagnosis of pneumococcal pneumonia caused by different serotypes.
The introduction of the seven-valent pneumococcal conjugate vaccine (PCV7) in 2000, which was later replaced by PCV10 and PCV13, changed the global epidemiology of pneumococcal disease. This was followed by a decrease in invasive pneumococcal disease caused by those PCV serotypes in children and adults, including bacteremic and nonbacteremic pneumonia (i.e., herd protection) (5, 6). However, the burden of CAP due to pneumococcal infection continues to be important for older adults, the immunosuppressed, and young children because of serotype replacement. Indeed, nonvaccine serotypes, such as 6C, 8, 15A, 23A, and 35B, have recently been reported to be emerging in some countries secondary to changes in carriage by children (7, 8). These recent changes in serotype distribution led us to question the effect on the sensitivity of the BinaxNOW PUA test.
In the present study, we had two main aims. First, we aimed to analyze the trends in the sensitivity of the PUA test among adults with proven or probable pneumococcal pneumonia over a 15-year period when PCVs were introduced for childhood immunization. Second, we aimed to analyze the sensitivity of the PUA test for the diagnosis of pneumococcal pneumonia caused by different serotypes.
MATERIALS AND METHODS
Hospital setting and study design.
This study was performed in a single hospital (Hospital Universitari de Bellvitge) located in a southern area of Barcelona, Spain. This is a teaching hospital without obstetric, pediatric, or burns units, serving a population of 600,000 adults. We performed a prospective, observational, laboratory-based study from January 2001 to December 2015. All pneumococcal isolates collected from sputum or invasive samples of adult patients (>18 years old) with pneumococcal pneumonia during this period were analyzed. Data for the PUA test at the onset of the pneumonia episode were recorded when available. Patients with a pneumococcal isolate and who underwent a PUA test were included in further analysis.
The analysis was divided into three periods according to when the vaccines were introduced: 2001 to 2005 (early PCV7), 2006 to 2010 (late PCV7), and 2011 to 2015 (early PCV13). PCV7 was first licensed for childhood vaccination on a voluntary basis in 2001 and reached an uptake rate of 50% by 2005. This vaccine was replaced by PCV13 in 2010, and the estimated proportion of vaccinated children was 60% in our area in 2012 (9).
Definitions.
Pneumococcal pneumonia was defined as the emergence of a new infiltrate on chest radiography plus clinical symptoms of pneumonia (i.e., fever, productive cough, dyspnea, and chest pain) plus isolation of S. pneumoniae from either a lower respiratory tract sample or a sterile specimen (e.g., blood, pleural effusion, or bronchoalveolar lavage fluid). Pneumococcal pneumonia was considered proven if patients met these clinical criteria and if a strain was isolated from a normally sterile site. Pneumococcal pneumonia was considered probable when pneumococcus was isolated from a sputum sample alone, provided that the patient had clinical symptoms.
Bacterial isolates and laboratory tests.
All available pneumococcal isolates collected from respiratory or normally sterile sites in adult patients with pneumococcal pneumonia were included. For the analysis, only patients who had a PUA test done at the onset of infection were included. We used only the BinaxNOW S. pneumoniae assay (Alere Inc.) throughout the study. The PUA test was requested at the discretion of attending physicians and was performed immediately on arrival at the microbiology laboratory, without assessing urine concentration. For sputum samples, only pneumococcal isolates collected from good quality sputum samples (<10 squamous cells and >25 leukocytes per low-power field) were included in the analysis. Pneumococcal isolates were identified by standard methodology (optochin susceptibility and bile solubility). Capsular typing was performed by PCR and/or Quellung reaction, as described previously (10, 11).
Statistical analysis.
Sensitivity was calculated using the number of positive tests as the numerator and the total number of pneumococcal pneumonia episodes with a PUA test performed (positive plus negative) as the denominator. All statistical analyses were performed using SPSS for Windows, version 14.0 (SPSS Inc., Chicago, IL, USA). We used the χ2 and Fisher exact tests to compare proportions, as appropriate. Two-sided P values of <0.05 were considered statistically significant.
To account for multiple comparisons, we applied the false-discovery rate suggested by Benjamini and Hochberg (12), using a maximum rate of 5%. We sorted all P values in ascending order, assigned each a rank, estimated the Benjamini-Hochberg critical value for each P value, and declared as statistically significant all P values below the largest P value less than or equal to the Benjamini-Hochberg critical value.
Data were managed and analyzed using R version 3.4.1 software (13).
Ethics.
Written informed consent was not considered necessary for the study because it was a retrospective analysis of pneumococcal isolates previously obtained for medical purposes and located in our laboratory. The data and the biological samples of the patients were anonymized for analysis and handling. Confidential information pertaining to individual patients was protected according to national guidance. The manuscript was reviewed for publication by the Clinical Research Ethics Committee of Bellvitge University Hospital (reference no. PR142/17).
RESULTS
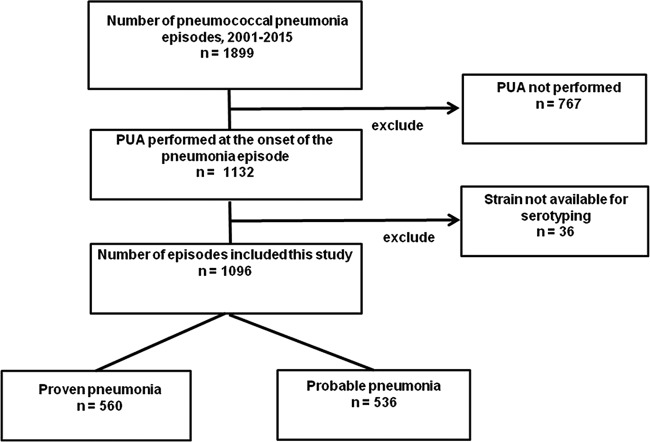
We collected 1,899 pneumococcal isolates from patients with pneumococcal pneumonia during the study period (Fig. 1). Of these, 767 (40.4%) were excluded because the BinaxNOW PUA was not performed and 36 (18.9%) because the strain was not available for serotyping.
FIG 1.
Selection flowchart for pneumococcal pneumonia episodes.
Among the 767 isolates excluded because the BinaxNOW PUA was not performed, the rates were 60.1% (297 of 494 episodes), 33.9% (292/862), and 32.8% (178/543) in the periods 2001 to 2005, 2006 to 2010, and 2011 to 2015, respectively. Finally, 1,096 episodes were included in the analysis, among which 536 were considered probable pneumococcal pneumonia and 560 were considered proven pneumococcal pneumonia. Among those with proven pneumonia, the positive isolates were collected from the following samples: blood and sputum in 95, blood and pleural effusion in 26, blood in 417, pleural effusion in 18, and bronchoalveolar lavage fluid in 4. The overall PUA test sensitivity was 72.0% (95% CI, 69.3% to 75.7%). Among all 1,096 episodes, 729 occurred in men, and the mean age was 63.7 ± 17.0 years (range, 18 to 97 years).
Table 1 shows that the overall sensitivity of the PUA test was 76.4% (95% CI, 70.5% to 82.4%) in 2001 to 2005, 77.9% (95% CI, 74.4% to 81.4%) in 2006 to 2010, and 60.5% (95% CI, 55.4% to 65.6%) in the last period (2011 to 2015). When we analyzed the cases with proven pneumonia, the sensitivity of PUA decreased (sensitivities of 83.2%, 86.5%, and 78.1% for 2001 to 2005, 2006 to 2010, and 2011 to 2015, respectively). In contrast, the sensitivity of the PUA test decreased significantly from 70.0% to 68.7% to 41.5% among patients with probable pneumococcal pneumonia in 2001 to 2005, 2006 to 2010, and 2011 to 2015, respectively. There were no significant differences in the sensitivity of the PUA test by age group (Table 1).
TABLE 1.
Sensitivity of urinary antigen test by period of time, sex, age, and pneumonia type
| Category | Total |
2001–2005 |
2006–2010 |
2011–2015 |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | Sensitivity, % (95% CI) | No. of isolates | Sensitivity, % (95% CI) | No. of isolates | Sensitivity, % (95% CI) | No. of isolates | Sensitivity, % (95% CI) | 2001–2005 vs 2006–2010 | 2006–2010 vs 2011–2015 | 2001–2005 vs 2011–2015 | |
| Overall | 1,096 | 72.0 (69.3–74.7) | 195 | 76.4 (70.5–82.4) | 547 | 77.9 (74.4–81.4) | 354 | 60.5 (55.4–65.6) | 0.673 | <0.001 | <0.001 |
| Age (yr) | |||||||||||
| <65 | 491 | 74.5 (70.7–78.4) | 101 | 80.2 (72.4–88.0) | 252 | 78.2 (73.1–83.3) | 138 | 63.8 (55.8–71.8) | 0.675 | 0.002 | 0.006 |
| ≥65 | 605 | 69.9 (66.3–73.6) | 94 | 72.3 (63.3–81.4) | 295 | 77.6 (72.9–82.4) | 216 | 58.3 (51.8–64.9) | 0.294 | <0.001 | 0.019 |
| Pneumococcal pneumonia | |||||||||||
| Proven | 560 | 83.2 (80.1–86.3) | 95 | 83.2 (75.7–90.7) | 282 | 86.5 (82.5–90.5) | 183 | 78.1 (72.1–84.1) | 0.418 | 0.018 | 0.323 |
| Probable | 536 | 60.3 (56.2–64.4) | 100 | 70.0 (61.0–79.0) | 265 | 68.7 (63.1–74.3) | 171 | 41.5 (34.1–48.9) | 0.808 | <0.001 | <0.001 |
The overall sensitivity of the PUA test for diagnosing pneumococcal pneumonia caused by each serotype is shown in Table 2. The sensitivities were significantly higher for episodes caused by serotypes that are included in the PCVs, including 9V (90.6%; 95% CI, 74.98% to 98.02%), 14 (86.8%; 95% CI, 74.66% to 94.52%), and 18C (100%; 95% CI, 71.5% to 100%).
TABLE 2.
Sensitivity of PUA test by serotype
| Category and serotypea | Total no. of isolates | Sensitivity (%) | P valueb |
|---|---|---|---|
| Overall | 1,096 | 72.0 | |
| PCV7 | 181 | 84.0 | 0.000 |
| 4 | 17 | 82.4 | 0.424 |
| 6B | 16 | 75.0 | 1.000 |
| 9V | 32 | 90.6 | 0.017 |
| 14 | 53 | 86.8 | 0.014 |
| 18C | 11 | 100.0 | 0.041 |
| 19F | 41 | 78.0 | 0.378 |
| 23F | 11 | 72.7 | 1.000 |
| Additional PCV13 | 500 | 74.6 | 0.078 |
| 1 | 77 | 79.2 | 0.143 |
| 3 | 226 | 69.0 | 0.266 |
| 5 | 22 | 68.2 | 0.688 |
| 6A | 7 | 85.7 | 0.680 |
| 7F | 79 | 81.0 | 0.064 |
| 19A | 89 | 79.8 | 0.088 |
| PCV13 | 681 | 77.1 | 0.000 |
| Non-PCV13 | 415 | 63.6 | <0.001 |
| 6C | 21 | 66.7 | 0.583 |
| 8 | 29 | 55.2 | 0.041 |
| 9L/N | 23 | 39.1 | 0.000 |
| 10A | 12 | 66.7 | 0.748 |
| 11A | 41 | 48.8 | 0.001 |
| 12F | 30 | 79.3 | 0.563 |
| 15A | 16 | 87.5 | 0.260 |
| 15B | 7 | 85.7 | 0.680 |
| 15C | 5 | 60.0 | 0.623 |
| 16F | 25 | 64.0 | 0.368 |
| 17F | 8 | 87.5 | 0.455 |
| 20 | 3 | 100.0 | 0.564 |
| 21 | 4 | 50.0 | 0.313 |
| 22F | 30 | 66.7 | 0.510 |
| 23A | 19 | 68.4 | 0.727 |
| 23B | 9 | 33.3 | 0.018 |
| 24F | 18 | 55.6 | 0.117 |
| 31 | 21 | 71.4 | 0.954 |
| 33F | 14 | 85.7 | 0.372 |
| 35F | 7 | 57.1 | 0.380 |
| 35B | 15 | 73.3 | 0.907 |
| NTc | 23 | 47.8 | 0.009 |
| Other | 35 | 68.6 | 0.647 |
PCV7, serotypes included in PCV7; additional PCV13, serotypes not included in PCV7 and included in PCV13; non-PCV13, serotypes not included in PCV13.
Significant results adjusted for multiple comparisons are underlined. Results for episodes caused by individual serotypes were compared with the remaining episodes.
NT, nontypeable.
Similarly, the sensitivities of the PUA tests were high for pneumonia caused by serotypes 7F (81.0%; 95% CI, 70.62% to 88.97%) and 19A (79.8%; 95% CI, 69.93% to 87.55%), both of which are included in PCV13. In contrast, the lowest sensitivities were for serotype 23B (33.3%; 95% CI, 7.49% to 70.07%), serotype 9L/N (39.1%; 95% CI, 19.71% to 61.46%), nontypeable strains (47.8%; 95% CI, 26.82% to 69.41%), serotype 11A (48.8%; 95% CI, 32.88% to 64.87%), and serotype 8 (55.2%; 95% CI, 35.69% to 73.55%). None of the latter serotypes is included in a PCV.
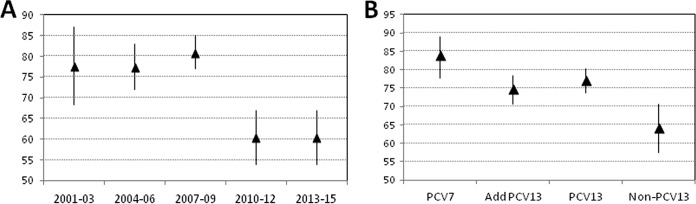
By serotype, the highest PUA test sensitivity was found among episodes due to PCV7 serotypes (84.0%; 95% CI, 78.6% to 89.3%), followed by those caused by additional PCV13 serotypes (74.6%; 95% CI, 70.8% to 78.4%), and the lowest sensitivity was found among episodes caused by non-PCV13 serotypes (63.6%, 95% CI, 59.0% to 68.2%) (Table 2). Figure 2A shows the progressive decrease in the sensitivity of the PUA test by period, and Fig. 2B shows the sensitivity of the PUA test by serotype group (i.e., PCV7, additional PCV13, and non-PCV13 serotypes).
FIG 2.
Sensitivity of the PUA test by period and grouped serotypes. (A) Sensitivity and 95% confidence intervals for the PUA test by period (i.e., 2001 to 2005, 2006 to 2010, and 2011 to 2015). (B) Sensitivity and 95% confidence intervals for the PUA test by grouped serotypes (i.e., PCV7 serotypes, PCV13 additional serotypes, and non-PCV13 serotypes).
Table 3 shows the serotype distribution in the three periods among the 1,096 episodes included in this study. Comparing the periods 2001 to 2005, 2006 to 2010, and 2011 to 2015, there was a decrease in the prevalence of serotypes 9V (3.1%, 3.7%, and 1.7%, respectively) and 14 (7.2%, 5.1%, and 3.1%, respectively); however, the prevalence of serotypes 23B (0%, 0.7%, and 1.4%, respectively), 9L/N (1.0%, 1.6%, and 3.4%, respectively), 11A (2.6%, 4.2%, and 3.7%, respectively), and 8 (1.5%, 1.5%, and 5.1%, respectively) increased. The overall decrease in PUA test sensitivity for diagnosing pneumococcal CAP could be due to changes in prevalence linked to the different PUA test sensitivities by serotype.
TABLE 3.
Prevalence of different serotypes among pneumonia episodes included in the studya
| Category and serotypeb | 2001–2005 |
2006–2010 |
2011–2015 |
|||
|---|---|---|---|---|---|---|
| No. of isolates (n = 195) | % | No. of isolates (n = 547) | % | No. of isolates (n = 354) | % | |
| PCV7 | 48 | 24.6 | 97 | 17.7 | 36 | 10.2d,e |
| 4 | 8 | 4.1 | 7 | 1.3c | 2 | 0.6d |
| 6B | 6 | 3.1 | 7 | 1.3 | 3 | 0.8 |
| 9V | 6 | 3.1 | 20 | 3.7 | 6 | 1.7 |
| 14 | 14 | 7.2 | 28 | 5.1 | 11 | 3.1 |
| 18C | 2 | 1.0 | 4 | 0.7 | 5 | 1.4 |
| 19F | 8 | 4.1 | 25 | 4.6 | 8 | 2.3 |
| 23F | 4 | 2.1 | 6 | 1.1 | 1 | 0.3 |
| Additional PCV13 | 107 | 54.9 | 254 | 46.4 | 139 | 39.3d |
| 1 | 17 | 8.7 | 51 | 9.3 | 9 | 2.5d,e |
| 3 | 54 | 27.7 | 93 | 17.0c | 79 | 22.3 |
| 5 | 15 | 7.7 | 6 | 1.1 | 1 | 0.3d |
| 6A | 2 | 1.0 | 5 | 0.9 | 0 | 0.0 |
| 7F | 13 | 6.7 | 48 | 8.8 | 18 | 5.1 |
| 19A | 6 | 3.1 | 51 | 9.3c | 32 | 9.0d |
| Non-PCV13 | 40 | 20.5 | 196 | 35.8c | 179 | 50.6d,e |
| 6C | 0 | 0.0 | 11 | 2.0 | 10 | 2.8 |
| 8 | 3 | 1.5 | 8 | 1.5 | 18 | 5.1e |
| 9L/N | 2 | 1.0 | 9 | 1.6 | 12 | 3.4 |
| 10A | 1 | 0.5 | 8 | 1.5 | 3 | 0.8 |
| 11A | 5 | 2.6 | 23 | 4.2 | 13 | 3.7 |
| 12F | 3 | 1.5 | 15 | 2.7 | 12 | 3.4 |
| 15A | 1 | 0.5 | 9 | 1.6 | 6 | 1.7 |
| 15B | 0 | 0.0 | 4 | 0.7 | 3 | 0.8 |
| 15C | 1 | 0.5 | 3 | 0.5 | 1 | 0.3 |
| 16F | 0 | 0.0 | 17 | 3.1c | 8 | 2.3 |
| 17F | 0 | 0.0 | 6 | 1.1 | 2 | 0.6 |
| 20 | 2 | 1.0 | 1 | 0.2 | 0 | 0.0 |
| 21 | 0 | 0.0 | 3 | 0.5 | 1 | 0.3 |
| 22F | 3 | 1.5 | 12 | 2.2 | 15 | 4.2 |
| 23A | 3 | 1.5 | 5 | 0.9 | 11 | 3.1e |
| 23B | 0 | 0.0 | 4 | 0.7 | 5 | 1.4 |
| 24F | 0 | 0.0 | 11 | 2.0 | 7 | 2.0 |
| 31 | 2 | 1.0 | 11 | 2.0 | 8 | 2.3 |
| 33F | 3 | 1.5 | 8 | 1.5 | 3 | 0.8e |
| 35F | 2 | 1.0 | 0 | 0.0 | 5 | 1.4e |
| 35B | 2 | 1.0 | 7 | 1.3 | 6 | 1.7 |
| NTf | 3 | 1.5 | 9 | 1.6 | 11 | 3.1 |
| Other | 4 | 2.1 | 12 | 2.2 | 19 | 5.4e |
Only pneumonia episodes for which the PUA test was performed reflected in this table.
PCV7, serotypes included in PCV7; additional PCV13, serotypes not included in PCV7 and included in PCV13; non-PCV13, serotypes not included in PCV13.
Statistically significant change (P < 0.0167) for 2001 to 2005 versus 2006 to 2010.
Statistically significant change (P < 0.0167) for 2001 to 2005 versus 2011 to 2015.
Statistically significant change (P < 0.0167) for 2006 to 2010 versus 2011 to 2015.
NT, nontypeable.
DISCUSSION
In this study, we show that changes in the pneumococcal serotype distribution that have occurred since the introduction of PCV may have affected the sensitivity of the BinaxNOW PUA test, though the overall sensitivity of 72.0% was consistent with previous studies and meta-analysis (4). The sensitivity of the PUA test was higher for proven pneumonia (83.2%) than for probable pneumonia (60.3%), which is explained by the fact that patients with proven pneumonia have higher C-polysaccharide levels in their blood and urine than patients with probable pneumonia (14). Although these results are consistent with previous observations, we observed a decrease in the sensitivity over the study period that should be confirmed with other PUA tests. For instance, the Immuview test has a higher proven sensitivity than BinaxNOW (15). There are at least three possible nonexclusive reasons for the secular change in PUA test sensitivity. The least likely explanation is that there was a change in test ordering practices, such that more patients who were less severely ill received the PUA test in the period from 2011 to 2015. The PUA test is more sensitive in patients with severe disease, so if this explanation was true, then a change in test ordering practices could have affected the apparent test performance. For the three periods, 2001 to 2005, 2006 to 2010, and 2011 to 2015, PUA tests were ordered for 40%, 65%, and 66% of patients with pneumococcal pneumonia, respectively. Given that PUA test sensitivity fell significantly from the period 2006 to 2010 to the period 2011 to 2015, when test ordering practices were similar, a change in test ordering practices is an unlikely explanation for the observed change in test sensitivity.
Second, differences in the C polysaccharide among strains could have resulted in differences in sensitivity. The C polysaccharide (teichoic acid) is a component of the pneumococcal cell wall that is excreted in the urine and detected by the PUA test. Although it is present in all pneumococcal strains, differences in its composition have been reported by serotype (16, 17). Consequently, the sensitivity of the PUA test for diagnosing pneumococcal pneumonia caused by different serotypes varied from 33.3% to 100% in our study. Moreover, this sensitivity was above 70% for episodes caused by all PCV13 serotypes, except for serotypes 3 and 5, reaching statistically significance for serotypes 9V, 14, and 18C; in contrast, the sensitivity was below 70% when pneumococcal pneumonia was caused by non-PCV13 serotypes.
Third, the introduction of PCVs appears to have changed the overall distribution of pneumococcal serotypes, causing a shift in the sensitivity of the PUA test. In fact, the decrease in the frequency of PCV13 serotypes that occurred in the last period of our study paralleled the overall decrease in PUA sensitivity (Tables 1 and 3). In Spain, PCV7 was licensed for the pediatric population in June 2001, followed by PCV10 and PCV13 in November 2009 and June 2010, respectively. However, the vaccine was not state subsidized, and coverage reached only 50% in 2005 to 2006 (18). After that, in the late PCV7 period, a decrease in PCV7 serotypes was observed among adults with pneumococcal disease. Over the study period, the frequency of pneumococcal pneumonia caused by serotypes with high PUA test sensitivity (i.e., 9V and 14) decreased, whereas that caused by serotypes associated with the lowest PUA test sensitivity (i.e., 23B, 9L/N, 11A, NT, and 8) increased. We therefore concluded that the introduction of PCVs was related to a decrease in pneumococcal pneumonia caused by serotypes with high sensitivity to the PUA test, leading to an overall decrease in the sensitivity of this useful instrument.
Another possible explanation for the decline in test sensitivity could be a change in the manufacturing process for BinaxNOW. However, to the best of our knowledge, neither the assay kit manufacturing process nor the reagents used in the kit changed over the study period.
The decrease in PUA test sensitivity could compromise the use of antibiotics with narrower spectrums for the treatment of CAP. The usefulness of the PUA test for selecting amoxicillin in the treatment of nonsevere CAP has been demonstrated in young immunocompetent patients, resulting in efficacy similar to that for antibiotics with broader spectrums of activity (19).
Despite our interesting results, this study has some limitations. First, this was a local study and must be extended to other geographical areas to facilitate generalization. Second, some authors have suggested that prior antibiotic therapy affects the sensitivity of the PUA test, but we did not have access to this information for our patients (20). Third, other urinary antigen tests with higher sensitivities (e.g., the Immuview test) could show different results and should also be applied.
In conclusion, our study shows differences in the sensitivity of the BinaxNOW PUA test when diagnosing pneumococcal pneumonia caused by different serotypes. These differences, together with changes in serotype distribution, were associated with an overall decrease in the sensitivity of the PUA test in Barcelona, Spain. Despite these results, the PUA test continues to be a very useful test when making an etiological diagnosis of CAP (3, 4).
ACKNOWLEDGMENTS
C.A. and J.L. received funding from Pfizer, unrelated to the present study. All other authors declare no conflicts of interest.
This study was supported by grants from Fondo de Investigaciones Sanitarias de la Seguridad Social (PI14/00627 and PI14/00580) and from Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Respiratorias (CIBERES CB06/06/0037), an initiative of the Instituto de Salud Carlos III, Madrid, Spain. Financial support was also provided by the European Regional Development Fund (ERDF).
REFERENCES
- 1.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America, American Thoracic Society. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viasus D, Simonetti AF, Garcia-Vidal C, Niubó J, Dorca J, Carratalà J. 2017. Impact of antibiotic de-escalation on clinical outcomes in community-acquired pneumococcal pneumonia. J Antimicrob Chemother 72:547–553. doi: 10.1093/jac/dkw441. [DOI] [PubMed] [Google Scholar]
- 3.Rosón B, Fernández-Sabé N, Carratalà J, Verdaguer R, Dorca J, Manresa F, Guidiol F. 2004. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis 38:222–226. doi: 10.1086/380639. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair A, Xie X, Teltscher M, Dendukuri N. 2013. Systematic review and meta-analysis of a urine-based pneumococcal antigen test for diagnosis of community-acquired pneumonia caused by Streptococcus pneumoniae. J Clin Microbiol 51:2303–2310. doi: 10.1128/JCM.00137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, Hadler J, Cieslak PR, Whitney CG, Active Bacterial Core Surveillance Team. 2005. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294:2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 6.Alari A, Chaussade H, Domenech De Cellès M, Le Fouler L, Varon E, Opatowski L, Guillemot D, Watier L. 2016. Impact of pneumococcal conjugate vaccines on pneumococcal meningitis cases in France between 2001 and 2014: a time series analysis. BMC Med 14:211. doi: 10.1186/s12916-016-0755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devine VT, Cleary DW, Jefferies JM, Anderson R, Morris DE, Tuck AC, Gladstone RA, O'Doherty G, Kuruparan P, Bentley SD, Faust SN, Clarke SC. 2017. The rise and fall of pneumococcal serotypes carried in the PCV era. Vaccine 35:1293–1298. doi: 10.1016/j.vaccine.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 8.Jayasinghe S, Menzies R, Chiu C, Toms C, Blyth CC, Krause V, McIntyre P. 2017. Long-term impact of a “3 + 0” schedule for 7- and 13-valent pneumococcal conjugate vaccines on invasive pneumococcal disease in Australia, 2002-2014. Clin Infect Dis 64:175–183. doi: 10.1093/cid/ciw720. [DOI] [PubMed] [Google Scholar]
- 9.Càmara J, Marimón JM, Cercenado E, Larrosa N, Quesada MD, Fontanals D, Cubero M, Pérez-Trallero E, Fenoll A, Liñares J, Ardanuy C. 2017. Decrease of invasive pneumococcal disease (IPD) in adults after introduction of pneumococcal 13-valent conjugate vaccine in Spain. PLoS One 12:e0175224. doi: 10.1371/journal.pone.0175224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. PCR deduction of pneumococcal serotypes. http://www.cdc.gov/streplab/pcr.html.
- 11.Fenoll A, Aguilar L, Vicioso MD, Gimenez MJ, Robledo O, Granizo JJ, Mendez C. 2010. Serotype distribution and susceptibility of Streptococcus pneumoniae isolates from pleural fluid in Spain from 1997 to 2008. Antimicrob Agents Chemother 54:5387–5390. doi: 10.1128/AAC.00217-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300. [Google Scholar]
- 13.R Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 14.Song JY, Eun BW, Nahm MH. 2013. Diagnosis of pneumococcal pneumonia: current pitfalls and the way forward. Infect Chemother 45:351–366. doi: 10.3947/ic.2013.45.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jørgensen CS, Uldum SA, Sørensen JF, Skovsted IC, Otte S, Elverdal PL. 2015. Evaluation of a new lateral flow test for detection of Streptococcus pneumoniae and Legionella pneumophila urinary antigen. J Microbiol Methods 116:33–36. doi: 10.1016/j.mimet.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Vialle S, Sepulcri P, Dubayle J, Talaga P. 2005. The teichoic acid (C-polysaccharide) synthesized by Streptococcus pneumoniae serotype 5 has a specific structure. Carbohydr Res 340:91–96. doi: 10.1016/j.carres.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson C, Jansson PE, SkovSørensen UB. 1999. The pneumococcal common antigen C-polysaccharide occurs in different forms. Mono-substituted or di-substituted with phosphocholine. Eur J Biochem 265:1091–1097. [DOI] [PubMed] [Google Scholar]
- 18.Ardanuy C, Tubau F, Pallares R, Calatayud L, Domínguez MA, Rolo D, Grau I, Martín R, Liñares J. 2009. Epidemiology of invasive pneumococcal disease among adult patients in Barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997-2007. Clin Infect Dis 48:57–64. doi: 10.1086/594125. [DOI] [PubMed] [Google Scholar]
- 19.Guchev IA, Yu VL, Sinopalnikov A, Klochkov OI, Kozlov RS, Stratchounski LS. 2005. Management of nonsevere pneumonia in military trainees with the urinary antigen test for Streptococcus pneumoniae: an innovative approach to targeted therapy. Clin Infect Dis 40:1608–1616. doi: 10.1086/429919. [DOI] [PubMed] [Google Scholar]
- 20.Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O'Brien KL, Adult Pneumococcal Burden Study Team. 2013. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One 8:e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]