ABSTRACT
The use of interferon gamma (IFN-γ) release assays (IGRAs) for the diagnosis of tuberculosis (TB) infection in children is still under debate because of concerns about the immature immune response in children. The aim of this study was to investigate quantitative values of the QuantiFERON-TB Gold In-Tube (QFT-IT) test, a commercially available IGRA, in a large cohort of children screened for TB infection. A retrospective analysis was conducted on samples from 517 children aged 0 to 14 years old at the Pediatric Unit of S. Orsola-Malpighi University Hospital of Bologna (Italy); quantitative responses to QFT-IT stimuli were analyzed according to diagnosis and age. Elevated IFN-γ values in the QFT-IT nil (background) tube were statistically associated with diagnosis of active TB. Quantitative IFN-γ response to Mycobacterium tuberculosis-specific antigens (TB Ag) was not significantly different in children with active TB compared to those with latent TB infection (LTBI), even though the median values were higher in the first group. When children were grouped by age, those less than 5 years old produced significantly higher levels of IFN-γ in response to TB Ag if they had active TB (median 10 IU/ml) than those with LTBI (median 1.96 IU/ml). IFN-γ response to mitogen increased with age. The overall rate of indeterminate results was low (3.9%), and no indeterminate QFT-IT values were observed in active or latent TB patients. In conclusion, quantitative QFT-IT values could provide further information to clinicians to manage TB in children, and these observations could be transferred to the new version of the test, QuantiFERON-TB Gold Plus, which to date lacks data from the pediatric population.
KEYWORDS: QuantiFERON-TB In-Tube, age, children, diagnosis, IFN-γ, tuberculosis
INTRODUCTION
Children infected with Mycobacterium tuberculosis are at a higher risk of progression to active tuberculosis (TB) than adults (1). Nevertheless, diagnosis of TB in children is challenging due to its nonspecific clinical presentation and its paucibacillary nature as well as the frequent extrapulmonary localization of the disease. Current pediatric guidelines recommend applying a combination of clinical, radiological, microbiological, and immunological approaches to improve the diagnostic yield (2–4).
Interferon gamma release assays (IGRAs) are immunological tests based on the in vitro detection of interferon gamma (IFN-γ) released by specifically sensitized T cells in response to M. tuberculosis antigens (ESAT-6, CFP-10, TB7.7) (5). IGRAs are used together with tuberculin skin tests (TSTs) for the diagnosis of latent tuberculosis infection (LTBI) (6). Several publications have shown that IGRAs overcome many drawbacks of TSTs; mainly, specificity is not affected by cross-reaction with bacillus Calmette-Guerin vaccination or non-tuberculous mycobacterial infections that could give rise to false positives (7–11). A particular advantage of in vitro testing is that stimulation reactions with negative and positive (mitogen stimulus) controls are carried out in parallel, primarily to evaluate test performance with respect to background signals and general T cell responsiveness (12).
A growing number of studies on the performance of IGRAs in children suggest that they are more accurate than TSTs even in this vulnerable population, thanks to better specificity and the presence of a control of immune reactivity (13, 14). IGRAs are not only tools for diagnosing LTBI but could be also used to integrate algorithms for active TB diagnosis. However, the routine use of IGRAs has not yet been extensively approved in children, due to concerns about their immature immune systems and discrepant results reported in different studies (15–20).
The commercially available IGRAs, the QuantiFERON-TB Gold In-Tube (QFT-IT) test and the new version, the QuantiFERON-TB Gold Plus test (Qiagen, Germany), measure IFN-γ release with an immunoenzymatic assay, and qualitative results (positive, negative, indeterminate) are reported according to specific cutoffs (21, 22).
Our previous publication comparing the performance of the QFT-IT to that of the TST in a large group of children assessed by reason for testing, BCG vaccination status, age, and diagnosis suggested the preferential use of QFT-IT as a supporting tool for diagnosis and management of TB even in infants, despite their immature immune systems (23). Here, we analyzed quantitative IFN-γ levels in background (nil) and in response to M. tuberculosis antigens (TB Ag) and mitogen of the QFT-IT test in the same population, according to diagnosis and age, in order to provide clinicians with additional information for the management of TB in children.
MATERIALS AND METHODS
Study design and population.
Retrospective analysis was conducted on samples from children aged 0 to 14 years old, referred over a 5-year period to the Pediatric Unit of the S. Orsola-Malpighi University Hospital in Bologna, Italy, a country with low TB endemicity that has high immigration rates from countries with middle/high TB prevalence.
The study population has been described in detail in our previous paper (23); briefly, subjects who had QFT-IT results suggestive of active TB, were found by contact tracing, recently arrived from a country where TB is endemic, and were routinely screened before immunosuppressive treatment were included. After giving informed consent, 30 children who had blood drawn for other reasons were also included as negative controls. Demographic and clinical information was collected from the medical files of the children, including origin of the family, place of birth, and BCG vaccination status.
The study protocol was approved by the Ethics Committee of S.Orsola-Malpighi University Hospital.
Diagnostic group definition.
According to our previously published work (23), children were categorized into 5 diagnostic groups according to the clinical outcome reported in their medical files. Briefly, children were defined as having active TB according to the diagnosis made by the physician following WHO criteria (2). This category included microbiologically confirmed cases, if diagnosis was based on culture and/or Xpert MTB/RIF assay (Cepheid, USA) positivity, and clinically confirmed cases, if diagnosis was based on clinical, pathological, and radiological findings consistent with the disease and no improvement was seen after a full course of antibiotics, followed by clinical improvement with anti-TB treatment. All children with a diagnosis of active TB were therefore followed up until recovery. Children were categorized as TB excluded if, after undergoing QFT-IT based on signs and symptoms suggestive of TB, active disease was excluded and another diagnosis was made at the end of investigation and treatment. According to Italian pediatric TB recommendations (24), before admission to school, children in contact with adults with active tuberculosis, children who had recently arrived from an area where TB is endemic, and those undergoing immunosuppressive therapy were referred to the Pediatric Unit and underwent a medical history investigation including BCG vaccination status, clinical assessment, chest X-ray, and immunological and microbiological assays. After exclusion of active TB, children were categorized at the first investigation and after 3 months as having LTBI based on the physician's decision after evaluating QFT-IT and TST results, risk of exposure, BCG status, and origin. All children diagnosed with LTBI were offered prophylaxis with isoniazid and followed up for 12 months. Children with risk factors for TB (contact tracing or recent arrival from a country where TB is endemic) who had a negative result at the first investigation and confirmed negativity after 3 months were defined as exposed.
Children were excluded from the analysis if the outcome was not specified or if they had a history of having been previously treated for TB.
Microbiological diagnosis of active TB.
When active TB was suspected, a multiple sampling approach was adopted according to Al-Aghbari et al. (25). Consecutive biological samples were collected from the respiratory tract (sputum, gastric lavage, aspiration of upper respiratory airways) and/or from extrapulmonary sites according to the suspected localization. Samples were examined by light smear microscopy (Ziehl-Neelsen staining) and the molecular assay Xpert MTB/RIF, which simultaneously detects the presence of the M. tuberculosis genome and resistance to rifampin.
M. tuberculosis was isolated using solid (Lowenstein-Jensen; Heipha Diagnostika Biotest, Germany) and liquid culture (MGIT; Becton Dickinson, USA). Drug susceptibility testing to first-line drugs (streptomycin, isoniazid, rifampin, ethambutol, pyrazinamide) was performed by the automatic MGIT 960 system (Becton Dickinson).
QuantiFERON-TB Gold In-Tube.
The QFT-IT assay (Cellestis, Melbourne, Australia) was performed and interpreted according to the manufacturer's instructions (21). Briefly, venous blood was collected in 3 distinct tubes: one containing M. tuberculosis-specific antigens (ESAT-6, CFP-10, and TB7.7), one with a nonspecific mitogen (phytohemagglutinin) as positive control of immune system reactivity, and an empty tube as negative control for IFN-γ background value (nil). The tubes were incubated at 37°C for 18 h, and after centrifugation, the IFN-γ released was measured by enzyme-linked immunosorbent assay and converted to IU/ml. Tubes that were not analyzed immediately were stored at 4°C. Positive results were defined as nil-corrected M. tuberculosis antigens (TB Ag) values above 0.35 IU IFN-γ/ml. If the nil-corrected mitogen value was <0.50 IU/ml and/or if the nil value was >8.0 IU/ml, the test was considered indeterminate. Test failure due to incorrect blood volume and time of blood draw was very low (<1%); failed samples were repeated.
Statistical analysis.
The quantitative analysis of QFT-IT results was carried out on background (nil)-corrected IFN-γ responses to M. tuberculosis antigens and mitogen. Since the QFT-IT test cannot accurately determine IFN-γ values >10 IU/ml, a value of 10 IU/ml was conventionally attributed to plateau values in all the analyses, as already adopted in the literature (26).
QFT-IT test values according to diagnostic group were compared using the Kruskal-Wallis test. Correlation between age and IFN-γ background levels, and in response to TB Ag and mitogen, was expressed by Pearson coefficient.
Statistical significance was set at P <0.05. Statistical analysis was performed using Stata/SE 14.1 software (College Station, TX, USA). All P values were two-tailed.
RESULTS
Study population and QFT-IT results.
A total of 517 children with an available QFT-IT result, including quantitative IFN-γ values in background (nil) and in response to TB Ag and mitogen, were included in the study. Characteristics of the study population have been described previously (23). Briefly, 288 (55.7%) were males, mean age 5.4 ± 4 years (range 0 to 14 years old); 215 (41.6%) were born in Italy into an Italian family, 197 (38.1%) were born in Italy into a family from a country with middle/high TB prevalence, and 98 (19.0%) were born in a country with middle/high TB prevalence. In 7 (1.3%) cases origin was unknown.
After clinical and laboratory evaluation, patients were categorized into 5 diagnostic groups: active TB, LTBI, TB excluded, uninfected after contact with an active TB case (defined as exposed), and negative controls.
Table 1 reports the qualitative QFT-IT results. Of the 517 children tested with QFT-IT, 79 (15.3%) were positive, 418 (80.8%) were negative, and 20 (3.9%) were indeterminate. According to diagnosis, no indeterminate QFT-IT results were found in patients diagnosed with active TB or LTBI, while indeterminate results were strongly associated with the category TB excluded (18 cases [90%]; P <0.0001).
TABLE 1.
Qualitative QFT-IT results and distribution according to diagnosis
| Diagnosis | No. of patients (%) | No. (%) of QFT-IT results determined to be: |
||
|---|---|---|---|---|
| Positive | Negative | Indeterminate | ||
| Active TB | 45 (8.7) | 42 (93.3) | 3 (6.7) | 0 |
| LTBI | 38 (7.4) | 34 (89.5) | 4 (10.5) | 0 |
| TB excluded | 159 (30.7) | 3 (1.9) | 138 (86.8) | 18 (11.3) |
| Exposed | 245 (47.4) | 0 | 244 (99.6) | 1 (0.4) |
| Negative controls | 30 (5.8) | 0 | 29 (96.7) | 1 (3.3) |
| Total | 517 | 79 (15.3) | 418 (80.8) | 20 (3.9) |
Quantitative analysis of QFT-IT nil, TB Ag, and mitogen values according to diagnosis.
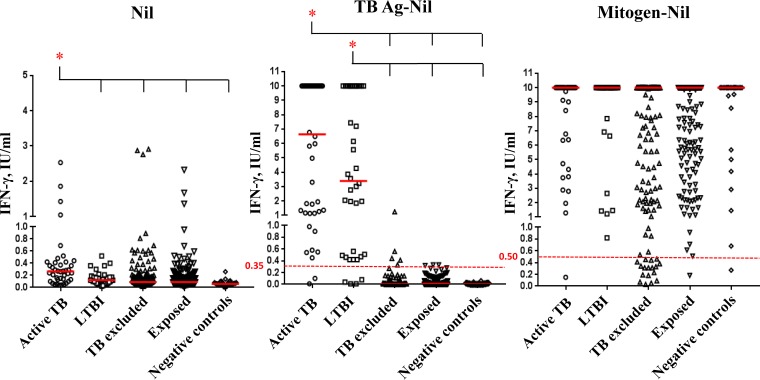
Distributions of IFN-γ levels in the background (nil) and in response to stimulation with nil-corrected M. tuberculosis antigens (TB Ag) and mitogen in the different diagnostic groups are shown in Fig. 1. Median values, interquartile range (IQR), and the overall statistical significance are reported in Table 2. IFN-γ levels in nil and in responses to TB Ag and mitogen were significantly different between diagnostic groups (P <0.0001, P = 0.0001, and P = 0.0023, respectively). In particular, median nil values were statistically higher in active TB (0.27 IU/ml) with respect to all the other diagnosis, including LTBI (0.12 IU/ml; P = 0.0010). Median IFN-γ responses to TB Ag were higher in cases of active TB (6.63 IU/ml) than in cases of LTBI (3.39 IU/ml), although the difference was not statistically significant.
FIG 1.
IFN-γ quantitative values in QFT-IT tubes according to diagnosis. Shown here are individual IFN-γ levels in background (nil) and in response to nil-corrected M. tuberculosis antigens (TB Ag) and nil-corrected mitogen in the different diagnostic groups. Median values are indicated as solid red lines. The cutoff values for positive and indeterminate QFT-IT are represented by dotted red lines at 0.35 and 0.50 IU/ml, respectively. Two nil values higher than 3 IU/ml were not reported for graphical reasons. *, significant differences.
TABLE 2.
Medians and interquartile ranges of QFT-IT IFN-γ quantitative values in QFT-IT tubes according to diagnosis
| Diagnosis | Median (IQR) QFT-IT IFN-γ quantitative values (IU/ml) in: |
||
|---|---|---|---|
| Nil | TB Ag-nil | Mitogen-nil | |
| Active TB | 0.27 (0.13–0.42) | 6.63 (1.19–10) | 10 (6.35–10) |
| LTBI | 0.12 (0.07–0.17) | 3.39 (0.49–10) | 10 (10–10) |
| TB excluded | 0.09 (0.05–0.17) | 0 (0–0.03) | 10 (2.90–10) |
| Exposed | 0.09 (0.06–0.16) | 0.01 (0–0.04) | 10 (7.35–10) |
| Negative controls | 0.06 (0.05–0.08) | 0 (0–0.01) | 10 (7.85–10) |
| Pa | <0.0001 | 0.0001 | 0.0023 |
aKruskal-Wallis P values.
Quantitative analysis of QFT-IT nil, TB Ag, and mitogen values according to age.
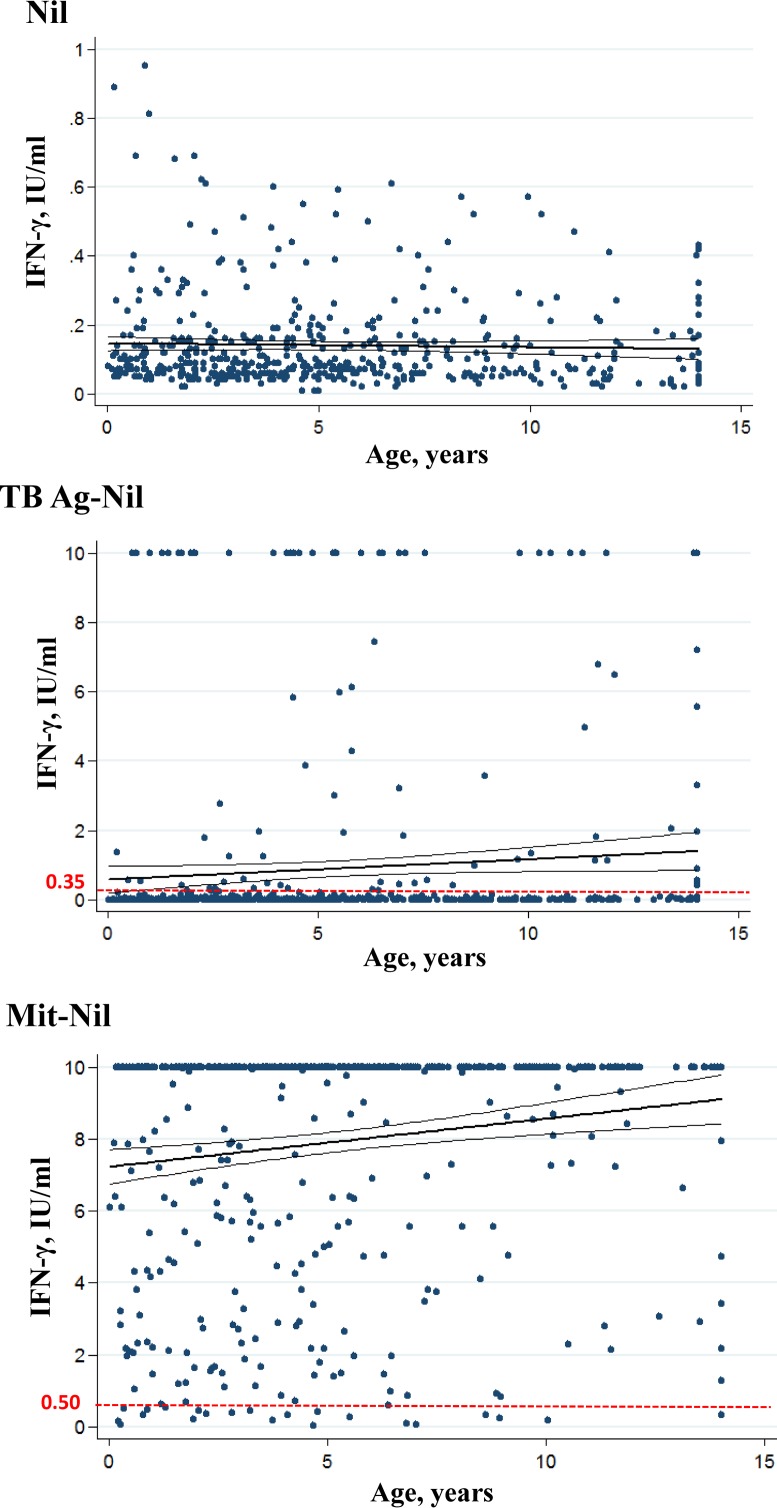
The linear regression analyses of IFN-γ levels in the background and in response to TB Ag and mitogen according to age are reported in Fig. 2. There was significant positive correlation between age and IFN-γ responses to TB Ag (Pearson's correlation coefficient = 0.087, 95% confidence interval [CI] 0.0001 to 0.1165, P = 0.050) and between age and IFN-γ responses to mitogen (Pearson's r = 0.16, 95% CI 0.0615 to 0.2060, P < 0.001). No significant correlation was observed between nil values and age.
FIG 2.
IFN-γ quantitative values in QFT-IT tubes according to age. Individual IFN-γ levels in background (nil), in response to nil-corrected M. tuberculosis antigens (TB Ag), and nil-corrected mitogen are plotted by age. The cutoff values for positive and indeterminate QFT-IT are represented by red dotted lines at 0.35 and 0.50 IU/ml, respectively. The bold lines indicate the linear regressions. The thinner lines indicate the 95% confidence intervals. For graphical reason nil values higher than 1 IU/ml (n = 12) were not reported.
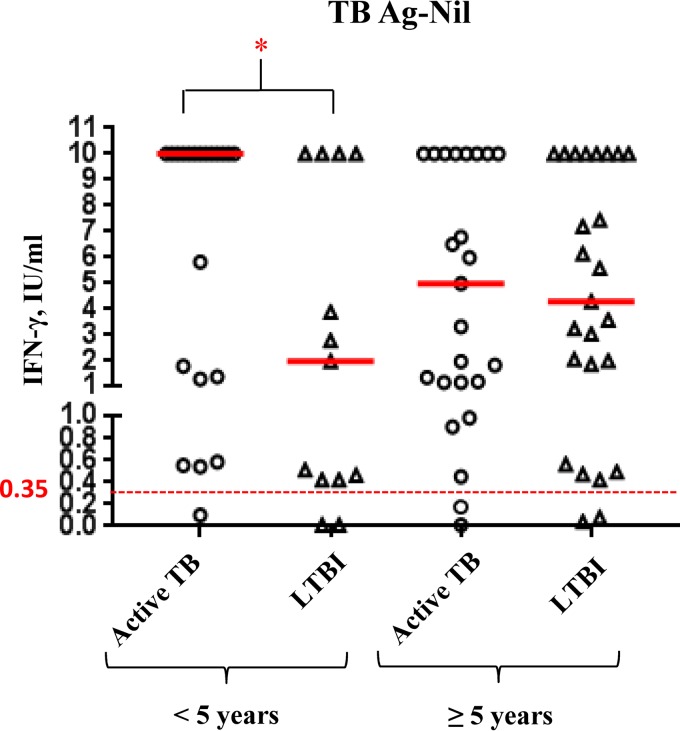
Quantitative values of TB Ag to discriminate between active and latent TB.
In order to evaluate whether the QFT-IT response to M. tuberculosis antigens could discriminate between latent and active TB, children were divided into 2 age groups: children younger than 5 years of age with active TB (n = 21) and LTBI (n = 13), and children over 5 with active TB (n = 24) and LTBI (n = 25). As shown in Fig. 3, the IFN-γ response to TB Ag significantly differed between active TB (median 10 IU/ml, IQR 1.31 to 10 IU/ml) and LTBI (median 1.96 IU/ml, IQR 0.42 to 10 IU/ml) in children under 5 (P = 0.0427). In contrast, in children more than 5 years old there was no significant difference between response to M. tuberculosis antigens regardless of diagnosis (active TB: median 4.97 IU/ml, IQR 1.14 to 10 IU/ml; LTBI: median 4.27 IU/ml, IQR 1.20 to 10 IU/ml).
FIG 3.
IFN-γ quantitative values in response to QFT-IT M. tuberculosis antigens in children with active TB and LTBI according to age groups. Individual IFN-γ levels in response to nil-corrected M. tuberculosis antigens (TB Ag) are reported in active TB and LTBI children divided into two age groups (≤5 years and >5 years old). Median values are indicated as solid red lines. The cutoff value for positive QFT-IT is represented by a dotted red line at 0.35 IU/ml. *, significant differences.
DISCUSSION
Children represent an important group that should be targeted for TB prevention, as they have a higher risk of progression to active disease and developing severe life-threatening TB compared to adults (27). Moreover, TB diagnosis in children is hampered by the paucibacillary nature of TB and its extrapulmonary localization and low level of microbiological confirmation (28, 29). IGRAs are immunological tests that could represent a promising diagnostic tool not only for LTBI but also for active TB. Previous reports showed that QFT-IT, a widespread and commercially available IGRA, correlates better than TST with TB exposure (30) and has overall sensitivity and specificity in diagnosing active TB of 80% and 79%, respectively (31).
Evaluation of IGRA performance in children remains limited due to small study sizes, resistance to phlebotomy, and difficulty in obtaining culture-confirmed results for reference (32). Sensitivity and specificity have been assessed in studies using either culture-confirmed cases or an exposure gradient as a surrogate for infection, but outcomes as well as proportion of indeterminate results are discordant, particularly in infants under 5 years (19, 20, 33, 34). In our previous report, we showed good sensitivity (93.3%) and specificity (99.3%) of QFT-IT in comparison with TST for diagnosis of active TB (23). However, caution in the use of IGRAs in children is based on the potentially immature immune system of infants which might not be capable of responding properly to antigenic stimuli ex vivo (15). Current evidence does not support the use of QFT-IT as an alternative to TST; however, different national guidelines recommend IGRAs in addition to TST in screening algorithms for TB infection (3, 24, 35).
Moreover, the role of quantitative QFT-IT values is under debate: the conventional cutoff value of response to M. tuberculosis antigens is 0.35 IFN-γ IU/ml, and it is unclear whether higher IFN-γ values represent more recent M. tuberculosis exposure, high mycobacterial-load inoculums, prolonged infection or simply reflect heterogeneity in human immune responses to M. tuberculosis. Recently, a prospective study by Andrews and colleagues analyzing serial QFT-IT results in order to define quantitative IFN-γ values predictive of TB in young children showed that conversion values >4 IU/ml were associated with an increased risk of TB disease (36).
In our study, we evaluated IFN-γ quantitative levels in the background (nil) and in response to stimulation with M. tuberculosis antigens and mitogen in the QFT-IT test according to diagnosis and age in a large group of children. Interestingly, nil values were higher in children with active TB than in those with all of the other diagnoses, independently of age, and this has never been shown before.
Quantitative IFN-γ response to M. tuberculosis-specific antigens was not significantly different in children with active TB compared to those with LTBI, even though the median values were higher in the first group, as already reported (26, 37, 38). However, when children were grouped by age, those less than 5 years old with active TB produced significantly higher levels of IFN-γ in response to TB Ag than those with LTBI. These results indicate that even younger children with active TB are capable of mounting a strong T cell response to M. tuberculosis antigens. Further studies could be useful to characterize M. tuberculosis-specific responses to distinguish between LTBI and active TB in younger (<5 years old) and older (>5 years old) children.
Linear dispersion of IFN-γ in response to mitogen showed that these values increased with age, suggesting that this response is significantly influenced by children's ages, as previously reported (39–41). However, most children responded to mitogen by secreting high levels of IFN-γ, as already demonstrated by Sali and colleagues (34). This was supported by the overall low rate of indeterminate results, equally distributed between age groups in the same cohort of patients (23). In particular, children with TB and those with LTBI did not exhibit significantly different responses to mitogen. Furthermore, no indeterminate results were observed in these two diagnostic groups, suggesting that children are able to produce a response to ensure a valid QFT-IT result. For one active TB case, the mitogen value was below the threshold, but the test was interpreted as positive because the TB antigen was 1.2 IU/ml. Interestingly, indeterminate results were mostly distributed among non-TB acute infections, with pneumonia accounting for 35% of cases, in agreement with other studies (34, 38). A detailed description of indeterminate cases has already been reported in the paper by Petrucci and colleagues (23).
We expect that the new generation of the QFT-IT test, QuantiFERON-TB Plus, could better define response to M. tuberculosis infection, by also detecting specific CD8+ T cell response through the presence of an additional M. tuberculosis antigen tube. Recent studies have reported higher M. tuberculosis-specific CD8+ T cell responses in patients with active TB disease compared to those with LTBI (42, 43), with recent M. tuberculosis exposure (44, 45) and with more severe disease (46, 47). Previously, higher M. tuberculosis-specific CD8+ T cell responses were observed in young children with TB (48), but no studies on QFT Plus performance in children have been performed to date.
In conclusion, this study suggests that QFT-IT could be an efficient and reliable test for the management of pediatric TB in routine clinical practice, even in young children. Furthermore, the background IFN-γ level and response to QFT-IT M. tuberculosis antigens could play a potential role in the differential diagnosis of latent and active TB in children. Our observations could be transferred to the new version of the test, QFT Plus, which has recently been shown to substantially agree with QFT-IT results and has similar sensitivity in active TB (49) but lacks data in children.
ACKNOWLEDGMENTS
This study was partially supported by the contribution of the Fondazione Del Monte of Bologna and Ravenna (ID ROL FdM/2400).
We thank Paola Monari and Sonia Bonora for technical support and Jackie Leeder, B.Sc., for English language editing.
REFERENCES
- 1.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. 2008. Paediatric tuberculosis. Lancet Infect Dis 8:498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2014. Guidance for national tuberculosis programmes on the management of tuberculosis in children, 2nd ed World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/112360/1/9789241548748_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 3.Berti E, Galli L, Venturini E, de Martini M, Chiappini E. 2014. Tuberculosis in childhood: a systematic review of national and international guidelines. BMC Infect Dis 14(Suppl 1):S3. doi: 10.1186/1471-2334-14-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar MK, Kumar P, Singh A. 2015. Recent advances in the diagnosis and treatment of childhood tuberculosis. J Nat Sci Biol Med 6:314–320. doi: 10.4103/0976-9668.159988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pai M, Kalantri S, Dheda K. 2006. New tools and emerging technologies for the diagnosis of tuberculosis: part I. Latent tuberculosis. Expert Rev Mol Diagn 6:413–422. doi: 10.1586/14737159.6.3.413. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2015. Guidelines on the management of latent tuberculosis infection. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/136471/1/9789241548908_eng.pdf?ua=1&ua=1. [PubMed] [Google Scholar]
- 7.Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. 2002. A meta-analysis of the effect of bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax 57:804–809. doi: 10.1136/thorax.57.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farhat M, Greenaway C, Pai M, Menzies D. 2006. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 10:1192–1204. [PubMed] [Google Scholar]
- 9.Menzies D, Pai M, Comstock G. 2007. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 146:340–354. [DOI] [PubMed] [Google Scholar]
- 10.Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, Lange C, Losi M, Markova R, Migliori GB, Nienhaus A, Ruhwald M, Wagner D, Zellweger JP, Huitric E, Sandgren A, Manissero D. 2011. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 37:88–99. doi: 10.1183/09031936.00115110. [DOI] [PubMed] [Google Scholar]
- 11.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. 2014. Gamma interferon release assays for detection of mycobacterium tuberculosis infection. Clin Microbiol Rev 27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goletti D, Sanduzzi A, Delogu G. 2014. Performance of the tuberculin skin test and interferon-γ release assays: an update on the accuracy, cutoff stratification, and new potential immune-based approaches. J Rheumatol Suppl 91:24–31. doi: 10.3899/jrheum.140099. [DOI] [PubMed] [Google Scholar]
- 13.Mandalakas AM, Detjen AK, Hesseling AC, Benedetti A, Menzies D. 2011. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis 15:1018–1032. doi: 10.5588/ijtld.10.0631. [DOI] [PubMed] [Google Scholar]
- 14.Sollai S, Galli L, de Martino M, Chiappini E. 2014. Systematic review and meta-analysis on the utility of interferon-gamma release assays for the diagnosis of Mycobacterium tuberculosis infection in children: a 2013 update. BMC Infect Dis 14(Suppl 1):S6. doi: 10.1186/1471-2334-14-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haustein T, Ridout DA, Hartley JC, Thaker U, Shingadia D, Klein NJ, Novelli V, Dixon GL. 2009. The likelihood of an indeterminate test result from a whole-blood interferon-gamma release assay for the diagnosis of Mycobacterium tuberculosis infection in children correlates with age and immune status. Pediatr Infect Dis J 28:669–673. doi: 10.1097/INF.0b013e3181a16394. [DOI] [PubMed] [Google Scholar]
- 16.Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, Hatherill M, Moyo S, Hanekom W, Mahomed H. 2011. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J 30:694–700. doi: 10.1097/INF.0b013e318214b915. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Xiao J, Miao Q, Feng WX, Wu XR, Yin QQ, Jiao WW, Shen C, Liu F, Shen D, Shen AD. 2011. Interferon gamma release assay in diagnosis of pediatric tuberculosis: a meta-analysis. FEMS Immunol Med Microbiol 63:165–173. doi: 10.1111/j.1574-695X.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 18.Cruz AT, Starke JR, Lobato MN. 2014. Old and new approaches to diagnosing and treating latent tuberculosis in children in low-incidence countries. Curr Opin Pediatr 26:106–113. doi: 10.1097/MOP.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiappini E, Bonsignori F, Mazzantini R, Sollai S, Venturini E, Mangone G, Cortimiglia M, Olivito B, Azzari C, Galli L, de Martino M. 2014. Interferon-gamma release assay sensitivity in children younger than 5 years is insufficient to replace the use of tuberculin skin test in western countries. Pediatr Infect Dis J 33:1291–1293. doi: 10.1097/INF.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 20.Laurenti P, Raponi M, de Waure C, Marino M, Ricciardi W, Damiani G. 2016. Performance of interferon-γ release assays in the diagnosis of confirmed active tuberculosis in immunocompetent children: a new systematic review and meta-analysis. BMC Infect Dis 16:131. doi: 10.1186/s12879-016-1461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiagen. 2013. QuantiFERON-TB Gold (QFT) ELISA package insert. Qiagen, Germany. http://www.hshc.com.tw/data/files/201612/o_1b2vd080mcmg16nh1c3gg8v1hsd9.pdf.
- 22.Qiagen. 2016. QuantiFERON-TB Gold Plus (QFT-Plus) package insert. Qiagen, Germany. http://www.quantiferon.com/us/products/quantiferon-tb-gold-plus-us/package-inserts/.
- 23.Petrucci R, Lombardi G, Corsini I, Bacchi Reggiani ML, Visciotti F, Bernardi F, Landini MP, Cazzato S, Dal Monte P. 2017. Quantiferon-TB Gold In-Tube improves tuberculosis diagnosis in children. Pediatr Infect Dis J 36:44–49. doi: 10.1097/INF.0000000000001350. [DOI] [PubMed] [Google Scholar]
- 24.Lancella L, Lo Vecchio A, Chiappini E, Tadolini M, Cirillo D, Tortoli E, de Martino M, Guarino A, Principi N, Villania A, Esposito S, Galli L, Italian Pediatric TB Study Group. 2015. How to manage children who have come into contact with patients affected by tuberculosis. J Clin Tuberc Other Mycobact Dis 1:1–12. doi: 10.1016/j.jctube.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Aghbari N, Al-Sonboli N, Yassin MA, Coulter JB, Atef Z, Al-Eryani A, Cuevas LE. 2009. Multiple sampling in one day to optimize smear microscopy in children with tuberculosis in Yemen. PLoS One 4:e5140. doi: 10.1371/journal.pone.0005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchi L, Galli L, Moriondo M, Veneruso G, Becciolini L, Azzari C, Chiappini E, de Martino M. 2009. Interferon-gamma release assay improves the diagnosis of tuberculosis in children. Pediatr Infect Dis J 28:510–514. doi: 10.1097/INF.0b013e31819abf6b. [DOI] [PubMed] [Google Scholar]
- 27.Seddon JA, Shingadia D. 2014. Epidemiology and disease burden of tuberculosis in children: a global perspective. Infect Drug Resist 7:153–165. doi: 10.2147/IDR.S45090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigouts L. 2009. Clinical practice: diagnosis of childhood tuberculosis. Eur J Pediatr 168:1285–1290. doi: 10.1007/s00431-009-0988-y. [DOI] [PubMed] [Google Scholar]
- 29.Cuevas LE. 2011. The urgent need for new diagnostics for symptomatic tuberculosis in children. Indian J Pediatr 78:449–455. doi: 10.1007/s12098-010-0354-0. [DOI] [PubMed] [Google Scholar]
- 30.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. 2011. Negative and positive predictive value of a whole-blood interferon-γ release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med 183:88–95. doi: 10.1164/rccm.201006-0974OC. [DOI] [PubMed] [Google Scholar]
- 31.Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, Bossink A, Dheda K, Diel R, Dominguez J, Lipman M, Nemeth J, Ravn P, Winkler S, Huitric E, Sandgren A, Manissero D. 2011. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 37:100–111. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- 32.Chiappini E, Bonsignori F, Accetta G, Boddi V, Galli L, Biggeri A, De Martino M. 2012. Interferon-γ release assays for the diagnosis of Mycobacterium tuberculosis infection in children: a literature review. Int J Immunopathol Pharmacol 25:335–343. doi: 10.1177/039463201202500203. [DOI] [PubMed] [Google Scholar]
- 33.Garazzino S, Galli L, Chiappini E, Pinon M, Bergamini BM, Cazzato S, Dal Monte P, Dodi I, Lancella L, Esposito S, Iughetti L, Montagnani C, De Martino M, Tovo PA, SITIP IGRA Study Group. 2014. Performance of interferon-γ release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age: a multicenter study of the Italian Society of Pediatric Infectious Diseases. Pediatr Infect Dis J 33:e226-31. doi: 10.1097/INF.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 34.Sali M, Buonsenso D, Goletti D, D'Alfonso P, Zumbo A, Fadda G, Sanguinetti M, Delogu G, Valentini P. 2015. Accuracy of QuantiFERON-TB Gold test for tuberculosis diagnosis in children. PLoS One 10:e0138952. doi: 10.1371/journal.pone.0138952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollock L, Basu RR, Kampmann B. 2013. How to use: interferon γ release assays for tuberculosis. Arch Dis Child Educ Pract Ed 98:99–105. doi: 10.1136/archdischild-2013-303641. [DOI] [PubMed] [Google Scholar]
- 36.Andrews JR, Nemes E, Tameris M, Landry BS, Mahomed H, McClain JB, Fletcher HA, Hanekom WA, Wood R, McShane H, Scriba TJ, Hatherill M. 2017. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med 5:282–290. doi: 10.1016/S2213-2600(17)30060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Critselis E, Amanatidou V, Syridou G, Spyridis NP, Mavrikou M, Papadopoulos NG, Tsolia MN. 2012. The effect of age on whole blood interferon-gamma release assay response among children investigated for latent tuberculosis infection. J Pediatr 161:632–638. doi: 10.1016/j.jpeds.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Blandinières A, de Lauzanne A, Guérin-El Khourouj V, Gourgouillon N, See H, Pédron B, Faye A, Sterkers G. 2013. QuantiFERON to diagnose infection by Mycobacterium tuberculosis: performance in infants and older children. J Infect 67:391–398. doi: 10.1016/j.jinf.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Connell TG, Tebruegge M, Ritz N, Bryant PA, Leslie D, Curtis N. 2010. Indeterminate interferon-gamma release assay results in children. Pediatr Infect Dis J 29:285–286. doi: 10.1097/INF.0b013e3181c4822f. [DOI] [PubMed] [Google Scholar]
- 40.Thomas B, Pugalenthi A, Patel H, Woltmann G, Bankart J, Hoskyns W. 2011. Concordance between tuberculin skin test and interferon-γ assay and interferon-γ response to mitogen in pediatric tuberculosis contacts. Pediatr Pulmonol 46:1225–1232. doi: 10.1002/ppul.21494. [DOI] [PubMed] [Google Scholar]
- 41.Tebruegge M, de Graaf H, Sukhtankar P, Elkington P, Marshall B, Schuster H, Patel S, Faust SN. 2014. Extremes of age are associated with indeterminate QuantiFERON-TB gold assay results. J Clin Microbiol 52:2694–2697. doi: 10.1128/JCM.00814-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiacchio T, Petruccioli E, Vanini V, Cuzzi G, Pinnetti C, Sampaolesi A, Antinori A, Girardi E, Goletti D. 2014. Polyfunctional T-cells and effector memory phenotype are associated with active TB in HIV-infected patients. J Infect 69:533–545. doi: 10.1016/j.jinf.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Petruccioli E, Vanini V, Chiacchio T, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2016. Modulation of interferon-gamma response to QuantiFERON-TB-plus detected by enzyme-linked immunosorbent assay in patients with active and latent tuberculosis infection. Int J Mycobacteriol 5(Suppl 1):S143–S144. doi: 10.1016/j.ijmyco.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 44.Lancioni C, Nyendak M, Kiguli S, Zalwango S, Mori T, Mayanja-Kizza H, Balyejusa S, Null M, Baseke J, Mulindwa D, Byrd L, Swarbrick G, Scott C, Johnson DF, Malone L, Mudido-Musoke P, Boom WH, Lewinsohn DM, Lewinsohn DA, Tuberculosis Research Unit. 2012. CD8+ T cells provide an immunologic signature of tuberculosis in young children. Am J Respir Crit Care Med 185:206–212. doi: 10.1164/rccm.201107-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barcellini L, Borroni E, Brown J, Brunetti E, Campisi D, Castellotti PF, Codecasa LR, Cugnata F, Di Serio C, Ferrarese M, Goletti D, Lipman M, Rancoita PM, Russo G, Tadolini M, Vanino E, Cirillo DM. 2016. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J 48:1411–1419. doi: 10.1183/13993003.00510-2016. [DOI] [PubMed] [Google Scholar]
- 46.Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, Dal Monte P, Di Serio C, Goletti D, Lombardi G, Lipman M, Rancoita PM, Tadolini M, Cirillo DM. 2016. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J 47:1587–1590. doi: 10.1183/13993003.02033-2015. [DOI] [PubMed] [Google Scholar]
- 47.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, Barcellini L, Palmieri F, Cirillo DM, Ippolito G, Goletti D. 2016. Characterization of the CD4 and CD8 T-cell response in the QuantiFERON-TB Gold Plus kit. Int J Mycobacteriol Suppl 5(Suppl 1):S25–S26. doi: 10.1016/j.ijmyco.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 48.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA. 2011. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petruccioli E, Vanini V, Chiacchio T, Cuzzi G, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2017. Analytical evaluation of QuantiFERON-Plus and QuantiFERON-Gold In-tube assays in subjects with or without tuberculosis. Tuberculosis (Edinb) 106:38–43. doi: 10.1016/j.tube.2017.06.002. [DOI] [PubMed] [Google Scholar]