LETTER
Mycobacterium tuberculosis isolates of the Beijing 94-32 cluster (also named the Central Asian/Russian Beijing strain) constitute an important component of the population structure of the pathogen in the countries of the Former Soviet Union (1–4). A variable-number tandem-repeat (VNTR)-based analysis suggested that this genotype could speculatively trace its origins to the northwestern regions of China (5).
Beijing 94-32 is the largest type within the VNTR-defined CC1 group (6) and falls within the East Europe 1 group as defined by whole-genome sequencing (WGS) (7). Our analysis of all CC1 isolates compiled in the work of Merker et al. (6) demonstrated that type 94-32 presents the largest node in the central position in the phylogenetic network (see Fig. S1 in the supplemental material), and we therefore suggest naming this clonal complex the Beijing 94-32 cluster.
The 94-32 cluster isolates were associated with multidrug-resistant/extremely drug-resistant tuberculosis in Russia (8) and in Uzbekistan (termed the Central Asia outbreak strain [2]), and in immigrants in Western Europe (9, 10). This justifies the interest in having a simple tool to rapidly detect this clinically and epidemiologically relevant strain.
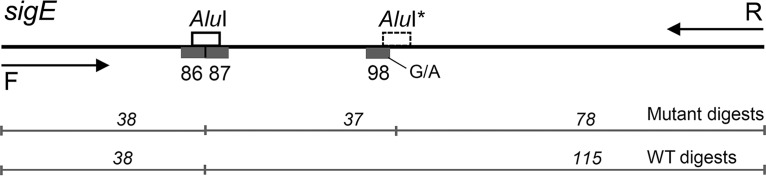
In this study, DNA of 19 Russian M. tuberculosis isolates of the Beijing genotype was subjected to WGS on the MiSeq platform (Illumina). The next-generation sequencing (NGS) data were deposited in the NCBI Sequence Read Archive (project number PRJNA305488). The fastq and vcf files were subjected to comprehensive bioinformatics analysis (see the legend of Fig. S2 in the supplemental material for details). In total, 24 single-nucleotide polymorphisms (SNPs) specific of the Beijing 94-32 cluster were found. We looked more closely at these SNPs in order to find one that could be a synonymous mutation in a coding gene sequence and would be practically suitable for PCR-restriction fragment length polymorphism (RFLP) analysis with a common and inexpensive restriction enzyme. We chose a mutation in the sigE gene at codon 98, CTG→CTA (position 1364706, G→A in the genome of reference strain H37Rv [RefSeq accession number NC_000962.3]), as a candidate SNP for subsequent analysis and assay development. This mutation creates an additional AluI site (AGCT) and thus can be detected by this restriction endonuclease (Fig. 1). The PCR-RFLP profile consists of two bands in the case of the wild-type allele (38 plus 115 bp) and three bands in the case of the mutant allele (being visualized as single strong band of two fragments of 38 plus 37 bp and one band of 78 bp) (Fig. 2).
FIG 1.
Schematic view of the sigE gene fragment targeted by the PCR-RFLP assay. Arrows show primers. The asterisk shows the AluI site created in the case of the codon 98 CTG→CTA mutation in a Beijing 94-32 cluster isolate. Gray boxes show particular codons. The permanent AluI site (codons 86 to 87) was observed in all genomes in the GMTV database and thus does not interfere with PCR-RFLP analysis of codon 98. F, forward; R, reverse; WT, wild type.
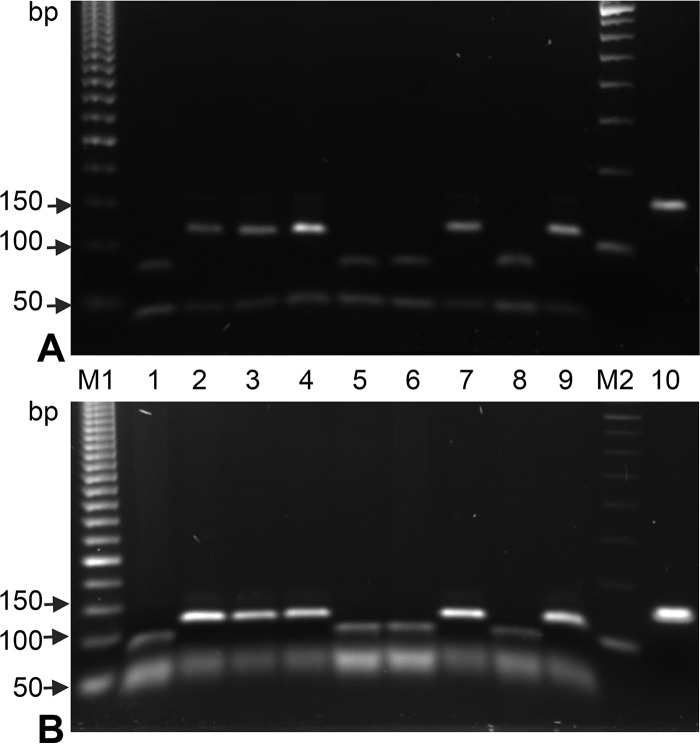
FIG 2.
Gel electrophoresis of the amplified sigE fragment and the products of its digestion by AluI separated in a 2% high-resolution 1:1 MetaPhor agarose-standard agarose gel (A) and 1.8% standard agarose gel (B). Lanes: M1, 50-bp DNA ladder; M2, 100-bp DNA ladder; 1, 5, 6, and 8, Beijing 94-32 cluster isolates with a sigE98 CTA mutant codon (37/38 plus 78 bp); 2 to 4, 7, and 9, other genotypes with a sigE98 CTG wild-type codon (38 plus 115 bp); 10, undigested PCR product (153 bp).
A portion of the sigE gene was amplified with primers SigF (5′-CCACCGGGGACAAGGCCAC) and SigR (5′-GGACCGACCGGAACACCCTG) under the following conditions: initial denaturation at 95°C for 4 min; 35 cycles of 94°C for 20 s, 66°C for 20 s, and 72°C for 15 s; and a final elongation at 72°C for 4 min. The 153-bp PCR product was digested by AluI (Roche) according to the manufacturer's instructions, and the digests were separated in a short-run agarose gel (either 2% MetaPhor agarose [Cambrex Bio Science Rockland, Inc.] plus standard agarose [Quantum Biotechnologies]) at 1:1 or 1.8% standard agarose) and photographed in UV light (Fig. 2A and B, respectively).
The specificity of the sigE98 CTG→CTA mutation was verified through a search of the GMTV database (http://mtb.dobzhanskycenter.org/) and analysis of the vcf files with the PhyTB tool (http://pathogenseq.lshtm.ac.uk/phytblive/index.php) (see the legend of Fig. S2 in the supplemental material for details).
Initial assessment and optimization of the PCR-RFLP assay were done with DNA samples of isolates with available NGS data. Experimental validation of the assay was performed with the M. tuberculosis DNA collections (342 isolates in total [Table S1]). DNA was extracted from bacterial cultures with a GenoLyse kit (Hain Lifescience) (Estonia collection), by the Qiagen protocol for DNA purification using the QIAamp spin procedure (Kazakhstan collection), or by the recommended cetyltrimethylammonium bromide (CTAB), phenol-chloroform extraction, and isopropanol precipitation-based procedure as described in reference 11 (all other collections). The Beijing collections came from distinct and partly contrasting settings: (i) Russia, where the 94-32 type is detected in half of the Beijing isolates, i.e., one-fourth of all circulating isolates (4, 8); (ii) Kazakhstan, where this type constitutes 70% of the population (1); (iii) China, where the Beijing family is dominant but type 94-32 is negligibly rare or absent (2, 5); and (iv) Brazil, where Beijing isolates have been imported recently from diverse sources and remain sporadic (12). Collections of the non-Beijing genotypes represented all major phylogenetic lineages of human M. tuberculosis. Our results demonstrate that this CTG→CTA sigE98 mutation is present only in the Beijing 94-32 cluster isolates.
The assay was performed successfully irrespective of the different protocols used for DNA extraction. In addition, we preliminarily tested the assay with cell lysates obtained by boiling/centrifugation of a small amount of wet bacterial mass. The PCR conditions were the same as those described above except for the number of PCR cycles, which was increased to 40 cycles, and dimethyl sulfoxide was added to the PCR mix, and good results were achieved in terms of both PCR yield and subsequent restriction endonuclease analysis. We are currently evaluating the assay directly on clinical samples as a part of a large-scale, long-term prospective study.
In conclusion, we developed an assay for rapid detection of isolates of the epidemiologically important Central Asian/Russian Beijing strain (94-32 cluster) that may serve for prospective screening and for retrospective assessment of large collections when comprehensive and time-consuming tools, such as multilocus VNTR and WGS, are not available or practical.
The test to detect Central Asian/Russian Beijing 94-32 cluster isolates developed presently may be used in parallel with a previously developed test to detect isolates of the Beijing B0/W148 cluster termed “successful Russian clone” (13) on strains identified as of the Beijing genotype. The combined use of these PCR-based assays will permit a rapid (1-day) detection of both the major and the epidemic clonal clusters of M. tuberculosis circulating in the countries of the Former Soviet Union and being spread by immigrants to different parts of the world.
Supplementary Material
ACKNOWLEDGMENTS
I.M., E.C., A.V., V.Z., and O.N. were supported by Russian Science Foundation grant 14-14-00292. E.C. was supported by Russian Foundation for Basic Research grant 16-34-60163. Y.S. was supported by Ministry of Education and Science of the Republic of Kazakhstan grant 3732/GF4.
We are grateful to the anonymous reviewer for valuable comments that helped to improve the paper.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01551-17.
REFERENCES
- 1.Skiba Y, Mokrousov I, Ismagulova G, Maltseva E, Yurkevich N, Bismilda V, Chingissova L, Abildaev T, Aitkhozhina N. 2015. Molecular snapshot of Mycobacterium tuberculosis population in Kazakhstan: a country-wide study. Tuberculosis (Edinb) 95:538–546. doi: 10.1016/j.tube.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Merker M, Feuerriegel S, Cox H, Barbier M, Kohl T, Borrell Farnov S, Gagneux S, Nübel U, Nikolayevskyy V, Supply P, Wirth T, Niemann S. 2016. Anticipating the second-line antibiotic era: drug resistant tuberculosis strain drives epidemic in Central Asia, p 48–49. In Program of the 37th Annual Congress of the European Society of Mycobacteriology. Agency Konsens Ltd, Groß-Umstadt, Germany. [Google Scholar]
- 3.Shitikov E, Kolchenko S, Mokrousov I, Bespyatykh J, Ischenko D, Ilina E, Govorun V. 2017. Evolutionary pathway analysis and unified classification of East Asian lineage of Mycobacterium tuberculosis. Sci Rep 7:9227. doi: 10.1038/s41598-017-10018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mokrousov I, Narvskaya O, Vyazovaya A, Millet J, Otten T, Vishnevsky B, Rastogi N. 2008. Mycobacterium tuberculosis Beijing genotype in Russia: in search of informative variable-number tandem-repeat loci. J Clin Microbiol 46:3576–3584. doi: 10.1128/JCM.00414-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin QQ, Liu HC, Jiao WW, Li QJ, Han R, Tian JL, Liu ZG, Zhao XQ, Li YJ, Wan KL, Shen AD, Mokrousov I. 2016. Evolutionary history and ongoing transmission of phylogenetic sublineages of Mycobacterium tuberculosis Beijing genotype in China. Sci Rep 6:34353. doi: 10.1038/srep34353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merker M, Blin C, Mona S, Duforet-Frebourg N, Lecher S, Willery E, Blum MG, Rüsch-Gerdes S, Mokrousov I, Aleksic E, Allix-Béguec C, Antierens A, Augustynowicz-Kopeć E, Ballif M, Barletta F, Beck HP, Barry CE III, Bonnet M, Borroni E, Campos-Herrero I, Cirillo D, Cox H, Crowe S, Crudu V, Diel R, Drobniewski F, Fauville-Dufaux M, Gagneux S, Ghebremichael S, Hanekom M, Hoffner S, Jiao WW, Kalon S, Kohl TA, Kontsevaya I, Lillebæk T, Maeda S, Nikolayevskyy V, Rasmussen M, Rastogi N, Samper S, Sanchez-Padilla E, Savic B, Shamputa IC, Shen A, Sng LH, Stakenas P, Toit K, Varaine F, Vukovic D, Wahl C, Warren R, Supply P, Niemann S, Wirth T. 2015. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat Genet 47:242–249. doi: 10.1038/ng.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo T, Comas I, Luo D, Lu B, Wu J, Wei L, Yang C, Liu Q, Gan M, Sun G, Shen X, Liu F, Gagneux S, Mei J, Lan R, Wan K, Gao Q. 2015. Southern East Asian origin and coexpansion of Mycobacterium tuberculosis Beijing family with Han Chinese. Proc Natl Acad Sci U S A 112:8136–8141. doi: 10.1073/pnas.1424063112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vyazovaya A, Mokrousov I, Solovieva N, Mushkin A, Manicheva O, Vishnevsky B, Zhuravlev V, Narvskaya O. 2015. Tuberculous spondylitis in Russia and prominent role of multidrug-resistant clone Mycobacterium tuberculosis Beijing B0/W148. Antimicrob Agents Chemother 59:2349–2357. doi: 10.1128/AAC.04221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Lago L, Martínez-Lirola M, García S, Herranz M, Mokrousov I, Comas I, Martínez-Priego L, Bouza E, García-de-Viedma D. 2016. Urgent implementation in a hospital setting of a strategy to rule out secondary cases caused by imported extensively drug-resistant Mycobacterium tuberculosis strains at diagnosis. J Clin Microbiol 54:2969–2974. doi: 10.1128/JCM.01718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioannidis P, van Soolingen D, Mokrousov I, Papaventsis D, Karabela S, Konstantinidou E, Marinou I, Nikolaou S, Kanavaki S, Mantadakis E, Samonis G, Anthony R, Vogiatzakis E. 8 July 2017. Multidrug-resistant/extensively drug-resistant tuberculosis in Greece: predominance of Mycobacterium tuberculosis genotypes endemic in the Former Soviet Union countries. Clin Microbiol Infect doi: 10.1016/j.cmi.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 11.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM, Small P. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 31:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes LL, Vasconcellos SE, Gomes HM, Elias AR, da Silva Rocha A, Ribeiro SC, Panunto AC, Ferrazoli L, da Silva Telles MA, Ivens de, Kritski AMAL, Mokrousov I, Manicheva OA, Lasunskaia E, Suffys PN. 2015. Genetic diversity of the Mycobacterium tuberculosis Beijing family in Brazil and Mozambique and relation with infectivity and induction of necrosis in THP-1 cells. Tuberculosis (Edinb) 95(Suppl 1):S190–S196. doi: 10.1016/j.tube.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Mokrousov I, Narvskaya O, Vyazovaya A, Otten T, Jiao WW, Gomes LL, Suffys PN, Shen AD, Vishnevsky B. 2012. Russian “successful” clone B0/W148 of Mycobacterium tuberculosis Beijing genotype: multiplex PCR assay for rapid detection and global screening. J Clin Microbiol 50:3757–3759. doi: 10.1128/JCM.02001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.