ABSTRACT
Effective concentrations of antibiotics in brain tissue are essential for antimicrobial therapy of brain infections. However, data concerning cerebral penetration properties of antibiotics for treatment or prophylaxis of central nervous system infections are rare. Six patients suffering subarachnoid hemorrhage and requiring cerebral microdialysis for neurochemical monitoring were included in this study. Free interstitial concentrations of cefuroxime after intravenous application of 1,500 mg were measured by microdialysis in brain tissue, as well as in plasma at steady-state (n = 6) or after single-dose administration (n = 1). At steady state, free area under the concentration-time curve from 0 to 24 h (AUC0–24) values of 389.0 ± 210.3 mg/liter·h and 131.4 ± 72.8 mg/liter·h were achieved for plasma and brain, respectively, resulting in a brain tissue penetration ratio (AUC0–24 brain/AUC0–24 free plasma) of 0.33 ± 0.1. Plasma and brain tissue concentrations at individual time points correlated well (R = 0.59, P = 0.001). At steady-state time over MIC (t>MIC) values of >40% of dosing interval were achieved up to an MIC of 16 mg/liter for plasma and 4 mg/liter for brain tissue. Although MIC90 values could not be achieved in brain tissue for relevant bacteria, current dosing strategies of cefuroxime might be sufficient to treat pathogens with MIC values up to 4 mg/liter. The activity of cefuroxime in brain tissue might be overestimated when relying exclusively on plasma levels. Although currently insufficient data after single dose administration exist, lower brain-plasma ratios observed after the first dose might warrant a loading dose for treatment and perioperative prophylaxis.
KEYWORDS: microdialysis, cefuroxime, brain, in vivo pharmacokinetics, drug tissue concentration, central nervous system infections
INTRODUCTION
Central nervous system (CNS) infections are serious complications of invasive neurosurgical procedures. Invasive monitoring devices in particular, such as external ventricular drainages, increase the risk of nosocomial bacterial ventriculitis and meningitis, with an incidence rate of up to 30% (1–4). Considering this high incidence rate and the devastating consequences of cerebral infections, antibiotic prophylaxis is commonly used in neurosurgical intensive care units (5, 6). There is no evidence that long-term antibiotic prophylaxis is superior to periprocedural antimicrobials for prevention of cerebral infections (7–9) and long-term administration increases the incidence of Clostridium difficile infections and select resistant or opportunistic pathogens (3, 4, 7, 9, 10). Although long-term prophylaxis is not recommended (4, 11), still 30 to 40% of intensive care units, dependent on specialty and geographic location, use antimicrobials for the duration of ventricular drainage (5, 6). The main reason for failure in treatment of bacterial CNS infection and development of bacterial resistance are subinhibitory antibiotic concentrations at the target site (12). The empirical antibiotic choice for treatment/prophylaxis of CNS infections is based on a broad-spectrum coverage of common pathogens and pharmacokinetic/pharmacodynamic (PK/PD) indices in plasma or cerebrospinal fluid (CSF). However, data concerning penetration into the brain—the ultimate target—are missing in most cases but are urgently needed for effective treatment.
Cerebral microdialysis is an established invasive method for neurochemical monitoring by sampling and analyzing extracellular concentrations of cerebral metabolites at the patient's bedside. The usefulness of this technique in neurointensive care units for patients suffering from head injury or subarachnoid hemorrhage has been reported in previous research (13, 14). In addition, cerebral microdialysis enables the measurement of free interstitial antibiotic concentrations within the brain tissue of a concomitant systemic antibiotic treatment, without the need for further invasive study-related procedures (15–18).
Since cefuroxime is a common antibiotic agent for perioperative antibiotic prophylaxis, this study seeks to determine cefuroxime brain-penetration properties by measuring concentration versus time profiles at steady state by use of microdialysis in patients suffering from subarachnoid hemorrhage.
RESULTS
Population.
Six patients suffering from aneurysmal subarachnoid hemorrhage (Hunt and Hess grading 3 to 5) were treated with intravenous cefuroxime as standard postoperative antibiotic prophylaxis. Patients' characteristics are shown in Table 1. The microdialysis probe was placed at a mean time interval of 2.8 ± 1.7 days (range, 1 to 6 days) after the initial bleeding. The probe was inserted into the brain parenchyma through a bolt system in three cases and tunnelated under the scalp due to decompressive craniectomy in three more cases. Postoperative computed tomography (CT) scans revealed the probe position in morphologically “normal” brain tissue and a mean depth from the dura to the catheters tip of 31.2 ± 5.3 mm. Measurement of the cefuroxime levels in steady state was performed at a mean dose of 11 ± 8.1 (range, doses 4 to 26) at a mean interval of 1.5 ± 0.5 days (range, 2 to 7 days) after probe insertion.
TABLE 1.
Patient characteristics
| Parametera | Mean ± SD |
|---|---|
| Demographics | |
| Age (yrs) | 52.7 ± 14.3 |
| Ht (cm) | 168.0 ± 10.6 |
| Wt (kg) | 75.3 ± 10.2 |
| BMI | 26.8 ± 4.5 |
| Sex | |
| Male | 1 |
| Female | 5 |
| Underlying disease | |
| Aneurysmal subarachnoid hemorrhage | 6 |
| Microdialysis probe | |
| Implantation after SAH (days) | 2.8 ± 1.7 |
| Depth (mm) | 31.2 ± 5.3 |
| Bolt | 3 |
| Tunnelated | 3 |
| Study day | |
| Days after subarachnoid hemorrhage | 4.3 ± 1.8 |
| Days after microdialysis implantation | 1.5 ± 0.5 |
| No. of cefuroxime applications | 11 ± 8.1 |
| Retrodialysis | |
| Individual recovery (%) | 31.7 ± 10.3 |
| Laboratory (study day) | |
| CRP (mg/dl) | 17.6 ± 6.7 |
| Leukocytes (G/liter) | 10.6 ± 5.9 |
| Creatinine (mg/dl) | 0.5 ± 0.1 |
| Creatinine-clearance (ml/min) | 149.3 ± 52.3 |
| Gamma-glutamyl transpeptidase (U/liter) | 128 ± 154.5 |
| Glutamic pyruvic transaminases (U/liter) | 22.7 ± 14.5 |
| Glutamic oxaloacetic transaminase (U/liter) | 29.5 ± 18 |
| Lactate dehydrogenase (U/liter) | 181 ± 25.3 |
| Alkaline phosphatase (U/liter) | 98.7 ± 80.8 |
| Albumin (g/liter) | 25.2 ± 2.3 |
| Multimodality monitoring (study period) | |
| Mean TCD flow velocities (cm/s) | 93.7 ± 57.6 |
| Mean BP head niveau (mm Hg) | 96.2 ± 12.4 |
| ICP (mm Hg) | 9.3 ± 3.6 |
| ptiO2 (mm Hg) | 25.0 ± 11.8 |
| Brain temp (°C) | 36.2 ± 0.6 |
| L/P ratio | 31.9 ± 5.8 |
BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; ICP, intracranial pressure; L/P ratio, lactate/pyruvate ratio; ptiO2, cerebral oxygen partial pressure; SAH, subarachnoid hemorrhage; TCD, transcranial Doppler.
Mean laboratory values on the day of study administration are shown in Table 1. All patients showed elevated infection parameters (CRP, 17.6 ± 6.7 mg/dl) and had hypoalbuminemia (25.2 ± 2.3 g/liter). Global renal und liver function was normal in all cases.
Multimodality monitoring data are also shown in Table 1. During the observation period, two patients had mild metabolic dysfunction. One patient had elevated transcranial Doppler (TCD) flow velocities (205 cm/s), indicating cerebral vasospasm. Mean blood pressure was elevated to 115 ± 18.1 mm Hg, resulting in only mild hypoxic cerebral oxygen partial pressure (ptiO2) levels of 19.7 ± 7.8 mm Hg and normal lactate/pyruvate (L/P) ratio of 27.4 ± 1.7. One patient without elevated TCD flow velocities (50 cm/s) showed mild hypoxia (ptiO2 = 17.3 ± 5.8 mm Hg) and an elevated L/P ratio (40.1 ± 2.4). There was no significant difference in the pharmacokinetic data between these two patients and patients without metabolic dysfunction. No correlation was found between cerebral AUC0–8 and any multimodality monitoring data (TCD flow velocities, ptiO2, L/P ratio, mean blood pressure, and intracranial pressure [ICP]). Retrodialysis was conducted at a mean interval of 2.2 ± 1.6 days (range, 1 to 5 days) after cefuroxime measurement. The individual relative recovery of cefuroxime was 31.7 ± 10.3%.
Pharmacokinetics.
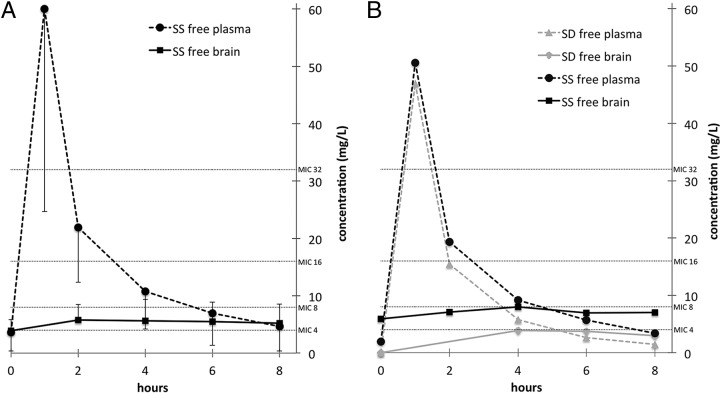
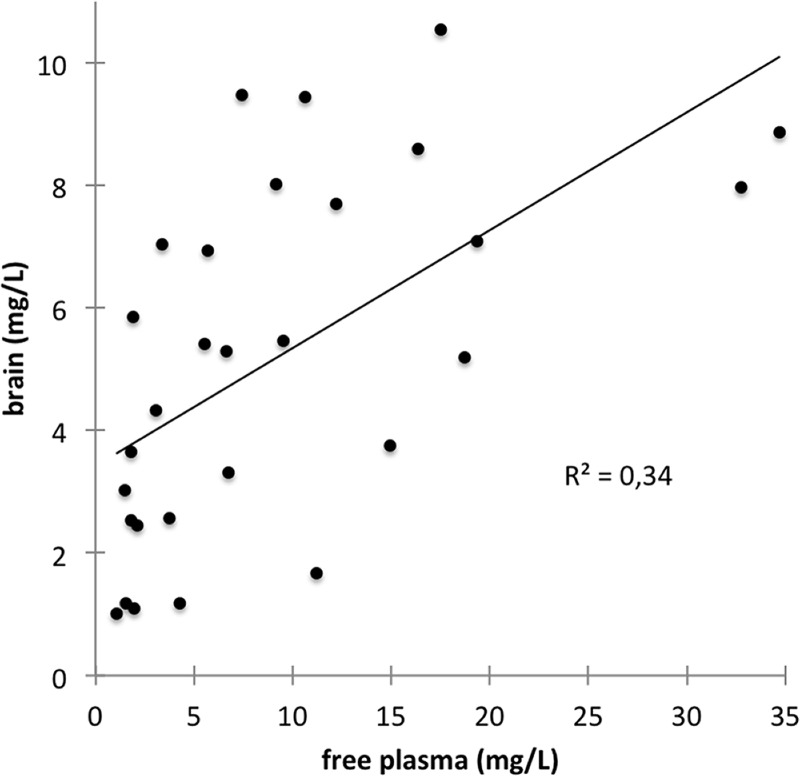
Figure 1A depicts mean free steady-state concentrations curves of cefuroxime in plasma and brain. Steady state was achieved in all cases, as indicated by plasma and cerebral concentrations that did not differ between baseline and end of dosing interval in both compartments (P = 0.12 for plasma and brain). Moreover, no correlation was found between number of doses and baseline concentrations in plasma or brain (P = 0.83 and P = 0.61, respectively). Free plasma and brain tissue concentrations at individual time points correlated well (R = 0.59, P = 0.001; Fig. 2). The coefficients of variation for AUCfree plasma and AUCbrain were 0.54 and 0.55, respectively. The Cbrain/Cfree plasma ratio 2 h after cefuroxime application was 0.3 ± 0.1, after 4 h it was 0.6 ± 0.2, after 6 h it was 0.9 ± 0.4, and after 8 h it was 1.3 ± 0.6.
FIG 1.
Free concentrations of cefuroxime in steady state in the plasma and brain of all patients (means ± the standard deviations) (A) and in one patient after the first dose and in steady state after dose 4 of intravenous infusion of 1,500 mg of cefuroxime (B). Horizontal lines indicate common MICs. SD, single dose; SS, steady state.
FIG 2.
Correlation of free plasma and brain tissue concentrations (R = 0.59, P = 0.001).
Pharmacokinetic parameters at steady-state are shown in Table 2. After intravenous infusion of 1,500 mg of cefuroxime over 60 min in steady state, a mean AUC0–24 of 567.9 ± 307.1 mg/liter·h in plasma and an AUC0–24 of 131.4 ± 72.8 mg/liter·h in brain tissue was achieved. For an estimated protein binding of cefuroxime of 31.5% (19), the free plasma AUC0–24 was 389.0 ± 210.3 mg/liter·h, which was significantly higher than in brain tissue (P = 0.03), resulting in a brain tissue penetration ratio (AUC0–24 brain/AUC0–24 free plasma) of 0.33 ± 0.1. The Cmax of free plasma concentration was 60.1 ± 35.4 mg/liter and was achieved after 1 h (tmax). The Cmax of brain tissue concentration was significantly lower (6.3 ± 3.3 mg/liter; P = 0.03) and with a tmax of 3.7 ± 1.5 h later than in plasma (P = 0.03). In two patients, the measurement of the last cefuroxime concentration in brain tissue was slightly higher than in the sample before. Therefore, t½brain was calculated only for 4 patients in which t1/2 in brain tissue was descriptively much longer than in plasma (t1/2 brain = 16.5 ± 9.0 h and t1/2 plasma = 3.1 ± 1.0 h). Free plasma AUC0–24 and t1/2 plasma significantly correlated with creatinine clearance (R = −0.85, P = 0.03; R = −0.88, P = 0.02), but cerebral AUC0–24 or t1/2 brain did not show any correlation with creatinine clearance.
TABLE 2.
Pharmacokinetic parameters of cefuroxime at steady statea
| Compartment | AUC (mg/liter·h) |
AUCbrain/AUCfree plasma | Cmax (mg/liter) | tmax (h) | t1/2 (h) | CL (liter/h) | VD (liter) | |
|---|---|---|---|---|---|---|---|---|
| AUC0–8 | AUC0–24 | |||||||
| Plasma total | 189.3 ± 102.4 | 567.9 ± 307.1 | 87.7 ± 51.6 | 1 | 3.1 ± 1.0 | 9.0 ± 4.8 | 35.3 ± 11.9 | |
| Plasma free | 129.7 ± 70.1 | 389.0 ± 210.3 | 60.1 ± 35.4 | 1 | 3.1 ± 1.0 | |||
| Brain | 43.8 ± 24.3 | 131.4 ± 72.8 | 0.3 ± 0.1 | 6.3 ± 3.3 | 3.7 ± 1.5 | 16.5 ± 9.0 | ||
AUC, area under the concentration-time curve; CL, total body clearance; Cmax, maximum concentration; tmax, time to maximum concentration; t1/2, terminal elimination half-life; VD, volume of distribution.
In one patient, both single dose and steady-state was measured (Fig. 1B). After a single dose intravenous infusion of 1,500 mg of cefuroxime over 60 min, the AUC0–8 values were 83.4 mg/liter·h for the free plasma concentration and 21.9 mg/liter·h for brain tissue, resulting in a brain tissue penetration ratio (AUC0–24 brain/AUC0–24 free plasma) of 0.26. The Cmax of the free plasma concentration was 46.9 mg/liter after 1 h (tmax), whereas the Cmax in the brain tissue concentration was 3.9 mg/liter and was reached after 4 h (tmax).
The steady-state AUC0–24/MIC and t>MIC values for pathogens with MICs of 2, 4, 8, 16, and 32 mg/liter in plasma and brain tissue are shown in Table 3. t>MIC8, t>MIC16, and t>MIC32 values were significantly longer in plasma than in brain tissue (P = 0.03, P = 0.03, and P = 0.04, respectively).
TABLE 3.
Pharmacokinetic/pharmacodynamic calculations of free plasma and brain tissue concentrations of cefuroxime for pathogens with MICs of 2 to 32 mg/liter
| Compartment and parametera | MIC |
||||
|---|---|---|---|---|---|
| 2 mg/liter | 4 mg/liter | 8 mg/liter | 16 mg/liter | 32 mg/liter | |
| Free plasma | |||||
| AUC0–24/MIC | 194.5 ± 105.2 | 97.3 ± 52.6 | 48.6 ± 26.3 | 24.3 ± 13.2 | 12.2 ± 6.6 |
| t>MIC (h) | 7.6 ± 0.8 | 6.5 ± 1.6 | 5.0 ± 2.3 | 3.0 ± 1.9 | 1.0 ± 0.8 |
| t>MIC (% of dosing interval) | 100 ± 10 | 80 ± 20 | 60 ± 30 | 40 ± 20 | 10 ± 10 |
| Brain tissue | |||||
| AUC0–24/MIC | 65.7 ± 36.4 | 32.9 ± 18.2 | 16.4 ± 9.1 | 8.2 ± 4.6 | 4.1 ± 2.3 |
| t>MIC (h) | 6.7 ± 3.3 | 5.0 ± 4.0 | 1.97 ± 3.06 | 0 | 0 |
| t>MIC (% of dosing interval) | 80 ± 40 | 60 ± 50 | 25 ± 38 | 0 | 0 |
AUC, area under the concentration-time curve; t>MIC, time over MICs.
DISCUSSION
This study delineates the pharmacokinetic profile of cefuroxime in plasma and brain tissue and demonstrates its penetration properties through the intact blood-brain barrier using cerebral microdialysis. Although all six patients suffered from severe subarachnoid hemorrhage, multimodality neuromonitoring did not indicate cerebral impairment, suggesting measurement of cefuroxime within healthy brain tissue.
Overall brain tissue penetration (AUC0–24 brain/AUC0–24 free plasma) was approximately 33%. However, when comparing cerebral cefuroxime concentration with free plasma levels at individual time points, the Cbrain/Cfree plasma ratio was only 26% 2 h after cefuroxime application but gradually increased thereafter. At the end of the dosing interval, the cerebral cefuroxime concentration was as high as 134% of the free plasma concentration, suggesting a delayed penetration through the blood-brain barrier and slower elimination in brain tissue than in plasma.
To overcome the observed fluctuations of plasma and tissue concentrations due to the short t1/2 of beta-lactam antibiotics, continuous application was previously suggested to be superior to intermittent short-term infusions (20–23). Randomized controlled trials have shown that administration of the same dose as continuous infusions may increase clinical effectiveness compared to an intermittent dosing regime (21). Particularly in critical care patients, intermittent bolus administration of standard doses resulted in underdosing for many patients (22). In contrast, continuous infusion and dose adaption for renal function are more likely to achieve sufficient concentrations. In an animal model, using microdialysis, continuous infusion of cefuroxime resulted in lower AUC values and a lower tissue penetration ratio, but significant longer t>MIC in plasma, subcutaneous tissue, and bone compared to short-term infusion (20). However, brain tissue levels seem to be independent of renal function and amplitude of fluctuation is not as high as in plasma. Therefore, it is still unknown whether the same effect that was observed for peripheral tissues will occur in interstitial brain tissue using continuous application. Further PK studies and modeling of data from several antibiotics seem warranted.
Although cefuroxime is widely used in the treatment of CNS infections, this is, to our knowledge, the first study to investigate cerebral cefuroxime concentrations in humans. One study measured cefuroxime in a rat's striatum after a single dose of the antibiotic (24). In this experimental setting, brain tissue penetration was only 4.2%, which is very low compared to the 26% observed in the present study after single-dose administration. Beside the potential differences between rodent and human brain in the study using rats cefuroxime was administered as intravenous bolus and not over 60 min as in our cohort.
Due to the limited access to the brain, CSF levels are often used as surrogate concentrations for interstitial brain levels (25). Therefore, the current dosage recommendations for CNS infections are based upon serum or CSF concentrations, which may significantly differ from the concentration within the interstitial brain (16). In traumatic brain injury patients receiving the same dosing regime as in our cohort, the cefuroxime levels after 1.5 and 6 h in plasma (2.0 to 61.7 mg/liter and 1.8 to 66.9 mg/liter, respectively) were comparable to our data. However, the CSF levels (0.35 to 1.76 mg/liter and 0.15 to 2.03 mg/liter after 1.5 and 6 h, respectively) were considerably lower than the interstitial brain tissue concentrations in our cohort (26). Other studies support that in patients without CNS infection CSF concentrations of cefuroxime are much lower than brain tissue concentrations, thereby failing to reflect cefuroxime concentrations within the cerebral interstitial space, the ultimate target site of antibiotic activity (26, 27). Since CNS infections cause an increase of the permeability of the blood-CSF/blood-brain barrier, CSF levels during the acute phase of bacterial meningitis were reported as high as in our cohort (27–30). Since our population did not suffer from meningitis, we cannot exclude that cefuroxime will achieve even higher concentration in brain tissue during CNS infection.
Since a steady state was reached in all individuals and the number of doses did not correlate with free plasma or brain tissue concentrations, differences in interindividual plasma levels may be explained by their strong correlation with renal function (22, 31). Free plasma and brain tissue concentrations correlated well within individual patients. As indicated by equal coefficients of variation, the variability of tissue concentrations was not higher than the variability in plasma. This may allow for at least limited extrapolation of interstitial brain tissue concentrations from plasma levels when other dosing regimens are applied.
For beta-lactams, the time that the unbound plasma/tissue concentration exceeds the MIC of a pathogen (t>MIC) best describes the antimicrobial effect and is associated with a better clinical outcome in the case of infection (no specific thresholds for prophylaxis are available) (32, 33). For cefuroxime, the required magnitudes of the t>MIC to achieve bacteriostatic and bactericidal effects were estimated to be 30 and 41%, respectively (34). In case comparable targets need to be achieved in brain tissue, the maximum MIC value for which a sufficient bacteriostatic and bactericidal t>MIC could be achieved was 4 mg/liter, while the MICs were 16 and 8 mg/liter, respectively, for plasma. Since the MIC50 is ≤4 mg/liter for the most frequent bacterial strains causing nosocomial CNS infections in neurocritical care patients (Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella pneumoniae, and Escherichia coli) (3, 28, 35–37), the t>MIC values were sufficiently high in plasma as well as in brain tissue for average pathogens, yet regarding MIC90 t>MIC values achieved in plasma might overestimate target side activity. Although the brain tissue concentration would support clinical breakpoints of 4 mg/liter, currently breakpoints of 8 mg/liter for K. pneumoniae and E. coli would only be supported by plasma concentrations (Table 3). These results demonstrate that whereas clinical breakpoints of cefuroxime are in accordance with the t>MIC values for plasma, the breakpoints might have to be reconsidered for brain, and higher cefuroxime dosages might be required to treat certain pathogens causing brain infections.
In our cohort, only one patient was monitored during single-dose administration of cefuroxime. As expected in plasma, an immediately sufficient concentration was achieved, exceeding an MIC of 8 mg/liter within the first 10 min and achieving a Cmax of 46.9 mg/liter after 1 h. In contrast, brain tissue levels showed a delayed Cmax of 3.9 mg/liter after 4 h, i.e., an MIC of 4 mg/liter was never reached and an MIC of 2 mg/liter was not achieved within the first 2 h after administration. Limited by the observation in only one subject, our data may indicate that sufficient brain tissue MIC will not be achieved after standard single-dose administration. Since antibiotic prophylaxis significantly reduces postcraniotomy infections and administration of antibiotics is recommended preprocedural to achieve sufficient concentrations prior to dura opening (4, 38, 39), a loading dose may overcome critical initial underdosing during neurosurgical procedures.
This study had several limitations. First of all, the population size was small. Steady-state data were available for six patients, and single-dose data were available for one patient. All patients had hypoalbuminemia on the day of study administration, which might have led to reduced protein binding. Thus, the free fraction of cefuroxime estimated by protein binding as described in the literature might have been underestimated. Sampling was performed in patients suffering from severe subarachnoid hemorrhage. Although multimodality monitoring did not indicate cerebral impairment at the site of the microdialysis probe, subclinical processes caused by the underlying disease, such as microcirculation constriction, inflammation, or blood-brain barrier disturbances, cannot be completely ruled out (40). Furthermore, differences in local perfusion cannot be excluded, even though the probe was always inserted at the same point and depth. To minimize local tissue reaction and potential leakage of the blood-brain barrier caused by the probe insertion, sampling was started at the earliest 1 day after probe implantation, as stated elsewhere (41). Measurement of cefuroxime concentrations was performed in macroscopically noninflamed cerebral tissue. Since the permeability of the blood-brain barrier is increased during CNS infection (30), the actual concentration in inflamed cerebral tissue cannot be extracted from our data.
Conclusion.
To our knowledge, this is the first study measuring cefuroxime concentrations within human brain tissue during steady-state. The overall brain tissue penetration was 33% ± 1%, and plasma levels correlated well with cerebral concentrations. Our data thus indicate that breakpoints based on plasma levels might overestimate target side activity, which might lead to clinical failure for intermediately susceptible pathogens with MICs of 8 mg/liter. Single-dose administration showed a delayed penetration, which might suggest benefit by a loading dose. Further research is required to prove whether the continuous administration of cefuroxime might improve target side PK/PD parameters in brain tissue as shown for plasma levels.
MATERIALS AND METHODS
Population.
The open-labeled, single-center phase 1 pharmacokinetic study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee (EK1031/2015; Eudract 2015-000121-37). Included were all consecutive patients older than 18 years between March 2016 and May 2017 at the neurosurgical intensive care unit of the Medical University Vienna requiring cerebral microdialysis as the standard care with a clinical indication for concomitant antimicrobial treatment with cefuroxime.
Multimodality monitoring.
Patients meeting the study inclusion criteria were by definition critically ill and under sedation and mechanical ventilation for either maximum cerebroprotection or for the management of associated extracerebral disorders. These patients routinely undergo invasive cerebral multimodality neuromonitoring, including the measurement of ICP, ptiO2, brain temperature, and microdialysis.
Using a two-lumen Bolt (Bolt kit PTO 2Ll; Raumedic AG, Helmbrechts, Germany), a NEUROVENT-PTO 2L catheter (Raumedic) for the measurement of ICP, ptiO2, and temperature, as well as a microdialysis probe, were placed into the brain parenchyma. In the case of decompressive craniectomy, the probes were tunnelated side by side. The probes were inserted at Kocher's point to monitor the watershed area between the ipsilateral territory of the middle cerebral artery and anterior cerebral artery. Positioning of the catheter was controlled with a CT scan 1 day after insertion, and the catheter depth was measured from the dura to the catheter's tip in coronal planes.
Intracranial hypertension was defined as ICP values of >20 mm Hg, and brain tissue hypoxia was defined as ptiO2 values of <20 mm Hg (42, 43). In addition, bedside TCD examinations were carried out on a daily basis. Acceleration of mean TCD flow velocities of more than 120 cm/s in the middle cerebral artery was assessed as cerebral vasospasm.
Microdialysis.
A microdialysis probe (70 MD catheter; M Dialysis AB, Stockholm, Sweden) with an external diameter of 0.6 mm, a membrane length of 10 mm, and a molecular mass cutoff of 20,000 Da was used. The probe was perfused at a flow rate of 0.3 μl per min using a microinfusion pump (107 microdialysis pump; M Dialysis) with perfusion fluid CSF (M Dialysis), which resembles the composition of artificial CNS (Na+, 147 mmol/liter; Ca2+, 1.2 mmol/liter; Mg2+, 0.85 mmol/liter; K+, 2.7 mmol/liter; Cl−, 153.8 mmol/liter; 305 mOsm/kg; pH 6). Microdialysis samples were collected in microvials (M Dialysis), which were changed every hour. Microdialysis sampling was started 3 h after probe insertion at the earliest to exclude neurochemical changes due to implantation trauma. A bedside microdialysis analyzer (ISCUSflex; M Dialysis), based on enzymatic reagents and colorimetric measurements, was used for immediate neurochemical analysis of the glucose, lactate, pyruvate, glycerol, and glutamate concentrations. The L/P ratio is the most sensitive marker of brain redox state and secondary ischemia. Brain metabolic dysfunction was defined as lactate/pyruvate ratio of >40 (42, 43).
Study medication.
The patients received 1,500 mg of cefuroxime (Cefuroxim Astro; 1,500 mg; Trockenstechampulle; Astro-Pharma GmbH, Vienna, Austria) in 100 ml of 0.9% saline solution (Physiologische Kochsalzlösung “Fresenius”; Infusionslösung; Fresenius Kabi Austria GmbH, Graz, Austria) over 60 min intravenously every 8 h using a perfusion pump. At least three applications were considered necessary to reach steady state. In addition, sampling was performed after the first dose for single-dose pharmacokinetic assessment in one patient.
Sampling.
Microdialysis samples were collected at baseline before the next antibiotic administration (−1 to 0 h) and between 1 and 2 h, 3 and 4 h, 5 and 6 h, and 7 and 8 h after the start of the infusion. The samples obtained in between were used for routine monitoring. Microvials for cefuroxime concentration determination were stored in microvial racks (M Dialysis) at −65°C for further analysis.
For the measurement of antibiotic plasma levels, 5 ml of venous blood was drawn from a venous catheter into lithium-heparin tubes (Vacuette lithium-heparin) at a baseline directly before starting the infusion, at 1 h (at the end of the infusion), and at 2, 4, 6, and 8 h after the start of the infusion. Samples were kept on ice for a maximum of 30 min until centrifugation. Blood samples were centrifuged at 4°C and 2,500 × g for 10 min; the cells were discharged, and plasma was obtained. Plasma was divided into two aliquots of approximately 1 ml and was shock frozen at below −65°C.
Retrodialysis.
Retrodialysis was performed to determine the individual in vivo probe recovery within 1 week after cefuroxime sampling. The microdialysis probe was perfused with a solution containing cefuroxime (Cin) at 200 μg/ml at the standard flow rate of 0.3 μl. To determine recovery by loss, after 1 h of equilibration two retrodialysis samples (each 60 min; Cout) were collected and stored in microvial racks (M Dialysis) and stored at −65°C. The Cin and Cout concentrations were determined by high-pressure liquid chromatography (HPLC). The individual relative recovery was calculated as the mean ratio of drug lost during passage (Cin − Cout) and drug entering the microdialysis probe (Cin). Interstitial cefuroxime concentrations were calculated as follows for each sample: interstitial concentration = 100 × (sample concentration/relative recovery).
Drug assay.
The concentration of cefuroxime in plasma and microdialysate was determined by HPLC. Frozen plasma and microdialysate samples were thawed at room temperature. After the addition of 300 μl of methanol to 100 μl of plasma, the samples were centrifuged (14,000 × g for 5 min), and 50 μl of the clear supernatant was injected onto the HPLC column. Microdialysate samples (10 μl) were injected onto the column without any previous precipitation procedure. The determination of cefuroxime was performed using a Dionex UltiMate 3000 system (Dionex Corp., Sunnyvale, CA) with UV detection at 275 nm. Chromatographic separation was carried out at 40°C on a Hypersil BDS-C18 column (5 μm, 250 × 4.6 mm [inner diameter]; Thermo Fisher Scientific, Inc., Waltham, MA), preceded by a Hypersil BDS-C18 precolumn (5 μm, 10 × 4.6 mm [inner diameter]). The mobile phase consisted of a continuous linear gradient, mixed from 10 mM ammonium acetate-acetic acid buffer (pH 5.0; mobile phase A) and acetonitrile (mobile phase B) at a flow rate of 1.0 ml/min. Mobile phase B linearly increased from 0% (0 min) to 15% at 11 min, increased to 80% at 11.5 min, and remained constant until 15.5 min. The percentage of acetonitrile was then decreased within 0.5 min to 0% in order to equilibrate the column for 9 min before application of the next sample. Quantification of cefuroxime was based on external calibration curves by spiking drug-free human plasma and microdialysate with standard solutions of cefuroxime to give a concentration range from 0.01 to 10 μg/ml. Linear least-squares regression curves excluding zero were fitted to these data with a weighting factor of 1/x2 using the GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA), leading to average correlation coefficients of 0.9997 and 0.9995 for plasma and microdialysate, respectively. The limit of quantification for cefuroxime in plasma was 0.04 μg/ml, while that in microdialysate was 0.03 μg/ml. The intraday variabilities for cefuroxime were 4.1 to 7.8% for plasma and 3.9 to 7.5% for microdialysate; the interday variabilities were 4.7 to 8.6% for plasma and 4.3 to 8.1% for microdialysate at cefuroxime concentrations of 0.1, 1.0, and 10 μg/ml. Accuracy was demonstrated for all three concentration levels, with recovery rates ranging from 97.9 to 101.1% for plasma and 96.4 to 103.3% for microdialysate. Cefuroxime plasma and microdialysate samples (0.1, 1, and 10 μg/ml) were stable at 7°C in the autosampler for up to 1 week with recovery rates of 98.4 to 99.7%.
Pharmacokinetics.
Pharmacokinetic data were analyzed with Kinetica 3.0 (InnaPhase, Philadelphia, PA) using noncompartmental analysis. The maximum concentration (Cmax), time to maximum concentration (tmax), terminal elimination half-life (t1/2), and area under the concentration-time curve from 0 to 8 h (AUC0–8) were obtained for cefuroxime in patients' plasma and interstitial brain tissue (after correction for individual recovery). The AUC from 0 to 24 h (AUC0–24) was calculated by multiplying AUC0–8 by 3. For plasma, the apparent volume of distribution (VD) and total body clearance (CL) were also calculated. Since microdialysis measures only the free fraction of cefuroxime within interstitial fluid, the plasma concentrations were also corrected for the known protein binding of cefuroxime of 31.5% and are described as “free plasma” concentrations (19, 44, 45).
Pharmacodynamics.
The most common bacterial strains causing nosocomial CNS infections in neurocritical care patients are the Gram-positive organisms Staphylococcus epidermidis (40 to 70%) and Staphylococcus aureus (5 to 10%) and the Gram-negative bacteria Klebsiella pneumonia (13 to 17%) and Escherichia coli (1 to 13%) (3, 28, 35–37). For PK/PD analysis, the MICs for 50 and 90% of the organisms (MIC50/90) from a global surveillance study of North American clinical isolates of these bacterial strains were used (MIC50/90 values: Staphylococcus aureus, 2/4 mg/liter; Staphylococcus epidermidis, 2/32 mg/liter; Klebsiella pneumonia, 2/16 mg/liter; Escherichia coli, 4/16 mg/liter) (46). For unbound plasma and brain concentrations, the AUC0–24/MIC and t>MIC were calculated as the best indicators for the antimicrobial effects of β-lactam (32). The t>MIC was directly determined by measuring strongly increased concentration versus time profiles. Results were compared to the clinical MIC breakpoints of the EUCAST database for cefuroxime (Staphylococcus aureus, 4 mg/liter; Staphylococcus epidermidis, not applicable; Klebsiella pneumoniae and Escherichia coli, 8 mg/liter) (47).
Statistics.
Statistical analysis was performed using SPSS Statistics 22 (IBM Corp., Armonk, NY). All data are presented as means ± the standard deviations. Wilcoxon signed-rank tests were performed for statistical intraindividual comparison of pharmacokinetic (Cmax, tmax, and t1/2) and pharmacodynamic data (t>MIC and Cmax/MIC) in plasma and brain tissue. To correlate the free plasma and brain tissue concentrations at individual time points, a Pearson correlation coefficient was used. Differences were considered to be statistically significant at a two-sided P value of <0.05.
ACKNOWLEDGMENT
This study was supported by funds of the Oesterreichische Nationalbank (Austrian Central Bank, Anniversary Fund, project 16446).
REFERENCES
- 1.Humphreys H, Jenks PJ. 2015. Surveillance and management of ventriculitis following neurosurgery. J Hosp Infect 89:281–286. doi: 10.1016/j.jhin.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Humphreys H, Jenks P, Wilson J, Weston V, Bayston R, Waterhouse C, Moore A, et al. 2017. Surveillance of infection associated with external ventricular drains: proposed methodology and results from a pilot study. J Hosp Infect 95:154–160. doi: 10.1016/j.jhin.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Beer R, Lackner P, Pfausler B, Schmutzhard E. 2008. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol 255:1617–1624. doi: 10.1007/s00415-008-0059-8. [DOI] [PubMed] [Google Scholar]
- 4.Fried HI, Nathan BR, Rowe AS, Zabramski JM, Andaluz N, Bhimraj A, Guanci MM, Seder DB, Singh JM. 2016. The insertion and management of external ventricular drains: an evidence-based consensus statement: a statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care 24:61–81. doi: 10.1007/s12028-015-0224-8. [DOI] [PubMed] [Google Scholar]
- 5.Cinibulak Z, Aschoff A, Apedjinou A, Kaminsky J, Trost HA, Krauss JK. 2016. Current practice of external ventricular drainage: a survey among neurosurgical departments in Germany. Acta Neurochir (Vienna) 158:847–853. doi: 10.1007/s00701-016-2747-y. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy PJ, Patil S, Conrad SA, Scott LK. 2010. International and specialty trends in the use of prophylactic antibiotics to prevent infectious complications after insertion of external ventricular drainage devices. Neurocrit Care 12:220–224. doi: 10.1007/s12028-009-9284-y. [DOI] [PubMed] [Google Scholar]
- 7.Alleyne CH, Hassan M, Zabramski JM. 2000. The efficacy and cost of prophylactic and perioprocedural antibiotics in patients with external ventricular drains. Neurosurgery 47:1124–1129. doi: 10.1097/00006123-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Saini NS, Dewan Y, Grewal S. 2012. Efficacy of periprocedural versus extended use of antibiotics in patients with external ventricular drains—a randomized trial. Indian J Neurotrauma 9:30–32. doi: 10.1016/j.ijnt.2012.04.005. [DOI] [Google Scholar]
- 9.Dellit TH, Chan JD, Fulton C, Pergamit RF, McNamara EA, Kim LJ, Ellenbogen RG, Lynch JB. 2014. Reduction in Clostridium difficile infections among neurosurgical patients associated with discontinuation of antimicrobial prophylaxis for the duration of external ventricular drain placement. Infect Control Hosp Epidemiol 35:589–590. doi: 10.1086/675828. [DOI] [PubMed] [Google Scholar]
- 10.Chauv S, Fontaine GV, Hoang QP, McKinney CB, Baldwin M, Buckel WR, Collingridge DS, Majercik S, Wohlt PD. 2016. Risk of resistant organisms and Clostridium difficile with prolonged systemic antibiotic prophylaxis for central nervous system devices. Neurocrit Care 25:128–132. doi: 10.1007/s12028-016-0254-x. [DOI] [PubMed] [Google Scholar]
- 11.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. 2007. Guidelines for the management of severe traumatic brain injury. IV. Infection prophylaxis. J Neurotrauma 24(Suppl 1):S26–S31. [DOI] [PubMed] [Google Scholar]
- 12.Gould IM, MacKenzie FM. 2002. Antibiotic exposure as a risk factor for emergence of resistance: the influence of concentration. Symp Ser Soc Appl Microbiol 2002:78S–84S. doi: 10.1046/j.1365-2672.92.5s1.10.x. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson PJ, Jalloh I, Helmy A, Carpenter KL, Rostami E, Bellander BM, Boutelle MG, Chen JW, Claassen J, Dahyot-Fizelier C, Enblad P, Gallagher CN, Helbok R, Hillered L, Le Roux PD, Magnoni S, Mangat HS, Menon DK, Nordström CH, O'Phelan KH, Oddo M, Perez Barcena J, Robertson C, Ronne-Engström E, Sahuquillo J, Smith M, Stocchetti N, Belli A, Carpenter TA, Coles JP, Czosnyka M, Dizdar N, Goodman JC, Gupta AK, Nielsen TH, Marklund N, Montcriol A, O'Connell MT, Poca MA, Sarrafzadeh A, Shannon RJ, Skjøth-Rasmussen J, Smielewski P, Stover JF, Timofeev I, Vespa P, Zavala E, Ungerstedt U. 2015. Consensus statement from the 2014 International Microdialysis Forum. Intensive Care Med 41:1517–1528. doi: 10.1007/s00134-015-3930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persson L, Valtysson J, Enblad P, Warme PE, Cesarini K, Lewen A, Hillered L. 1996. Neurochemical monitoring using intracerebral microdialysis in patients with subarachnoid hemorrhage. J Neurosurg 84:606–616. doi: 10.3171/jns.1996.84.4.0606. [DOI] [PubMed] [Google Scholar]
- 15.Pan YF, Feng J, Cheng QY, Li FZ. 2007. Intracerebral microdialysis technique and its application on brain pharmacokinetic-pharmacodynamic study. Arch Pharm Res 30:1635–1645. doi: 10.1007/BF02977335. [DOI] [PubMed] [Google Scholar]
- 16.Nau R, Sörgel F, Eiffert H. 2010. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahyot-Fizelier C, Timofeev I, Marchand S, Hutchinson P, Debaene B, Menon D, Mimoz O, Gupta A, Couet W. 2010. Brain microdialysis study of meropenem in two patients with acute brain injury. Antimicrob Agents Chemother 54:3502–3504. doi: 10.1128/AAC.01725-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caricato A, Pennisi M, Mancino A, Vigna G, Sandroni C, Arcangeli A, Antonelli M. 2006. Levels of vancomycin in the cerebral interstitial fluid after severe head injury. Intensive Care Med 32:325–328. doi: 10.1007/s00134-005-0015-3. [DOI] [PubMed] [Google Scholar]
- 19.Kratochwil NA, Huber W, Müller F, Kansy M, Gerber PR. 2002. Predicting plasma protein binding of drugs: a new approach. Biochem Pharmacol 64:1355–1374. doi: 10.1016/S0006-2952(02)01074-2. [DOI] [PubMed] [Google Scholar]
- 20.Tøttrup M, Bibby BM, Hardlei TF, Bue M, Kerrn-Jespersen S, Fuursted K, Søballe K, Birke-Sørensen H. 2015. Continuous versus short-term infusion of cefuroxime: assessment of concept based on plasma, subcutaneous tissue, and bone pharmacokinetics in an animal model. Antimicrob Agents Chemother 59:67–75. doi: 10.1128/AAC.03857-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasiakou SK, Sermaides GJ, Michalopoulos A, Soteriades ES, Falagas ME. 2005. Continuous versus intermittent intravenous administration of antibiotics: a meta-analysis of randomised controlled trials. Lancet Infect Dis 5:581–589. doi: 10.1016/S1473-3099(05)70218-8. [DOI] [PubMed] [Google Scholar]
- 22.Carlier M, Noë M, Roberts JA, Stove V, Verstraete AG, Lipman J, De Waele JJ. 2014. Population pharmacokinetics and dosing simulations of cefuroxime in critically ill patients: non-standard dosing approaches are required to achieve therapeutic exposures. J Antimicrob Chemother 69:2797–2803. doi: 10.1093/jac/dku195. [DOI] [PubMed] [Google Scholar]
- 23.Vondracek TG. 1995. Beta-lactam antibiotics: is continuous infusion the preferred method of administration? Ann Pharmacother 29:415–424. doi: 10.1177/106002809502900413. [DOI] [PubMed] [Google Scholar]
- 24.Tsai TH, Cheng FC, Chen KC, Chen YF, Chen CF. 1999. Simultaneous measurement of cefuroxime in rat blood and brain by microdialysis and microbore liquid chromatography: application to pharmacokinetics. J Chromatogr B Biomed Sci Appl 735:25–31. doi: 10.1016/S0378-4347(99)00410-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Van Natta K, Yeo H, Vilenski O, Weller PE, Worboys PD, Monshouwer M. 2009. Unbound drug concentration in brain homogenate and cerebral spinal fluid at steady state as a surrogate for unbound concentration in brain interstitial fluid. Drug Metab Dispos 37:787–793. doi: 10.1124/dmd.108.024125. [DOI] [PubMed] [Google Scholar]
- 26.Kossmann T, Hans V, Stocker R, Imhof HG, Joos B, Trentz O, Morganti-Kossmann MC. 1996. Penetration of cefuroxime into the cerebrospinal fluid of patients with traumatic brain injury. J Antimicrob Chemother 37:161–167. doi: 10.1093/jac/37.1.161. [DOI] [PubMed] [Google Scholar]
- 27.Sirinavin S, Chiemchanya S, Visudhipan P, Lolekha S. 1984. Cefuroxime treatment of bacterial meningitis in infants and children. Antimicrob Agents Chemother 25:273–275. doi: 10.1128/AAC.25.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullins AK, Abdel-Rahman SM. 2013. Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatr Drugs 15:93–117. doi: 10.1007/s40272-013-0017-5. [DOI] [PubMed] [Google Scholar]
- 29.Netland A, Müller C, Andrew E. 1981. Concentration of cefuroxime in cerebrospinal fluid in patients with bacterial meningitis. Scand J Infect Dis 13:273–275. doi: 10.3109/inf.1981.13.issue-4.06. [DOI] [PubMed] [Google Scholar]
- 30.Müller C, Netland A, Dawson AF, Andrew E. 1980. The penetration of cefuroxime into the cerebrospinal fluid through inflamed and noninflamed meninges. J Antimicrob Chemother 6:279–283. doi: 10.1093/jac/6.2.279. [DOI] [PubMed] [Google Scholar]
- 31.Viberg A, Lannergård A, Larsson A, Cars O, Karlsson MO, Sandström M. 2006. A population pharmacokinetic model for cefuroxime using cystatin C as a marker of renal function. Br J Clin Pharmacol 62:297–303. doi: 10.1111/j.1365-2125.2006.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labreche MJ, Graber CJ, Nguyen HM. 2015. Recent updates on the role of pharmacokinetics-pharmacodynamics in antimicrobial susceptibility testing as applied to clinical practice. Clin Infect Dis 61:1446–1452. doi: 10.1093/cid/civ498. [DOI] [PubMed] [Google Scholar]
- 33.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (t>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen EI, Cars O, Friberg LE. 2011. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob Agents Chemother 55:4619–4630. doi: 10.1128/AAC.00182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Citerio G, Signorini L, Bronco A, Vargiolu A, Rota M, Latronico N, Investigators ILCCS. 2015. External ventricular and lumbar drain device infections in ICU patients: a prospective multicenter Italian study. Crit Care Med 43:1630–1637. doi: 10.1097/CCM.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 36.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES. 2008. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery 62(Suppl 2):S688–S700. doi: 10.1227/01.neu.0000316273.35833.7c. [DOI] [PubMed] [Google Scholar]
- 37.Leverstein-van Hall MA, Hopmans TE, van der Sprenkel JW, Blok HE, van der Mark WA, Hanlo PW, Bonten MJ. 2010. A bundle approach to reduce the incidence of external ventricular and lumbar drain-related infections. J Neurosurg 112:345–353. doi: 10.3171/2009.6.JNS09223. [DOI] [PubMed] [Google Scholar]
- 38.Alotaibi AF, Hulou MM, Vestal M, Alkholifi F, Asgarzadeh M, Cote DJ, Bi WL, Dunn IF, Mekary RA, Smith TR. 2016. The efficacy of antibacterial prophylaxis against the development of meningitis after craniotomy: a meta-analysis. World Neurosurg 90:597–603. doi: 10.1016/j.wneu.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 39.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA, et al. 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 70:195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 40.Macdonald RL. 2014. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 10:44–58. doi: 10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- 41.Hammarlund-Udenaes M. 2017. Microdialysis as an important technique in systems pharmacology: a historical and methodological review. AAPS J 19:1294–1303. doi: 10.1208/s12248-017-0108-2. [DOI] [PubMed] [Google Scholar]
- 42.Messerer M, Daniel RT, Oddo M. 2012. Neuromonitoring after major neurosurgical procedures. Minerva Anestesiol 78:810–822. [PubMed] [Google Scholar]
- 43.Schmidt JM, Ko S-B, Helbok R, Kurtz P, Stuart RM, Presciutti M, Fernandez L, Lee K, Badjatia N, Connolly ES, Claassen J, Mayer SA. 2011. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke 42:1351–1356. doi: 10.1161/STROKEAHA.110.596874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dellamonica P. 1994. Cefuroxime axetil. Int J Antimicrob Agents 4:23–36. doi: 10.1016/0924-8579(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 45.Foord RD. 1976. Cefuroxime: human pharmacokinetics. Antimicrob Agents Chemother 9:741–747. doi: 10.1128/AAC.9.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoban DJ, Bouchillon SK, Johnson JL, Zhanel GG, Butler DL, Saunders KA, Miller LA, Poupard JA, et al. 2003. Comparative in vitro potency of amoxicillin-clavulanic acid and four oral agents against recent North American clinical isolates from a global surveillance study. Int J Antimicrob Agents 21:425–433. doi: 10.1016/S0924-8579(03)00038-4. [DOI] [PubMed] [Google Scholar]
- 47.EUCAST. 2007. Antimicrobial wild-type distributions of microorganisms: cefuroxime. EUCAST, Copenhagen, Denmark: https://mic.eucast.org/Eucast2/. [Google Scholar]