ABSTRACT
Staphylococcus aureus causes systemic infections with high morbidity and mortality, and the emergence of drug-resistant strains is a rapidly growing clinical concern. Novel therapeutic agents are required to tackle S. aureus infections. P128 is a bacteriophage-derived chimeric ectolysin with potent and rapid bactericidal activity against S. aureus. In the present study, the efficacy of P128 was evaluated in a newly developed rat model of S. aureus bacteremia. Prior to in vivo testing, P128 was shown to be stable in whole blood by incubation in rat blood for up to 6 h and testing its bactericidal activity against the methicillin-resistant S. aureus isolate USA300. Rats succumbed to intravenous challenge with 109 CFU of S. aureus USA300, resulting in 80 to 100% mortality by day 14. Evaluation of the bacterial load in various organs at 96 h postinfection revealed high bacterial counts in the kidney, and this correlated with the presence of renal abscesses. Treatment of infected animals with P128 either by intravenous bolus administration via tail vein or by 1-h infusion via the jugular vein at 2 h postinfection resulted in the dose-dependent survival of rats. P128 treatment also resulted in very few or no abscesses in the kidneys. These data show that P128 is stable in the physiological milieu and that intravenous treatment with P128 is highly effective in rescuing rats from S. aureus bacteremia. P128 can be a novel therapeutic option for treatment of S. aureus systemic infections.
KEYWORDS: Staphylococcus aureus bacteremia, antibiotic resistance, renal abscesses, phage lysins, ectolysin, P128
INTRODUCTION
Staphylococcus aureus is a ubiquitous pathogen that colonizes human nares, skin, and gastrointestinal tract and is a leading cause of both community- and hospital-acquired infections (1). Approximately 20 to 30% of the human population can be colonized with S. aureus in the nares (2). S. aureus is a causative agent of bacteremia, infective endocarditis, osteomyelitis, skin and soft tissue infections, pneumonia, and device-related infections (3–5). Infections caused by S. aureus remain a major public health problem worldwide and are considered difficult to treat. S. aureus is widely known for its ability to rapidly develop antibiotic resistance, and the indiscriminate use of antibiotics has led to the emergence of resistance to standard-of-care antibiotics, creating a need for novel and effective drugs against S. aureus. Development of new antibiotics that belong to already-existing classes leads to rapid development of resistance observed for currently used drugs in the same class (6). This calls for the development of novel classes of antibacterial agents.
Phage-derived peptidoglycan hydrolases that enzymatically degrade the bacterial cell wall have demonstrated potent antibacterial activity (7). These enzymes are called “lysins,” and they are classified into two types: ectolysins and endolysins. Ectolysins are phage structure-associated muralytic (cell wall-degrading) enzymes that aid entry of the phage DNA into bacterial cells at the initiation of phage infection, and endolysins are hydrolases that degrade the cell wall and aid in the release of progeny phages during the end of the phage lytic life cycle. With the increasing emergence of antibiotic resistance in S. aureus, cell wall hydrolases represent potential alternatives against S. aureus (8–10). P128 is a 27-kDa bacteriophage-derived ectolysin with potent antistaphylococcal activities. It is a chimeric protein comprising the tail-associated muralytic enzyme (ectolysin) of phage K and the staphylococcal cell wall targeting domain (SH3b) of lysostaphin (11). P128 has been shown to be effective against clinical strains of S. aureus, including methicillin-sensitive S. aureus, methicillin-resistant S. aureus (MRSA), vancomycin-resistant S. aureus, and linezolid-resistant S. aureus (12). In vivo efficacy of P128 has been shown against mupirocin-resistant MRSA in a rat nasal colonization model (11). P128 possesses potent antibiofilm activity and shows synergistic activity in combination with standard-of-care antibiotics against both planktonic and biofilm-embedded strains of S. aureus (12).
Although mouse models of infection are routinely used, rats offer many advantages in modeling human diseases. They are considered an excellent model to simulate cardiovascular diseases and cognitive disorders and for use in cancer research. One of the big advantages of rats is their size compared to that of mice. It is not only easy to perform surgical procedures, but also important substructures of the organs involved in lesions are proportional in size (13). The standard mouse peritonitis model used to evaluate antibacterials has drawbacks. Factors unique to the mouse peritoneum are not considered during the extrapolation of data to clinical conditions in humans. Because of its small size and limited blood volume, it is not possible to sample the same animal over longer durations and also, considering the rapid course of infection, mice are less relevant to clinical situations (14).
In the present study, we report a rat model of bacteremia with characteristics similar to the more rapidly fatal mouse model but presenting relevance to the clinical situations in humans. We demonstrate the efficacy of P128 in this model. To our knowledge, this is the first report of a rat model for bacteremia. Successful treatment of S. aureus-infected rats using this model demonstrates that P128 may be a viable option for the treatment of systemic S. aureus infections.
RESULTS
In vitro activity of P128 in whole blood.
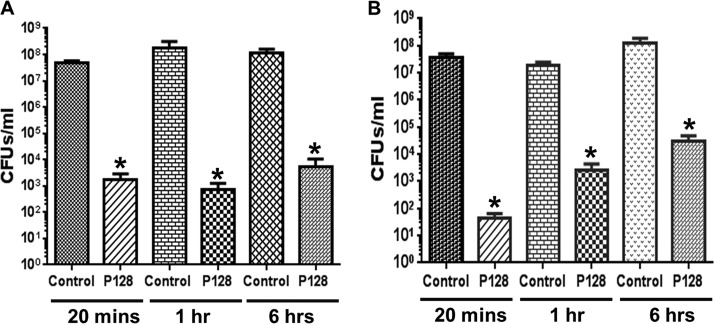
In order to verify that P128 is active in the complex and multifactorial milieu of whole blood, prior to testing its in vivo efficacy, P128 was incubated in rat blood for 20 min, 60 min, and 6 h after which its bactericidal activity was tested. In this test, a difference of 3 orders of magnitude in CFU compared to cell controls (99.9% reduction in CFU) is considered significant with respect to the bactericidal activity of P128. Both in saline (Fig. 1A) and blood (Fig. 1B), a 3-log reduction was seen up to 6 h, which is statistically significant (P < 0.05).
FIG 1.
Activity of P128 in rat blood. P128 retains activity against MRSA USA300 in saline (A) and in whole blood (B) as measured by a ≥99.9% reduction in CFU/ml in comparison to controls (n = 3). Results are expressed as mean residual CFU/ml ± the SE. *, significantly different from cell control (P < 0.05).
Minimum lethal dose of S. aureus bacteria.
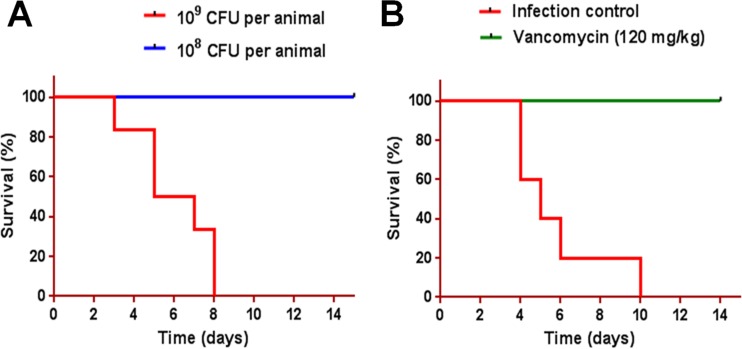
The minimum lethal dose of S. aureus bacteria was considered to be the bacterial inoculum that resulted in 80 to 100% mortality by day 14. Challenging animals with 109 CFU resulted in 100% mortality by day 14, whereas 108 CFU per animal did not result in any mortality (Fig. 2A). Treatment with vancomycin resulted in 100% survival (Fig. 2B).
FIG 2.
Establishment of minimum lethal dose of S. aureus USA300 strain. (A) Wistar albino rats (n = 6 per group) were challenged with 108 or 109 CFU of MRSA USA300 per animal and monitored for survival for 14 days. (B) Wistar albino rats (n = 5 per group) were challenged with 109 CFU of MRSA USA300 per animal and treated 2 h postinfection with vancomycin at 120 mg/kg administered subcutaneously (two doses at 12-h interval), and survival was monitored for 14 days.
Course of MRSA USA300 systemic infection.
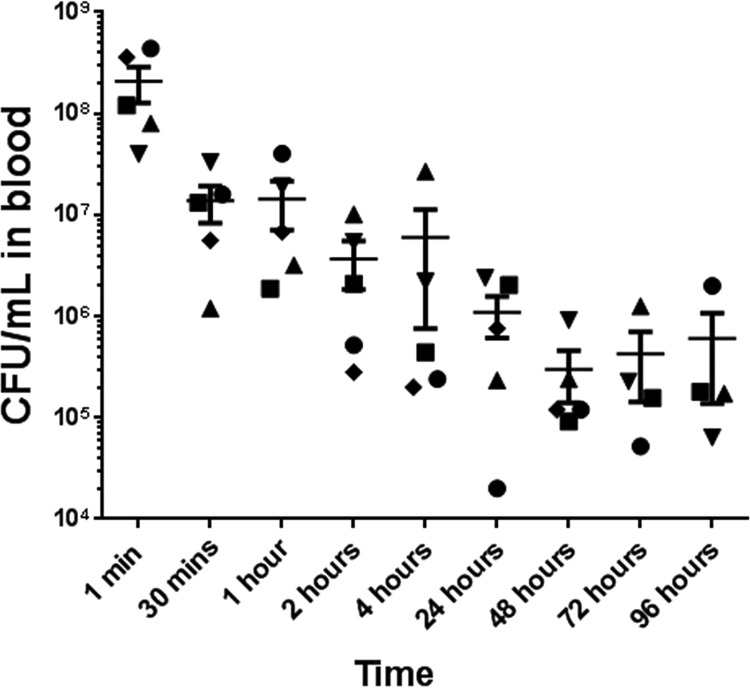
CFU counts were enumerated by sampling blood from animals at different time points following the bacterial challenge. At the first minute after challenge, CFU counts detected in blood were in the range of 2 × 108 ± 8 × 107 CFU/ml (Fig. 3). The counts reduced by 1 to 3 orders of magnitude by 4 h. Over the next 4 days, the CFU counts in the blood remained between 2 × 104 CFU/ml and 2 × 106 CFU/ml (Fig. 3).
FIG 3.
Bacterial load in blood at different time points after infection. Blood samples were drawn at different time points from rats challenged with 109 CFU of MRSA USA300 per animal to determine bacterial counts, expressed as the mean CFU/ml ± the SE in individual animals. Each animal is represented by a different shape. n = 4/group at 72 and 96 h; n = 5/group at all other time points.
Bacterial burden in tissues.
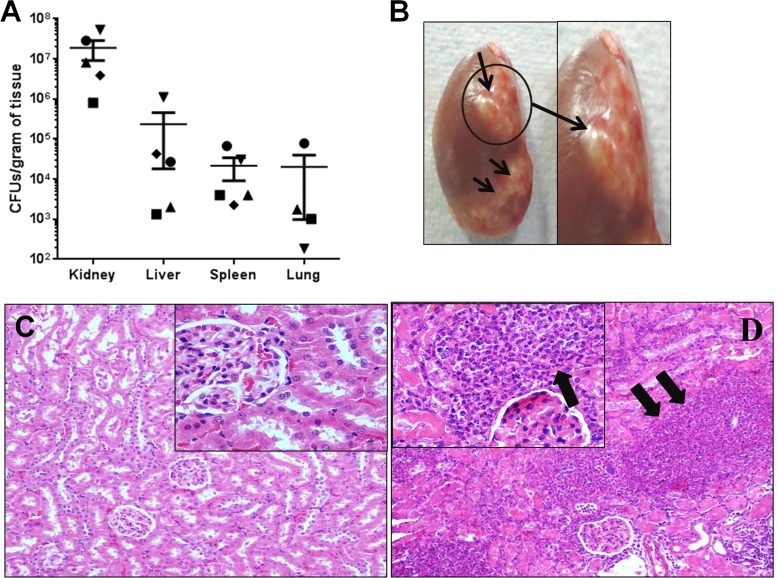
At 96 h postinfection, all of the test organs evaluated, i.e., kidney, liver, spleen, and lung tissues, showed the presence of bacteria. The CFU counts (means ± the standard errors [SE]) in the kidney, liver, spleen, and lungs were 1.89 × 107 ± 9.84 × 106, 2.37 × 105 ± 2.19 × 105, 2.16 × 104 ± 1.26 × 104, and 2.02 × 104 ± 1.93 × 104, respectively (Fig. 4A). During gross necropsy, diffuse abscesses were found in the kidneys (Fig. 4B). Histopathology revealed normal kidney architecture in control animals (Fig. 4C), whereas in animals infected with S. aureus, there was massive infiltration of inflammatory cells in the abscess lesions (Fig. 4D).
FIG 4.
Bacterial burden in tissues and renal abscesses. Wistar rats were challenged with 109 CFU per animal i.v. and euthanized at 96 h postinfection. (A) Liver, spleen, kidney, and lung tissues were homogenized and plated to determine the CFU. Data represent individual CFU counts and means ± the SE for each organ. Each animal is represented by a different shape. (B) Renal abscesses (arrows) were observed at 96 h postinfection. (C and D) Kidneys from control animals showed normal renal histology (C), whereas histological findings after i.v. challenge with S. aureus USA300 included massive infiltration of inflammatory cells and accumulation of inflammatory fluid, marked by arrows (D). The original magnification of panels C and D was ×400.
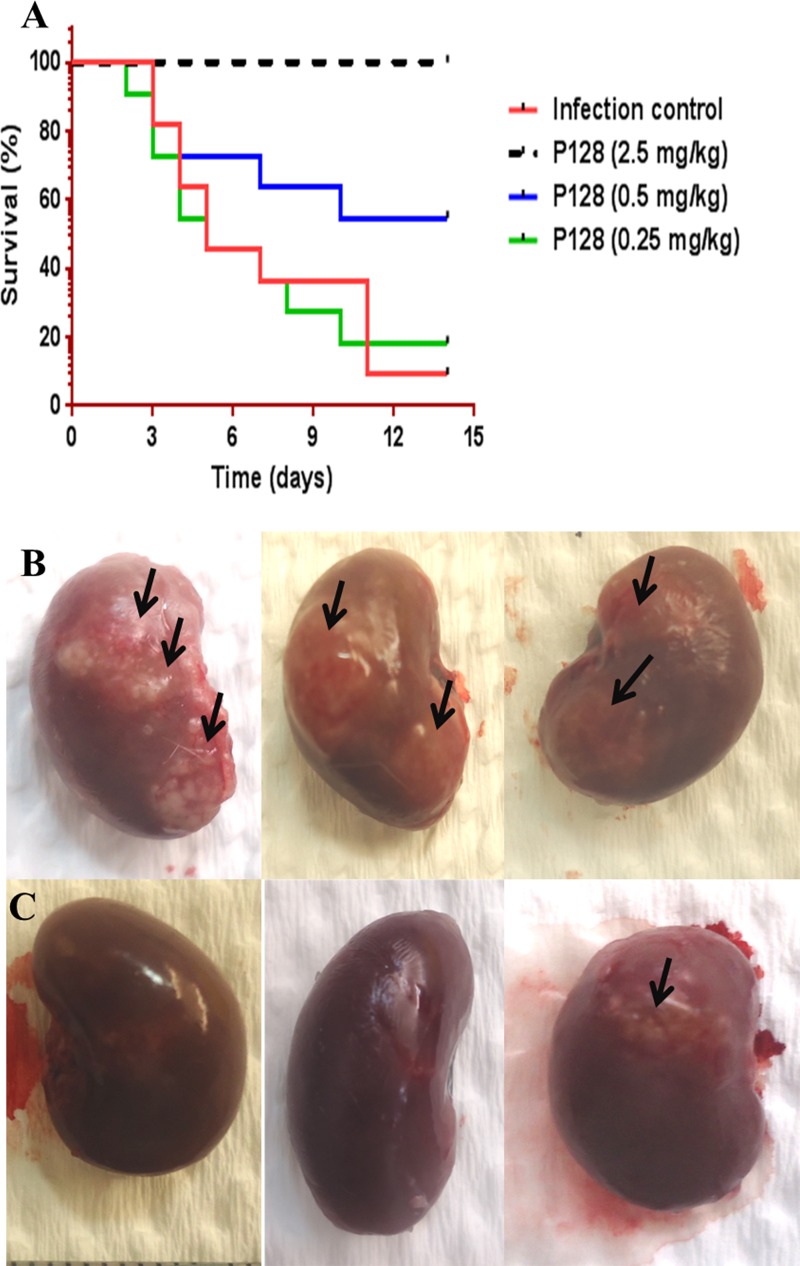
Effect of P128 intravenous (i.v.) bolus treatment on survival and renal abscesses.
In the control group, >90% mortality was observed by day 14. P128 treatment resulted in dose-dependent improvement in survival. The percent survival in animals treated with P128 at 0.25 mg/kg was comparable to that of the saline-treated group. Treatment with P128 at 0.5 mg/kg resulted in 54% survival by day 14, and 100% survival was observed in animals treated with P128 at 2.5 mg/kg (Fig. 5A). One subset of animals was euthanized at 96 h postinfection and evaluated for renal abscesses. In saline-treated animals, diffuse abscesses were observed (Fig. 5B), whereas P128 treatment (2.5 mg/kg) resulted in few or no abscesses (Fig. 5C).
FIG 5.
Efficacy of P128 after i.v. bolus administration. Wistar rats were challenged i.v. with 109 CFU per animal and treated 2 h postinfection with either saline or various doses of P128. (A) Survival was monitored for 14 days. P128 treatment resulted in dose-dependent survival with 100% survival observed at 2.5 mg/kg (P < 0.0001). (B and C) Diffuse renal abscesses (indicated by arrows) were found in animals treated with saline (B), whereas P128 treatment (2.5 mg/kg) resulted in few or no abscesses (C).
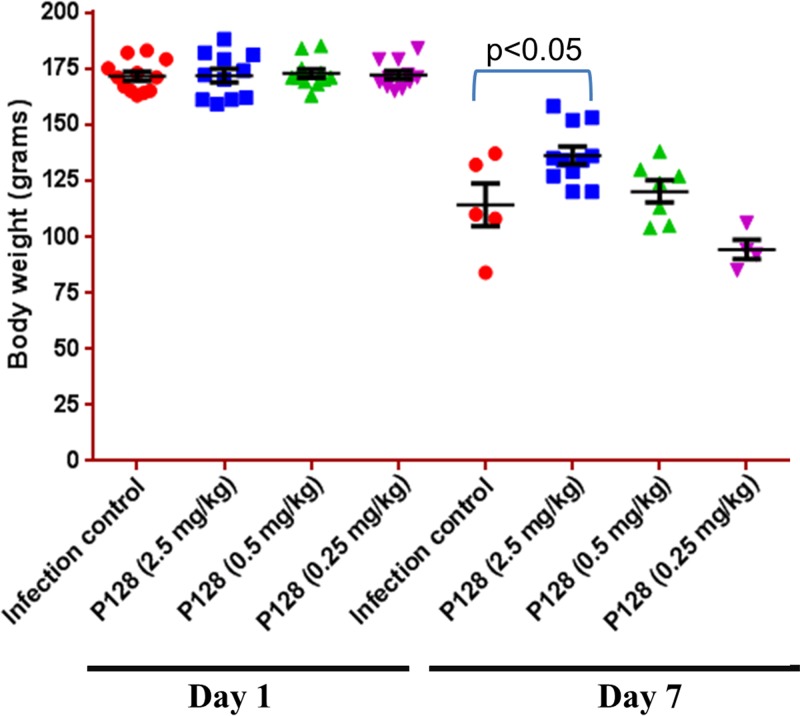
Effect of P128 treatment on body weight.
On day 1, the body weights (means ± the SE) of animals treated with saline, P128 (2.5 mg/kg), P128 (0.5 mg/kg), and P128 (0.25 mg/kg) were 171.5 ± 2.04 g, 171.7 ± 3.02 g, 172.7 ± 1.98 g, and 172.0 ± 1.84 g, respectively (Fig. 6). On day 7, the body weights (means ± the SE) of saline-, P128 (2.5 mg/kg)-, P128 (0.5 mg/kg)-, and P128 (0.25 mg/kg)-treated animals were 114.2 ± 9.04 g, 136.2 ± 3.91 g, 120.1 ± 4.93 g, and 94.2 ± 4.37 g, respectively (Fig. 6). On day 7, in comparison to control animals, P128-treated (2.5 mg/kg) animals showed significantly higher mean body weights (Fig. 6).
FIG 6.
Body weights of saline- and P128-treated animals. Wistar rats were challenged i.v. with 109 CFU per animal and treated 2 h postinfection with either saline or various doses of P128. Body weights were recorded on days 1 and 7. Data are represented as means ± the SE. n = 10 or 11 on day 1, and n = 4 to 10 on day 7.
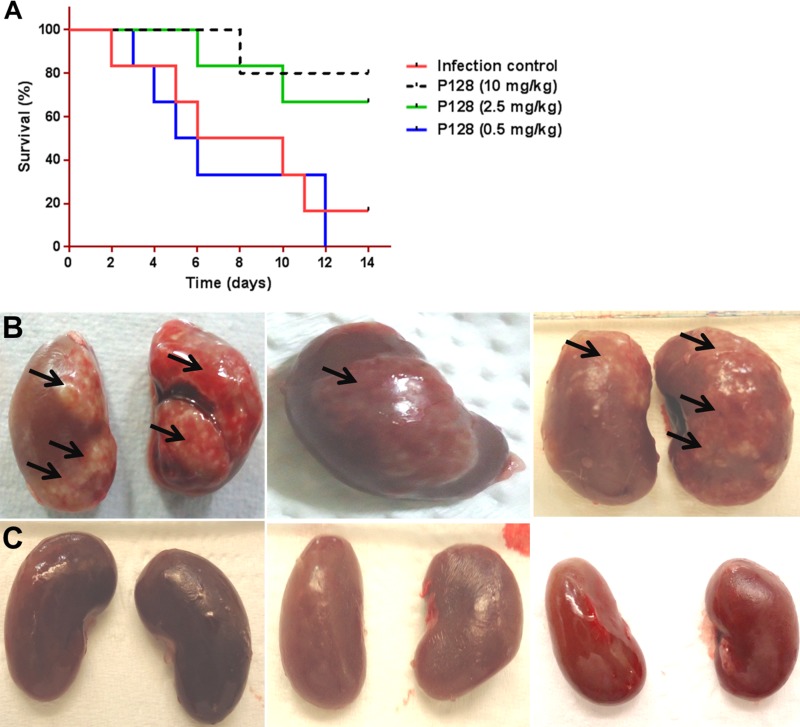
Efficacy of P128 after i.v. infusion.
P128 was evaluated at 10, 2.5, and 0.5 mg/kg by i.v. infusion over 1 h after challenging animals with 109 CFU i.v. In this subset of animals, infusion was started at 2 h after bacterial challenge, and the animals were monitored for survival. A total of 84% of the untreated animals died in the control group by day 14. Infusion of P128 at 10 and 2.5 mg/kg resulted in 84 and 66% survival, respectively, whereas 0.5 mg/kg did not protect the animals and the mortality remained comparable to that of the infection control group (Fig. 7A). In the second subset of animals that were infused with either saline or P128 at 10 mg/kg starting immediately after infection to compare renal abscesses, diffuse abscesses were found throughout the kidney (Fig. 7B) in the saline-infused animals, whereas in the P128-treated animals (Fig. 7C) renal abscesses were either pinpoint sized and few in number or entirely absent.
FIG 7.
Efficacy of P128 after i.v. infusion. Wistar rats (n = ≥5) were challenged i.v. with 109 CFU per animal and treated 2 h postinfection with a 1-h i.v. infusion of either saline or various doses of P128. (A) Survival was monitored for 14 days. P128 resulted in dose-dependent survival with 80% survival at 10 mg/kg (P < 0.04). (B and C) Renal abscesses (arrows) found in animals 96 h after bacterial challenge and administration of a single dose of saline (B) or P128 at 10 mg/kg per animal (C).
DISCUSSION
Staphylococcus aureus is a major cause of bacteremia with significant morbidity and mortality (15). S. aureus bacteremia is associated with complications such as infective endocarditis, osteomyelitis, pneumonia, and foreign body infections which require long-term hospitalization (16). Extensive use of antibiotics for several decades has led to the emergence of antibiotic resistance (17). In this study, we report the efficacy of a novel antistaphylococcal recombinant protein, P128, against MRSA USA300 both under in vitro conditions and in vivo in a rat model of systemic infection.
Biological fluids and physiological microenvironments can influence antibacterial properties of therapeutic molecules. Some antimicrobial peptides that show potent antibacterial activity under in vitro conditions in media are inactivated in plasma and serum (18, 19). Human serum inhibits the antibacterial effect of lysostaphin (20). Therapeutic proteins are also subjected to degradation and inactivation by peptidases or proteases present in biological fluids. Therefore, we considered it important to evaluate the stability and activity of P128 after exposure to whole blood in vitro, before administering the protein into systemic circulation in vivo. Whole rat blood had no inhibitory effect on P128 activity after incubation for 6 h. Based on complete retention of activity in vitro in whole blood, P128 was evaluated by using i.v. dosing in rats.
The mouse peritonitis model has been extensively used for preclinical evaluation of antimicrobial drugs, with mortality the most common efficacy endpoint. Rapid progression of disease culminating in death, often within 24 h, makes the mouse model less relevant to clinical conditions in human infections. Because of their small size and low blood volume, studying the course of infection and monitoring the efficacy of test antibacterial agents in the same mouse is not possible (14). The rat offers many advantages over the mouse as an animal model of infection. Because of their larger size, performing surgical procedures in rats is technically easier than in mice. Their larger blood volume enables serial blood sampling and monitoring of status in the same animal over the course of the disease (13). It also minimizes the number of animals required and helps in complying with the principle of 3Rs (replacement, reduction, and refinement) in animal use. Staphylococcal infections such as endocarditis, osteomyelitis, and wound infections have been modeled in rats (21–23). In most of these models, the endpoints have been reduction in CFU counts in specific target organs such as heart, bones, and wounds (24, 25). S. aureus infections are characterized by the formation of abscesses in soft tissues (26). We developed a rat model of S. aureus bacteremia with renal abscesses that is reproducible and presents with gradual progression of disease, leading to mortality over several days and thus more closely simulating human infection.
Intravenous challenge with S. aureus typically results in bacteremia associated with colonization of bacteria in various organs such as the kidney, liver, and spleen. Abscesses in renal tissues have been reported both in humans and laboratory animal models (27, 28). Although not unique, abscess formation and persistence in host tissues are characteristic features in S. aureus infections (26). Antibiotics have been shown to be ineffective in resolving abscesses because of stationary-phase bacteria, low pH, high protein content, the large number of bacteria, and the presence of bacterial antimicrobial agent-deactivating enzymes within abscesses (29).
In our study, after i.v. administration of 109 CFU of S. aureus USA300 in rats, 2.1 × 108 ± 8.0 × 107 CFU/ml of bacteria could be recovered in blood at 1 min. By 4 h, CFU counts in blood had decreased (to ∼5.00 × 106 CFU/ml), possibly due to dissemination to various organs as reported in the literature (28). By 96 h postinfection, bacterial counts in the blood were ∼5 × 105 CFU/ml, whereas bacterial counts in the kidney were 1.9 × 107 CFU/g of tissue. This high bacterial burden in the kidney correlated with development of abscesses, indicating that S. aureus USA300 localized mainly in the kidney and disseminated into blood and other tissues. Abscesses caused by S. aureus mature over weeks, rupture, and release the contents into peritoneal cavity, leading to new lesions (30).
When mortality was assessed over a period of 14 days, 80 to 100% mortality was observed with i.v. challenge of MRSA USA300 at 109 CFU per animal. Under similar infection conditions in mice, animals succumb to infection rapidly, generally within 24 h, whereas in this model infection is established gradually, leading to mortality over a period of 2 weeks. This is clinically relevant and enabled us to monitor the disease progression and efficacy of P128 in the same animal over the course of disease.
Single i.v. bolus administration of P128 at 2.5 mg/kg per animal resulted in 100% survival of rats. Body weight is a vital indicator of health status in laboratory animals. During infection, physiological changes affect feed and water intake resulting in the loss of body weight (31–33). On day 7 postinfection, there was a significant difference in the mean body weight between saline-treated and P128 (2.5 mg/kg)-treated animals that was presumably due to less severe infection and faster recovery in the P128-treated group. In clinical settings, treatments for bacteremia are often administered by i.v. infusion. We therefore tested P128 for efficacy in this model by i.v. infusion through the jugular vein. This mode of dosing also resulted in dose-dependent survival in rats. Further studies are being done to determine the pharmacokinetic/pharmacodynamic index driving the efficacy by bolus and infusion routes.
Several cases of renal abscesses in S. aureus infections have been reported in human beings (34–36). In a retrospective study, S. aureus was found to be the second most common etiological agent of renal and perinephric abscesses (37). Therefore, in addition to monitoring the dose-to-survival ratio, we evaluated renal abscesses in the untreated and P128-treated animals. Saline-treated animals showed diffuse abscesses in both of the kidneys, whereas in P128-treated animals, either there were no abscesses at all, or very few small pin-point abscesses were discernible. We presume that P128, through its bactericidal effect, contributes to reduction in abscess formation and protects animals from mortality. The probability that P128 acts in the renal tissue warrants further investigation.
Because P128 is a phage-derived protein, it is expected to be immunogenic, leading to the production of antidrug antibodies both in animals and human beings. The impact of antidrug antibodies on the efficacy of P128 has been evaluated. Hyperimmune serum raised against P128 did not exert an inhibitory effect on the activity of the protein (38).
To conclude, we report the efficacy of P128 in a rat model of bacteremia. A single dose of P128 delivered by either i.v. bolus or i.v. infusion resulted in protection from mortality in this model, demonstrating its therapeutic potential. P128 merits clinical development for the treatment of bacteremia in humans.
MATERIALS AND METHODS
P128 protein.
P128 protein of >95% purity was used in all of the studies. Recombinant P128 was expressed in Escherichia coli and purified as described previously (11).
Bacterial strains.
Community associated mupirocin-resistant MRSA USA300 was obtained from BEI Resources. Glycerol stocks of bacterial strains were maintained at −70°C and revived for preparation of challenge doses.
Animals.
All procedures involving animals were approved by the Institutional Animal Ethics Committee. Female Wistar albino rats, 8 to 9 weeks of age, were used for the study. Animals were housed in individually ventilated cages with free access to water, fed ad libitum, and maintained under a 12:12-h light/dark cycle at 19 to 21°C and 55 to 75% relative humidity.
P128 activity in whole blood.
The stability of P128 in rat blood (n = 3) was evaluated by CFU reduction assay after preincubation of the protein in whole blood (38). P128 was diluted into whole blood to a final concentration of 10 μg/ml and incubated for 6 h. Aliquots were assayed for bactericidal activity at 20 min, 60 min, and 6 h by adding USA300 cells (5 × 107 CFU/ml), followed by incubation for an additional 2 h at 37°C. Bacterial cells incubated in whole blood without P128 served as controls. Residual CFU at the end of the assay were enumerated by plating on Luria-Bertani (LB) agar. Each experiment was performed in duplicate and repeated three times.
Preparation of challenge dose.
All of the animal experiments were performed with MRSA strain USA300. The bacterial strain was revived from the glycerol stock onto LB agar plates and incubated at 37°C overnight. Colonies of the bacteria from LB agar plates were resuspended in LB broth and grown for 3 h at 37°C/120 rpm in a shaker-incubator. Bacterial lawns were prepared from these cultures on nutrient agar plates containing 5% sheep blood and incubated at 37°C overnight. The lawn culture was harvested, suspended in sterile saline, and centrifuged at 8,000 rpm for 10 min. Cell pellets were washed three times with sterile saline, and the optical density at 600 nm of the cell suspension was adjusted to obtain a final suspension of 2.5 ± 0.5 × 1010 CFU/ml.
Challenge dose administration.
Animals weighing 140 to 180 g of body weight at the beginning of the study were challenged with exponential-phase bacterial inocula prepared as described above. To determine the minimum lethal dose, a cell suspension of ∼108 or 109 CFU was administered in 200 μl of saline, i.v., via the tail vein. Survival was monitored for 14 days postinfection. To validate the model, animals challenged with 109 CFU of MRSA USA300 were treated subcutaneously with vancomycin (120 mg/kg, two doses separated by a 12-h interval on the day of infection).
Characterization of course of infection and bacteremia.
Blood samples were collected from rats at 1, 30, 60, 120, and 240 min and at 24, 48, 72, and 96 h after the i.v. administration of the bacterial inoculum (∼109 CFU per animal). A 50-μl portion of blood was collected from the tail vein into sterile tubes containing 450 μl of sterile saline. Appropriate dilutions were prepared and plated on LB agar for CFU enumeration. At 96 h postinfection, the animals were euthanized, and gross necropsy was performed. Liver, kidney, spleen, and lung tissues were collected into sample vials containing 2 ml of sterile saline. The tissues were macerated to homogeneity to release the bacteria, and appropriate dilutions were prepared and plated for determination of the bacterial load in the organs. Subsets of the kidneys were fixed in 10% formalin and processed for routine histopathology. Tissue sections were stained with hematoxylin and eosin and examined by light microscopy.
Efficacy of P128 after an i.v. bolus.
Rats were challenged i.v. with 109 CFU of MRSA USA300, and the animals were treated with different doses (2.5, 0.5, or 0.25 mg/kg per animal) of P128 (200 μl) administered i.v. 2 h after infection. The untreated group was administered 200 μl of sterile saline. Survival was monitored for 14 days. The experiment was repeated twice, with each treatment group consisting of 11 to 12 rats.
Efficacy of P128 after i.v. infusion.
A bacterial suspension (109 CFU in 200 μl normal saline) was injected i.v. into rats, and 1 min later blood samples were collected via the tail vein for enumeration of CFU in blood. Animals were then anesthetized with ketamine and xylazine and were catheterized by cannulating the jugular vein using aseptic surgical procedures. Infusion of P128 was initiated within 5 min after bacterial challenge or 2 h after bacterial challenge. P128 (10, 2.5, or 0.5 mg/kg in 1 ml of saline) or 1 ml of saline was infused over 1 h (flow rate = 16.6 μl/min). After the infusion was completed, the catheter was removed, the jugular vein was occluded to prevent bleeding, and the skin incision was sutured. Animals were monitored for survival for 14 days. Subsets of animals assigned for evaluation of renal abscesses were infused with sterile saline (control group) or 10 mg/kg of P128 (treatment group) over 1 h beginning immediately after infection. After 96 h, animals were euthanized and gross necropsy was done to evaluate renal abscesses.
Statistical methods.
Data are presented as means ± the SE. Statistical analysis was performed by one-way analysis of variance with Tukey's post hoc test (GraphPad Prism Software, San Diego, CA). Survival curves were analyzed by log-rank (Mantel-Cox) test. A P value of ≤0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We thank J. Ramachandran, T. S. Balaganesh, and M. Jayasheela for support, encouragement, and suggestions during the study. We thank Amy Percy, Aradhana Vipra, Candida Braithwaite, Umender Sharma, and Vivek Paul for reviewing the manuscript. We acknowledge BEI Resources, Manassas, VA, for providing S. aureus isolate USA300. We thank G. B. Manjunatha Redd, National Institute of Veterinary Epidemiology and Disease Informatics, and B. P. Shankar, Institute of Animal Health and Veterinary Biologicals, Bangalore, India, for providing technical assistance for the histopathology work.
S.C. planned the experiments, participated in conducting experiments, analyzed the data, and wrote the manuscript. M.D., R.C., S.K., and A.J. executed the experiments. B.S. contributed to planning of experiments, data analysis, and manuscript writing. All of the authors have reviewed the manuscript.
REFERENCES
- 1.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Torok ME, Walker S, Wertheim HF, Wilson P, Llewelyn MJ. 2011. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis 11:208–222. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 2.von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 3.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 4.Boucher H, Miller LG, Razonable RR. 2010. Serious infections caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 51(Suppl 2):S183–S197. doi: 10.1086/653519. [DOI] [PubMed] [Google Scholar]
- 5.Daum RS. 2007. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 357:380–390. [DOI] [PubMed] [Google Scholar]
- 6.Fair RJ, Tor Y. 2014. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem 6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan DM. 2007. Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications. Rec Pat Biotechnol 1:113–122. doi: 10.2174/187220807780809463. [DOI] [PubMed] [Google Scholar]
- 8.Fischetti VA. 2010. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol 300:357–362. doi: 10.1016/j.ijmm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmelcher M, Donovan DM, Loessner MJ. 2012. Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, Yu J, Wei H. 2014. Engineered bacteriophage lysins as novel anti-infectives. Front Microbiol 5:542. doi: 10.3389/fmicb.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul VD, Rajagopalan SS, Sundarrajan S, George SE, Asrani JY, Pillai R, Chikkamadaiah R, Durgaiah M, Sriram B, Padmanabhan S. 2011. A novel bacteriophage tail-associated muralytic enzyme (TAME) from phage K and its development into a potent antistaphylococcal protein. BMC Microbiol 11:226. doi: 10.1186/1471-2180-11-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair S, Desai S, Poonacha N, Vipra A, Sharma U. 2016. Antibiofilm activity and synergistic inhibition of Staphylococcus aureus biofilms by bactericidal protein P128 in combination with antibiotics. Antimicrob Agents Chemother 60:7280–7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iannaccone PM, Jacob HJ. 2009. Rats! Dis Model Mech 2:206–210. doi: 10.1242/dmm.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zak O, Sand MA. 1999. Handbook of animal models on infection. Academic Press, San Diego, CA. [Google Scholar]
- 15.Yilmaz M, Elaldi N, Balkan II, Arslan F, Batirel AA, Bakici MZ, Gozel MG, Alkan S, Celik AD, Yetkin MA, Bodur H, Sinirtas M, Akalin H, Altay FA, Sencan I, Azak E, Gundes S, Ceylan B, Ozturk R, Leblebicioglu H, Vahaboglu H, Mert A. 2016. Mortality predictors of Staphylococcus aureus bacteremia: a prospective multicenter study. Ann Clin Microbiol Antimicrob 15:7. doi: 10.1186/s12941-016-0122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troidle L, Eisen T, Pacelli L, Finkelstein F. 2007. Complications associated with the development of bacteremia with Staphylococcus aureus. Hemodial Int 11:72–75. doi: 10.1111/j.1542-4758.2007.00156.x. [DOI] [PubMed] [Google Scholar]
- 17.Shorr AF. 2007. Epidemiology of staphylococcal resistance. Clin Infect Dis 45(Suppl 3):S171–S176. doi: 10.1086/519473. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen LT, Chau JK, Perry NA, de Boer L, Zaat SA, Vogel HJ. 2010. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS One 5:e12684. doi: 10.1371/journal.pone.0012684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kromminga A, Schellekens H. 2005. Antibodies against erythropoietin and other protein-based therapeutics: an overview. Ann N Y Acad Sci 1050:257–265. doi: 10.1196/annals.1313.027. [DOI] [PubMed] [Google Scholar]
- 20.Sygmunt WA, Browder HP, Tavormina PA. 1966. Influence of blood and serum on the antistaphylococcal activity of lysostaphin. J Bacteriol 91:725–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hady WA, Bayer AS, Xiong YQ. 2012. Experimental endocarditis model of methicillin-resistant Staphylococcus aureus (MRSA) in rat. J Vis Exp 2012:e3863. doi: 10.3791/3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucke M, Schmidmaier G, Sadoni S, Wildemann B, Schiller R, Stemberger A, Haas NP, Raschke M. 2003. A new model of implant-related osteomyelitis in rats. J Biomed Mater Res B Appl Biomater 67:593–602. doi: 10.1002/jbm.b.10051. [DOI] [PubMed] [Google Scholar]
- 23.Mendes JJ, Leandro CI, Bonaparte DP, Pinto AL. 2012. A rat model of diabetic wound infection for the evaluation of topical antimicrobial therapies. Comp Med 62:37–48. [PMC free article] [PubMed] [Google Scholar]
- 24.Poeppl W, Tobudic S, Lingscheid T, Plasenzotti R, Kozakowski N, Lagler H, Georgopoulos A, Burgmann H. 2011. Daptomycin, fosfomycin, or both for treatment of methicillin-resistant Staphylococcus aureus osteomyelitis in an experimental rat model. Antimicrob Agents Chemother 55:4999–5003. doi: 10.1128/AAC.00584-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy TM, Deitz JM, Petersen PJ, Mikels SM, Weiss WJ. 2000. Therapeutic efficacy of GAR-936, a novel glycylcycline, in a rat model of experimental endocarditis. Antimicrob Agents Chemother 44:3022–3027. doi: 10.1128/AAC.44.11.3022-3027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi SD, Malachowa N, DeLeo FR. 2015. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol 185:1518–1527. doi: 10.1016/j.ajpath.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BE, Seol HY, Kim TK, Seong EY, Song SH, Lee DW, Lee SB, Kwak IS. 2008. Recent clinical overview of renal and perirenal abscesses in 56 consecutive cases. Korean J Intern Med 23:140–148. doi: 10.3904/kjim.2008.23.3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. 2009. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J 23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bamberger DM. 1996. Outcome of medical treatment of bacterial abscesses without therapeutic drainage: review of cases reported in the literature. Clin Infect Dis 23:592–603. doi: 10.1093/clind/23.1.592. [DOI] [PubMed] [Google Scholar]
- 30.Cheng AG, DeDent AC, Schneewind O, Missiakas D. 2011. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol 19:225–232. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137. doi: 10.1016/S0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 32.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granger JI, Ratti PL, Datta SC, Raymond RM, Opp MR. 2013. Sepsis-induced morbidity in mice: effects on body temperature, body weight, cage activity, social behavior, and cytokines in brain. Psychoneuroendocrinology 38:1047–1057. doi: 10.1016/j.psyneuen.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baradkar VP, Mathur M, Kumar S. 2009. Renal and perinephric abscess due to Staphylococcus aureus. Indian J Pathol Microbiol 52:440–441. doi: 10.4103/0377-4929.55022. [DOI] [PubMed] [Google Scholar]
- 35.Brandeis JM, Baskin LS, Kogan BA, Wara D, Dorenbaum A. 1995. Recurrent Staphylococcus aureus renal abscess in a child positive for the human immunodeficiency virus. Urology 46:246–248. doi: 10.1016/S0090-4295(99)80201-5. [DOI] [PubMed] [Google Scholar]
- 36.Dougherty FE, Gottlieb RP, Gross GW, Denison MR. 1991. Neonatal renal abscess caused by Staphylococcus aureus. Pediatr Infect Dis J 10:463–466. doi: 10.1097/00006454-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Liu XQ, Wang CC, Liu YB, Liu K. 2016. Renal and perinephric abscesses in West China Hospital: 10-year retrospective-descriptive study. World J Nephrol 5:108–114. doi: 10.5527/wjn.v5.i1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George SE, Chikkamadaiah R, Durgaiah M, Joshi AA, Thankappan UP, Madhusudhana SN, Sriram B. 2012. Biochemical characterization and evaluation of cytotoxicity of antistaphylococcal chimeric protein P128. BMC Res Notes 5:280. doi: 10.1186/1756-0500-5-280. [DOI] [PMC free article] [PubMed] [Google Scholar]