ABSTRACT
Therapies for human African trypanosomiasis and Chagas disease, caused by Trypanosoma brucei and Trypanosoma cruzi, respectively, are limited, providing minimal therapeutic options for the millions of individuals living in very poor communities. Here the effects of 10 novel quinolines are evaluated in silico and by phenotypic studies using in vitro and in vivo models. Absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties revealed that most molecules did not infringe on Lipinski's rules, which is a prediction of good oral absorption. These quinolines showed high probabilities of Caco2 permeability and human intestinal absorption and low probabilities of mutagenicity and of hERG1 inhibition. In vitro screens against bloodstream forms of T. cruzi demonstrated that all quinolines were more active than the reference drug (benznidazole [Bz]), except for DB2171 and DB2192, with five (DB2187, DB2131, DB2186, DB2191, and DB2217) displaying 50% effective concentrations (EC50s) of <3 μM (4-fold lower than that of Bz). Nine quinolines were more effective than Bz (2.7 μM) against amastigotes, showing EC50s ranging from 0.6 to 0.1 μM. All quinolines were also highly active in vitro against African trypanosomes, showing EC50s of ≤0.25 μM. The most potent and highly selective candidates for each parasite species were tested in in vivo models. Results for DB2186 were promising in mice with T. cruzi and T. brucei infections, reaching a 70% reduction of the parasitemia load for T. cruzi, and it cured 2 out of 4 mice infected with T. brucei. DB2217 was also active in vivo and cured all 4 mice (100% cure rate) with T. brucei infection.
KEYWORDS: Trypanosoma cruzi, Trypanosoma brucei, experimental chemotherapy, quinolines, in vitro, in vivo, in silico
INTRODUCTION
Currently, more than 1 billion people live in poverty, without access to basic sanitation, favoring the emergence and development of various diseases. The WHO grouped 20 pathologies caused by viruses, fungi, bacteria, protozoans, and helminths, named neglected tropical diseases, that have a severe impact in on public health programs of developing countries but present low interest and investments for the development of early diagnostic tools and safer/potent therapies by most pharmaceutical companies (https://www.dndi.org/) (1, 2).
Human African trypanosomiasis (HAT), or sleeping sickness, is a lethal disease in sub-Saharan Africa caused by two subspecies of Trypanosoma brucei: Trypanosoma brucei gambiense is endemic in western and central Africa, and Trypanosoma brucei rhodesiense is most prevalent in eastern and southern Africa (3). Both parasite subspecies are transmitted by the bite of an infected tsetse fly (genus Glossina). Clinical presentations vary according to the subspecies and the disease stage. The symptoms of the hemolymphatic stage are mostly nonspecific and include fever, headache, and swelling of the lymph nodes. In the second, meningoencephalitic stage, the trypomastigotes infect the central nervous system in addition to the blood and lymph system. Neurological symptoms such as mental confusion and emotional lability as well as convulsions and alteration of the circadian rhythm, a characteristic giving the disease its name, accompany the second stage. Sleeping sickness is fatal, if left untreated (3).
Chagas disease (CD), also a neglected tropical disease, is endemic in 21 countries in Latin America, constituting a continuing serious public health problem and presenting a chronic progressive pathology that affects more than 6 million to 8 million people worldwide (https://www.dndi.org/). CD is caused by the protozoan Trypanosoma cruzi, and its transmission occurs primarily via bug triatomine vectors and may also include other routes such as blood transfusion, congenital transmission (both of which are declining due to public health measures adopted by countries where the disease is endemic), laboratory accidents, and ingestion of food and drinks contaminated with the feces of and/or entire triatomines containing infective forms of the parasite (4). Current treatment of CD is based on two nitroheterocyclic drugs, nifurtimox (Nif) and the 2-nitroimidazole benznidazole (Bz), introduced into clinical therapy over 5 decades ago (5). Recent clinical trials (6, 7) performed on chronically infected patients to evaluate the azole inhibitors of CYP51 (prodrug of ravuconazole and posaconazole) and a nitroderivative (fexinidazole) showed high rates of therapeutic failure despite their excellent activity in vitro and in vivo using experimental models (mouse and canine models), arguing for the generation of more predictive in vitro and in vivo data (5, 8, 9).
For HAT, a total of five drugs are available. However, treatment recommendations fall back to one option for each subspecies and disease stage. Pentamidine (T. b. gambiense) and suramin (T. b. rhodesiense) are used as first-line treatments for first-stage disease, and melarsoprol (T. b. rhodesiense) and a combination of eflornithine and nifurtimox (T. b. gambiense) are used for second-stage disease (1). The main general limitations of the current therapies for both HAT and CD include considerable adverse effects, high costs, the requirement for long periods of exposure, the occurrence of natural and acquired resistant parasites, and treatment failures, especially in the later pathological stages (9). These findings underscore the urgent need to search for new trypanocidal agents with characteristics for each target product profile (for CD and HAT) (https://www.dndi.org/) (4, 10). In this context, many compounds have been tested in vitro and in vivo, but until now, only a few candidates have been found (5, 11–13).
The present work with quinolines is based on a high-throughput phenotypic screening of a library of 700,000 compounds by the Genomics Institute of the Novartis Research Foundation. It yielded over a hundred different scaffolds that were nontoxic to human cells and were active (3.6 μM or lower) against T. brucei (14). One of the initial hits, 2-(2-benzamido)ethyl-4-phenylthiazole, has been extensively explored, and a number of compounds that were highly active against T. brucei in vitro were discovered (15). However, these compounds were only moderately effective in the STIB900 mouse model of T. b. rhodesiense infections, which was attributed, at least in part, to poor metabolic stability (15). We undertook an exploration of N-(2-phenylquinolin-7-yl)benzamides and related compounds, which retain similar geometric relationships between the amide unit and the thiazole nitrogen atom hypothesized to be important for activity. In this quinoline system, the “ethylamine link” of the original thiazole hit is incorporated into the quinoline ring and may improve metabolic stability. While our study was in progress, excellent in vivo results against both early- and late-stage T. brucei infections in mice for a benzothiazole analogue of the initial hit were reported by that same group (15).
Thus, in this work, we investigate the phenotypic activities of 10 novel quinolines through whole-cell-based assays in vitro by assaying different parasite forms (trypomastigotes and amastigotes) and strains (discrete typing unit [DTU] II and VI) of T. cruzi in addition to exploring their biological activities against bloodstream forms of T. b. rhodesiense in vitro. Furthermore, the toxicity profiles of these quinolines were studied by using different mammalian cells and by their predictive pharmacological properties evaluated by pKCSM. Finally, the most promising compounds were moved to animal models of T. b. rhodesiense and T. cruzi infections, with the aim to contribute to the identification of novel therapeutic options for these severe neglected pathologies.
RESULTS
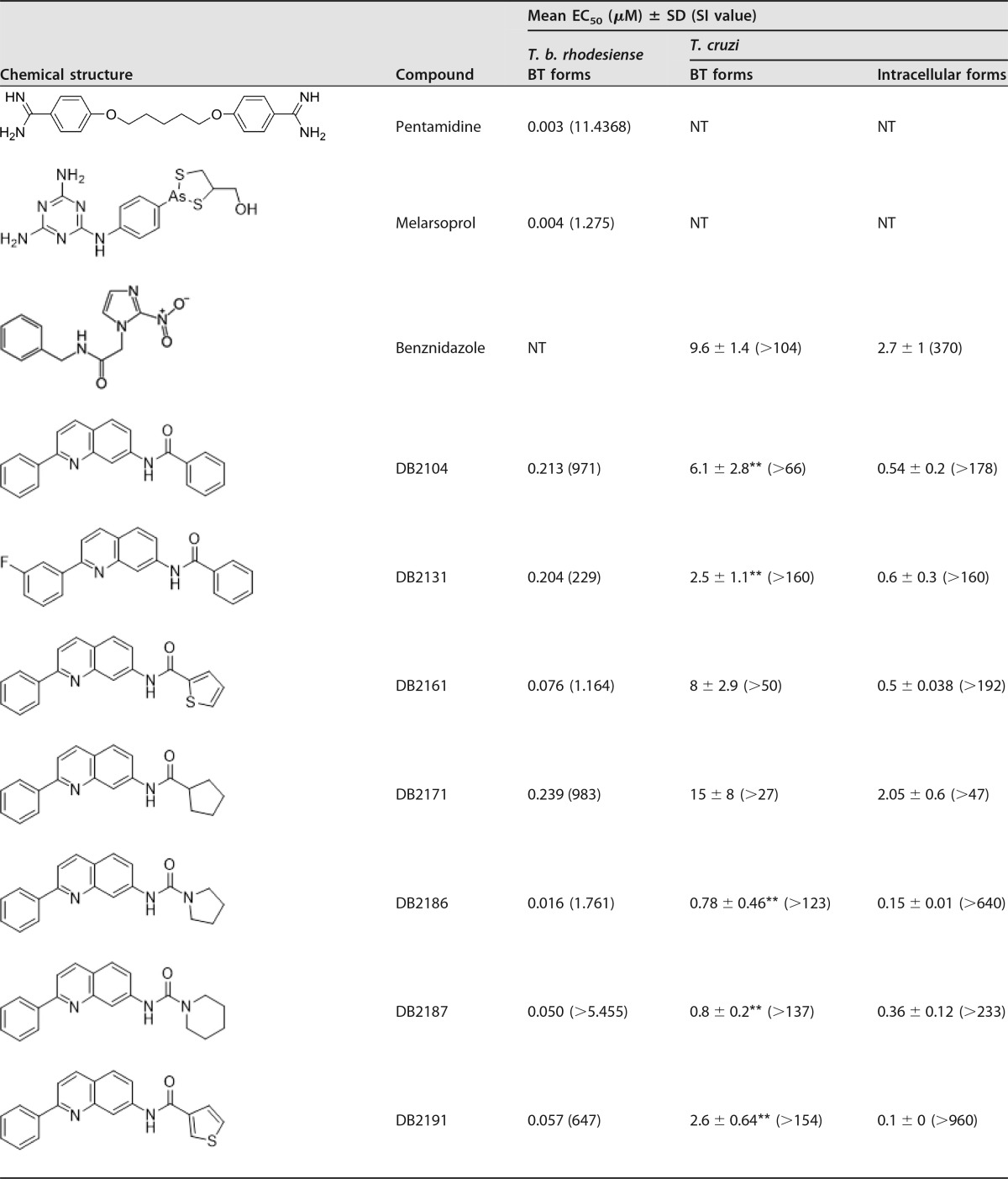
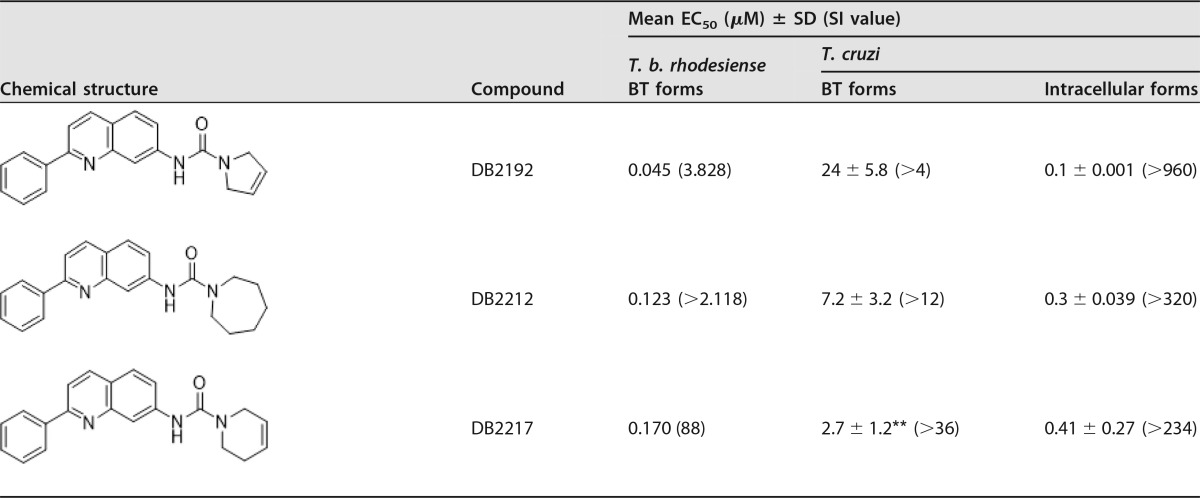
A phenotypic in vitro study using the 10 quinoline derivates (Table 1) was performed on T. cruzi and T. brucei parasites. Considering that all active drug candidates for T. cruzi must also be assessed against the relevant intracellular forms (16), the initial step consisted of analyses of intracellular forms (Tulahuen strain transfected with β-galactosidase [DTU VI] and Y strain [DTU II]). Our findings for the Tulahuen strain showed that all quinolines were more potent than Bz when infected L929 cells were incubated for 96 h at 37°C, with 50% effective concentrations (EC50s) ranging from 0.1 μM up to 2.05 μM and selectivity indices (SI) ranging from 48 up to 960 (Table 1). By screening against intracellular forms of the Y strain lodged inside cardiac cells (CC), the trypanocidal efficacy of quinolines was confirmed, as DB2187 exhibited a low EC50 (1.03 ± 0.3 μM) (data not shown).
TABLE 1.
Antitrypanosomal activities of novel quinolines against bloodstream trypomastigotes of T. brucei and intracellular and bloodstream forms of T. cruzi and corresponding selective indicesa
**, P < 0.05 as determined by ANOVA of the studied compound and Bz; SI, selectivity index; NT, not tested.
Following 24 h of incubation with trypomastigote forms of T. cruzi (Y strain [DTU II]), except for DB2171 and DB2192, all quinolines presented higher trypanocidal activity than that of Bz, exhibiting EC50s of ≤8 μM (Table 1). Among these quinolines, DB2187 and its analogue DB2186 were the most effective (EC50 of ≤0.8 μM), being about 12-fold more potent than the reference drug. All molecules also showed high trypanocidal activity against T. brucei bloodstream forms, with EC50s ranging from 0.016 to 0.239 μM, and strong selectivity for this parasite, with selectivity indices ranging from 88 to 5,455 (Table 1). The cytotoxicity data for the studied quinolines using colorimetric assays with PrestoBlue (cardiac cells) and alamarBlue (L929 cultures) showed that all molecules were tolerated, with no detectable toxicity at concentrations of up to 96 μM after 24 to 96 h of incubation (data not shown). The lack of mammalian host toxicity was confirmed when DB2104, DB2131, DB2161, DB2171, and DB2191 were tested (up to 48 h) using higher concentrations on cardiac cells (up 400 μM) (data not shown). Cytotoxicity against L6 cells was also low and varied from 15 μM (DB2217) to >270 μM (DB2187). Fifty percent lethal concentrations (LC50s) of each compound on L6 cells can be deduced from the SI of T. b. rhodesiense (Table 1).
For both parasite species (T. brucei and T. cruzi), DB2186 was the most potent molecule from this series, without inhibiting mammalian cells. Absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties of quinolines predicted by using the pKCSM tool revealed that DB2186, DB2187, DB2192, and DB2217 did not infringe on Lipinski's rule of five, which is a prediction of good oral absorption (Table 2). The quinolines showed a good probability of permeability on Caco2 cells, with values above the adopted threshold of 0.9; probabilities of human intestinal absorption of >89%, with an even better oral absorption profile than that of Bz; and a positive prediction to be metabolized by CYP3A4 (Table 3). These quinolines have a low probability of mutagenicity and no prediction to inhibit hERG1, although they all show the possibility of inhibiting hERG2 and a hepatotoxicity profile similar to that of Bz (Table 4). Evaluation of hepatic markers in biochemical analyses in vivo using mouse models of acute toxicity demonstrated no alterations of the plasma levels of alanine aminotransferase (ALT) (except for DB2192), aspartate aminotransferase (AST), urea, and creatine kinase (CK) in addition to no major clinical signs (except for losses of animal weight) when mice were given up to 200 mg/kg of body weight of DB2187 and its derivatives DB2186, DB2191, and DB2192 and monitored up to 48 h (data not shown). Next, based on the excellent phenotypic findings and lack of preliminary acute toxicity indications, DB2187 and derivatives were moved to in vivo antiparasitic analyses using mouse models.
TABLE 2.
Physicochemical parameters and Lipinski's rule of fivea
| Compound | Water solubility (mg/liter) | No. of donors | No. of acceptors | LogP | MW |
|---|---|---|---|---|---|
| DB2104 | 2.394 | 2 | 4 | 5.154 | 324.383 |
| DB2131 | 1.041 | 2 | 4 | 5.293 | 342.373 |
| DB2161 | 1.416 | 3 | 4 | 5.215 | 330.412 |
| DB2171 | 2.775 | 2 | 4 | 5.030 | 316.404 |
| DB2186 | 3.887 | 2 | 4 | 4.529 | 317.392 |
| DB2187 | −5.9 | 1 | 2 | 4.9 | 333.419 |
| DB2191 | 1.416 | 3 | 4 | 5.215 | 330.412 |
| DB2192 | 5.356 | 2 | 4 | 4.305 | 315.376 |
| DB2212 | 0.934 | 2 | 4 | 5.309 | 345.446 |
| DB2217 | 2.587 | 2 | 4 | 4.695 | 329.403 |
| Bz | 376.248 | 1 | 5 | 0.11 | 260.253 |
MW, molecular weight; LogP, partition coefficient.
TABLE 3.
In silico ADMEa
| Parameter | Value for compound |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DB2104 | DB2131 | DB2161 | DB2171 | DB2186 | DB2187 | DB2191 | DB2192 | DB2212 | DB2217 | Bz | |
| Absorption | |||||||||||
| Caco2 cell permeability (log cm/s) | 1.491 | 1.163 | 1.773 | 1.765 | 1.373 | 1.21 | 1.773 | 1.361 | 1.158 | 1.384 | 0.479 |
| Intestinal absorption (human) (%) | 94.747 | 89.969 | 89.46 | 90.659 | 90.877 | 91.515 | 89.46 | 91.353 | 90.1 | 90.964 | 68.885 |
| Skin permeability (logKp) | −2.763 | −3.167 | −3.118 | −3.092 | −3.214 | −3.012 | −3.118 | −3.238 | −3.198 | −3.23 | −2.893 |
| Distribution | |||||||||||
| Vss (human) (liters/kg) | 0.345 | 5.521 | 5.140 | 6.124 | 4.721 | −0.115 | 5.140 | 4.624 | 5.508 | 4.989 | 0.787 |
| Fraction unbound (human) | 0 | 0.278 | 0.292 | 0.3 | 0.321 | 0.064 | 0.292 | 0.331 | 0.275 | 0.307 | 0.503 |
| BBB permeability | 0.264 | 0.265 | 0.241 | 0.254 | 0.201 | 0.269 | 0.241 | 0.2 | 0.227 | 0.213 | −0.619 |
| CNS permeability | −1.013 | −2.778 | −2.687 | −2.687 | −2.691 | −1.639 | −2.687 | −2.691 | −2.782 | −2.737 | −2.995 |
| Metabolism | |||||||||||
| CYP2D6 substrate | No | No | No | No | No | No | No | No | No | No | No |
| CYP3A4 substrate | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| CYP1A2 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| CYP2C19 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| CYP2C9 inhibitor | Yes | No | No | No | No | No | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No | No | No |
| Excretion | |||||||||||
| Total clearance (ml/min/kg) | 3.793 | 6.998 | 12.023 | 10.162 | 8.433 | 0.545 | 12.023 | 6.714 | 6.823 | 6.368 | 4.217 |
Vss, volume of distribution at steady state; BBB, blood-brain barrier; CNS, central nervous system; logKp, skin permeability constant (cm/h).
TABLE 4.
In silico toxicitya
| Parameter | Value for compound |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DB2104 | DB2131 | DB2161 | DB2171 | DB2186 | DB2187 | DB2191 | DB2192 | DB2212 | DB2217 | Bz | |
| AMES toxicity | No | No | No | No | No | No | No | No | No | No | Yes |
| Max tolerated dose (human) (mol/kg) | 17.418 | 1.393 | 1.758 | 1.807 | 1.758 | 0.784 | 1.758 | 1.766 | 1.330 | 1.535 | 9.638 |
| hERG1 inhibitor I | No | No | No | No | No | No | No | No | No | No | No |
| hERG2 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Oral rat acute toxicity (LD50) (mol kg−1) | 2.535 | 2.906 | 2.924 | 2.719 | 2.817 | 2.856 | 2.924 | 2.783 | 2.885 | 2.819 | 2.454 |
| Oral rat chronic toxicity (LOAEL) (mg/kg of body wt/day) | 289.068 | 46.345 | 61.094 | 80.168 | 47.973 | 1.878 | 61.094 | 43.451 | 46.345 | 42.756 | 44.566 |
| Hepatotoxicity | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Skin sensitization | No | No | No | No | No | No | No | No | No | No | No |
| Tetrahymena pyriformis toxicity (pIGC50 [μg/liter]) | 5.929 | 29.923 | 33.884 | 31.405 | 31.842 | 1.256 | 33.884 | 31.333 | 28.642 | 30.479 | 16.866 |
| Minnow toxicity (LC50 [mM]) | 0.586 | 4.732 | 3.365 | 4.325 | 6.039 | 0.739 | 3.365 | 7.244 | 3.524 | 5.534 | 44.566 |
LD50, 50% lethal dose; LOAEL, lowest observed adverse effect level; pIGC50, negative logarithm of the concentration required to inhibit 50% growth (in log µg/liter).
Male mice inoculated with 104 bloodstream forms (T. cruzi Y strain) and treated (intraperitoneally [i.p.]) at 5 and 8 days postinfection (dpi) using nontoxic concentrations of DB2187 gave a maximum reduction of parasitemia of 38% at the peak (8 dpi) in comparison to the vehicle group but failed to protect against mortality induced by parasite infection (Table 5). The administration of nontoxic concentrations of DB2186, DB2191, and DB2192 at 25 mg/kg/day for five consecutive days in T. cruzi-infected male and female mice showed that DB2186 was the most active compound, reaching 25 and 70% reductions of the blood parasitemia loads, respectively, when 25 mg/kg was given for five consecutive days, while Bz completely suppressed parasitism (Table 5). Regarding the effects on mortality induced by experimental T. cruzi infection, infected female and male mice treated with the vehicle only displayed 50 and 17% survival rates, respectively, whereas all the Bz-treated animals were alive. The tested quinolines were not able to provide significant protection against mortality (Table 5).
TABLE 5.
Antitrypanosomal activities of quinolines in mouse models of T. cruzi infection (Y strain) using 25 mg/kg (i.p.) for five consecutive days starting at parasitemia onset (5 dpi)
| Compound | Mouse gender | % parasite variation at parasitemia peak (8 dpi) | % cumulative mortality at 30 days posttherapy |
|---|---|---|---|
| Benznidazolea | Female | 100 | 0 |
| Male | 100 | 0 | |
| Vehicle | Female | 0 | 50 |
| Male | 0 | 83 | |
| DB2187b | Male | −38 | 100 |
| DB2186 | Female | −25 | 75 |
| Male | −70 | 50 | |
| DB2191 | Female | −9 | 50 |
| Male | −27 | 100 | |
| DB2192 | Female | +74 | 33 |
| Male | +28 | 100 |
Benznidazole was tested at 100 mg/kg p.o.
DB2187 was tested at 20 mg/kg i.p. at 5 and 8 dpi.
The quinolines were also evaluated for their antitrypanosomal efficacy in T. b. rhodesiense-infected mice. In the preliminary experiment, the compounds were tested on small groups of 2 mice treated at 40 mg/kg/day i.p. for three consecutive days. Six compounds showed activity on the level of a strong reduction of parasitemia (>98%) in at least one of the two mice and were well tolerated at the tested dosage (Table 6). These compounds were further tested in 4 infected mice at a higher dosage of 50 mg/kg/day i.p. for four consecutive days. DB2186 and DB2217 were the best molecules and cured 2 of 4 infected mice and all 4 infected mice, respectively (Table 6).
TABLE 6.
Antitrypanosomal activities of quinolines in a mouse model of T. b. rhodesiense STIB900 infection
| Compound | % parasite reduction after treatment with 3 doses of 40 mg/kg i.p. |
No. of mice cured with 4 doses of 50 mg/kg i.p./no. of mice testedb | |
|---|---|---|---|
| 1 day | 3 days | ||
| Pentamidinea | 100 | 100 | 4/4a |
| 100 | 100 | ||
| DB2104 | >98 | >98 | NT |
| >98 | >98 | ||
| DB2131 | >98 | 100 | 0/4 |
| 98 | >98 | ||
| DB2161 | >98 | 100 | 0/4 |
| >98 | >98 | ||
| DB2171 | >98 | 99 | 0/4 |
| >98 | >98 | ||
| DB2186 | 100 | >98 | 2/4 |
| 100 | >98 | ||
| DB2187 | >98 | 100 | 0/4 |
| 98 | >98 | ||
| DB2191 | >98 | >98 | NT |
| >98 | >98 | ||
| DB2192 | >98 | >98 | NT |
| >98 | >98 | ||
| DB2212 | >98 | >98 | NT |
| >98 | >98 | ||
| DB2217 | >98 | >98 | 4/4 |
| >98 | 100 | ||
Pentamidine was tested at 3 doses of 4 mg/kg i.p. and at 4 doses of 1 mg/kg i.p. and cured all infected mice.
NT, not tested.
DISCUSSION
Most drug development programs for neglected diseases are time-consuming (often more than 10 years) and highly expensive (more than $1 billion) and get only limited attention by the pharmaceutical industry. Until now, no vaccine has been available for Chagas disease and for HAT, and the current therapies available have strong liabilities; thus, novel therapeutic options are urgently needed. Drug discovery and development strategies include phenotypic screening of synthetic and natural molecules, assessment of combination therapies, repurposing of medicines, and drug development toward selective parasite targets (11, 17, 18). Our goal was to investigate the biological effects in vitro and in vivo of the 10 novel quinoline derivatives (Table 1) on T. cruzi and T. brucei infections. We conducted different analyses that included computational and cell-based screening as well as mouse models of trypanosome infections. As reported previously, in silico analyses have the advantages of low cost, fast processing, and the fact that compounds can be evaluated without synthesizing them, allowing large libraries to be explored (22). However, computational screens often fail to simulate the full complexity of biological systems and need to be complemented with experimental studies (19). Here, the antiparasitic activities of the novel quinolines were explored, considering different aspects of the drug discovery cascade performed in in silico, in vitro (whole-cell-based), and in vivo (the most biologically realistic) assays, according to current strategies for hit and lead identification for novel anti-T. cruzi and anti-T. brucei drugs. Our findings demonstrated the promising in vitro activities of these compounds against both bloodstream trypomastigotes (T. cruzi and T. brucei) and intracellular forms (T. cruzi) of these parasites, with most molecules exhibiting greater potency than the reference drug for Chagas disease (Bz). The quinolines also have the potential to be developed for T. brucei, although they were less potent than the highly toxic compound melarsoprol or the T. b. gambiense first-stage drug pentamidine. The quinoline DB2186 was very active against both trypanosome species regardless of the parasite form, displaying quite high selectivity indices that were even superior to those reported previously for novel hits for CD and HAT (11, 12, 20). Regarding T. cruzi screens, the quinolines were active against parasite strains from different DTUs (DTUs II and VI for the Y and Tulahuen strains, respectively), furthermore showing low toxicity toward mammalian host cells, including primary cultures of cardiac cells that provide, in a more sensitive manner, the potential for in vivo cardiotoxicity. These are very critical data since the heart represents an important target for T. cruzi infection and inflammation (21). In fact, plasma biochemical analyses of quinoline-treated mice confirmed the low-cardiotoxicity profile determined by CK measurements. Thus, in vitro whole-cell-based screening associated with theoretical analyses of the ADMET properties and mouse models of acute toxicity were used to select potential drug candidates to proceed to in vivo efficacy evaluations (22). The in silico properties of the novel quinolines were evaluated by using the pKCSM tool, and the overall findings predicted good oral absorption, a high probability of permeability in Caco2 cells, good human intestinal absorption, and low probabilities of mutagenicity and inhibition of hERG1. The preliminary acute murine toxicity assays failed to demonstrate increased levels of hepatic lesion markers such as ALT and AST, except for DB2192 (statistically significant enhancement of ALT levels, indicative of hepatic damage). To evaluate efficacy in vivo against T. cruzi infection, both female and male mouse models were used, and our data confirmed the higher susceptibility of male mice to T. cruzi experimental infection than of female mice (25). The findings also demonstrated that DB2186 was the most promising candidate for T. cruzi infection as well as for T. brucei infection in murine models. It is important to consider that DB2186 exhibited consistently high selectivity indices for T. cruzi (123 and 640 for bloodstream trypomastigote [BT] and intracellular forms) and T. brucei (1,761 for BT). It is interesting to note that DB2171 is much less active than two close analogues, DB2186 and DB2192, pointing to the importance of the urea fragment for activity compared to the simple amide unit.
The limited water solubility of the quinolines may have impaired a more successful in vivo result, which also was not improved by the use of other vehicles, including cyclodextrin and carboxymethylcellulose (data not shown). It is possible that minor structural modifications can improve their solubility, allowing further animal studies. The present set of results provides a basis for the development of novel quinoline derivatives, following medicinal chemistry approaches, presenting better solubility and improved potency, thus contributing to the identification of more effective and safe medicines to treat neglected tropical diseases such as Chagas disease and HAT.
MATERIALS AND METHODS
Compounds.
The synthesis and characterization of the 10 quinolines (Table 1) can be found in the supplemental material. For T. cruzi assays, Bz (2-nitroimidazole; Laboratório Farmacêutico do Estado de Pernambuco [LAFEPE], Brazil) was used as a reference drug, and stock solutions were prepared in dimethyl sulfoxide (DMSO), with the final concentrations of the solvent never exceeding 0.6% and 10% in assays in vitro and in vivo, respectively, which are not toxic to the parasite, mammalian cells, and mice. For T. b. rhodesiense, pentamidine and melarsoprol were used as reference drugs. Pentamidine (Sigma) was dissolved in DMSO, and melarsoprol (Arsobal; Aventis) was dissolved in water.
Computational assessment of drug-like properties.
Absorption, distribution, metabolism, excretion, and toxicity (ADMET and Lipinski's rule of five) properties of the studied quinolines were evaluated by using the pKCSM approach, which uses graph-based signatures to develop predictive ADMET (22, 24).
Parasites. (i) T. cruzi.
BT forms of the Y strain were obtained from blood samples of infected albino Swiss mice at the peak of parasitemia. The purified parasites were resuspended in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum as reported previously (25, 26). Trypomastigotes of the Tulahuen strain expressing the Escherichia coli β-galactosidase gene were collected from the supernatant of T. cruzi-infected L929 cultures as reported previously (16, 27).
(ii) T. brucei.
T. b. rhodesiense strain STIB900, a derivative of strain STIB704, was isolated from a patient in Ifakara, Tanzania, in 1982 (28). Bloodstream forms were used for in vitro screening as well as for the acute mouse infection model, which mimics the first stage of HAT.
Mammalian cell cultures.
For toxicity assays on mammalian cells, primary cultures of cardiac cells (CC) obtained from mouse embryos were plated onto 96-well plates previously coated with 0.01% gelatin (25). L929 cell lineages were obtained as described previously by Romanha et al. (16). L6 cells (rat skeletal myoblast; ATCC CRL-1458) were maintained in RPMI 1640 medium supplemented with 2 mM l-glutamine, 5.95 g/liter HEPES, 2 g/liter NaHCO3, and 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2 (29).
In vitro cytotoxicity tests.
CC were incubated for 24 h at 37°C with different concentrations of each compound (up to 400 μM) diluted in DMEM; the morphology, cell density, and spontaneous contractibility were then evaluated by light microscopy; and cellular viability was determined by the PrestoBlue test as reported previously (27). L929 cells were incubated for 96 h at 37°C with different concentrations of each compound (up to 96 μM) diluted in RPMI, and cellular viability was determined by the alamarBlue test as reported previously (27). The results were expressed according to the manufacturer's instructions, and the LC50 value, which corresponds to the concentration that reduces cellular viability by 50%, was determined. Cytotoxicity assays performed by using L6 cells were conducted with a 72-h compound exposure time as previously reported (29). The selectivity index (SI) was expressed as the ratio between the LC50 values obtained on host or L6 cells and the EC50 obtained for the parasites.
Trypanocidal activity.
For T. cruzi assays, BT of the Y strain (DTU II) (30) (5 × 106 BT per ml) were incubated for 2 and 24 h at 37°C in RPMI in the presence or absence of serial dilutions of the compounds (up to 32 μM). After compound incubation, the death rates of parasites were determined by light microscopy through the direct quantification of the number of live parasites by using a Neubauer chamber, and the EC50 (the compound concentration that reduces the number of parasites by 50%) was calculated (22). For assaying intracellular forms of the Y strain (DTU II), the most promising compound was further evaluated with infection of primary cultures of CC (using a ratio of 10 BT to 1 host cell). After 24 h of parasite interaction, the cultures were rinsed and incubated with the compounds for 48 h. After fixation with Bouin solution and staining with Giemsa solution, the percentage of CC infection and the mean number of parasites per infected cell were calculated by light microscopy for the determination of EC50s of the infection index (II) (II = % infected host cells × mean number of parasites per cell) (26). Culture-derived trypomastigotes of T. cruzi (Tulahuen strain expressing β-galactosidase [DTU VI]) were used to infect L929 cultures at a ratio of 10:1 (parasites/host cell). After 2 h, the cultures were washed and cultivated for another 48 h for the establishment of infection. The compounds were then added at increasing nontoxic concentrations to mammalian host cells, followed by maintenance at 37°C for 96 h for the determination of EC50s. After the addition of 50 μl of the substrate (CPRG [chlorophenol red glycoside] at 500 mM) in 0.5% Nonidet P40 and incubation at 37°C for 18 h, the absorbance at 570 nm was measured, and results were expressed as percent inhibition of the infection rate (16). For T. brucei assays, bloodstream forms of T. b. rhodesiense strain STIB900 were incubated in minimum essential medium (MEM) for 72 h in the presence of 3-fold serial dilutions at 37°C in a humidified atmosphere containing 5% CO2. Parasite viability was assessed with the viability marker resazurin after a 3-day drug exposure time as previously reported (29).
Mouse acute toxicity.
In order to determine the no-observed-adverse-effect level (NOAEL), increasing doses of the tested compounds (up to 200 mg/kg of body weight) were injected by the i.p. route individually into female Swiss mice (21 to 23 g; n = 2 per assay of the tested compounds). Treated animals were inspected for toxic and subtoxic symptoms according to Organization for Economic Cooperation and Development (OECD) guidelines (33). Forty-eight hours after compound injection, the NOAEL values were determined as reported previously (31). Biochemical analyses performed 48 h after compound exposure were performed at the ICTB platform (Fiocruz/RJ) as reported previously (17, 31).
Mouse infection and treatment.
For acute T. cruzi infection models, male and female Swiss Webster mice (18 to 20 g) obtained from the animal facilities of ICTB were housed at a maximum of 7 per cage, kept in a specific-pathogen-free (SPF) room at 20°C to 24°C under a 12-h-light/12-h-dark cycle, and provided with sterilized water and chow ad libitum. The animals were allowed to acclimate for 7 days before the start of the experiments. Infection was performed by i.p. injection of 104 bloodstream trypomastigotes (Y strain). Age-matched noninfected mice were maintained under identical conditions (26). Quinolines were first dissolved in DMSO and then freshly diluted with sterile distilled water. The stock solution of Bz was prepared in sterile distilled water with 3% Tween 80 (Sigma-Aldrich). The animals were divided into the following groups (n > 3 per group): uninfected (noninfected and nontreated), untreated (infected but treated with the vehicle only), and treated (infected and treated with the compounds). Therapy was performed through the administration of 5 to 20 mg/kg at parasitemia onset (5 dpi) and the peak of parasitemia (8 dpi) according to an animal model reported previously (23). Alternatively, the most promising quinoline derivatives were administered for five consecutive days, starting at 5 dpi, using up to 25 mg/kg/day (i.p.) and 100 mg/kg/day Bz (orally [p.o.]). For all assays, only mice with positive parasitemia were used in the infected groups. Parasitemia levels in T. cruzi assays were individually checked by direct microscopic counting of parasites in 5 μl of blood, and mortality rates were checked daily until 30 days posttreatment and expressed as a percentage of cumulative mortality (CM) as described previously (27).
For T. brucei models, efficacy experiments were performed as previously reported (32), with modifications to soften the stringency of the mouse model of infection, and in line with the 3R principles for animal testing (reduce, refine, and replace), the number of mice was reduced in the primary in vivo screen. Female NMRI mice were infected i.p. with 104 T. b. rhodesiense STIB900 bloodstream trypanosomes. Experimental groups of two mice were treated with the new test compounds at 40 mg/kg i.p. on three consecutive days from day 1 to day 3 postinfection (total dose of 120 mg/kg i.p.). A control group was infected but remained untreated. The tail blood of all mice was checked microscopically for parasitemia reduction 24 h and 96 h after the last dose. Parasite reduction in mice treated with the experimental compounds was compared with that in the untreated control mice. Mice were euthanized at 96 h posttreatment if parasites were still detected in the tail blood. Aparasitemic mice were further examined twice per week for 30 days, or mice were euthanized after parasitemia relapses were detected. Mice that remained aparasitemic until day 30 were considered cured. The data from the follow-up efficacy study for compounds that achieved a parasite reduction of at least 98% in one of the two treated mice were comparable to those of groups of 4 infected mice treated for four consecutive days with a higher dosage of 50 mg/kg i.p. (total dose of 200 mg/kg i.p.). Pentamidine was used as a positive drug control, and it cured mice at 3× 4 mg/kg and 4× 1 mg/kg i.p.
Statistical analyses.
Statistical analyses were performed by analysis of variance (ANOVA) with the level of significance set at a P value of ≤0.05.
Ethics.
All animal procedures performed at Fiocruz were carried out in accordance with the guidelines established by the Committee of Ethics for the Use of Animals (CEUA LW16/14). All protocols and procedures using animal models of T. brucei infection were reviewed and approved by the local veterinary authorities of the Canton Basel-Stadt, Switzerland.
Supplementary Material
ACKNOWLEDGMENTS
The present study was supported by grants from the Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação Oswaldo Cruz, PDTIS, PAEF/CNPq/Fiocruz, and CAPES. M.N.C.S. is a research fellow of the CNPq and CNE Research. This work was also supported, in part, by National Institutes of Health grant no. AI06420 and by The Bill and Melinda Gates Foundation through a subcontract with the CPDD (to D.W.B. and R.B.).
We thank the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ for use of its facilities.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01936-17.
REFERENCES
- 1.World Health Organization. 2017. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. World Health Organization, Geneva, Switzerland: http://www.who.int/neglected_diseases/2010report/en/. [Google Scholar]
- 2.Mackey TK, Liang BA, Cuomo R, Hafen R, Brouwer KC, Lee DE. 2014. Emerging and reemerging neglected tropical diseases: a review of key characteristics, risk factors and the policy and innovation environment. Clin Microbiol Rev 4:949–979. doi: 10.1128/CMR.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Büscher P, Cecchi G, Jamonneau V, Priotto G. 2017. Human African trypanosomiasis. Lancet 390:2397–2409. doi: 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- 4.Chatelain E. 2015. Chagas disease drug discovery: toward a new era. J Biomol Screen 1:22–35. doi: 10.1177/1087057114550585. [DOI] [PubMed] [Google Scholar]
- 5.Chatelain E, Konar N. 2015. Translational challenges of animal models in Chagas disease drug development: a review. Drug Des Devel Ther 9:4807–4823. doi: 10.2147/DDDT.S90208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina I, Salvador F, Sánchez-Montalvá A. 2015. The use of posaconazole against Chagas disease. Curr Opin Infect Dis 5:397–407. doi: 10.1097/QCO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 7.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guh LF, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S, BENEFIT Investigators . 2015. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med 14:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 8.Devine W, Thomas SM, Erath J, Bachovchin KA, Lee PJ, Leed SE, Rodriguez A, Sciotti RJ, Mensa-Wilmot K, Pollastri MP. 2017. Antiparasitic lead discovery: toward optimization of a chemotype with activity against multiple protozoan parasites. ACS Med Chem Lett 3:350–354. doi: 10.1021/acsmedchemlett.7b00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatelain E. 2017. Chagas disease research and development: is there light at the end of the tunnel? Comput Struct Biotechnol J 15:98–103. doi: 10.1016/j.csbj.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Don R, Ioset JR. 2013. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology 141:140–146. doi: 10.1017/S003118201300142X. [DOI] [PubMed] [Google Scholar]
- 11.Field MC, Horn D, Fairlamb AH, Ferguson MA, Gray DW, Read KD, De Rycker M, Torrie LS, Wyatt PG, Wyllie S, Gilbert IH. 2017. Anti-trypanosomatid drug discovery: an ongoing challenge and a continuing need. Nat Rev Microbiol 4:217–231. doi: 10.1038/nrmicro.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenan M, Chaplin JH. 2015. A new era for Chagas disease drug discovery? Prog Med Chem 54:185–230. doi: 10.1016/bs.pmch.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Bahia MT, Nascimento AF, Mazzeti AL, Marques LF, Gonçalves KR, Mota LW, Diniz LDF, Caldas IS, Talvani A, Shackleford DM, Koltun M, Saunders J, White KL, Scandale I, Charman SA, Chatelain E. 2014. Antitrypanosomal activity of fexinidazole metabolites, potential new drug candidates for Chagas disease. Antimicrob Agents Chemother 58:4362–4370. doi: 10.1128/AAC.02754-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatipaka HB, Gillespie JR, Chatterjee AK, Norcross NR, Hulverson MA, Ranade RM, Nagendar P, Creason SA, McQueen J, Duster NA, Nagle A, Supek F, Molteni V, Wenzler T, Brun R, Glynne R, Buckner FS, Gelb MH. 2014. Substituted 2-phenylimidazopyridines: a new class of drug leads for human African trypanosomiasis. J Med Chem 57:828–835. doi: 10.1021/jm401178t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patrick DA, Wenzler T, Yang S, Weiser PT, Wang MZ, Brun R, Tidwell RR. 2016. Synthesis of novel amide and urea derivatives of thiazol-2-ethylamines and their activity against Trypanosoma brucei rhodesiense. Bioorg Med Chem 24:2451–2465. doi: 10.1016/j.bmc.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romanha AJ, de Castro SL, Soeiro MNC, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Freitas LH Jr, Romero LI, Bahia MT, Lotrowska M, Soares M, Andrade SG, Armstrong T, Degrave W, Andrade ZA. 2010. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz 105:233–238. doi: 10.1590/S0074-02762010000200022. [DOI] [PubMed] [Google Scholar]
- 17.Soeiro MNC, Werbovetz K, Boykin DW, Wilson WD, Wang MZ, Hemphill A. 2013. Novel amidines and analogues as promising agents against intracellular parasites: a systematic review. Parasitology 140:929–951. doi: 10.1017/S0031182013000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peña I, Pilar Manzano M, Cantizani J, Kessler A, Alonso-Padilla J, Bardera AI, Alvarez E, Colmenarejo G, Cotillo I, Roquero I, de Dios-Anton F, Barroso V, Rodriguez A, Gray DW, Navarro M, Kumar V, Sherstnev A, Drewry DH, Brown JR, Fiandor JM, Julio Martin J. 2015. New compound sets identified from high throughput phenotypic screening against three kinetoplastid parasites: an open resource. Sci Rep 5:8771. doi: 10.1038/srep08771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams K, Bilsland E, Sparkes A, Aubrey W, Young M, Soldatova LN, De Grave K, Ramon J, de Clare M, Sirawaraporn W, Oliver SG, King RD. 2015. Cheaper faster drug development validated by the repositioning of drugs against neglected tropical diseases. J R Soc Interface 104:20141289. doi: 10.1098/rsif.2014.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsuno K, Burrows JN, Duncan K, Hooft van Huijsduijnen R, Kaneko T, Kita K, Mowbray CE, Schmatz D, Warner P, Slingsby BT. 2015. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov 11:751–758. doi: 10.1038/nrd4683. [DOI] [PubMed] [Google Scholar]
- 21.Rassi A Jr, Marin Neto JA, Rassi A. 2017. Chronic Chagas cardiomyopathy: a review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Mem Inst Oswaldo Cruz 3:224–235. doi: 10.1590/0074-02760160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nefertiti ASG, Batista MM, Da Silva PB, Torres-Santos EC, Cunha EF Jr, Green J, Kumar A, Farahat AA, Boykin DW, Soeiro MNC. 2017. Anti-parasitic effect of novel amidines against Trypanosoma cruzi: phenotypic and in silico absorption, distribution, metabolism, excretion and toxicity analysis. Parasitol Open 5:e5. doi: 10.1017/pao.2017.5. [DOI] [Google Scholar]
- 23.Guedes-da-Silva FH, Batista DG, da Silva CF, Meuser MB, Simões-Silva MR, de Araújo JS, Ferreira CG, Moreira OC, Britto C, Lepesheva GI, Soeiro MDN. 2015. Different therapeutic outcomes of benznidazole and VNI treatments in different genders in mouse experimental models of Trypanosoma cruzi infection. Antimicrob Agents Chemother 12:7564–7570. doi: 10.1128/AAC.01294-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pires DE, Blundell TL, Ascher DB. 2015. pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem 9:4066–4072. doi: 10.1021/acs.jmedchem.5b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meirelles MN, De Araujo-Jorge TC, Miranda CF, De Souza W, Barbosa HS. 1986. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur J Cell Biol 41:198–206. [PubMed] [Google Scholar]
- 26.Batista DG, Pacheco MG, Kumar A, Branowska D, Ismail MA, Hu L, Boykin DW, Soeiro MN. 2010. Biological, ultrastructural effect and subcellular localization of aromatic diamidines in Trypanosoma cruzi. Parasitology 2:251–259. doi: 10.1017/S0031182009991223. [DOI] [PubMed] [Google Scholar]
- 27.Timm BL, da Silva PB, Batista MM, da Silva FHG, da Silva CF, Tidwell RR, Patrick DA, Jones SK, Bakunov SA, Bakunova SM, Soeiro MNC. 2014. In vitro and in vivo biological effect of novel arylimidamide derivatives against Trypanosoma cruzi. Antimicrob Agents Chemother 7:3720–3726. doi: 10.1128/AAC.02353-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brun R, Schumacher R, Schmid C, Kunz C, Burri C. 2001. The phenomenon of treatment failures in human African trypanosomiasis. Trop Med Int Health 6:906–914. doi: 10.1046/j.1365-3156.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- 29.Bakunov SA, Bakunova SM, Wenzler T, Ghebru M, Werbovetz KA, Brun R, Tidwell RR. 2010. Synthesis and antiprotozoal activity of cationic 1,4-diphenyl-1-H-1,2,3-triazoles. J Med Chem 53:254–272. doi: 10.1021/jm901178d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. 2009. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 7:1051–1054. doi: 10.1590/S0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 31.Da Silva CF, Batista DDG, Oliveira GM, de Souza EM, Hammer ER, da Silva PB, Daliry A, Araujo JS, Britto C, Rodrigues AC, Liu Z, Farahat AA, Kumar A, Boykin DW, Soeiro MDNC. 2012. In vitro and in vivo investigation of the efficacy of arylimidamide DB1831 and its mesylated salt form—DB1965—against Trypanosoma cruzi infection. PLoS One 1:e30356. doi: 10.1371/journal.pone.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenzler T, Yang S, Patrick DA, Braissant O, Ismail MA, Tidwell RR, Boykin DW, Wang MZ, Brun R. 2014. In vitro and in vivo evaluation of 28DAP010, a novel diamidine for treatment of second-stage African sleeping sickness. Antimicrob Agents Chemother 8:4452–4463. doi: 10.1128/AAC.02309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Organisation for Economic Co-operation and Development. 2017. OECD guidelines for the testing of chemicals. OECD, Paris, France: http://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.