ABSTRACT
Carbapenem-resistant Enterobacteriaceae are urgent threats to global human health. These organisms produce β-lactamases with carbapenemase activity, such as the metallo-β-lactamase NDM-1, which is notable due to its association with mobile genetic elements and the lack of a clinically useful inhibitor. Here we examined the ability of copper to inhibit the activity of NDM-1 and explored the potential of a copper coordination complex as a mechanism to efficiently deliver copper as an adjuvant in clinical therapeutics. An NDM-positive Escherichia coli isolate, MS6192, was cultured from the urine of a patient with a urinary tract infection. MS6192 was resistant to antibiotics from multiple classes, including diverse β-lactams (penicillins, cephalosporins, and carbapenems), aminoglycosides, and fluoroquinolones. In the presence of copper (range, 0 to 2 mM), however, the susceptibility of MS6192 to the carbapenems ertapenem and meropenem increased markedly. In standard checkerboard assays, copper decreased the MICs of ertapenem and meropenem against MS6192 in a dose-dependent manner, suggesting a synergistic mode of action. To examine the inhibitory effect of copper in the absence of other β-lactamases, the blaNDM-1 gene from MS6192 was cloned and expressed in a recombinant E. coli K-12 strain. Analysis of cell extracts prepared from this strain revealed that copper directly inhibited NDM-1 activity, which was confirmed using purified recombinant NDM-1. Finally, delivery of copper at a low concentration of 10 μM by using the FDA-approved coordination complex copper-pyrithione sensitized MS6192 to ertapenem and meropenem in a synergistic manner. Overall, this work demonstrates the potential use of copper coordination complexes as novel carbapenemase adjuvants.
KEYWORDS: metallo-β-lactamase, carbapenem-resistant Enterobacteriaceae, copper, urinary tract infection, antibiotic resistance
INTRODUCTION
Carbapenems (ertapenem, doripenem, imipenem, and meropenem) are β-lactam antibiotics with broad-spectrum activity (1, 2). They are generally used as a last resort for treating infections caused by cephalosporin-resistant Enterobacteriaceae. Hence, it is alarming that resistance to carbapenems, primarily in Gram-negative bacteria, has now emerged and disseminated worldwide, leading to high rates of treatment failure and increased complications (3–6). Carbapenem-resistant Enterobacteriaceae (CRE), including Escherichia coli and Klebsiella pneumoniae, are frequently associated with hospital-acquired lung infections, urinary tract infections (UTIs), bloodstream infections, and device-related infections, with UTIs, including catheter-associated UTIs, the most common infections acquired in nosocomial settings (7). Combined with the asymptomatic carriage of CRE (8, 9) and the potential for transmission of resistance via mobile genetic elements (10, 11), it is not surprising that CRE are recognized as some of the most urgent threats to global human health today (12).
Mechanisms of carbapenem resistance in CRE frequently involve the expression of carbapenemases, which are broad-spectrum β-lactamases that hydrolyze carbapenems with high catalytic efficiency. These carbapenemases are diverse and include the Ambler class A (e.g., KPC) and class D (e.g., OXA-48) serine hydrolases, as well as class B metallo-β-lactamases (MBLs) (e.g., VIM, IMP, and NDM) (13). One approach to combat carbapenem resistance would be to develop adjuvants that inhibit carbapenemases, thus restoring susceptibility to carbapenems and ultimately extending the use of these antibiotics. For the serine-dependent lactamases, this strategy is best exemplified by the clinical use of clavulanic acid and tazobactam as β-lactam adjuvants that inhibit the activity of extended-spectrum β-lactamases (ESBLs) and restore the susceptibility of ESBL-positive strains to β-lactams (14). However, such inhibitors are ineffective against MBLs (15, 16). MBLs require up to two zinc ions for activity and thus they are inhibited by reagents that disrupt zinc binding, through either complete chelation (e.g., EDTA) or partial coordination (e.g., thiol-containing compounds) (14, 15, 17). Despite their effectiveness in vitro, these inhibitors have not proven to be clinically useful (14, 15).
Here we present evidence for a possible approach to inactivate MBLs with copper ions. Using the New Delhi metallo-β-lactamase 1 (NDM-1) enzyme as our model, we show that copper ions inhibit the activity of this MBL in vitro and enhance the susceptibility of MBL-positive isolates of E. coli to carbapenems. Using pyrithione, a FDA-approved antifungal agent that exerts an antimicrobial effect by acting as a copper delivery molecule (18), we also provide proof of the concept that copper coordination complexes have the potential to be used clinically as carbapenem adjuvants.
RESULTS
Copper ions potentiate the antibacterial activity of carbapenems against NDM-positive E. coli.
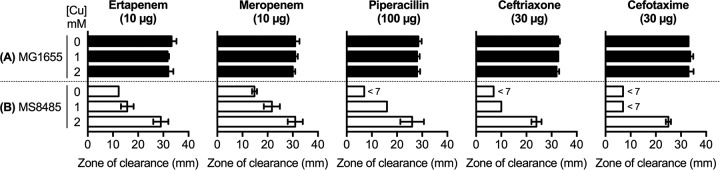
MS6192 is a NDM-positive, carbapenem-resistant isolate of E. coli that is also resistant to cephalosporins, fluoroquinolones, and aminoglycosides (Table 1). To assess the effects of copper ions on this antibiotic resistance profile, we first employed a modified disc diffusion assay on solid medium containing copper sulfate (0 to 2 mM). At those concentrations, copper alone did not inhibit the growth of MS6192 but it led to dose-dependent increases in the zones of clearance around carbapenem (ertapenem and meropenem) discs (Table 2). This potentiating effect of copper was also observed with 11 additional NDM-positive clinical isolates (see Table S1 in the supplemental material), but only strain MS6192 was selected for further study. The zones of clearance around other antibiotic discs, including other β-lactams, remained unchanged (Table 2), suggesting that the potentiating effect of copper was specific to carbapenems. Standard checkerboard assays confirmed that addition of copper decreased the MICs of carbapenems against strain MS6192 in a dose-dependent manner (Fig. 1A). The fractional inhibitory concentration (FIC) index values were 0.17 ± 0.13 for the combination of copper and ertapenem and 0.11 ± 0.06 for the combination of copper and meropenem, suggesting that the interactions between copper and carbapenems were synergistic (FIC index values of <0.5) (Fig. S1A).
TABLE 1.
Antibiotic resistance profile of E. coli strain MS6192
| Antibiotic class and name | MIC (μg/ml) |
|---|---|
| β-Lactams | |
| Penicillins | |
| Ampicillin | ≥32 |
| Amoxicillin-clavulanic acid | ≥32 |
| Ticarcillin-clavulanic acid | ≥128 |
| Piperacillin-tazobactam | ≥128 |
| Cephalosporins | |
| Cefazolin (first generation) | ≥64 |
| Cefoxitin (second generation) | ≥64 |
| Ceftazidime (third generation) | ≥64 |
| Ceftriaxone (third generation) | ≥64 |
| Cefepime (fourth generation) | ≥64 |
| Carbapenems | |
| Meropenem | ≥16 |
| Aminoglycosides | |
| Amikacin | ≥64 |
| Gentamicin | ≥16 |
| Tobramycin | ≥16 |
| Fluoroquinolones | |
| Norfloxacin | ≥16 |
| Ciprofloxacin | ≥4 |
| Others | |
| Nitrofurantoin | 128 |
| Trimethoprim | ≥16 |
| Trimethoprim-sulfamethoxazole | ≥32 |
TABLE 2.
Effects of copper ions on the resistance of E. coli strain MS6192 to antibiotics, as determined by disc diffusion assays
| Antibiotic class and name | Amount (μg) | Zone of clearance (mm)a |
||
|---|---|---|---|---|
| 0 mM copper sulfate | 1 mM copper sulfate | 2 mM copper sulfate | ||
| β-Lactams | ||||
| Carbapenems | ||||
| Ertapenem | 10 | 8 | 11 | 26 |
| Meropenem | 10 | 11 | 17 | 32 |
| Penicillins | ||||
| Ampicillin | 10 | <7 | <7 | <7 |
| Cephalosporins | ||||
| Ceftriaxone | 30 | <7 | <7 | <7 |
| Cefotaxime | 30 | <7 | <7 | <7 |
| Monobactams | ||||
| Aztreonam | 30 | <7 | <7 | <7 |
| Aminoglycosides | ||||
| Gentamicin | 10 | <7 | <7 | <7 |
| Tobramycin | 10 | <7 | <7 | <7 |
| Fluoroquinolones | ||||
| Ciprofloxacin | 5 | <7 | <7 | <7 |
Values are representative of three independent experiments. The diameter of the discs was 7 mm, and a value of <7 mm indicates that no zone of clearance was observed around the disc.
FIG 1.
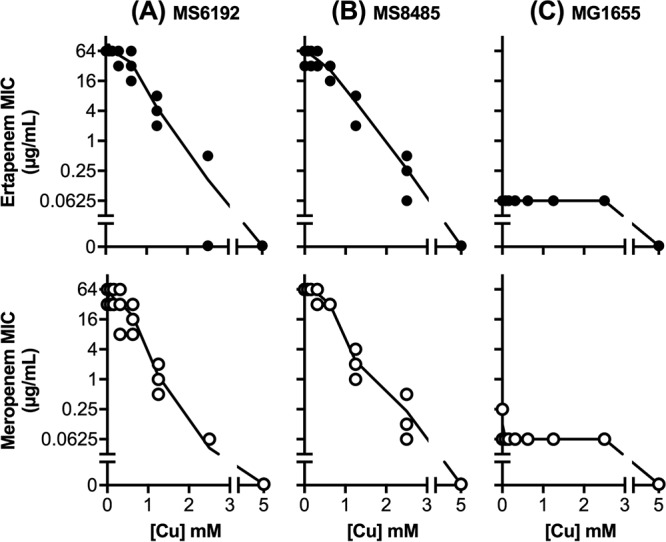
Effects of subinhibitory amounts of copper ions (0 to 2.5 mM) on the MIC values of ertapenem (top) and meropenem (bottom) against E. coli strains MS6192 (A), MS8485 (B), and MG1655 (C), as determined by standard checkerboard assays. Data shown were from three independent replicates. A MIC value of 0 μg/ml indicates that growth was inhibited in the absence of the carbapenem.
Copper ions inhibit the activity of NDM-1 carbapenemase.
Excess copper ions are known to inactivate a variety of metalloenzymes (19–22). Because the observed potentiating effect of copper was specific to carbapenems (Table 2), we hypothesized that this metal affected the activity of NDM-1. To test this idea, strain MS6192 was cultured without or with copper sulfate (2 mM) to mid-exponential phase, and total β-lactamase activities in cell extracts were measured using nitrocefin as the substrate. As predicted, growth in copper-rich medium led to a decrease in lactamase activity (Fig. 2A). However, a partial reduction was achieved (∼50%) (Fig. 2A), likely because MS6192 possesses multiple β-lactamase enzymes (NDM-1, CTX-M-15, and OXA-1), some of which are not MBLs and thus may be insensitive to copper.
FIG 2.
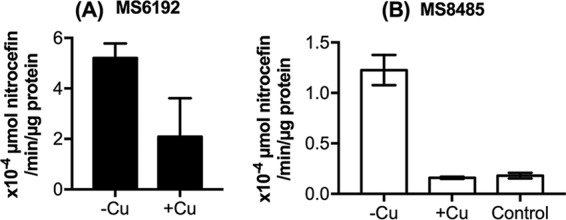
Effects of copper ions on β-lactamase activity in E. coli strains MS6192 (A) and MS8485 (B). Bacteria were cultured to the mid-exponential phase without (-Cu) or with (+Cu) subinhibitory amounts of copper (2 mM) and were lysed by sonication. As a negative control, MG1655 was also cultured without any copper (Control). Lactamase activities were measured in cell extracts and averaged from three independent replicates. Error bars represent standard deviations (SDs).
To simplify this analysis, we cloned the blaNDM-1 gene of MS6192 under an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter and transformed this plasmid (pSU2718::blaNDM-1) into the K-12 strain MG1655. The resulting NDM-positive recombinant strain MS8485 was resistant to penicillin, cephalosporins, and carbapenems (Fig. 3), as expected from the broad-spectrum activity of NDM-1. Disc diffusion and checkerboard assays confirmed that addition of copper to the culture medium restored the susceptibility of MS8485 to all β-lactams to levels that were comparable to those of MG1655 (Fig. 1 and 3). The mode of action was again synergistic, with FIC index values of 0.11 ± 0.03 for ertapenem and 0.11 ± 0.04 for meropenem (Fig. S1B). These results were consistent with loss of NDM-1 activity in the presence of copper. Indeed, cell extracts of copper-treated MS8485 did not display appreciable NDM-1 activity when tested using nitrocefin as the substrate (Fig. 2B).
FIG 3.
Effects of subinhibitory amounts of copper ions (0 to 2 mM) on the resistance of E. coli strains MG1655 (A) and MS8485 (B) to β-lactam antibiotics, as determined by disc diffusion assays. Diameters of the zones of clearance were averaged from three independent replicates. Error bars represent SDs. The diameter of the discs was 7 mm, and a value of <7 mm indicated that no zone of clearance was observed around the disc.
As a control, we measured the production of NDM-1 in MS8485 by immunoblot analysis. Expression of the blaNDM-1 gene in strain MS8485 was induced by IPTG but, to our surprise, copper treatment led to a reduction in the amount of NDM-1 enzyme (Fig. S2). This may account, at least partially, for the loss of β-lactamase activity in copper-treated cells (Fig. 2B). Similar observations were made using strain MS6192 (Fig. 2A; also see Fig. S2), but the blaNDM-1 gene in this strain is expressed from its native promoter. It is possible that copper exerts an effect at the step of enzyme folding, maturation, or secretion.
Although our immunoblot assays confirmed the observed production (albeit reduced) of NDM-1 in copper-treated MS8485 (Fig. S2), we did not detect appreciable NDM-1 activity above background levels (Fig. 2B). Thus, we tested the possibility that copper also directly inhibited the activity of NDM-1 by measuring its kinetic properties in cell extracts of MS8485 prepared following culture in copper-free medium. Addition of copper (0 to 80 μM) to the reaction buffer led to a dose-dependent decrease in NDM-1 activity (Fig. 4A). The data were best fitted to a noncompetitive model of inhibition (R2 = 0.97) with an apparent inhibition constant (Ki) of 47 ± 5 μM in these cell extracts (Fig. 4A; also see Fig. S3A). A noncompetitive mode of inhibition was confirmed by repeating these measurements using purified recombinant NDM-1 (Fig. 4B; also see Fig. S3B). A lower Ki of 3.7 ± 0.3 μM (R2 = 0.99) was obtained, confirming that copper strongly inhibits NDM-1 activity. A noncompetitive mode of inhibition by Cu(II) with a similar magnitude was recently reported for the MBL AIM-1 (23).
FIG 4.
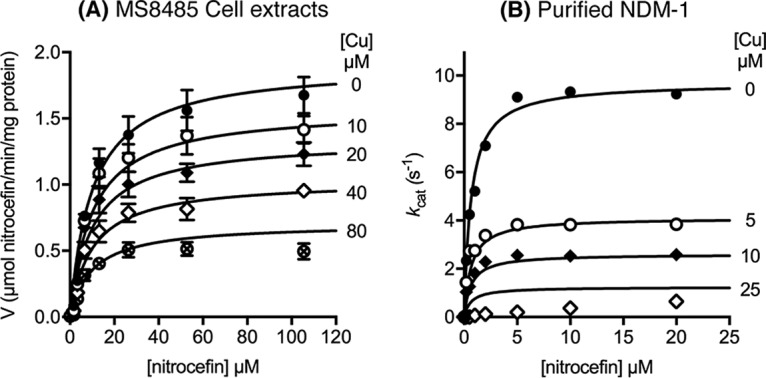
Direct inhibitory effects of copper ions on NDM-1 activity. The activity of MS8485 cell extracts (A) or purified NDM-1 (B) was measured in the presence of copper sulfate, using nitrocefin as the substrate. The concentrations of copper sulfate are indicated to the right of each curve. Each data point was averaged from three independent replicates. Error bars represent standard errors of the mean (SEMs). Data were fitted to noncompetitive and competitive models of enzyme inhibition (see Fig. S3 in the supplemental material).
The susceptibility of NDM-positive E. coli to carbapenems is enhanced using a copper coordination complex.
Ionic copper salts are lipid insoluble and so are poorly membrane permeable. As a consequence, high doses are often required to achieve antibacterial effects in vitro (e.g., >2 mM copper sulfate in our assays), hampering the development of copper-based antibiotics in clinical medicine. We and others have used small (<500-Da) lipophilic compounds with high binding affinities for copper to act as membrane-permeable carriers of copper ions (24–26). These copper coordination complexes are under investigation as clinical therapeutic agents (27–29), and some are potent antibacterial agents, at least in vitro (24–26). One such carrier, pyrithione (Fig. 5A), has been marketed for decades as an antifungal agent in health care and consumer products. Pyrithione is usually supplied in a zinc-coordinated form, but its action relies on transmetalation by trace exogenous copper ions and subsequent delivery of antimicrobial copper (18).
FIG 5.
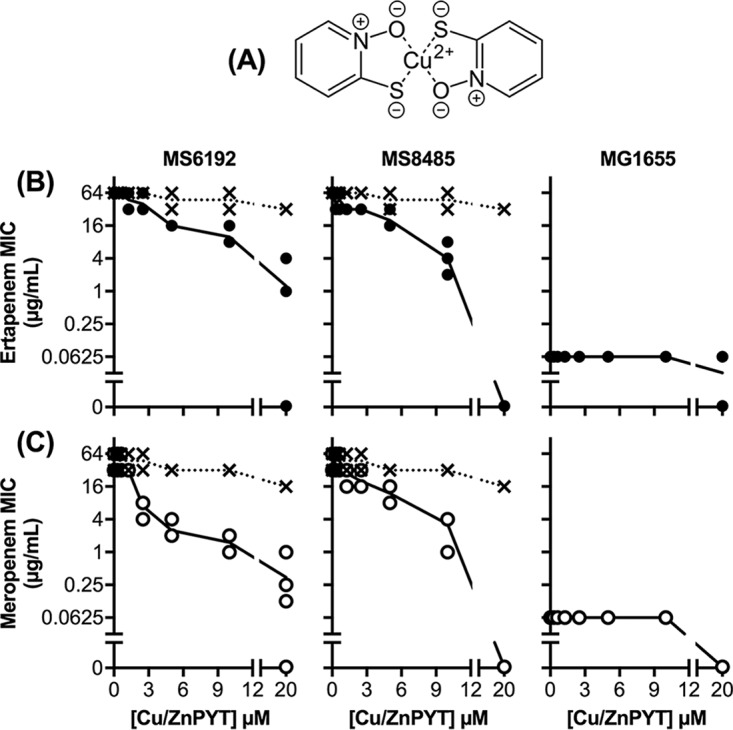
Structure of copper-pyrithione complex (A) and the effects of subinhibitory amounts of copper-pyrithione (0 to 20 μM) (solid lines) and zinc-pyrithione (0 to 20 μM) (dotted lines) on the MIC values of ertapenem (B) and meropenem (C) against E. coli strains MS6192, MS8485, and MG1655, as determined by standard checkerboard assays. Data shown were from three independent replicates. A MIC value of 0 μg/ml indicates that growth was inhibited in the absence of added carbapenem. PYT, pyrithione.
To determine whether pyrithione can deliver copper ions to CRE and inhibit NDM-1 carbapenemase activity, we repeated our checkerboard assays in the presence of copper-loaded pyrithione (0 to 20 μM). As anticipated, the copper-pyrithione complex increased the susceptibility of MS6192 and MS8485 to ertapenem and meropenem (Fig. 5B and C). The FIC index values were 0.18 ± 0.07 (ertapenem) and 0.13 ± 0.05 (meropenem) for MS6192 and 0.28 ± 0.11 (ertapenem) and 0.17 ± 0.08 (meropenem) for MS8485 (Fig. S4), again suggesting that the mode of interaction was synergistic. Copper was required for this synergy, as zinc-loaded pyrithione had little effect on carbapenem resistance (Fig. 5B and C). It must be noted that, in contrast to copper ions (Fig. 1), copper-pyrithione did not decrease the MIC values of carbapenems against MS8485 to MG1655 levels (Fig. 5). At 20 μM, copper-pyrithione completely suppressed the growth of E. coli even in the absence of carbapenems (Fig. 5B and C). Therefore, the potentiating effect of copper-pyrithione is a combination of its direct antibacterial action and the inhibition of carbapenemase activity.
DISCUSSION
The antibacterial properties of copper have been recognized for millennia, and simple ionic salts and complexes of copper were used to control bacterial infections in the preantibiotic age (30). It is now established that excess copper ions poison bacterial cells by inactivating key metalloenzymes, particularly those containing solvent-accessible iron (19) and zinc (20). This is a consequence of the high relative affinity of copper for these metal binding sites, which leads to metal exchange and displacement of the cognate but more weakly binding metals (21). Here we showed that copper ions, as Cu(II), can directly inactivate the metalloenzyme NDM-1. The precise mechanism remains to be elucidated, but we propose that copper may disrupt binding of one or both of the zinc ions in the active site of NDM-1. This model is in agreement with a recent study using the MBL AIM-1, which demonstrated that two Cu(II) ions bind to the enzyme in close proximity (23). Alternatively, copper ions may bind to an allosteric site outside the zinc-containing pocket. Both scenarios (summarized in Fig. 6) would be consistent with the observed noncompetitive mode of enzyme inhibition, but detailed structural and biochemical studies of the purified enzyme will be required to describe the molecular basis of this inhibition.
FIG 6.
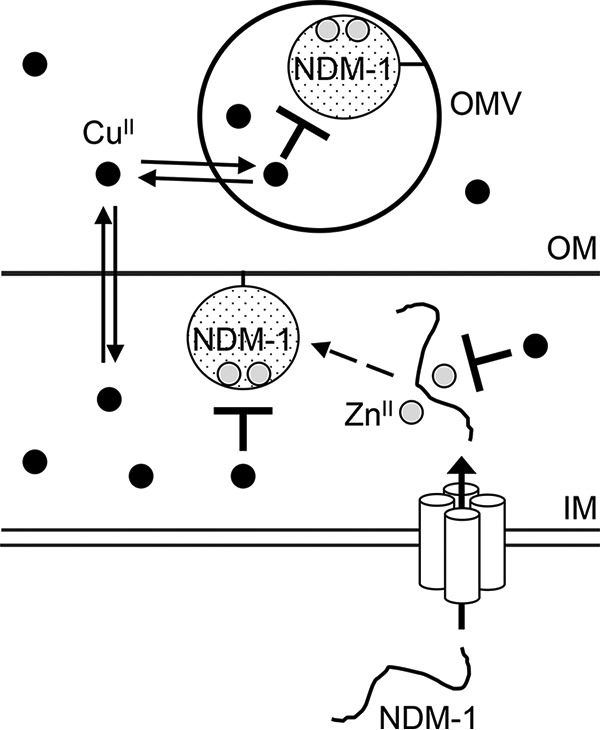
General schematic of the effects of copper ions on NDM-1 activity. Our data indicate that copper can directly inhibit the carbapenemase activity of NDM-1 and also may affect NDM-1 synthesis, maturation, or stability. Cu(II) and Zn(II) ions are depicted by black circles and light gray circles, respectively. IM, inner membrane; OM, outer membrane; OMV, outer membrane vesicle.
Our immunoblot results indicated that copper may also affect the synthesis, maturation (enzyme folding and zinc site assembly), or stability of NDM-1. Although NDM-1 is anchored to the outer membrane and secreted in outer membrane vesicles (31), it is folded and metallated in the periplasm (32). These latter processes are universal to all MBLs, including the VIM, IMP, and AIM carbapenemases, and they can also be disrupted by excess copper (23, 33, 34). Indeed, a recent study suggested that NDM-1 enzymes lacking the zinc centers are degraded in the cell (31).
In our experiments, copper was supplied in the growth medium as Cu(II) ions. Inactivation of NDM-1 via the various potential routes described above would rely on diffusion of these Cu(II) ions into the cytoplasm, periplasm, or outer membrane vesicles. However, the concentration of copper required to restore susceptibility of MS6192 to carbapenems (∼2 mM) in our study was high and unlikely to be tolerated physiologically. We were able to reduce this amount by 2 orders of magnitude to 10 μM by coordinating the copper ion to pyrithione, a FDA-approved antifungal agent that acts as a membrane-permeable carrier of copper. Pyrithione and its zinc-coordinated form are currently approved for topical administration. While most formulations in consumer goods and health care products contain up to 2% of this compound, it is not known whether the copper form is equally tolerated. However, a variety of other copper carriers are currently being investigated for their therapeutic potential (24–26). The results presented here provide an early proof of concept that ligand- or carrier-mediated delivery of copper ions to bacterial cells, in this case to target NDM-1 carbapenemase activity, is possible. Our data also support an emerging role of metal ions in enhancing the action of antibiotics. For example, silver can potentiate vancomycin activity by disrupting multiple bacterial cellular processes, including disulfide bond formation, metabolism, and iron homeostasis (35).
The ability of copper to inhibit NDM-1 carbapenemase activity also provides an opportunity to develop therapeutic agents that work in concert with the host innate immune system. Although the availability and location of copper in the human body are tightly regulated, copper is mobilized in response to inflammation, leading to increased copper concentrations at the site of infection. For instance, mobilization of copper occurs in infected macrophages (36). Infections involving a variety of pathogens also result in increased copper levels in the serum, liver, and spleen of animals (37, 38). In the case of E. coli, particularly uropathogenic strains, it has been shown that copper levels are elevated (to ∼0.3 μM) in the urine of patients with UTIs, compared to healthy controls (39, 40). Thus, delivery of membrane-permeable copper carriers such as pyrithione into the urinary tract may allow us to exploit this host-derived copper and enhance its action against CRE.
MATERIALS AND METHODS
Bacterial strains, reagents, and culture conditions.
E. coli MS6192 is a NDM-1-positive strain that was isolated from the urine of a patient with a UTI. E. coli MG1655 is a K-12 strain and is susceptible to all antibiotics. All strains were propagated from frozen glycerol stocks on Luria-Bertani (LB) agar at 37°C. Liquid cultures were prepared in LB broth and grown at 37°C with shaking at 200 rpm. Antibiotic discs (Sensi-Discs) were purchased from BD Biosciences (Australia). Copper(II) sulfate (product no. C8027), zinc(II) pyrithione (product no. H6377), ertapenem sodium (product no. SML1238), and meropenem trihydrate (product no. M2574) were purchased from Sigma (Australia). Stocks of reagents were prepared in deionized water except for zinc pyrithione, which was dissolved in dimethyl sulfoxide (DMSO). Copper(II) pyrithione was prepared by adding equimolar amounts of copper sulfate to a solution of zinc(II) pyrithione.
Cloning and expression of the blaNDM-1 gene in MG1655.
The blaNDM-1 gene from MS6192 was amplified with primers 7414 (5′-TGATAAGGATCCATTCAGCTTTCACCCATTGG-3′) and 7415 (5′-TCGAAAAAGCTTGATGGCAGATTGGGGGTGA-3′) and cloned between the BamHI and HindIII sites of pSU2718. The resulting plasmid, pSU2718::blaNDM-1, was transformed into E. coli MG1655 by electroporation to generate the NDM-1-positive strain MS8485. MS8485 was cultured in the presence of chloramphenicol (30 μg/ml) and IPTG (0.1 mM) to maintain the plasmid and to promote NDM-1 expression, respectively.
Antibiotic susceptibility assays.
The antimicrobial susceptibility profile of MS6192 was determined using the Vitek 2 automated AST-N426 card (bioMérieux). The Etest was used to determine MICs for meropenem, imipenem, and ertapenem. Disc diffusion assays were performed by seeding LB agar containing copper sulfate (0 to 2 mM) or copper pyrithione (0 to 20 μM) with bacterial suspensions at an optical density at 600 nm (OD600) of 0.18 (∼1.5 × 108 CFU/ml, equivalent to a McFarland standard of 0.5). Zones of clearance around antibiotic discs were measured after incubation at 37°C for 24 h. Checkerboard assays were also performed in LB broth. Bacterial suspensions were prepared to an OD600 of 0.001 (∼5 × 105 CFU/ml), and exactly 100 μl was dispensed into each well of a U-bottomed, 96-well, microtiter plate. To each well were also added 50 μl of LB broth containing ertapenem or meropenem (0 to 64 μg/ml) and 50 μl of LB broth containing copper sulfate (0 to 5 mM) or copper pyrithione (0 to 20 μM). Turbidity in each well was measured using a microtiter plate reader after incubation at 37°C for 24 h. The MIC was defined as the lowest concentration of agent that completely inhibited bacterial growth. The FIC for each agent was defined as its MIC in combination divided by its MIC alone. The FIC index was the sum of the FIC values for the two agents.
Overexpression and purification of recombinant NDM-1.
To obtain the pure NDM-1 enzyme, the coding sequence for NDM-1 (residues 27 to 270) was amplified from strain MS6192 using primers 7456 (5′-TACTTCCAATCCAATGCGATGCCCGGTGAAATCC-3′) and 7457 (5′-TTATCCACTTCCAATGTCAGCGCAGCTTGTCG-3′) containing ligation-independent cloning (LIC) overhangs. The gene product was cloned into the pAL vector encoding a N-terminal His6 tag followed by a thioredoxin (TRX) domain and a TEV protease cleavage site. NDM-1 was expressed overnight at 37°C in the E. coli BL21(DE3) host in the presence of 0.5 mM IPTG. Cells were lysed in Tris-Cl buffer (25 mM Tris, 150 mM NaCl, 20 μM ZnCl2 [pH 7.5]) by sonication, and NDM-1 protein was purified on a Ni-nitrilotriacetic acid (NTA) HisTrap column (GE Healthcare) by using the same buffer and a gradient of 0 to 400 mM imidazole. The N-terminal His6 tag was cleaved with TEV protease and repurified by elution from a Ni-NTA HisTrap column.
Assays of NDM-1 activity.
To prepare cell extracts, bacteria (50 ml) were cultured without or with copper sulfate (2 mM) to the mid-exponential phase (OD600 of ∼0.4 to 0.5), centrifuged (5,000 × g for 10 min), washed with phosphate-buffered saline (PBS), resuspended in 500 μl of HEPES buffer (50 mM HEPES [pH 7.4]), lysed by sonication (five 10-s bursts at 10 W each), and clarified by centrifugation (20,000 × g for 5 min). β-Lactamase activity in these cell extracts was measured by monitoring the hydrolysis of nitrocefin (0 to 250 μM) in HEPES buffer (50 mM HEPES [pH 7.4]) at 35°C. Copper sulfate (0 to 100 μM) was added to the nitrocefin solution immediately before addition of the cell extracts to initiate hydrolysis. Absorbance values at 485 nm (ε of 17.5 mM−1 cm−1) were monitored continuously for 2 min in a spectrophotometer. Initial rates (up to 30 s) were normalized to total protein concentrations as determined by the bicinchoninic acid (BCA) assay. Data were fitted to the Michaelis-Menten equation, which incorporates terms describing either noncompetitive or competitive inhibition, using the software package Prism 7 (GraphPad).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia (grants GNT1033799 and GNT1084778) and the Queensland-Emory Development Alliance. M.A.S. is supported by an NHMRC Senior Research Fellowship (grant GNT1106930), G.S. by an Australian Research Council Future Fellowship (grant FT120100694), D.L.P. by an NHMRC Practitioner Fellowship, and N.M. by a President of Ireland Young Researcher Award from Science Foundation Ireland. WMS is the recipient of a Senior Research Career Scientist Award from the VA Medical Research Service.
The contents of this article are solely the responsibility of the authors and do not necessarily reflect the official views of the Department of Veterans Affairs.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02280-17.
REFERENCES
- 1.Bush K, Bradford PA. 2016. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6:a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chibabhai V, Nana T, Bosman N, Thomas T, Lowman W. 2017. Were all carbapenemases created equal? Treatment of NDM-producing extensively drug-resistant Enterobacteriaceae: a case report and literature review. Infection doi: 10.1007/s15010-017-1070-8. [DOI] [PubMed] [Google Scholar]
- 4.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215(Suppl 1):S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meletis G. 2016. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis 3:15–21. doi: 10.1177/2049936115621709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tangden T, Giske CG. 2015. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Intern Med 277:501–512. doi: 10.1111/joim.12342. [DOI] [PubMed] [Google Scholar]
- 7.National Nosocomial Infections Surveillance System. 2004. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 32:470–485. doi: 10.1016/j.ajic.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Akova M, Daikos GL, Tzouvelekis L, Carmeli Y. 2012. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin Microbiol Infect 18:439–448. doi: 10.1111/j.1469-0691.2012.03823.x. [DOI] [PubMed] [Google Scholar]
- 9.Wiener-Well Y, Rudensky B, Yinnon AM, Kopuit P, Schlesinger Y, Broide E, Lachish T, Raveh D. 2010. Carriage rate of carbapenem-resistant Klebsiella pneumoniae in hospitalised patients during a national outbreak. J Hosp Infect 74:344–349. doi: 10.1016/j.jhin.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Diene SM, Rolain JM. 2014. Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species. Clin Microbiol Infect 20:831–838. doi: 10.1111/1469-0691.12655. [DOI] [PubMed] [Google Scholar]
- 11.Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AK, Carroll J, Scheld WM, Hazen KC, Sifri CD. 2011. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. mBio 2:e00204-11. doi: 10.1128/mBio.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 13.Bush K. 2013. The ABCD's of β-lactamase nomenclature. J Infect Chemother 19:549–559. doi: 10.1007/s10156-013-0640-7. [DOI] [PubMed] [Google Scholar]
- 14.Drawz SM, Bonomo RA. 2010. Three decades of β-lactamase inhibitors. Clin Microbiol Rev 23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGeary RP, Schenk G, Guddat LW. 2014. The applications of binuclear metallohydrolases in medicine: recent advances in the design and development of novel drug leads for purple acid phosphatases, metallo-β-lactamases and arginases. Eur J Med Chem 76:132–144. doi: 10.1016/j.ejmech.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Mitic N, Miraula M, Selleck C, Hadler KS, Uribe E, Pedroso MM, Schenk G. 2014. Catalytic mechanisms of metallohydrolases containing two metal ions. Adv Protein Chem Struct Biol 97:49–81. doi: 10.1016/bs.apcsb.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Hinchliffe P, Gonzalez MM, Mojica MF, Gonzalez JM, Castillo V, Saiz C, Kosmopoulou M, Tooke CL, Llarrull LI, Mahler G, Bonomo RA, Vila AJ, Spencer J. 2016. Cross-class metallo-β-lactamase inhibition by bisthiazolidines reveals multiple binding modes. Proc Natl Acad Sci U S A 113:E3745–E3754. doi: 10.1073/pnas.1601368113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeder NL, Kaplan J, Xu J, Youngquist RS, Wallace J, Hu P, Juhlin KD, Schwartz JR, Grant RA, Fieno A, Nemeth S, Reichling T, Tiesman JP, Mills T, Steinke M, Wang SL, Saunders CW. 2011. Zinc pyrithione inhibits yeast growth through copper influx and inactivation of iron-sulfur proteins. Antimicrob Agents Chemother 55:5753–5760. doi: 10.1128/AAC.00724-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tottey S, Patterson CJ, Banci L, Bertini I, Felli IC, Pavelkova A, Dainty SJ, Pernil R, Waldron KJ, Foster AW, Robinson NJ. 2012. Cyanobacterial metallochaperone inhibits deleterious side reactions of copper. Proc Natl Acad Sci U S A 109:95–100. doi: 10.1073/pnas.1117515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster AW, Osman D, Robinson NJ. 2014. Metal preferences and metallation. J Biol Chem 289:28095–28103. doi: 10.1074/jbc.R114.588145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djoko KY, Phan MD, Peters KM, Walker MJ, Schembri MA, McEwan AG. 2017. Interplay between tolerance mechanisms to copper and acid stress in Escherichia coli. Proc Natl Acad Sci U S A 114:6818–6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selleck C, Larrabee JA, Harmer J, Guddat LW, Mitic N, Helweh W, Ollis DL, Craig WR, Tierney DL, Monteiro Pedroso M, Schenk G. 2016. AIM-1: an antibiotic-degrading metallohydrolase that displays mechanistic flexibility. Chemistry 22:17704–17714. doi: 10.1002/chem.201602762. [DOI] [PubMed] [Google Scholar]
- 24.Djoko KY, Goytia MM, Donnelly PS, Schembri MA, Shafer WM, McEwan AG. 2015. Copper(II)-bis(thiosemicarbazonato) complexes as antibacterial agents: insights into their mode of action and potential as therapeutics. Antimicrob Agents Chemother 59:6444–6453. doi: 10.1128/AAC.01289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haeili M, Moore C, Davis CJ, Cochran JB, Shah S, Shrestha TB, Zhang Y, Bossmann SH, Benjamin WH, Kutsch O, Wolschendorf F. 2014. Copper complexation screen reveals compounds with potent antibiotic properties against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 58:3727–3736. doi: 10.1128/AAC.02316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speer A, Shrestha TB, Bossmann SH, Basaraba RJ, Harber GJ, Michalek SM, Niederweis M, Kutsch O, Wolschendorf F. 2013. Copper-boosting compounds: a novel concept for antimycobacterial drug discovery. Antimicrob Agents Chemother 57:1089–1091. doi: 10.1128/AAC.01781-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C. 2014. Advances in copper complexes as anticancer agents. Chem Rev 114:815–862. doi: 10.1021/cr400135x. [DOI] [PubMed] [Google Scholar]
- 28.Helsel ME, Franz KJ. 2015. Pharmacological activity of metal binding agents that alter copper bioavailability. Dalton Trans 44:8760–8770. doi: 10.1039/C5DT00634A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan C, White AR. 2012. Copper complexes as therapeutic agents. Metallomics 4:127–138. doi: 10.1039/C2MT00174H. [DOI] [PubMed] [Google Scholar]
- 30.Dollwet HHA, Sorenson JRJ. 1985. Historic uses of copper compounds in medicine. Trace Elem Med 2:80–87. [Google Scholar]
- 31.Gonzalez LJ, Bahr G, Nakashige TG, Nolan EM, Bonomo RA, Vila AJ. 2016. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-β-lactamase. Nat Chem Biol 12:516–522. doi: 10.1038/nchembio.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran-Barrio J, Limansky AS, Viale AM. 2009. Secretion of GOB metallo-β-lactamase in Escherichia coli depends strictly on the cooperation between the cytoplasmic DnaK chaperone system and the Sec machinery: completion of folding and Zn(II) ion acquisition occur in the bacterial periplasm. Antimicrob Agents Chemother 53:2908–2917. doi: 10.1128/AAC.01637-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tottey S, Waldron KJ, Firbank SJ, Reale B, Bessant C, Sato K, Cheek TR, Gray J, Banfield MJ, Dennison C, Robinson NJ. 2008. Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature 455:1138–1142. doi: 10.1038/nature07340. [DOI] [PubMed] [Google Scholar]
- 34.Hu Z, Gunasekera TS, Spadafora L, Bennett B, Crowder MW. 2008. Metal content of metallo-β-lactamase L1 is determined by the bioavailability of metal ions. Biochemistry 47:7947–7953. doi: 10.1021/bi8004768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ. 2013. Silver enhances antibiotic activity against Gram-negative bacteria. Sci Transl Med 5:190ra81. doi: 10.1126/scitranslmed.3006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achard ME, Stafford SL, Bokil NJ, Chartres J, Bernhardt PV, Schembri MA, Sweet MJ, McEwan AG. 2012. Copper redistribution in murine macrophages in response to Salmonella infection. Biochem J 444:51–57. doi: 10.1042/BJ20112180. [DOI] [PubMed] [Google Scholar]
- 37.Chaturvedi KS, Henderson JP. 2014. Pathogenic adaptations to host-derived antibacterial copper. Front Cell Infect Microbiol 4:3. doi: 10.3389/fcimb.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Djoko KY, Ong CL, Walker MJ, McEwan AG. 2015. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J Biol Chem 290:18954–18961. doi: 10.1074/jbc.R115.647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subashchandrabose S, Hazen TH, Brumbaugh AR, Himpsl SD, Smith SN, Ernst RD, Rasko DA, Mobley HL. 2014. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A 111:18327–18332. doi: 10.1073/pnas.1415959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyre AN, Kavanagh K, Kock ND, Donati GL, Subashchandrabose S. 2017. Copper is a host effector mobilized to urine during urinary tract infection to impair bacterial colonization. Infect Immun 85:e01041-16. doi: 10.1128/IAI.01041-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.