ABSTRACT
Intermittent, three-times-weekly oral antibiotic therapy is recommended for the initial treatment of noncavitary nodular bronchiectatic (NB) Mycobacterium avium complex (MAC) lung disease. However, intermittent therapy is not recommended for patients who have been previously treated. We evaluated 53 patients with recurrent noncavitary NB MAC lung disease who underwent antibiotic treatment for ≥12 months with daily therapy (n = 26) or intermittent therapy (n = 27) between January 2008 and December 2015. Baseline characteristics were comparable between daily therapy and intermittent therapy groups. Sputum culture conversion rates did not differ between daily therapy (21/26, 81%) and intermittent therapy (22/27, 82%) groups. Compared to the etiologic organism at the time of previous treatment, recurrent MAC lung disease was caused by the same MAC species in 38 patients (72%) and by a different MAC species in 15 patients (28%). Genotype analysis in patients with sequenced paired isolates revealed that 86% (12/14) of cases with same species recurrence were due to reinfection with a new MAC genotype. In conclusion, most recurrent noncavitary NB MAC lung disease cases were caused by reinfection rather than relapse. Intermittent antibiotic therapy is a reasonable treatment strategy for recurrent noncavitary NB MAC lung disease.
KEYWORDS: Mycobacterium avium complex, Mycobacterium avium, Mycobacterium intracellulare, recurrence, treatment outcome
INTRODUCTION
Mycobacterium avium complex (MAC) lung disease is the most common form of lung disease caused by nontuberculous mycobacteria (NTM), and its incidence and prevalence are increasing worldwide (1–3). MAC lung disease usually has two major clinical phenotypes: fibrocavitary and nodular bronchiectatic (NB) (4–7). The fibrocavitary form is characterized by cavitary lesions that occur predominantly in the upper lobes and usually develops in older males with underlying lung disease, such as previous pulmonary tuberculosis and/or chronic obstructive pulmonary disease (4–7). The NB form occurs predominantly in postmenopausal, nonsmoking females (4–7) and can present as bilateral bronchiectasis with multiple nodules and tree-in-bud opacities on high-resolution computed tomography (HRCT) (8, 9).
Macrolide-based combination antibiotic therapy is recommended as the initial therapy for MAC lung disease, and the current guidelines published by the American Thoracic Society and Infectious Diseases Society of America in 2007 recommend different antibiotic regimens according to clinical phenotype: intermittent, three-times-weekly oral administration of three drugs (macrolide, ethambutol [EMB], and rifamycin) for noncavitary NB MAC lung disease versus daily oral drugs with or without administration of parenteral drugs, such as streptomycin or amikacin, for cavitary MAC lung disease (4). For previously treated disease, however, the guidelines recommend more aggressive therapy with three oral drugs daily plus parenteral drug administration regardless of clinical phenotype (4). Intermittent therapy is not recommended for patients who have been previously treated (4).
Recurrence of MAC lung disease is not uncommon after successful treatment completion, especially in patients with NB MAC lung disease (10–15). In addition, evidence is mounting that the majority of recurrences are due to reinfection by new MAC genotypes rather than true relapse with the same MAC genotypes (10, 14–17). These data suggest that an aggressive strategy with daily oral drugs plus parenteral drugs for all patients with recurrent MAC lung disease might not be necessary and that intermittent oral antibiotic therapy might be more appropriate in patients with recurrent NB MAC lung disease.
However, no published data are available regarding the treatment outcomes of intermittent antibiotic therapy for patients with recurrent NB MAC lung disease. We hypothesized that the treatment outcomes would not be different between the daily therapy group and the intermittent therapy in recurrent NB MAC lung disease. The purpose of the present study was to compare the clinical efficacy of intermittent antibiotic therapy with daily therapy in patients with recurrent noncavitary NB MAC lung disease.
RESULTS
Baseline characteristics.
The characteristics of the 53 study patients are shown in Table 1. None of the patients tested positive for human immunodeficiency virus. The median age was 63 years (interquartile range [IQR], 54 to 71 years), and most patients (76%) were female. There were no significant differences in age, sex, body mass index, smoking history, underlying conditions, or etiologic organism between the daily therapy and intermittent therapy groups. Positivity of the sputum AFB smear at treatment initiation (31% versus 30%) and the time interval between the initial treatment completion and diagnosis of recurrent MAC lung disease (12.3 months; IQR, 6.0 to 29.8 months versus 21.3 months; IQR, 12.3 to 29.5 months) did not differ between the daily therapy and intermittent therapy groups (Table 1). The most common etiological organism of recurrent MAC lung disease was M. intracellulare (n = 29, 55%), followed by M. avium (n = 23, 43%), and mixed infection with M. avium and M. intracellulare (n = 1, 2%). Compared to the etiologic organism at the time of previous treatment, recurrent MAC lung disease was caused by the same MAC species in 38 patients (72%) and by a different MAC species in 15 patients (28%) (Table 2).
TABLE 1.
Baseline characteristics of study patients
| Characteristicsa | No. (%) or median (IQR)b |
P | ||
|---|---|---|---|---|
| Total (n = 53) | Daily therapy (n = 26) | Intermittent therapy (n = 27) | ||
| Age (yrs) | 63 (54–71) | 64 (54–71) | 63 (53–71) | 0.727 |
| Sex, female | 40 (76) | 21 (81) | 19 (70) | 0.526 |
| Body mass index, kg/m2 | 19.6 (18.3–21.0) | 20.2 (18.4–21.2) | 19.3 (18.0–20.9) | 0.333 |
| Never smoker | 45 (85) | 24 (92) | 21 (78) | 0.250 |
| Underlying condition | ||||
| Bronchiectasis | 53 (100) | 26 (100) | 27 (100) | NA |
| Previous tuberculosis | 18 (34) | 9 (35) | 9 (33) | 0.999 |
| Chronic obstructive lung disease | 3 (6) | 0 (0) | 3 (11) | 0.236 |
| Diabetes mellitus | 4 (8) | 1 (4) | 3 (11) | 0.610 |
| Chronic heart disease | 3 (6) | 1 (4) | 2 (7) | 0.999 |
| Etiologic organism | 0.999 | |||
| M. avium | 23 (43) | 11 (42) | 12 (44) | |
| M. intracellulare | 29 (55) | 14 (54) | 15 (56) | |
| M. avium and M. intracellulare | 1 (2) | 1 (4) | 0 (0) | |
| Positive sputum AFB smear | 16 (30) | 8 (31) | 8 (30) | 0.999 |
| ESR (mm/h) | 31 (16–57) | 42 (9–58) | 25 (17–57) | 0.801 |
| CRP (mg/dl) | 0.10 (0.04–0.52) | 0.11 (0.04–1.40) | 0.09 (0.04–0.24) | 0.442 |
| Pulmonary function test | ||||
| FEV1 (%) | 83 (66–90) | 86 (71–94) | 79 (63–89) | 0.181 |
| FVC (%) | 83 (71–91) | 88 (69–97) | 82 (69–90) | 0.239 |
| Time interval between initial treatment completion and recurrence (mos) | 16.8 (8.2–29.6) | 12.3 (6.0–29.8) | 21.3 (12.3–29.5) | 0.188 |
Abbreviations: AFB, acid-fast bacilli; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Data are presented as numbers (%) or as medians (interquartile ranges). NA, not applicable.
TABLE 2.
Etiologic organism
| Etiology at previous treatment | Etiology at recurrence (no. of patients) |
|||
|---|---|---|---|---|
| M. avium | M. intracellulare | M. avium and M. intracellulare | Total | |
| M. avium | 18 | 9 | 1 | 28 |
| M. intracellulare | 3 | 20 | 0 | 23 |
| M. avium and M. intracellulare | 2 | 0 | 0 | 2 |
| Total | 23 | 29 | 1 | 53 |
Antibiotic treatment.
All patients were treated with a combination of antibiotics consisting of a macrolide, EMB, and rifampin (RIF) (Table 3). Azithromycin (AZM) was the macrolide initially used in more than half of the daily therapy group (14/26, 54%) and in all patients in the intermittent therapy group (27/27, 100%). Clarithromycin (CLR) was replaced with AZM in five (5/26, 19%) patients in the daily therapy group, while AZM was replaced with CLR in one (1/27, 4%) patient in the intermittent therapy group during the treatment period. In the daily therapy group, streptomycin or kanamycin was used in six patients (23%) for a median of 3.0 months (IQR, 2.3 to 3.5 months). The median of total treatment duration was longer in the daily therapy group (23.2 months; IQR, 8.5 to 26.5 months) than in the intermittent therapy group (19.3 months; IQR, 16.5 to 23.0 months; P = 0.023).
TABLE 3.
Antibiotic treatment regimen
| Regimena | No. (%) or median (IQR)b |
P | ||
|---|---|---|---|---|
| Total (n = 53) | Daily therapy (n = 26) | Intermittent therapy (n = 27) | ||
| Macrolide | 53 (100) | 26 (100) | 27 (100) | NA |
| CLR | 7 | 7 | 0 | |
| AZM | 40 | 14 | 26 | |
| CLR, followed by AZM | 5 | 5 | 0 | |
| AZM, followed by CLR | 1 | 0 | 1 | |
| EMB | 53 (100) | 26 (100) | 27 (100) | NA |
| RIF | 53 (100) | 26 (100) | 27 (100) | NA |
| Aminoglycoside | 6 (11) | 6 (23) | 0 | 0.010 |
| Streptomycin | 4 | 4 | 0 | |
| Kanamycin | 2 | 2 | 0 | |
| Total treatment duration (mos) | 20.1 (17.3–25.9) | 23.2 (18.5–26.5) | 19.3 (16.5–23.0) | 0.023 |
Abbreviations: AZM, azithromycin; CLR, clarithromycin; EMB, ethambutol; RIF, rifampin.
Data are presented as numbers (%) or as medians (interquartile ranges). NA, not applicable.
Antibiotic treatment was modified in six patients during the study period. EMB was discontinued in one patient in the daily therapy group due to visual disturbances after 1 month of EMB treatment, and RIF was discontinued in five patients in the intermittent therapy group due to gastrointestinal intolerance after a median of 5.5 months (IQR, 1.9 to 12.1 months) of RIF treatment, but there were no significant differences in antibiotic treatment modifications between the two groups.
Treatment outcomes.
After 12 months of antibiotic treatment, there were no differences in symptom improvement (92 versus 85%; P = 0.416) or HRCT improvement (69 versus 70%; P = 0.608) between the daily therapy and intermittent therapy groups (Table 4).
TABLE 4.
Treatment outcomes
| Treatment outcomea | No. (%) or median (IQR)b |
P | ||
|---|---|---|---|---|
| Total (n = 53) | Daily therapy (n = 26) | Intermittent therapy (n = 27) | ||
| Symptomatic response | 0.416 | |||
| Improved | 47 (88) | 24 (92) | 23 (85) | |
| Unchanged | 3 (6) | 2 (8) | 1 (4) | |
| Worsened | 3 (6) | 0 (0) | 3 (11) | |
| Radiographic response on HRCT | 0.608 | |||
| Improved | 37 (70) | 18 (69) | 19 (70) | |
| Unchanged | 12 (22) | 7 (27) | 5 (19) | |
| Worsened | 4 (8) | 1 (4) | 3 (11) | |
| Sputum culture conversion | 43 (81) | 21 (81) | 22 (82) | 0.999 |
| Time to culture conversion (months) | 1.4 (1.0–8.4) | 1.6 (1.1–5.0) | 1.3 (1.0–10.1) | 0.920 |
| Development of macrolide resistance | 2 (4) | 2 (8) | 0 (0) | 0.236 |
| Redevelopment of NTM lung disease | 11/43 (26) | 6/21 (29) | 5/22 (23) | 0.736 |
| Caused by same species | 6/43 (14) | 2/21 (10) | 4/22 (18) | |
| M. avium → M. avium | 4 | 1 | 3 | |
| M. intracellulare → M. intracellulare | 2 | 1 | 1 | |
| Caused by different species | 5/43 (12) | 4/21 (19) | 1/22 (5) | |
| M. avium → M. intracellulare | 1 | 1 | 0 | |
| M. avium → M. kansasii | 1 | 1 | 0 | |
| M. avium → M. avium/M. massiliense | 1 | 1 | 0 | |
| M. intracellulare → M. abscessus | 2 | 1 | 1 | |
Abbreviations: HRCT, high-resolution computed tomography; NTM, nontuberculous mycobacteria.
Data are presented as numbers (%) or as medians (interquartile ranges).
Forty-three (81%) patients achieved sputum culture conversion and maintained negative cultures for more than 12 months. The culture conversion rates were not different between the daily therapy (81%) and intermittent therapy groups (82%, P = 0.999), and it showed similar clinical efficacy of both treatment strategies (Table 4). Further analysis demonstrated that the crude and adjusted proportions of culture conversion also did not differ between the daily therapy and intermittent therapy groups (Table 5).
TABLE 5.
Crude and adjusted proportions and odds ratios for culture conversiona
| Variable | Crude or adjusted % proportion (95% CI) of culture conversion |
% differences of proportionb (95% CI) |
ORc (95% CI) | P | |
|---|---|---|---|---|---|
| Daily therapy | Intermittent therapy | ||||
| Crude state | 80.8 (51.5–94.3) | 81.5 (52.6–94.6) | −0.7 (−21.8–20.4) | 1.048 (0.265–4.148) | 0.947 |
| Adjusted stated | 93.9 (62.9–99.3) | 91.0 (52.5–98.9) | 2.9 (−11.3–17.2) | 0.652 (0.071–5.967) | 0.689 |
Abbreviations: OR, odds ratio; CI, confidence interval.
Calculated as the proportion of culture conversion in patients with daily therapy − the proportion of culture conversion in patients with intermittent therapy. Therefore, positive values mean that daily therapy was more effective than intermittent therapy, and negative values mean the opposite.
Calculated using ratio of odds of culture conversion in daily therapy to those in intermittent therapy. Therefore, values greater than 1 mean that daily therapy was more effective than intermittent therapy, and values less than 1 mean the opposite.
Adjusted for age, sex, body mass index, smoking, etiologic organism (M. avium versus M. intracellulare), sputum smear positivity, forced expiratory volume in 1 s, and choice of macrolide (clarithromycin vs. azithromycin).
In addition, there was no difference in median time to culture conversion between the daily therapy group (1.6 months; IQR, 1.1 to 5.0 months) and intermittent therapy group (1.3 months; IQR, 1.0 to 10.1 months; P = 0.920) (Table 4). Among 10 patients who had persistent positive sputum cultures after 12 months of antibiotic therapy, macrolide resistance developed in two patients who received daily therapy, whereas the other eight patients remained susceptible to CLR.
Of the 43 patients who completed treatment for recurrent MAC lung disease (21 patients in the daily therapy group and 22 patients in the intermittent therapy group), 11 (26%) redeveloped NTM lung during the median follow-up period of 16.6 months (IQR, 5.1 to 32.2 months) up to June 30, 2017. This rate was not different between the daily therapy group (6/21, 29%) and the intermittent therapy group (5/22, 23%, P = 0.736). In these 11 patients, MAC lung disease from the same species recurred in six (55%) cases (four cases of M. avium and two cases of M. intracellulare), and NTM lung disease from a different NTM species developed in five (45%) cases (Table 4).
Genotyping of paired isolates.
As described previously, recurrent MAC lung disease was caused by the same species in 38 patients among the 53 patients in the study. Among these 38 patients, paired MAC isolates from the time of the previous initial treatment episode and the time of diagnosis of recurrent MAC lung disease were available for genotype analysis in 14 (37%) patients (eight patients infected with M. avium and six patients infected with M. intracellulare). A total of 30 isolates from 14 patients were examined, including two isolates from two patients who developed a second recurrence of MAC lung disease caused by the same species after treatment for recurrent MAC lung disease. The morphotype of each single colony was classified macroscopically as smooth or rough. If the isolates included colonies of both morphotypes, single colonies of each type were analyzed separately. Finally, 98 single colonies were sampled from these isolates, and the median number of single colonies per patients was seven (range, 4 to 15). All initial and recurrent isolates were susceptible to clarithromycin and had no rrl mutations (see Table S1 in the supplemental material).
Among the 14 patients, most cases were caused by reinfections with new MAC genotypes different from the initial MAC genotypes (12/14, 86%); only two cases of relapse were caused by the same MAC genotypes that caused the original disease (2/14, 14%) (see Fig. S1 in the supplemental material). In addition, two patients with a second recurrence were reinfected with additional new MAC genotypes that differed from the genotypes of both the initial infection and the first recurrence (see Table S1 and Fig. S1 in the supplemental material).
DISCUSSION
To the best of our knowledge, this is the first study to evaluate the clinical efficacy of intermittent antibiotic therapy for recurrent noncavitary NB MAC lung disease. One of the most important findings in our study was that patients who received intermittent therapy had similar treatment response rates compared to patients in the daily therapy group with respect to symptomatic improvement, HRCT improvement, culture conversion and maintenance, and time to culture conversion. In addition, the proportion of culture conversion was not different between daily and intermittent therapy groups. This constitutes important information that could aid in determining the most appropriate treatment strategy for recurrent MAC lung disease.
The treatment success rate for patients with previous treatment history for MAC lung disease is significantly lower (38 to 69%) than that for newly treated MAC patients (68 to 91%) for both daily antibiotic therapy (18, 19) and intermittent antibiotic therapy (20–23). Based on these results, current guidelines recommend very aggressive therapy composed of three oral drugs daily plus parenteral injection of streptomycin or amikacin in patients who have been treated previously, regardless of clinical phenotype, although intermittent therapy is recommended as the initial therapy for noncavitary NB MAC lung disease (4). In all these previous studies, however, a substantial proportion (more than 50% in some studies) of enrolled patients had fibrocavitary MAC lung disease, and some patients even had macrolide-resistant MAC lung disease (18–23). In the present study, which focused on patients with recurrent noncavitary NB MAC lung disease, sputum culture conversion rates were higher than 80% in both intermittent therapy and daily therapy groups, comparable to those obtained in patients with newly treated NB MAC lung disease (10, 15, 24).
Previous studies found that 22 to 50% of patients with MAC lung disease experience recurrence of MAC lung disease after successful treatment (10–15). The majority of these recurrences were reported to be due to reinfection rather than true relapse, especially in patients with NB MAC lung disease (10, 11, 15). Wallace et al. found that patients with NB MAC lung disease frequently suffer from multiple or repeated infections, whereas patients with fibrocavitary MAC lung disease are frequently infected with a single strain (16). In their subsequent study, they analyzed the genotypes of isolates in patients with NB MAC lung disease and showed that most infections were from new MAC strains after completing initial therapy (10, 17). In our recent study, which included 481 MAC patients, we found that the proportion of reinfections was higher in patients with NB MAC disease (82%) than in those with fibrocavitary MAC disease (40%) (15).
In the present study, more than one-fourth (15/53, 28%) of patients with recurrent NB MAC lung disease experienced recurrence due to infection with a MAC species different from the MAC organism present at the initial treatment, and genotype analysis performed in cases with recurrence caused by the same MAC species demonstrated that most of the analyzed cases were due to reinfections with new MAC genotypes (12/14, 86%), although the genotype analysis results were available for only one-third (14/38, 37%) of these patients. All of these findings suggest that a large proportion of our recurrent cases was likely caused by new MAC genotypes rather than true relapse caused by the same MAC genotypes.
Given the high reinfection rate by new genotypes in recurrent NB MAC lung disease, universal aggressive daily therapy with oral and parenteral agents might not be warranted for managing patients with recurrent noncavitary NB MAC lung disease. The results of our study strongly suggest that intermittent oral antibiotic therapy without parenteral drug administration, rather than more toxic daily oral therapy plus parenteral drug administration, might be more appropriate for these patients.
The present study had several limitations. First, this study was conducted at a single referral center with specialized NTM clinics. Second, we did not use a prospective randomized controlled design to compare the clinical efficacy of daily versus intermittent therapy. Thus, there is a possibility that not enough patients were included to confirm the noninferiority of intermittent therapy compared to the daily therapy. Third, since patients were treated with daily therapy or intermittent therapy during different time periods, according to our treatment protocols, there could be selection bias between the groups in this retrospective study. Fourth, genotyping data were not available for a significant proportion of patients with recurrent disease caused by the same MAC species. Thus, we could not differentiate between persistent infection with the initial MAC strain and reinfection with a new MAC strain in these patients. Fifth, M. chimaera, new species closely related to M. intracellulare, was not differentiated in this study. However, M. chimaera is relatively rare in South Korea (25–27).
Lastly, the follow-up duration after treatment of recurrent disease was relatively short.
In conclusion, the majority of recurrent noncavitary NB MAC lung disease cases were caused by reinfection by either a different MAC species or the same MAC species with a different genotype. Considering that distinguishing between relapse and reinfection in recurrent cases requires molecular typing methods that are not routinely available in clinical practice in most countries, intermittent three-times-weekly oral antibiotic therapy could be a reasonable treatment strategy for recurrent noncavitary NB MAC lung disease. In light of our findings, current treatment guidelines may need to be reevaluated, although further clinical studies are recommended.
MATERIALS AND METHODS
Study population.
Consecutive patients who had a history of previous treatment of MAC lung disease and for whom antibiotic treatment for recurrent noncavitary NB MAC lung disease was initiated between January 2008 and December 2015 were identified using the database of the NTM Registry of Samsung Medical Center (a 1,979-bed referral hospital in Seoul, South Korea). All patients met the diagnostic criteria for NTM lung disease (4). Data were from an ongoing institutional review board-approved prospective observational cohort study to investigate NTM lung disease, and written informed consent was obtained from all participants (clinicaltrials.gov identifier NCT00970801) (15, 24).
Radiologic phenotypes were classified according to the main features on chest X-ray and HRCT. HRCT scans were available for all patients at the time of initiation of treatment for recurrent noncavitary NB MAC lung disease. All patients had characteristic chest X-ray and HRCT findings, such as the presence of multifocal bronchiectasis, clusters of small nodules, and absence of cavities on chest HRCT (8, 9).
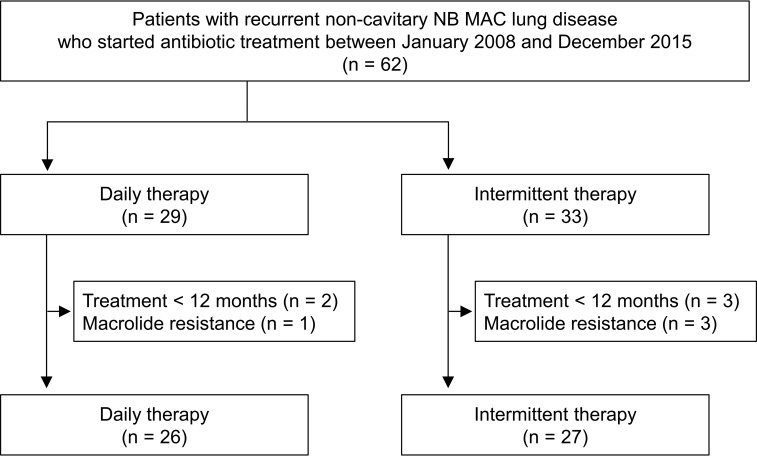
During the study period, 99 patients with recurrent MAC lung disease started antibiotic treatment. After excluding 17 patients with fibrocavitary form, 14 patients with cavitary NB form, and six patients with unclassifiable form of MAC lung disease, 62 patients with recurrent noncavitary NB MAC lung disease who initiated antibiotic therapy were identified. All patients had persistent respiratory symptoms and radiographic progression associated with MAC lung disease. In our institution, intermittent therapy was introduced for initial treatment of all patients with noncavitary NB MAC lung disease in January 2011 (24), and intermittent therapy was also applied in patients with recurrent noncavitary NB MAC lung disease beginning in January 2012. Of 62 patients, 29 (47%) received daily therapy and 33 (53%) patients received intermittent therapy for recurrent disease during the study period. After excluding patients who received less than 12 months of antibiotic treatment (n = 5) and patients with macrolide-resistant MAC lung disease (n = 4), 53 patients with recurrent noncavitary NB MAC lung disease were included in the final study, comprising 26 patients with daily therapy and 27 patients with intermittent therapy (Fig. 1).
FIG 1.
Study population. NB, nodular bronchiectatic form; MAC, M. avium complex.
Antibiotic treatment.
All patients received standardized combination antibiotic treatment, which consisted of an oral macrolide (CLR or AZM), EMB, and RIF (4). The daily therapy regimen included an oral macrolide (1,000 mg CLR or 250 mg AZM), 15 mg/kg EMB, and 450 mg RIF (body weight < 50 kg) or 600 mg RIF (body weight ≥ 50 kg). Streptomycin or kanamycin was administered intramuscularly at 10 to 15 mg/kg (500 to 1,000 mg) three times a week in the daily therapy group for the first several months, at the discretion of the attending physician, especially in patients with acid-fast bacilli smear-positive sputum (24). The regimen for intermittent therapy included an oral macrolide (1,000 mg CLR or 500 mg AZM), 25 mg/kg EMB, and 600 mg RIF three times weekly. Patients were generally treated until culture negative for 12 months (24).
Treatment outcomes.
After 12 months of treatment, we evaluated improvement in symptoms, HRCT findings, and conversion of a sputum culture. Symptomatic responses were determined by the attending physician, and radiographic responses were evaluated based on the radiologists' interpretation of HRCT scans (24). HRCT scans were available in all patients after 12 months of treatment. Sputum examinations were performed at 1, 3, and 6 months after initiation of antibiotic treatment and then at 2- to 3-month intervals during treatment. Sputum culture conversion was defined as three consecutive negative cultures, and the time to culture conversion was defined as the date of the first negative culture (24). The clinical efficacy of antibiotic therapy was determined based on the sputum negative sputum culture conversion and maintenance of negative cultures for more than 12 months (4).
Microbiological examinations.
Clinical isolates were identified using PCR-restriction fragment length polymorphism analysis of the rpoB gene or reverse-blot hybridization assay of the rpoB gene (24, 28). Drug susceptibility testing was performed using the broth microdilution method (29). Isolates with a MIC of 32 μg/ml or greater for clarithromycin were considered resistant to macrolides (29).
Mutations in the 23S rRNA gene (rrl) were detected by PCR sequencing as described previously (30). Mycobacterial genotyping was performed using repetitive sequence-based PCR (rep-PCR), which was standardized according to the DiversiLab Mycobacterium kit protocol (bioMérieux, Marcy l'Étoile, France) (31). Reports of rep-PCR were generated based on the Kullback-Leibler method, and isolates with identical profiles or >97% similarity were regarded as indistinguishable (32). In patients with recurrent MAC lung disease for whom initial and recurrent MAC isolates were stored, we distinguished between “relapse” with the same MAC genotype and “reinfection” with a new MAC genotype.
Statistical analyses.
All data are presented as numbers (percentages) for categorical variables and medians (interquartile ranges [IQRs]) for continuous variables. Data were compared using Pearson χ2 tests or Fisher exact tests for categorical variables and Mann-Whitney U tests for continuous variables. To compare the outcomes of daily therapy versus intermittent therapy, an adjustment for confounding factors, including age, sex, body mass index, smoking, etiologic organism (M. avium versus M. intracellulare), sputum smear positivity, forced expiratory volume in 1 s, and choice of macrolide (clarithromycin versus azithromycin), was conducted. The crude and adjusted proportion of patients with culture conversion was calculated by logistic regression analysis. All statistical analyses were performed using PASW (version 18.0; SPSS Inc., Chicago, IL), and a two-sided P < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Soohyun Ahn, a statistician in Samsung Medical Center, for help with the statistical analysis.
C.L.D. received grants from Insmed, Inc., not associated with the submitted work. Otherwise, we have no conflicts of interest to declare.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (NRF-2015R1A2A1A01003959) and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI15C2778). The sponsor had no role in the design of the study, collection, and analysis of the data, or preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01812-17.
REFERENCES
- 1.Prevots DR, Marras TK. 2015. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stout JE, Koh WJ, Yew WW. 2016. Update on pulmonary disease due to nontuberculous mycobacteria. Int J Infect Dis 45:123–134. doi: 10.1016/j.ijid.2016.02.308. [DOI] [PubMed] [Google Scholar]
- 3.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Daza R, Daley CL, Dekhuijzen PN, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, Folkvardsen DB, Gibbons N, Gómez-Mampaso E, Gonzalez R, Hoffmann H, Hsueh PR, Indra A, Jagielski T, Jamieson F, Jankovic M, Jong E, Keane J, Koh WJ, Lange B, Leao S, Macedo R, Mannsåker T, Marras TK, Maugein J, Milburn HJ, Mlinkó T, Morcillo N, Morimoto K, Papaventsis D, Palenque E, Paez-Peña M, Piersimoni C, Polanová M, Rastogi N, Richter E, Ruiz-Serrano MJ, Silva A, da Silva MP, Simsek H, van Soolingen D, Szabó N, Thomson R, Tórtola Fernandez T, Tortoli E, Totten SE, Tyrrell G, Vasankari T, Villar M, Walkiewicz R, Winthrop KL, Wagner D. 2013. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Philley JV, Griffith DE. 2015. Treatment of slowly growing mycobacteria. Clin Chest Med 36:79–90. doi: 10.1016/j.ccm.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Ryu YJ, Koh WJ, Daley CL. 2016. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians' perspectives. Tuberc Respir Dis (Seoul) 79:74–84. doi: 10.4046/trd.2016.79.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon YS, Koh WJ. 2016. Diagnosis and treatment of nontuberculous mycobacterial lung disease. J Korean Med Sci 31:649–659. doi: 10.3346/jkms.2016.31.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee G, Lee KS, Moon JW, Koh WJ, Jeong BH, Jeong YJ, Kim HJ, Woo S. 2013. Nodular bronchiectatic Mycobacterium avium complex pulmonary disease. Natural course on serial computed tomographic scans. Ann Am Thorac Soc 10:299–306. doi: 10.1513/AnnalsATS.201303-062OC. [DOI] [PubMed] [Google Scholar]
- 9.Lee G, Kim HS, Lee KS, Koh WJ, Jeon K, Jeong BH, Ahn J. 2013. Serial CT findings of nodular bronchiectatic Mycobacterium avium complex pulmonary disease with antibiotic treatment. AJR 201:764–772. doi: 10.2214/AJR.12.9897. [DOI] [PubMed] [Google Scholar]
- 10.Wallace RJ Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, York DS, Shepherd S, Griffith DE. 2014. Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest 146:276–282. doi: 10.1378/chest.13-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BY, Kim S, Hong Y, Lee SD, Kim WS, Kim DS, Shim TS, Jo KW. 2015. Risk factors for recurrence after successful treatment of Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 59:2972–2977. doi: 10.1128/AAC.04577-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min J, Park J, Lee YJ, Kim SJ, Park JS, Cho YJ, Yoon HI, Lee CT, Lee JH. 2015. Determinants of recurrence after successful treatment of Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis 19:1239–1245. doi: 10.5588/ijtld.14.0139. [DOI] [PubMed] [Google Scholar]
- 13.Jarand J, Davis JP, Cowie RL, Field SK, Fisher DA. 2016. Long-term follow-up of Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest 149:1285–1293. doi: 10.1378/chest.15-0543. [DOI] [PubMed] [Google Scholar]
- 14.Boyle DP, Zembower TR, Qi C. 2016. Relapse versus reinfection of Mycobacterium avium complex pulmonary disease. patient characteristics and macrolide susceptibility. Ann Am Thorac Soc 13:1956–1961. doi: 10.1513/AnnalsATS.201605-344BC. [DOI] [PubMed] [Google Scholar]
- 15.Koh WJ, Moon SM, Kim SY, Woo MA, Kim S, Jhun BW, Park HY, Jeon K, Huh HJ, Ki CS, Lee NY, Chung MJ, Lee KS, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J 50:1602503. doi: 10.1183/13993003.02503-2016. [DOI] [PubMed] [Google Scholar]
- 16.Wallace RJ Jr, Zhang Y, Brown BA, Dawson D, Murphy DT, Wilson R, Griffith DE. 1998. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med 158:1235–1244. doi: 10.1164/ajrccm.158.4.9712098. [DOI] [PubMed] [Google Scholar]
- 17.Wallace RJ Jr, Zhang Y, Brown-Elliott BA, Yakrus MA, Wilson RW, Mann L, Couch L, Girard WM, Griffith DE. 2002. Repeat positive cultures in Mycobacterium intracellulare lung disease after macrolide therapy represent new infections in patients with nodular bronchiectasis. J Infect Dis 186:266–273. doi: 10.1086/341207. [DOI] [PubMed] [Google Scholar]
- 18.Wallace RJ Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. 1996. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med 153:1766–1772. doi: 10.1164/ajrccm.153.6.8665032. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka E, Kimoto T, Tsuyuguchi K, Watanabe I, Matsumoto H, Niimi A, Suzuki K, Murayama T, Amitani R, Kuze F. 1999. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med 160:866–872. doi: 10.1164/ajrccm.160.3.9811086. [DOI] [PubMed] [Google Scholar]
- 20.Griffith DE, Brown BA, Murphy DT, Girard WM, Couch L, Wallace RJ Jr. 1998. Initial (6-month) results of three-times-weekly azithromycin in treatment regimens for Mycobacterium avium complex lung disease in human immunodeficiency virus-negative patients. J Infect Dis 178:121–126. doi: 10.1086/515597. [DOI] [PubMed] [Google Scholar]
- 21.Griffith DE, Brown BA, Cegielski P, Murphy DT, Wallace RJ Jr. 2000. Early results (at 6 months) with intermittent clarithromycin-including regimens for lung disease due to Mycobacterium avium complex. Clin Infect Dis 30:288–292. doi: 10.1086/313644. [DOI] [PubMed] [Google Scholar]
- 22.Griffith DE, Brown BA, Girard WM, Griffith BE, Couch LA, Wallace RJ Jr. 2001. Azithromycin-containing regimens for treatment of Mycobacterium avium complex lung disease. Clin Infect Dis 32:1547–1553. doi: 10.1086/320512. [DOI] [PubMed] [Google Scholar]
- 23.Lam PK, Griffith DE, Aksamit TR, Ruoss SJ, Garay SM, Daley CL, Catanzaro A. 2006. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 173:1283–1289. doi: 10.1164/rccm.200509-1531OC. [DOI] [PubMed] [Google Scholar]
- 24.Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, Ki CS, Lee NY, Shin SJ, Daley CL, Koh WJ. 2015. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 191:96–103. doi: 10.1164/rccm.201408-1545OC. [DOI] [PubMed] [Google Scholar]
- 25.Kim SY, Park HY, Jeong BH, Jeon K, Huh HJ, Ki CS, Lee NY, Han SJ, Shin SJ, Koh WJ. 2015. Molecular analysis of clinical isolates previously diagnosed as Mycobacterium intracellulare reveals incidental findings of “Mycobacterium indicus pranii” genotypes in human lung infection. BMC Infect Dis 15:406. doi: 10.1186/s12879-015-1140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon SM, Kim SY, Jhun BW, Lee H, Park HY, Jeon K, Huh HJ, Ki CS, Lee NY, Shin SJ, Koh WJ. 2016. Clinical characteristics and treatment outcomes of pulmonary disease caused by Mycobacterium chimaera. Diagn Microbiol Infect Dis 86:382–384. doi: 10.1016/j.diagmicrobio.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Kim SY, Shin SH, Moon SM, Yang B, Kim H, Kwon OJ, Huh HJ, Ki CS, Lee NY, Shin SJ, Koh WJ. 2017. Distribution and clinical significance of Mycobacterium avium complex species isolated from respiratory specimens. Diagn Microbiol Infect Dis 88:125–137. doi: 10.1016/j.diagmicrobio.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, Shin SJ, Kwon OJ. 2012. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M. avium complex lung disease. Chest 142:1482–1488. doi: 10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. 2nd ed CLSI document No. M24-A2, Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 30.Moon SM, Park HY, Kim SY, Jhun BW, Lee H, Jeon K, Kim DH, Huh HJ, Ki CS, Lee NY, Kim HK, Choi YS, Kim J, Lee SH, Kim CK, Shin SJ, Daley CL, Koh WJ. 2016. Clinical characteristics, treatment outcomes, and resistance mutations associated with macrolide-resistant Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 60:6758–6765. doi: 10.1128/AAC.01240-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Healy M, Huong J, Bittner T, Lising M, Frye S, Raza S, Schrock R, Manry J, Renwick A, Nieto R, Woods C, Versalovic J, Lupski JR. 2005. Microbial DNA typing by automated repetitive-sequence-based PCR. J Clin Microbiol 43:199–207. doi: 10.1128/JCM.43.1.199-207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mougari F, Raskine L, Ferroni A, Marcon E, Sermet-Gaudelus I, Veziris N, Heym B, Gaillard JL, Nassif X, Cambau E. 2014. Clonal relationship and differentiation among Mycobacterium abscessus isolates as determined using the semiautomated repetitive extragenic palindromic sequence PCR-based DiversiLab system. J Clin Microbiol 52:1969–1977. doi: 10.1128/JCM.03600-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.