ABSTRACT
Levofloxacin is increasingly used in the treatment of multidrug-resistant tuberculosis (MDR-TB). There are limited pediatric pharmacokinetic data to inform dose selection for children. Children routinely receiving levofloxacin (250-mg adult tablets) for MDR-TB prophylaxis or disease in Cape Town, South Africa, underwent pharmacokinetic sampling following receipt of a dose of 15 or 20 mg/kg of body weight given as a whole or crushed tablet(s) orally or via a nasogastric tube. Pharmacokinetic parameters were estimated using nonlinear mixed-effects modeling. Model-based simulations were performed to estimate the doses across weight bands that would achieve adult exposures with 750-mg once-daily dosing. One hundred nine children were included. The median age was 2.1 years (range, 0.3 to 8.7 years), and the median weight was 12 kg (range, 6 to 22 kg). Levofloxacin followed 2-compartment kinetics with first-order elimination and absorption with a lag time. After inclusion of allometric scaling, the model characterized the age-driven maturation of clearance (CL), with the effect reaching 50% of that at maturity at about 2 months after birth and 100% of that at maturity by 2 years of age. CL in a typical child (weight, 12 kg; age, 2 years) was 4.7 liters/h. HIV infection reduced CL by 16%. By use of the adult 250-mg formulation, levofloxacin exposures were substantially lower than those reported in adults receiving a similar dose on a milligram-per-kilogram basis. To achieve adult-equivalent exposures at a 750-mg daily dose, higher levofloxacin pediatric doses of from 18 mg/kg/day for younger children with weights of 3 to 4 kg (due to immature clearance) to 40 mg/kg/day for older children may be required. The doses of levofloxacin currently recommended for the treatment of MDR-TB in children result in exposures considerably lower than those in adults. The effects of different formulations and formulation manipulation require further investigation. We recommend age- and weight-banded doses of 250-mg tablets of the adult formulation most likely to achieve target concentrations for prospective evaluation.
KEYWORDS: NONMEM, allometric scaling, dosing recommendations, fluoroquinolones, maturation, pediatric, population PK modeling
INTRODUCTION
The fluoroquinolones, with their potent antimycobacterial activity, are increasingly important medications for tuberculosis (TB) prevention and treatment, particularly for multidrug-resistant (MDR) TB (i.e., TB caused by Mycobacterium tuberculosis strains resistant to both isoniazid and rifampin), against which the fluoroquinolones are a key component of current regimens. Levofloxacin, the l-isomer and the more active component of the ofloxacin racemate (1, 2), is the fluoroquinolone most commonly used in young children with TB, partly due to the availability of smaller, child-friendly formulations. Levofloxacin also has the advantage of having a reduced QT-prolonging effect compared to that of other fluoroquinolones (like moxifloxacin) (3), making it more suitable for use in explorations of the effects of increased doses and its combined use with the novel and repurposed anti-TB drugs bedaquiline, delamanid, pretomanid, and clofazimine, all of which can cause QT interval prolongation (4). Levofloxacin is also increasingly used as a substitute for isoniazid in the treatment of isoniazid-resistant TB and is being evaluated for the treatment of tuberculous meningitis. Data from an animal model of latent TB infection and from observational studies in children suggest a potential role for the fluoroquinolones in MDR-TB-preventive therapy as well, and two phase III clinical trials (the TB-CHAMP trial in South Africa [International Standard Randomized Controlled Trial number ISRCTN92634082] and the V-QUIN trial in Vietnam [trial identifier, ACTRN12616000215426]) comparing levofloxacin and placebo are ongoing (5, 6). Given these broad indications for the use of levofloxacin against TB, it is likely to be more widely used in children affected by TB (7, 8).
There are currently limited data on levofloxacin pharmacokinetics (PK) in children with TB. The fluoroquinolones are concentration-dependent antibiotics, with the area under the concentration-time curve (AUC) and maximum plasma concentrations (Cmax) being considered the pharmacokinetic parameters most closely correlated with efficacy (9). Levofloxacin is rapidly absorbed after oral administration with a bioavailability of >90% and is mainly renally eliminated (10). The doses of levofloxacin in children that result in exposures approximating those in adults receiving the current World Health Organization (WHO)-recommended dose of 750 mg once daily for the treatment of TB are not well defined (11). The levofloxacin dose currently recommended by WHO for MDR-TB treatment in children is 15 to 20 mg/kg of body weight/day divided into two doses daily in children aged ≤5 years and 10 to 15 mg/kg/day once daily in children aged >5 years; however, published data supporting this recommendation are limited (11). A small study of 22 South African children aged 0 to 8 years with MDR-TB disease or exposure who received levofloxacin at 15 mg/kg once daily showed lower exposures (median Cmax, 6.79 mg/liter; median AUC, 32.9 mg · h/liter) than healthy adults after a standard 750-mg dose (mean Cmax, 9.3 mg/liter; mean AUC, 101 mg · h/liter) (10, 12). On the basis of data from a study of 50 children receiving levofloxacin for MDR-TB treatment or preventive therapy in the Federated States of Micronesia and the Republic of the Marshall Islands, a 15- to 20-mg/kg once-daily dose was recently recommended (13). However, few young children were included in that study, with the study including only three children younger than 2 years of age, the age range during which renal function is rapidly maturing (13). Data for HIV-infected children also remain limited.
The objective of this study was to describe the population pharmacokinetics of levofloxacin among children receiving levofloxacin for MDR-TB and to use this information in simulations to predict the dosing that would achieve the exposures seen in adults receiving the currently recommended 750-mg dose.
RESULTS
Study population and pharmacokinetic samples.
The study included 109 children. The median age was 2.1 years (range, 0.3 to 8.7 years), and the median weight was 12 kg (range, 6 to 22 kg); 16 were HIV infected and on antiretroviral therapy (ART) containing either lopinavir-ritonavir (n = 13) or efavirenz (n = 3). Patient characteristics are shown in Table 1. A total of 662 samples were available for analysis (3 participants contributed data from more than one sampling occasion), with the levofloxacin concentrations in 36 (5.4%) samples being below the limit of quantification of the assay, and all of these were observed in the predose sample.
TABLE 1.
Characteristics of children with MDR-TB disease or exposure treated with levofloxacina
| Characteristic | Value |
|---|---|
| No. (%) of children of the following ethnicity: | |
| Black | 69 (63.3) |
| Mixed race | 40 (36.7) |
| No. (%) of male children | 56 (51.4) |
| Median (range) age (yr) | 2.1 (0.32–8.65) |
| Median (range) wt (kg) | 12.4 (5.88–21.8) |
| Median (range) wt-for-age Z-score (WHO) | −0.39 (−4.18–3.32)b |
| Median (range) ht-for-age Z-score (WHO) | −1.31 (−4.70–1.45)c |
| Median (range) wt-for-length Z-score (WHO) | 0.60 (−4.88–4.33)d |
| No. (%) of children with the following MDR-TB disease status: | |
| MDR-TB disease (treatment) | 71 (65.1) |
| MDR-TB exposure (preventive therapy) | 38 (34.9) |
| Median (range) levofloxacin total dose (mg) | 212 (88.5–435) |
| Median (range) levofloxacin dose (mg/kg) | 15 (10–21.4) |
| Median (range) creatinine clearance (ml/min)e | 119 (66.6–181) |
| No. (%) of children administered drug by the following procedure: | |
| Whole tablet, orally | 7 (6.4) |
| Crushed tablet, orally | 12 (11) |
| Crushed tablet, nasogastric tube | 90 (82.6) |
| No. (%) of children HIV infected | 16 (14.7) |
Data are for 109 children. WHO, World Health Organization.
Twelve children had a Z-score of <−2.
Thirty-three children had a Z-score of <−2.
Two children had a Z-score of <−2.
The revised formula of Schwartz et al. (47) was used; the serum creatinine concentration was measured only in the patients treated for TB disease (n = 71, 65.1%).
Pharmacokinetic model.
The pharmacokinetics of levofloxacin was well described using 2-compartment disposition kinetics (with respect to a 1-compartment model, the change in the objective function value [ΔOFV] was 43.8; 2 degrees of freedom [df]; P < 0.001), first-order elimination and absorption, and inclusion of an absorption lag time (ΔOFV, 68.5; 2 df; P < 0.001). The final parameter estimates of the model, along with their precisions, are shown in Table 2.
TABLE 2.
PK parameter values for levofloxacin in children with MDR-TB disease or exposured
| PK parameter | Typical value (95% CI) | Variability as CV% (95% CI) [eta shrinkage (%)] |
|---|---|---|
| CL (liters/h)a | 4.70 (4.37, 5.00) for HIV− | BSV: 15.2 (10.6, 19.0) [24] |
| Vc (liters)a | 19.2 (10.9, 21.8) | |
| Q (liters/h)a | 0.796 (0.332, 4.76) | |
| Vp (liters)a | 3.40 (2.53, 38.7) | |
| Tlag (h) | 0.242 (0.0385, 0.654) for oral dosing | BOV: 130 (30.9, 303) [76] |
| ka (1/h) | 1.61 (0.855, 2.78) | BOV: 64.8 (43.4, 80.9) [43] |
| F | 1 (fixed) | BOV: 21.8 (13.7, 28.4) [10] |
| HIV+ on CL (%) | −15.9 (−26.6, −5.93) | |
| NGT on Tlag (%) | −85.6 (−99.3, −34.6) | |
| Scaling of BOV in F for unobserved doses (fold)b | 4.48 (3.31, 7.08) | |
| PMAGE50 (mo) | 10.6 (7.55, 12.9) | |
| γ | 3.39 (1.42, 4.98) | |
| Additive error (mg/liter)c | 0.0160, i.e., 20% of LLOQ (fixed) | |
| Proporational error (%) [epsilon shrinkage (%)] | 11.6 (10.0, 12.7) [29] |
All clearance and volume parameters have been scaled with allometric scaling. The typical values reported here refer to a 12-kg child aged 2 years. Age affects clearance, since maturation was used. At 2 years after birth, maturation is predicted to be 97.9% complete.
This is a multiplicative factor increasing the BOV in bioavailability for all predose concentrations, which follow an unobserved dose.
The estimate of the additive error hit the stipulated lower boundary (20% of LLOQ), so it was fixed to this value.
Values in parentheses are empirical 95% confidence intervals obtained with a 500-sample nonparametric bootstrap. The PK parameter variability was included either as between-subject variability or between-occasion variability, assuming a lognormal distribution. It is reported here as the approximate coefficient of variation (in percent). CL, clearance; Vc, central volume of distribution; Q, intercompartmental clearance; Vp, peripheral volume of distribution; Tlag, absorption lag time; ka, absorption rate constant; F, bioavailability; PK, pharmacokinetic; PMAGE50, postmenstrual age at which 50% maturation is reached; γ, shape factor for the maturation function; BSV, between-subject variability; BOV, between-occasion variability; HIV+ and HIV− HIV-infected and uninfected children, respectively; NGT, drug administration with a nasogastric tube.
After the inclusion of allometric scaling with total body weight, which substantially improved the fit (ΔOFV, 118), the model could characterize the effect of age, as shown in Fig. 1 (ΔOFV, 18.5; 2 df; P < 0.001): the model estimated that levofloxacin clearance (CL) reaches 50% maturity at about 2 months after birth and is nearly fully mature around 2 years of age. The use of fat-free mass (14) for allometric scaling did not provide meaningful improvements to the fit.
FIG 1.
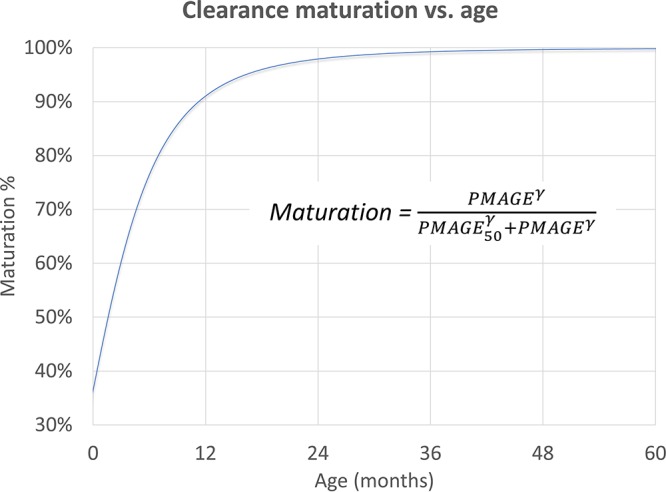
Maturation function of levofloxacin clearance. The percent maturation achieved versus postnatal age, assuming a standard duration of gestation (9 months), is shown.
The model estimated the typical value of CL in a 12-kg, 2-year-old child to be 4.7 liters/h. The use of a nasogastric tube (NGT) for drug administration was found to increase the speed of absorption by shortening the absorption lag time (ΔOFV, 11.4; 1 df; P < 0.001), but no significant effect on bioavailability was detected when either the tablet was crushed or an NGT was used. Additionally, HIV-infected children had a 16% lower CL (ΔOFV, 9.3; 1 df; P < 0.01) and, hence, higher exposure. This difference could not be ascribed to a particular ART regimen, due to the small sample size of this subgroup. Creatinine clearance was tested as an alternative to weight and age as a predictor of CL in the subset for which serum creatinine concentration measurements were available but did not provide a better model fit. No significant differences were found between children receiving treatment for MDR-TB and those receiving preventive treatment, and there was no effect of undernutrition or ethnicity.
After adjustment for all the predictors mentioned above, the model still identified a moderate random between-subject variability (BSV) in CL, a large between-occasion variability (BOV) in the absorption parameters, and a moderate BOV in bioavailability. The model significantly improved (ΔOFV, 51.4; 1 df; P < 0.001) when the estimate of the greater variability (∼4.5-fold) in the bioavailability of the unobserved doses was allowed. This additional parameter was included to adjust for the greater uncertainty expected to affect the information on the timing of doses and accurate dosing procedures on the days prior to the day of the visit when the dose was observed to have been given and samples were collected for pharmacokinetic analysis (Table 2).
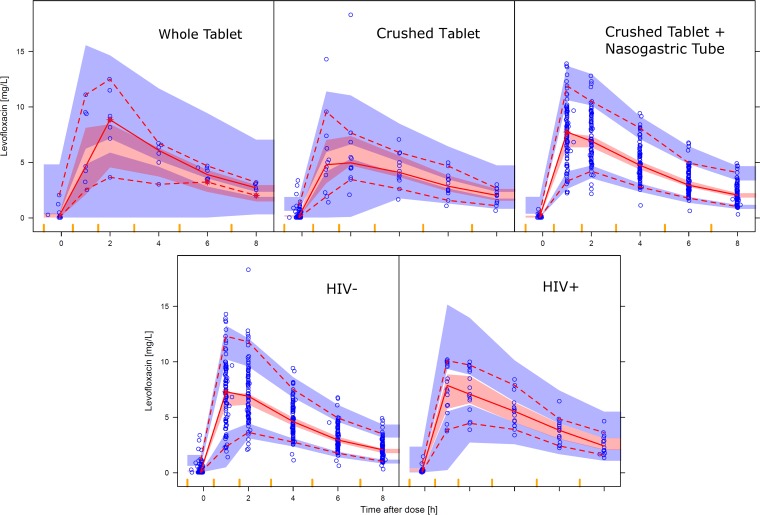
The model fit the data adequately, as shown in the visual predictive check (VPC) in Fig. 2, which also highlights the differences between the pharmacokinetic profiles obtained using different administration procedures and the effect of HIV infection.
FIG 2.
Visual predictive check of the levofloxacin concentration versus time after dose, stratified by either the administration method (top) or HIV infection status (bottom). The solid and dashed lines represent the 50th, 5th, and 95th percentiles of the observed data, while the shaded areas represent the model-predicted 95% confidence intervals for the same percentiles. The dots are the observed concentrations.
Simulated exposures.
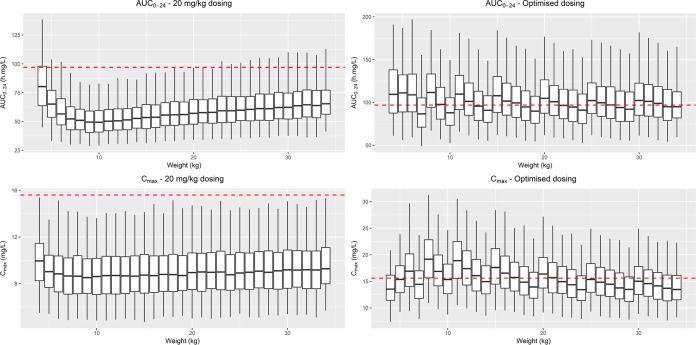
The final model was used to simulate the exposures achieved in children with different weights and ages, and these were compared with the values for adults. We first explored the expected AUC and Cmax in children receiving levofloxacin at a 20-mg/kg dose (the high end of the currently used dosing range of 15 to 20 mg/kg). The resulting exposures (AUC and Cmax) across different weights are shown in Fig. 3. These simulations show that the AUC achieved in children after dosing of a 20-mg/kg dose using the 250-mg adult formulation was considerably lower than the chosen target for all age groups. Additionally, a fixed dose on a milligram-per-kilogram basis achieved a progressively lower AUC as age and weight decreased down to ∼9 kg (for children <∼1 year of age), at which point the effect of maturation reversed this trend. Cmax was also lower than that previously reported in adults. We then evaluated an alternative dosing scheme, shown in Table 3, designed to more closely approximate the proposed target exposures in adults. To attain an AUC in children similar to that in adults, our model predicts that children in the weight band of between 8 and 11 kg may require considerably higher doses of this formulation of up to almost 40 mg/kg on a milligram-per-kilogram basis compared with the dose in adults. Children below 2 years of age, in whom the maturation of clearance is still incomplete, would require a slightly lower dose on a milligram-per-kilogram basis compared with the dose in adults, and the weight bands for very young children must be narrow to provide appropriate doses, given the substantial impact of body size and age on the pharmacokinetics of levofloxacin in younger children.
FIG 3.
Simulated levofloxacin steady-state AUC from time zero to 24 h (AUC0–24) (top) and Cmax (bottom) versus body weight. (Left) The concentrations achieved with dosing at 20 mg/kg; (right) the suggested optimized dosing. The dashed lines for Cmax (15.55 mg/liter) are the median values observed by Peloquin et al. (15) with dosing at 1,000 mg daily, while the dashed lines for AUC (96.8 mg · h/liter) represent the median exposure from the same study, after rescaling of the dose from 1,000 mg to 750 mg daily, the dose currently recommended for the treatment of tuberculosis in adults.
TABLE 3.
Suggested optimized weight-banded dosing of once-daily levofloxacin in children to approximate an adult 750-mg once-daily dosea
| Wt band (kg) | No. of 250-mg tablets | Daily dose (mg) | Median daily dose (mg/kg) |
|---|---|---|---|
| 3-<4 | 0.25 | 62.5 | 18 |
| 4-<5 | 0.5 | 125 | 28 |
| 5-<6 | 0.75 | 187.5 | 34 |
| 6-<8 | 1 | 250 | 36 |
| 8-<11 | 1.5 | 375 | 39 |
| 11-<15 | 2 | 500 | 38 |
| 15-<20 | 2.5 | 625 | 36 |
| 20-<25 | 3 | 750 | 33 |
| 25-<30 | 3.5 | 875 | 32 |
| 30-<35 | 4 | 1,000 | 31 |
The target was a steady-state AUC0–24 of 96.8 mg · h/liter (rescaled from 1,000 mg to 750 mg from Peloquin et al. [15]).
DISCUSSION
Among children who routinely receive the standard adult formulation of levofloxacin for the treatment or prevention of MDR-TB at currently recommended doses, exposures were substantially lower than those for adults receiving levofloxacin at the standard 750-mg dose for the same indication. This was true even in young children in whom renal function is still immature (15). As fluoroquinolones are considered the most important component of MDR-TB regimens, underdosing of these drugs is particularly concerning (16).
Our findings are generally consistent with those described in previous reports (13, 17) of the pharmacokinetics of levofloxacin in children (also determined using a standard 250-mg formulation), which showed lower exposures in children than in adults when both groups were dosed at the same milligram-per-kilogram level. Only part of the difference between the exposures in children and adults could be explained by the nonlinear effect of differences in body size described by allometric scaling. After standardizing the value of CL to a 70-kg individual with allometric scaling, our estimate is higher than the values previously reported for pediatric populations (13, 17) and, in keeping with the findings of other studies, is higher than the CL values reported in studies with adults (Table 4) (15, 18, 19). Why the levofloxacin concentrations in our study were lower than expected after adjustment for body size is unclear. Compared to other studies with pediatric populations, our CL values are greater than any of the values previously reported and are closest to those reported by Chien et al. (17) for the youngest cohorts in their study (<2 years old). As differences in body composition could play a role, we tested fat-free mass (FFM) as a descriptor of size for allometric scaling, but this did not significantly improve the model. Although we did not identify a significant effect of the use of crushed tablets versus whole tablets in the model, our study may have been underpowered to detect a difference, as only 7 of 109 children received whole tablets. Differences in bioavailability between formulations are increasingly recognized, and this is an important potential explanation for the variability in exposures between those found in previous work and those found in our study. This possibility should be evaluated in future work, as it has important practical implications. Other explanations could be differences in the ethnicities of the patients evaluated, differences between the drug formulations used our study and those used in previous studies, or the concomitant use of other antituberculosis drugs. Novel dispersible levofloxacin formulations (e.g., a 100-mg scored dispersible pediatric formulation [Macleods, India]) which are currently undergoing prequalification by WHO and which will be used in several trials for the treatment of MDR-TB in children should be evaluated, since bioavailability may vary substantially by formulation (20).
TABLE 4.
Comparison of scaled AUC and CL of levofloxacin in South African children with MDR-TB treatment or exposure with previously reported values in adults and children
| Author (reference or source) | No. of patients | Country | Median wt (kg) | Median and/or range age (yr) | Route of administration | Dose |
AUC (mg · h/liter) | AUC scaled to 1,000 mg or 20 mg/kg | CL (liters/h) scaled to a 70-kg adulta | |
|---|---|---|---|---|---|---|---|---|---|---|
| mg | mg/kg | |||||||||
| Preston et al. (19) | 272 | USA | 77.5 | 47 | Oral | 500 | ∼6.5 | 72.53 | 145 | 8.35 |
| Thwaites et al. (18) | 15 | Vietnam | 48 | 33 | Intravenous | 1000 | ∼20.8 | 155 | 155 | 8.56 |
| Peloquin et al. (15) | 10 | Brazil | 56 | 44 | Oral | 1000 | ∼17.9 | 129 | 129 | 9.16 |
| Mase et al. (13) | 50 | Pacific Islands | 1–15 | Oral gel | ∼14 | ∼55 | ∼80 | 11.6 | ||
| Chien et al. (17) | 8 | USA | 11 | 0.5–2 | Oral | 7 | 25.8 | 13.7 | ||
| 8 | USA | 15.6 | 2–5 | Oral | 7 | 25.9 | 15.4 | |||
| 8 | USA | 26.1 | 5–10 | Oral | 7 | 29.0 | 13.7 | |||
| 8 | USA | 42.5 | 10–12 | Oral | 7 | 37.3 | 11.7 | |||
| 8 | USA | 60.7 | 12–16 | Oral | 7 | 41.1 | 12.2 | |||
| Denti et al. (this study) | 109 | South Africa | 12 | 2.4 (0.6–8.6) | Oral | 17.7 | 45 | 51 | 18.8 | |
Allometric scaling was applied with the exponent 3/4 to scale the value from the median weight in the original study to 70 kg.
Our study was unique in that a large number of very young children were included, and we were able to characterize the developmental pharmacokinetics of levofloxacin. Specifically, levofloxacin CL was estimated to reach 50% of the value at maturity at about 2 months after birth. Seventeen children in our cohort were younger than 1 year of age. Since the youngest participants were about 4 months of age, the predicted exposure for infants under age 4 months is a model extrapolation and should be interpreted with caution. However, the parameter values for the maturation of levofloxacin clearance were estimated with reasonable precision and they are similar to those reported for the maturation of glomerular filtration rate (21). This is consistent with the fact that levofloxacin’s main route of elimination is renal excretion. It is important to appropriately dose the youngest children, as these children are at the highest risk of progression to TB disease following M. tuberculosis infection; have the highest risk of disseminated TB in hard-to-reach compartments, such as the cerebrospinal fluid (CSF); and have the highest rates of TB-related mortality (22).
HIV infection was associated with a lower clearance of levofloxacin. The effect was small and is unlikely to be clinically significant. As all HIV-infected children were on ART, we could not separate the effects of HIV infection and ART. The lower levofloxacin clearance could be related to a drug-drug interaction, possibly with ritonavir, which has broad effects on metabolizing and transport enzymes. However, our study had an insufficient power to ascribe this finding to a single antiretroviral drug. Recently, high levels of immune activation have been associated with reduced isoniazid clearance in adults coinfected with M. tuberculosis and HIV (23). We did not routinely measure markers of inflammation in the children to explore this hypothesis, which may deserve further evaluation in the future. A previous study in adults failed to identify a significant increase in levofloxacin concentrations when levofloxacin was given to HIV-infected patients treated with efavirenz or nelfinavir, but that study had no HIV-infected control group, and the comparison was instead made with historical data (24). The lack of an effect of the drug administration method on the overall bioavailability of levofloxacin is reassuring, given the limited availability of child-friendly levofloxacin formulations and the frequent need to crush levofloxacin tablets intended for use by adults for administration to young children. However, only 7 of the 109 children in this study received whole tablets, and those were the oldest children. This limits the power of our analysis to investigate this effect. Confirmation in other studies is necessary. Furthermore, the pharmacokinetics of a novel dispersible levofloxacin formulation should be evaluated in children, to inform the safe and effective dosing of levofloxacin for the treatment of MDR-TB.
The structure of our pharmacokinetic model, which has 2 compartments and delayed first-order absorption, differs from that of most previous models, which have 1 compartment and different models of absorption. These differences are likely due to different sampling schedules: the beta phase of the pharmacokinetic profile predicted by our model did not affect the concentration during first hours after the dose, but it improved our description of the minimum concentration at 24 h postdosing.
To achieve exposures similar to those in adults receiving the routine daily 750-mg dose used for the treatment of TB, the dose in children would need to be substantially increased from the currently recommended doses. In general, small children may need doses much higher on a milligram-per-kilogram basis (up to 40 mg/kg) than the 10- to 15-mg/kg dose used in adults. However, for infants (age, <1 year), who may have an immature clearance function, the dose increase needed to achieve the pharmacokinetic targets is more modest (18 to 28 mg/kg). Different formulations (e.g., dispersible tablets) may, however, have different dosing requirements.
The use of higher doses of levofloxacin in children may have implications for safety, particularly if similar exposures produce more serious adverse effects in children than in adults. Our most immediate concern would be for central nervous system (CNS) toxicity. Hallucinations, serious sleep disturbances, severe headaches, and other clinically significant CNS events, such as intracranial hypertension, have been described in children and adults receiving fluoroquinolones. These have particularly been observed with doses higher than those that were previously recommended but that were still within the range of the doses required to achieve the target concentrations in adults after the administration of 750-mg doses (25–28).
As can be seen from the simulations, if children are dosed once daily to target AUC values similar to adults on the current 750-mg dose, this will also result in Cmax values in children substantially higher than those in adults; the Cmax values in some children will exceed those observed in adults on a 1,000-mg dose. The relationship between Cmax and some adverse effects of concern (such as CNS side effects or muscle or joint toxicity) is poorly characterized. As the Cmax values expected with the once-daily simulated doses exceed what has been shown to be safe in adults to date, these doses would need to be carefully assessed in children before they are recommended for routine use for either the treatment or prevention of MDR-TB. For MDR-TB prevention, the risk/benefit ratio needs to be even more carefully considered, given the fact that well children are treated to prevent future MDR-TB disease. Although the QT interval-prolonging effect of levofloxacin appears to be less of a concern, the very high peak concentrations achieved with the simulated once-daily doses in children might still pose a risk of QT prolongation that would need to be assessed carefully. Splitting of the total daily dose into twice-daily doses would be a way to preserve the AUC without producing such a high Cmax; however, in this situation, the balance between potentially (but perhaps only modestly) improved safety and the burden to drug treatment programs and/or the impact on adherence to the treatment regimen must be considered. The relationship between pharmacokinetic parameters (Cmax versus AUC) and other toxicities, such as the CNS effects, has not been characterized. Therefore, splitting of the daily dose to reduce the expected Cmax may not eliminate the risk of adverse effects if they are, in fact, more closely associated with the AUC. Exploration of the use of higher doses of levofloxacin would therefore need to be carried out thoughtfully with careful safety monitoring. It should also be noted that the use of higher levofloxacin doses for the treatment of MDR-TB in adults is being explored in a phase II trial (the Opti-Q trial; ClinicalTrials.gov registration number NCT01918397); depending on the results from that study, target levofloxacin exposures may be higher than the current target exposures, which would impact dosing in the pediatric population.
Favorable outcomes are reported in 75 to 90% of children with MDR-TB (29, 30). This is much better than that in adults with MDR-TB, where globally only 50% are successfully treated (31). However, there are a number of reasons for the further optimization of levofloxacin doses in children with MDR-TB disease. First, there is scope for improving successful MDR-TB treatment outcomes further in children beyond the current 75 to 90% rate. Second, increasing evidence shows that low drug exposures are associated with poor TB treatment outcomes (including death and treatment failure) in children (32, 33), and low drug exposures may increase the risk of acquired drug resistance (34). Third, tuberculous meningitis is a relatively frequent form of TB in young children and is associated with devastating morbidity and mortality. A recently published analysis showed that doses of levofloxacin considerably higher than those currently used in children, approximately 19 to 33 mg/kg, are needed to obtain optimal concentrations in the CSF (35). Lastly, revised WHO guidance provided in 2016 recommended avoidance of the use of injectable medications in children with clinically diagnosed MDR-TB; optimization of the exposures to the fluoroquinolone in the regimen is especially important to ensure the potency of injectable-sparing MDR-TB treatment regimens (16) Additionally, the extrapolation of efficacy to children from the results of ongoing or planned trials of treatments for TB in adults, many of which contain levofloxacin, is, in principle, dependent on achieving similar drug exposures in children (36, 37).
There are limitations to the approach of using adult exposures as targets to define pediatric doses for anti-TB drugs. However, in the absence of other more optimal, validated approaches to defining doses in children, we believe that it remains a useful strategy. The use of pharmacokinetic (PK)/pharmacodynamic (PD) targets, such as the AUC/MIC, is an option. However, exposures in plasma may not reflect exposures at the site of disease. Differences in drug penetration into lesions or macrophages, which may not be reflected in the levels of plasma exposure, may have important implications for efficacy. These are potentially important limitations to the targeting of PK/PD indices. Interesting work on the development of hollow-fiber models of pediatric TB to establish drug targets has recently been undertaken (38); however, in the absence of validation of the exposure targets generated by these hollow-fiber models, we believe that it is premature to define doses on the basis of that work. Therefore, despite the limitations, the use of exposures in adults associated with efficacy remains a reasonable approach to establishing pediatric doses for anti-TB drugs. We would argue that TB disease in children differs from that in adults both in severity and in type (extrapulmonary versus pulmonary). Although a small proportion of children, mostly older children and adolescents, develop cavitating, adult-type TB, the majority of children develop less severe, paucibacillary disease, which is likely to be more responsive to treatment. It is therefore possible that children may be successfully treated with less intense treatment. However, what less intense treatment means needs to be carefully defined. We can be confident that the doses in children resulting in exposures approximating those seen with efficacious doses in adults would be as efficacious or more efficacious in children. It may be that exposures lower than those needed in adults with TB may still be efficacious in children; however, in the absence of a clear definition of “lower,” targeting of adult exposures remains a reasonable approach. One approach would be to maintain good exposures early in treatment to ensure a rapid response to treatment and a rapid reduction in organism burden but then to reduce the intensity of treatment by shortening the treatment duration in children. Such a strategy is being evaluated in the SHINE trial (International Standard Randomized Controlled Trial number ISRCTN63579542), which is a study of 4 versus 6 months of treatment for nonsevere intrathoracic TB in children.
A limitation of our study was the lack of older children, as moxifloxacin was routinely used in children 8 years of age and older in the local setting. However, pharmacokinetics is easier to predict in older children than in younger children. Another limitation is the lack of clear documentation of previous dosing times prior to the day on which samples were collected for pharmacokinetic analysis. Incomplete data on creatinine values, which were not collected for all children, limited our ability to assess whether renal function could explain some of the variability in clearance. Finally, we cannot explain the differences between our data and those published previously, such as higher values for clearance, and we cannot rule out the possibility of a contribution of the formulation used. We were unable to include different levofloxacin formulations in this study. This may need further evaluation, and the levofloxacin doses derived from our simulations should not be used routinely in children before additional careful evaluations have been completed.
In the large cohort of young South African children treated for MDR-TB described here, levofloxacin doses of 15 to 20 mg/kg resulted in exposures (Cmax and AUC) lower than those seen in adults receiving the standard 750-mg daily dose for the same indication, even in those very young children with immature renal function. Considerably higher levofloxacin doses will thus be needed for children. Model-derived dose recommendations are provided and should be explored prospectively, with special attention being given to safety.
MATERIALS AND METHODS
Study design.
This was a prospective observational intensive pharmacokinetic study.
Setting and population.
The study was performed in Cape Town, South Africa. Children were eligible if they were 0 to 14 years of age, were routinely treated with levofloxacin for the treatment of pulmonary or extrapulmonary MDR-TB disease or exposure, had been on TB treatment and antiretroviral therapy (ART) for at least 2 weeks if they were HIV infected, and provided written informed consent and assent. Children with body weights of <5 kg or with hemoglobin concentrations of <8 g/dl were excluded or enrollment was deferred. Children were diagnosed with confirmed or probable TB on the basis of signs, symptoms, microbiology, and radiography. In addition, the following was required: isolation from the child of an M. tuberculosis strain with resistance to both isoniazid and rifampin (i.e., confirmed MDR-TB disease), exposure to a case of infectious MDR-TB (probable MDR-TB), or clinical evidence of the failure of adherent first-line TB treatment (possible MDR-TB) (39). Children with MDR-TB disease were treated according to local and international recommendations with at least four drugs confirmed or likely to be effective against the infecting strain plus pyrazinamide and ethambutol. Local guidelines recommended the use of levofloxacin for children <8 years of age and moxifloxacin for adults and children >8 years of age on the basis of the available drug formulations (250 mg levofloxacin; Austel, South Africa). In our setting, high-risk contacts of an infectious MDR-TB source case, including children aged <5 years and HIV-infected children aged <14 years, without evidence of TB disease were prescribed preventive therapy against MDR-TB consisting of levofloxacin, ethambutol, and high-dose isoniazid daily for 6 months. All children in the study were routinely tested for HIV by an HIV-specific enzyme-linked immunosorbent assay if they are ≥18 months of age and by a PCR for HIV DNA if they are <18 months of age. All HIV-infected children in the study received ART on the basis of local recommendations, including lopinavir-ritonavir (children <3 years of age) or efavirenz (children >3 years of age) in combination with two nonnucleoside reverse transcriptase inhibitors, usually lamivudine (3TC) with abacavir or zidovudine.
Levofloxacin dosing and pharmacokinetic sampling.
Children received standard doses of 10 to 15 mg/kg once daily during the beginning of the study (2012 and 2013), according to the dose used for routine care at the time. Following interim analyses showing low drug exposures with these doses, the dose used for routine care was increased to 15 to 20 mg/kg once daily (2013 to 2017) (12). Pharmacokinetic sampling was performed between 2 and 16 weeks after the start of the anti-TB treatment. On the day of sampling, after an overnight fast, a levofloxacin dose of 15 mg/kg (during 2012 and 2013) or 20 mg/kg (from 2013 to 2015) (exact milligram-to-kilogram dosing to 0.1 mg adjusted to body weight) was administered by the study team together with all other anti-TB medications in the child's MDR-TB regimen. Medications were administered either as whole tablets (for older children able to swallow pills) or as tablets crushed and dissolved in water and given either orally or via a nasogastric tube (NGT), depending on what the child would tolerate. One hour after the anti-TB medication was dosed, a standard meal was offered. For HIV-infected children, antiretrovirals were also administered at that time. Blood samples were collected predose and then at 1, 2, 4, 6, and 8 h after levofloxacin dosing.
Levofloxacin assay.
A method for the quantification of levofloxacin in plasma was validated and consisted of protein precipitation using acetonitrile, followed by high-performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) detection. The calibration curve fit a linear (weighted by 1/concentration) regression over the range of 0.0781 to 5.00 mg/liter on the basis of the peak area ratios for levofloxacin. Levofloxacin-d8 was used as the internal standard. The lower limit of quantification (LLOQ) was set at the concentration of the lowest validated standard for levofloxacin, 0.0781 mg/liter. Inter- and intraday coefficients of variation were below 7% for all quality control samples. Dilutions were performed using a validated dilution procedure for samples with concentrations greater than the upper limit of the assay.
Population pharmacokinetic model development.
The data were interpreted using nonlinear mixed-effects modeling (NONMEM software, version 7.3) (40) and an algorithm with first-order conditional estimation with a eta-epsilon interaction. Summary statistics were calculated using the R program, and model development was assisted and graphic diagnostics were generated using the R program (41) and the Perl-Speaks-NONMEM, Xpose4, and Pirana programs (42). Several structural models were tested, including models with 1- and 2-compartment dispositions with first-order elimination and first-order absorption with and without the use of a lag time or a chain-of-transit compartment to model the onset of absorption (43). Random between-subject variability (BSV) and between-occasion variability (BOV) were assumed to have a lognormal distribution, and the error model included both an additive component and a proportional component. Concentrations below the LLOQ of the assay were imputed as LLOQ/2, as suggested in the M6 method of Beal (44), and the additive error component was constrained to be at least 20% of the LLOQ. The effect of differences in body size on the pharmacokinetic parameters was described using allometric scaling, with exponents fixed to 3/4 for clearance parameters and 1 for volumes of distribution (45), while the effect of growth and development was tested with a maturation function using postmenstrual age (i.e., postnatal age plus the estimated gestational age) (46), as shown in the following equation: ), where PMAGE denotes postmenstrual age, PMAGE50 is the value of PMAGE at which 50% of the maturation is complete, and γ is a parameter changing the shape of the relationship. Since no information on the actual gestational age of the children was available, the postmenstrual age was assumed to be the postnatal age plus 9 months.
Additionally, other covariate effects were explored, including HIV infection status, MDR-TB disease versus exposure status, ethnicity, undernutrition, the method of drug administration (crushed tablets via an NGT, crushed tablets orally, whole tablets orally), and coadministration of antituberculosis or antiretroviral drugs (the regimens are shown in Table S1 in the supplemental material). Creatinine clearance (estimated using the serum creatinine concentration and the revised formula of Schwartz et al. [47]) was also explored as a covariate; however, it was available only for children receiving MDR-TB treatment and not those receiving preventive therapy. Additionally, since administration of the dose 1 day prior to the day of sampling for pharmacokinetic analysis was not observed by the study team and the exact time and method (crushed versus whole tablets) of dose administration were not always accurately recorded, the inclusion of a larger BOV for the absorption and bioavailability pharmacokinetic parameters was tested for the pharmacokinetic profiles following a nonobserved dose (i.e., all the predose concentrations) (Table 2). Model development was guided by the use of the change in the objective function value (ΔOFV), considering decreases of 3.84 points to be significant at a P value of <0.05 for the inclusion of one additional parameter (denoted as the number of degrees of freedom [df]), by inspection of goodness-of-fit plots, and by the use of visual predictive checks (48). The precision of the final parameter estimates was evaluated using a nonparametric bootstrap with replacement (n = 500).
Levofloxacin dosing simulations.
Simulations from the final model were used to optimize the doses across different weight bands. To account for the effect of age (in addition to weight), simulations were performed using in silico patients with combinations of age and weight relevant for the population for whom the dosing guidelines are designed. To increase the granularity of the simulations and reflect the fact that children affected by TB generally have weights lower than those of children in the general population, we used a model of weight for age developed with children with TB to generate in silico patients: 100 males and 100 females at each 1-month age step from 0 to 18 years of age (49). Those in the weight range of 4 to 35 kg were retained for the simulations. All in silico children were assumed to be born at term, to be HIV uninfected, and to be dosed every 24 h with whole or crushed tablets without an NGT. Values of AUC and Cmax were collected.
The pharmacokinetic target was the AUC expected in adults with normal renal function treated for TB with levofloxacin at the currently recommended 750-mg daily dose. The AUC target was set at 96.8 mg · h/liter, a value that was obtained after rescaling the median AUC reported by Peloquin et al. (15) from 1,000 mg (reported in the study) to a 750-mg daily dose, since the pharmacokinetics of levofloxacin has been reported to be linear in this dose range (50). The study by Peloquin et al. (15) was chosen as it provides the only current data on levofloxacin pharmacokinetics in adults treated for TB. Reassuringly, the AUC rescaled to 750 mg is similar to the value reported to be obtained with a 750-mg dose in healthy adults without TB (mean AUC, 101 mg · h/liter) (10). As there may be implications for toxicity, we compared the simulated Cmax levels with the original Cmax values from the same study (median Cmax, 15.55 mg/liter) (15), without any rescaling of the dose, since these adult TB patients received 1,000 mg and levofloxacin was reported to be well tolerated during a 7-day dosing period. The optimized dosing regimen was designed to achieve a median AUC in each 1-kg weight band within a 20% tolerance of the target while ensuring that the median Cmax would not exceed by 20% the value previously observed in adults receiving the well-tolerated 1,000-mg dose. Finally, the doses in each weight band were adjusted to account for the fact that levofloxacin is currently available as a 250-mg tablet, which can be split in half or into quarters, if needed.
Ethical considerations.
Informed consent was provided by the parent(s) or legal guardian of all participants, and assent was provided by all participants aged 7 years and older. Ethics approval for the study was provided by the Health Research Ethics Committees of Stellenbosch University (N11/03/59) and the University of Cape Town (397/2011).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge all the children who participated in the study, their caregivers, and Maxwell Chirehwa's help with the creation of the figures.
The research reported in this publication was supported by The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health under award number R01HD06916 (to A.C.H.). A.C.H. (the SaRCHI Chair in Pediatric Tuberculosis), H.S.S., and H.M.M. (grant 90729) receive funding from the South Africa National Research Foundation (NRF). The University of Cape Town (UCT) Clinical PK Laboratory is supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) at UCT was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632), The Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute of Mental Health grant AI068632. The Division of Clinical Pharmacology at the University of Cape Town gratefully acknowledges Novartis Pharma for its support of the development of pharmacometrics skills in Africa.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01521-17.
REFERENCES
- 1.Hayakawa I, Atarashi S, Yokohama S, Imamura M, Sakano K, Furukawa M. 1986. Synthesis and antibacterial activities of optically active ofloxacin. Antimicrob Agents Chemother 29:163–164. doi: 10.1128/AAC.29.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu KP, Lafredo SC, Foleno B, Isaacson DM, Barrett JF, Tobia AJ, Rosenthale ME. 1992. In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob Agents Chemother 36:860–866. doi: 10.1128/AAC.36.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noel GJ, Goodman DB, Chien S, Solanki B, Padmanabhan M, Natarajan J. 2004. Measuring the effects of supratherapeutic doses of levofloxacin on healthy volunteers using four methods of QT correction and periodic and continuous ECG recordings. J Clin Pharmacol 44:464–473. doi: 10.1177/0091270004264643. [DOI] [PubMed] [Google Scholar]
- 4.Noel GJ, Natarajan J, Chien S, Hunt TL, Goodman DB, Abels R. 2003. Effects of three fluoroquinolones on QT interval in healthy adults after single doses. Clin Pharmacol Ther 73:292–303. doi: 10.1016/S0009-9236(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 5.Lanoix JP, Betoudji F, Nuermberger E. 2014. Novel regimens identified in mice for treatment of latent tuberculosis infection in contacts of patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 58:2316–2321. doi: 10.1128/AAC.02658-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bamrah S, Brostrom R, Dorina F, Setik L, Song R, Kawamura LM, Heetderks A, Mase S. 2014. Treatment for LTBI in contacts of MDR-TB patients, Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis 18:912–918. doi: 10.5588/ijtld.13.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodd PJ, Sismanidis C, Seddon JA. 2016. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis 16:1193–1201. doi: 10.1016/s1473-3099(16)30132-3. [DOI] [PubMed] [Google Scholar]
- 8.Seddon JA, Fred D, Amanullah F, Schaaf HS, Starke JR, Keshavjee S, Burzynski J, Furin JJ, Swaminathan S, Becerra MC. 2015. Post-exposure management of multidrug-resistant tuberculosis contacts: evidence-based recommendations. Policy brief no. 1. Harvard Medical School, Center for Global Health Delivery, Dubai, United Arab Emirates: http://www.ghd-dubai.hms.harvard.edu Accessed 3 May 2017. [Google Scholar]
- 9.Shandil RK, Jayaram R, Kaur P, Gaonkar S, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharath S, Balasubramanian V. 2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother 51:576–582. doi: 10.1128/AAC.00414-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration 2008. Levofloxacin label. U.S. Food and Drug Administration, Rockville, MD: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021721s020_020635s57_020634s52_lbl.pdf Accessed 17 April 2014. [Google Scholar]
- 11.World Health Organization 2014. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/pmdt_companionhandbook/en/ Accessed 8 August 2016. [PubMed] [Google Scholar]
- 12.Thee S, Garcia-Prats AJ, McIlleron HM, Wiesner L, Castel S, Norman J, Draper HR, van der Merwe PL, Hesseling AC, Schaaf HS. 2014. Pharmacokinetics of ofloxacin and levofloxacin for prevention and treatment of multidrug-resistant tuberculosis in children. Antimicrob Agents Chemother 58:2948–2951. doi: 10.1128/AAC.02755-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mase SR, Jereb JA, Gonzalez D, Martin F, Daley CL, Fred D, Loeffler AM, Menon LR, Bamrah Morris S, Brostrom R, Chorba T, Peloquin CA. 2016. Pharmacokinetics and dosing of levofloxacin in children treated for active or latent multidrug-resistant tuberculosis, Federated States of Micronesia and Republic of the Marshall Islands. Pediatr Infect Dis J 35:414–421. doi: 10.1097/INF.0000000000001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Sallami HS, Goulding A, Grant A, Taylor R, Holford N, Duffull SB. 2015. Prediction of fat-free mass in children. Clin Pharmacokinet 54:1169–1178. doi: 10.1007/s40262-015-0277-z. [DOI] [PubMed] [Google Scholar]
- 15.Peloquin CA, Hadad DJ, Molino LP, Palaci M, Boom WH, Dietze R, Johnson JL. 2008. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 52:852–857. doi: 10.1128/AAC.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. 2016. WHO treatment guidelines for drug-resistant tuberculosis: 2016 update. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/250125/1/9789241549639-eng.pdf Accessed 30 May 2016. [Google Scholar]
- 17.Chien S, Wells TG, Blumer JL, Kearns GL, Bradley JS, Bocchini JA Jr, Natarajan J, Maldonado S, Noel GJ. 2005. Levofloxacin pharmacokinetics in children. J Clin Pharmacol 45:153–160. doi: 10.1177/0091270004271944. [DOI] [PubMed] [Google Scholar]
- 18.Thwaites GE, Bhavnani SM, Chau TT, Hammel JP, Torok ME, Van Wart SA, Mai PP, Reynolds DK, Caws M, Dung NT, Hien TT, Kulawy R, Farrar J, Ambrose PG. 2011. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother 55:3244–3253. doi: 10.1128/AAC.00064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preston SL, Drusano GL, Berman AL, Fowler CL, Chow AT, Dornseif B, Reichl V, Natarajan J, Wong FA, Corrado M. 1998. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob Agents Chemother 42:1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blomberg B, Spinaci S, Fourie B, Laing R. 2001. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis. Bull World Health Organ 79:61–68. [PMC free article] [PubMed] [Google Scholar]
- 21.Holford N, Heo YA, Anderson B. 2013. A pharmacokinetic standard for babies and adults. J Pharm Sci 102:2941–2952. doi: 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 22.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, Enarson DA, Donald PR, Beyers N. 2004. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era state of the art. Int J Tuberc Lung Dis 8:392–402. [PubMed] [Google Scholar]
- 23.Vinnard C, Ravimohan S, Tamuhla N, Ivaturi V, Pasipanodya J, Srivastava S, Modongo C, Zetola NM, Weissman D, Gumbo T, Bisson GP. 2017. Isoniazid clearance is impaired among human immunodeficiency virus/tuberculosis patients with high levels of immune activation. Br J Clin Pharmacol 83:801–811. doi: 10.1111/bcp.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villani P, Viale P, Signorini L, Cadeo B, Marchetti F, Villani A, Fiocchi C, Regazzi MB, Carosi G. 2001. Pharmacokinetic evaluation of oral levofloxacin in human immunodeficiency virus-infected subjects receiving concomitant antiretroviral therapy. Antimicrob Agents Chemother 45:2160–2162. doi: 10.1128/AAC.45.7.2160-2162.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upton C. 1994. Sleep disturbance in children treated with ofloxacin. BMJ 309:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tome AM, Filipe A. 2011. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf 34:465–488. doi: 10.2165/11587280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Seddon JA, Hesseling AC, Finlayson H, Fielding K, Cox H, Hughes J, Godfrey-Faussett P, Schaaf HS. 2013. Preventive therapy for child contacts of multidrug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis 57:1676–1684. doi: 10.1093/cid/cit655. [DOI] [PubMed] [Google Scholar]
- 28.van der Laan LE, Schaaf HS, Solomons R, Willemse M, Mohamed N, Baboolal SO, Hesseling AC, van Toorn R, Garcia-Prats AJ. 2016. Probable levofloxacin-associated secondary intracranial hypertension in a child with multidrug-resistant tuberculosis. Pediatr Infect Dis J 35:706–708. doi: 10.1097/INF.0000000000001137. [DOI] [PubMed] [Google Scholar]
- 29.Harausz E, Garcia-Prats AJ, Schaaf HS, Hesseling AC, Furin J, Menzies R, Law SC, Seddon J. 2015. Global treatment outcomes in children with paediatric MDR-TB: systematic review and meta-analysis. Abstr 46th World Conf Lung Health, Cape Town, South Africa. International Union against TB and Lung Disease, Paris, France https://www.theunion.org/what-we-do/journals/ijtld/body/Abstract_Book_2015-Web.pdf. [Google Scholar]
- 30.Seddon JA, Hesseling AC, Godfrey-Faussett P, Schaaf HS. 2014. High treatment success in children treated for multidrug-resistant tuberculosis: an observational cohort study. Thorax 69:458–464. doi: 10.1136/thoraxjnl-2013-203900. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. 2015. Global tuberculosis report 2015, World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf Accessed 13 February 2016. [Google Scholar]
- 32.Ramachandran G, Kumar AK, Kannan T, Bhavani PK, Kumar SR, Gangadevi NP, Banurekha VV, Sudha V, Venkatesh S, Ravichandran N, Kalpana S, Mathevan G, Sanjeeva GN, Agarwal D, Swaminathan S. 2016. Low serum concentrations of rifampicin and pyrazinamide associated with poor treatment outcomes in children with tuberculosis related to HIV status. Pediatr Infect Dis J 35:530–534. doi: 10.1097/INF.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 33.Swaminathan S, Pasipanodya JG, Ramachandran G, Hemanth Kumar AK, Srivastava S, Deshpande D, Nuermberger E, Gumbo T. 2016. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 63:S63–S74. doi: 10.1093/cid/ciw471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savic RM, Ruslami R, Hibma JE, Hesseling A, Ramachandran G, Ganiem AR, Swaminathan S, McIlleron H, Gupta A, Thakur K, van Crevel R, Aarnoutse R, Dooley KE. 2015. Pediatric tuberculous meningitis: model-based approach to determining optimal doses of the anti-tuberculosis drugs rifampin and levofloxacin for children. Clin Pharmacol Ther 98:622–629. doi: 10.1002/cpt.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunne J, Rodriguez WJ, Murphy MD, Beasley BN, Burckart GJ, Filie JD, Lewis LL, Sachs HC, Sheridan PH, Starke P, Yao LP. 2011. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics 128:e1242–e1249. doi: 10.1542/peds.2010-3487. [DOI] [PubMed] [Google Scholar]
- 37.Burman WJ, Cotton MF, Gibb DM, Walker AS, Vernon AA, Donald PR. 2008. Ensuring the involvement of children in the evaluation of new tuberculosis treatment regimens. PLoS Med 5:e176. doi: 10.1371/journal.pmed.0050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava S, Deshpande D, Pasipanodya JG, Thomas T, Swaminathan S, Nuermberger E, Gumbo T. 2016. A combination regimen design program based on pharmacodynamic target setting for childhood tuberculosis: design rules for the playground. Clin Infect Dis 63:S75–S79. doi: 10.1093/cid/ciw472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seddon JA, Perez-Velez CM, Schaaf HS, Furin JJ, Marais BJ, Tebruegge M, Detjen A, Hesseling AC, Shah S, Adams LV, Starke JR, Swaminathan S, Becerra MC, Sentinel Project on Pediatric Drug-Resistant Tuberculosis. 2013. Consensus statement on research definitions for drug-resistant tuberculosis in children. J Pediatr Infect Dis Soc 2:100–109. doi: 10.1093/jpids/pit012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beal S, Sheiner L, Boeckmann A, Bauer R. 2009. NONMEM users guides (1989-2009). Icon Development Solutions, Ellicott City, MD. [Google Scholar]
- 41.R Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: http://www.r-project.org/ Accessed 13 June 2017. [Google Scholar]
- 42.Keizer RJ, Karlsson MO, Hooker A. 2013. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2:e50. doi: 10.1038/psp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savic RM, Jonker DM, Kerbusch T, Karlsson MO. 2007. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34:711–726. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 44.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28:481–504. doi: 10.1023/A:1012299115260. [DOI] [PubMed] [Google Scholar]
- 45.Anderson BJ, Holford NH. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 46.Anderson BJ, Holford NHG. 2013. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child 98:737–744. doi: 10.1136/archdischild-2013-303720. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. 2009. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karlsson M, Holford N. 2008. A tutorial on visual predictive checks, abstr 1434. Abstr Ann Meet Popul Approach Group Eur https://www.page-meeting.org/?abstract=1434. [Google Scholar]
- 49.Svensson EM, Yngman G, Denti P, McIlleron H, Kjellsson MC, Karlsson MO. 4 August 2017. Evidence-based design of fixed-dose combinations: principles and application to pediatric anti-tuberculosis therapy. Clin Pharmacokinet. doi: 10.1007/s40262-017-0577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fish DN, Chow AT. 1997. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet 32:101–119. doi: 10.2165/00003088-199732020-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.