ABSTRACT
Invasive methicillin-resistant Staphylococcus aureus (MRSA) treated with vancomycin (VAN) is associated with reduced VAN susceptibility and treatment failure. VAN combination therapy is one strategy to improve response, but comprehensive assessments of combinations to prevent resistance are limited. This study identifies optimal combinations to prevent the emergence of VAN-intermediate Staphylococcus aureus (VISA). Two standard MRSA and two heterogeneous VISA (hVISA) strains were exposed for 28 days in vitro to VAN alone, VAN with cefazolin (CFZ), fosfomycin, gentamicin, meropenem, rifampin, piperacillin-tazobactam (TZP), or trimethoprim-sulfamethoxazole. In addition to VAN susceptibility testing, cell wall thickness (CWT), carotenoid content, and membrane fluidity were determined for Mu3. VAN plus any β-lactam limited the VAN MIC increase to 1 to 4 mg/liter throughout the 28-day exposure, with CFZ and TZP being the most effective agents (VAN MIC = 1 to 2 mg/liter). Similar MIC trends occurred with the lipo-/glycopeptide agents daptomycin and telavancin, where β-lactam combinations with VAN prevented MIC increases to these agents as well. Combinations with non-β-lactams were ineffective in preventing VAN MIC increases with VAN MICs of 4 to 16 mg/liter emerging during weeks 2 to 4 of treatment. VAN plus β-lactam decreased CWT significantly, whereas VAN plus other antibiotics significantly increased the CWT. No correlation was observed between carotenoid content or membrane fluidity and antibiotic exposure. Only the combination exposures of VAN plus β-lactam suppress the development of VISA. Rational selection of VAN plus β-lactam should be further explored as a long-term combination treatment of MRSA infections due to their ability to suppress VAN resistance.
KEYWORDS: glycopeptides, vancomycin resistance, antimicrobial combinations, Staphylococcus aureus
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) has spread rapidly around the world and has become an important and common pathogen in both hospital and community settings. It is now a major causative agent of several invasive bacterial infections, including pneumonia, skin and soft tissue infection, osteomyelitis, septic joints, bloodstream infections, and infective endocarditis, among others (1).
Vancomycin (VAN) has been considered the frontline therapy for empirical and definitive treatment of severe MRSA infections for many years. Although VAN resistance remains rare, isolates with reduced susceptibility to VAN have appeared, including VAN-intermediate Staphylococcus aureus (VISA), heterogeneous VAN-intermediate Staphylococcus aureus (hVISA), and vancomycin-tolerant strains. VAN treatment can select for expansion of this small subpopulation, resulting in VAN treatment failure and a transition to a homogeneous VISA population. These isolates have been associated with poor response to VAN therapy in patients and are frequently more resistant to secondary treatment options (2–6). VAN treatment failure is a significant clinical concern, and strategies to prevent the emergence and spread of these organisms are needed.
Despite these reduced susceptibility phenotypes, VAN still remains the principal antimicrobial agent of choice in the treatment of MRSA (7). In addition, it is often used initially in patients with hVISA since the identification of these isolates appear as susceptible by standard testing (1). To improve VAN clinical outcomes in the treatment of MRSA, many studies have focused on the in vitro or in vivo activities of VAN combination strategies which demonstrate synergy, antagonism, or indifference. These include VAN plus rifampin, gentamicin, fosfomycin, linezolid, or a variety β-lactams (8–19). However, relatively few studies have comprehensively studied these combinations for their ability to delay or prevent reduced susceptibility to VAN over prolonged exposures. This study identifies optimal combinations with VAN to prevent the emergence of VISA from either VAN-susceptible or hVISA isolates.
RESULTS
In vitro antibiotic susceptibility.
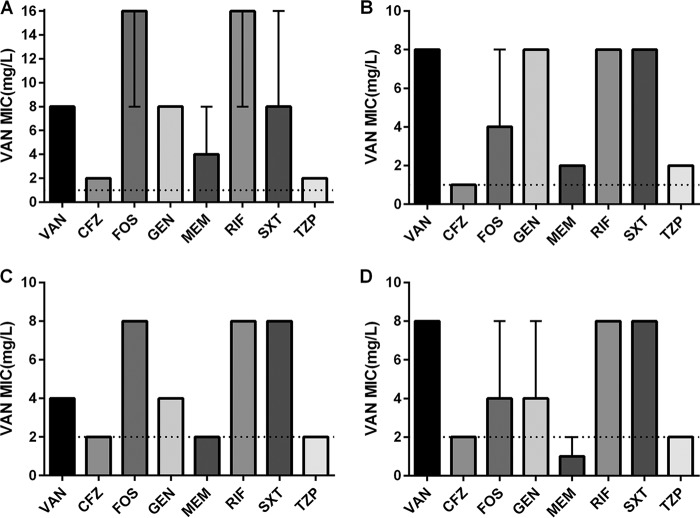
The VAN susceptibility for all four isolates after 4 weeks of serial passages with VAN or with combination exposures are presented in Fig. 1. Among all four isolates tested, VAN alone resulted in VISA at day 28. VAN combinations with fosfomycin (FOF), gentamicin (GEN), rifampin (RIF), or trimethoprim-sulfamethoxazole (SXT) were ineffective in preventing VISA development and in some cases hastened the development of reduced VAN susceptibility compared to VAN alone. The β-lactam combinations of cefazolin (CFZ), meropenem (MEM), or piperacillin-tazobactam (TZP) with VAN largely prevented VISA emergence in all isolates with the exception of VAN+MEM in Mu3. Overall, the β-lactam combinations were effective in preventing any change in VAN susceptibility, including strains with an initial VAN MIC of 1 mg/liter (Mu3 and ATCC 33591) or 2 mg/liter (W1082 and W217).
FIG 1.
VAN susceptibility at day 28 for all four isolates following VAN or combination exposures. Data represent the medians and ranges (when applicable) of the triplicate replicates for each exposure. The dashed lines indicate the initial VAN MICs for each isolate at the beginning of the experiment (day 0). (A) hVISA Mu3; (B) ATCC 33591; (C) hVISA W1082; (D) W217.
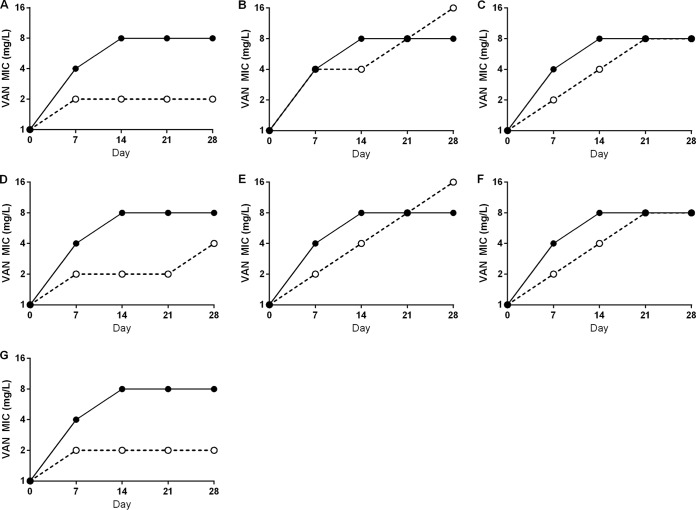
The susceptibility changes over the entire 4-week experiment with VAN alone or with secondary antibiotic combinations are represented in the prototypical hVISA Mu3 (Fig. 2). VAN exposure alone quickly resulted in the development of VISA (VAN MICs of 4 mg/liter) at the first 7-day time point tested during passage. All of the combination exposures prevented VAN resistance at day 7, except VAN+FOF. Although resistance development was delayed, the combinations VAN+GEN, VAN+RIF, and VAN+SXT were unable to prevent VAN resistance development from hVISA with VAN MICs of 4 mg/liter emerging by day 14. Among all of the combinations of VAN and β-lactams, VAN+CFZ and VAN+TZP prevented the development of VAN resistance throughout the 28-day exposure (MIC = 2 mg/liter), but VAN+MEM produced strains with VAN MICs of 4 to 8 mg/liter between days 21 to 28. At the end of the 28-day exposure, exposure to VAN alone or VAN+FOF, GEN, RIF, or SXT increased the Mu3 VAN MIC to 8 to 16 mg/liter.
FIG 2.
VAN MIC development in Mu3 during 28-day antibiotic exposures in vitro. Solid line, VAN alone; dashed line, VAN plus a secondary antibiotic. (A) VAN+CFZ; (B) VAN+FOF; (C) VAN+GEN; (D) VAN+MEM; (E) VAN+RIF; (F) VAN+SXT; (G) VAN+TZP. Data points are the median MIC values from three independent replicates of each exposure.
The MICs of daptomycin (DAP), linezolid (LZD), and telavancin (TLV), as well as all of the secondary antibiotics used in passage, were determined against all four strains at days 0 and 28 with the results represented by Mu3 in Table 1. The combination of VAN and any β-lactam exposure tested resulted in minimal DAP and TLV MIC increases (<1-fold) and in some cases paradoxically reduced MICs to DAP and TLV. In contrast, the non-β-lactam combinations increased the MICs of DAP and TLV. These increases resulted in strains that had become nonsusceptible to DAP and/or TLV with MICs greater than at least one doubling of the MIC breakpoints of 1 and 0.12 mg/liter, respectively. Development of LZD MIC changes was distinct from development of VAN, DAP, or TLV MIC changes. The combinations VAN+TZP and VAN+MEM resulted in a doubling of the median LZD MICs at the end of exposure, whereas VAN alone and other VAN combination exposures did not alter the median LZD MIC. No changes to the MIC of any secondary antibiotic used in each passage were observed in any combination except VAN+RIF, which increased the RIF MIC 512-fold (Table 1) Similar results were found for the other three strains in this study (data not shown).
TABLE 1.
Mu3 MIC values before and after 28-day antibiotic exposures
| Treatment | Median MIC (mg/liter)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DAP | LZD | TLV | CFZ | TZP | MEM | GEN | SXT | FOF | RIF | |
| Mu3 | 1 (1) | 2 (2) | 0.125 (0.125) | >256 (>256) | >256/4 (256/4) | 128 (128) | >256 (>256) | 0.064 (0.064) | >256 (>256) | 0.03 (0.03) |
| VAN | 4 (4) | 2 (2–4) | 0.25 (0.25) | ND | ND | ND | ND | ND | ND | ND |
| VAN+CFZ | 1 (1) | 2 (2) | 0.19 (0.19) | >256 (>256) | ND | ND | ND | ND | ND | ND |
| VAN+TZP | 1 (1) | 4 (4) | 0.125 (0.125) | ND | >256/4 (>256/4) | ND | ND | ND | ND | ND |
| VAN+MEM | 0.5 (0.5–1) | 4 (4) | 0.047 (0.032–0.047) | ND | ND | 256 (256) | ND | ND | ND | ND |
| VAN+GEN | 4 (4–8) | 2 (2–4) | 0.25 (0.25) | ND | ND | ND | 256 (256–>256) | ND | ND | ND |
| VAN+SXT | 4 (4) | 2 (2) | 0.5 (0.5–0.75) | ND | ND | ND | ND | 0.125 (0.125) | ND | ND |
| VAN+FOF | 8 (2–16) | 2 (2–4) | 0.38 (0.38) | ND | ND | ND | ND | ND | >256 (>256) | ND |
| VAN+RIF | 4 (4–8) | 2 (2) | 0.38 (0.38–0.5) | N | ND | ND | ND | ND | ND | 4 (0.5–16) |
Ranges are indicated in parentheses. ND, not determined.
Since the β-lactam antibiotics were effective in combination with VAN in preventing the emergence of VISA over 28 days, we were interested in the effect of these exposures on the susceptibility to ceftaroline (CPT), a recently developed cephalosporin antibiotic with activity against MRSA through inhibition of penicillin-binding protein 2a (PBP2a). The CPT MICs at initiation (day 0) and at 28 days for all four isolates passaged with antibiotics are presented in Table 2. VAN alone resulted in high VAN MICs and often lower CPT MICs at 4 weeks, which is consistent with the noted see-saw effect with glycopeptides and β-lactams (20). Interestingly, the CFZ combination had little effect on CPT MICs, whereas MEM and TZP combinations often increased CPT MICs. The resulting CPT MICs from MEM or TZP exposure were noted to be intermediate (2 mg/liter) or resistant (≥4 mg/liter).
TABLE 2.
CPT susceptibility determined by broth dilution and Etest before and after 28-day antibiotic exposures of VAN alone or in combination with β-lactams in Mu3, W1082, W217, and ATCC 33591
| Treatment | Median CPT MIC (mg/liter)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mu3 |
W1082 |
W217 |
ATCC 33591 |
|||||
| Broth | Etest | Broth | Etest | Broth | Etest | Broth | Etest | |
| Initial MIC (day 0) | 2 | 3 | 1 | 1.5 | 1 | 1 | 1 | 1 |
| VAN | 0.5 | 0.75 (0.5–0.75) | 1 | 1.5 (1–1.5) | 0.5 | 0.75 (0.75–1) | 1 (0.5–1) | 0.75 (0.5–1) |
| VAN+CFZ | 2 | 2 (2–3) | 1 | 1.5 | 1 | 1.5 | 1 | 1.5 |
| VAN+MEM | 4 (2–4) | 3 (2–4) | 1 | 1.5 (1.5–2) | 2 (1–2) | 2 (1.5–2) | 1 (1–2) | 1.5 (1.5–2) |
| VAN+TZP | 4 | 3 | 1 | 1 | 1 (1–2) | 1.5 | 2 (1–2) | 2 |
The data represent the medians of three replicate passages. Ranges are indicated in parentheses.
CWT.
In order to assess the relationship between reduced VAN susceptibility and cell wall thickening, the cell wall thickness (CWT) of Mu3 with each antibiotic exposure was examined after the 28-day study (Table 3 and Fig. S1 in the supplemental material). The CWT increased significantly after the 28-day VAN only exposure. Of the combinations tested, only VAN+CFZ and VAN+TZP exposures prevented cell wall thickening. When we compared CWT values for the 28-day passage with VAN versus VAN plus combination antibiotics, all β-lactam combinations resulted in significantly reduced CWT, while the other combinations had cultures containing cells with CWT similar to VAN passage alone. As expected, a significantly positive correlation between the CWT and the VAN MIC (r = 0.939) was observed.
TABLE 3.
Mu3 strain characteristics after 28-day antibiotic exposures
| Treatment | Median VAN MIC (mg/liter)a | Mean ± SDb |
||
|---|---|---|---|---|
| CWT (nm) | Carotenoid content (ng/mg) | Membrane fluidity (p) | ||
| No antibiotic | 1 (1) | 52.1 ± 6.5 | 13.3 ± 1.2 | 0.213 ± 0.009 |
| VAN | 8 (8) | 60.1 ± 6.7‡ | 13.6 ± 1.6 | 0.215 ± 0.011 |
| VAN+CFZ | 2 (2) | 52.0 ± 4.9** | 13.8 ± 1.2 | 0.209 ± 0.015 |
| VAN+TZP | 2 (2–4) | 53.9 ± 6.0** | 16.1 ± 1.0*‡ | 0.208 ± 0.008* |
| VAN+MEM | 4 (4–8) | 58.3 ± 8.0*‡ | 14.3 ± 0.9 | 0.201 ± 0.012**‡ |
| VAN+GEN | 8 (8) | 63.5 ± 8.7**‡ | 14.3 ± 0.7 | 0.214 ± 0.010 |
| VAN+SXT | 8 (8–16) | 64.3 ± 9.6**‡ | 11.8 ± 2.1 | 0.224 ± 0.014*† |
| VAN+FOF | 16 (8–16) | 66.4 ± 6.7**‡ | 16.2 ± 1.6*† | 0.214 ± 0.012 |
| VAN+RIF | 16 (8–16) | 68.5 ± 8.8**‡ | 21.2 ± 3.9**‡ | 0.217 ± 0.017 |
Ranges are indicated in parentheses.
Asterisks indicate statistically significant differences between the VAN single exposure and combination exposures (*, P < 0.05; **, P < 0.001). Dagger indicate statistically significant differences between the any exposure and Mu3 progenitor strain (†, P < 0.05; ‡, P < 0.001). Lower polarization index (p) values indicate a greater degree of membrane fluidity.
Carotenoid content and membrane fluidity.
The carotenoid content and membrane fluidity of Mu3 after antibiotic exposure was tested to determine a potential correlation with VAN resistance (Table 3). The hVISA Mu3 exposed to the combinations VAN+FOF, VAN+RIF, and VAN+TZP led to a significantly higher carotenoid content compared to both Mu3 and VAN alone. As for membrane fluidity, VAN+SXT obtained a lower degree of fluidity compared to both Mu3 and VAN alone, whereas VAN+MEM resulted in enhanced membrane fluidity. However, no significant correlation was observed between carotenoid content (r = 0.267) or membrane fluidity (r = 0.680) and VAN MIC.
DISCUSSION
The causes of reduced susceptibility to VAN in patients with complex MRSA infections are often multifactorial, but this reduced susceptibility can be attributed in part to suboptimal and/or prolonged VAN exposure. Although combination therapy is one proposed strategy to prevent the loss of VAN activity, this approach has not been evaluated in a comprehensive fashion with commonly used combinations. In this study, antibiotics in combination with VAN significantly altered the trend toward the emergence of VISA (Fig. 1 and 2). Traditionally used combination therapies with VAN, such as GEN, RIF, and SXT, were not effective in preventing VAN resistance. However, the combinations of VAN plus β-lactams prevented the emergence of VAN resistance significantly during the 28 days of study, particularly the combinations VAN+CFZ and VAN+TZP. These results were consistent in four total strains regardless of hVISA background or initial VAN MIC.
The combination of VAN with β-lactams has displayed synergy against S. aureus both in vivo and in vitro (12–19). In addition, a phenomenon termed the “see-saw effect” was observed in some studies where a VAN MIC increase is accompanied by a β-lactam MIC decrease in select clinical MRSA, VISA, and VRSA strains, as well as in vitro-derived VISA (20–26). The mechanism for this is not completely known, and not all S. aureus demonstrate this phenomenon. However, there is evidence pointing toward a contribution of the VraSR two-component regulatory system on the seesaw effect, leading to enhanced sensitization of β-lactam antibiotics (27, 28). In our study, we evaluated CPT susceptibility following VAN alone and combined with the β-lactams in the study. Our results support this seesaw effect with CPT, since the MIC increased up to 4-fold when VISA (VAN MIC 8 mg/liter) developed with VAN exposure alone. We are currently evaluating what regulatory adaptations coincide with this effect.
The four isolates tested displayed reduced susceptibility to VAN, which can be related to several phenotype changes. One of the most common changes is the thickened cell wall, which correlates with VISA development (29, 30). Similarly, significant cell wall thickening of the in vitro-derived VISA strains under the selective pressure of VAN alone or any combination was observed in our study; a thicker cell wall correlated with a higher VAN MIC (r = 0.939). It is evident that the combination of VAN and β-lactams limited cell wall thickening; however, in all of the VAN+β-lactam combinations, only VAN+CFZ maintained the same CWT as Mu3 and prevented the VAN MIC in all replicates from increasing beyond 2 mg/liter. To assess other phenotypic changes previously observed in VISA, we tested the carotenoid content and membrane fluidity of the in vitro derived stains compared to the progenitor hVISA strain Mu3. Several studies (31, 32) have shown that in vitro-derived DAP-resistant MRSA strains frequently display excess carotenoids and decreased membrane fluidity as the DAP MIC increases in parallel with increasing VAN MICs. Of note, we did observe that exposure to VAN alone and VAN plus the secondary antibiotics resulted in DAP nonsusceptible MRSA (DAP MIC > 1 mg/liter) in parallel with increasing VAN MICs in Mu3 unless a β-lactam was present during the exposure. Similar observations were observed in the additional strains tested (data not shown) There was no correlation between VAN MICs and carotenoid content or membrane fluidity. Overall, our data confirm that the thickened cell wall may play a pivotal role in the VAN-intermediate resistance and the combination of VAN and β-lactams can limit the cell wall thickening. This evidence supports the combination of VAN with β-lactams not only for antimicrobial synergy purposes but also to prevent the mechanism associated with the transition to VISA.
Although the mechanism of DAP activity is different than VAN in that it acts as a functional cationic peptide on the cell membrane, several studies have identified a similar synergistic effect with DAP plus β-lactams (24, 33). This includes the ability of oxacillin to prevent the development of DAP resistance during prolonged exposure simulations (25). Recent evidence suggests that inhibition of PBP1 is important for maximal synergistic activity (34, 35). In our study with VAN, penicillin-binding protein inhibition specificity did not appear to reflect a more potent suppression of VAN resistance. CFZ and TZP, nonspecific inhibitors of S. aureus PBPs, prevented any increase in VAN MIC beyond 2 mg/liter, the breakpoint for susceptibility. MEM, which has affinity for PBP1/PBP2, was less effective against Mu3 (VAN MICs increases to 4 to 8 mg/liter) but showed results similar to those for other β-lactams in the other isolates. In addition, FOF, which has been shown to sharply reduce the expression of PBP1 (36), was not effective at suppressing VISA at any point during the exposure. These results support the existing literature that the mechanisms for β-lactam synergy with VAN versus DAP are different (34, 37).
Combining VAN with a second antibiotic may be an alternative strategy to improve clinical outcomes. The majority of VAN combination therapy research has focused on the combinations of VAN plus β-lactams that typically have demonstrated synergy (9–16). In contrast, few recent studies have compared this approach to other combinations using such agents as RIF, GEN, and SXT, and these findings have been inconsistent (38). In our study, the combinations of RIF, SXT, and FOS in combination with VAN hastened the development of VISA compared to VAN alone in two of the four strains tested and therefore appears to be strain dependent. Although the mechanisms for this are currently being explored, we note that the susceptibility to SXT and RIF increased 2- and 128-fold in Mu3, respectively.
Since complicated infections caused by MRSA are associated with long-term treatment and relapse, combination therapy may play an additional role beyond synergy to delay or prevent this transition. In this regard, it is significant that our findings show that the VAN-induced transition to VISA during the 28-day exposure can be delayed or prevented by the addition of β-lactams but not by the addition of other antibiotics. Clinical studies of this combination have been promising, primarily with piperacillin-tazobactam with vancomycin, for empirical coverage leading to improved outcomes (13). Although minimal clinical data are available with vancomycin combined with cefazolin, the ongoing CAMERA-2 study includes cefazolin as one of three β-lactam combinations with vancomycin and may provide clarity on the optimal approach (39). However, the phenomenon of β-lactam-induced vancomycin resistance (BIVR) has been described in the literature; this may conflict with applying our findings to all MRSA. In surveillance studies, BIVR, which is strain and concentration dependent, occurs in 6 to 20% of MRSA (40–42). This phenotype has been explored mostly in isolates from Japan, and studies of recent isolates are lacking. The mechanism for BIVR is attributed to depletion of free vancomycin when combined with the β-lactams ceftizoxime or imipenem (43). Further, testing for BIVR appears to lack consistency and standardization (44), so the clinical significance of this effect remains unknown and difficult to study.
We noted that the VAN combinations also altered susceptibility to other MRSA therapies such as DAP, LZD, TLV, and CPT (Tables 1 and 2). For example, only the β-lactam agents combined with VAN largely prevented collateral DAP and TLV resistance (MICs of >1.0 and >0.12 mg/liter, respectively). In particular, VAN+MEM reduced DAP MIC by 1-fold and TLV MIC by >2-fold compared to the initial MICs. This is consistent with MEMs ability to affect DAP activity (40), but this is the first time to our knowledge that this has been shown to possibly be related to TLV. These results also suggest that MEM or TZP exposures could alter the activity of CPT, by a mechanism that is under investigation (A. Rosato et al., unpublished results), since we noted that VAN combinations with these agents after 28 days resulted in CPT MICs up to 4 mg/liter. We are currently exploring how these secondary β-lactam agents may induce mutations in PBPs that could result in this observed reduced CPT effectiveness phenotype.
In conclusion, this study clearly demonstrated that combination treatment with VAN could significantly impact the transition to VISA in vitro. The combination of VAN and β-lactams most effectively prevented the emergence of VAN resistance, although some exceptions were noted with MER. In contrast, combination treatment with other agents, including VAN+FOF, VAN+GEN, VAN+RIF, and VAN+SXT, at best delayed VISA emergence only in the first week of treatment. Our study is limited to the in vitro environment and the concentrations of antibiotic tested, but it provides a comprehensive assessment of commonly used combinations with VAN in multiple isolates. The combination of VAN plus β-lactams should be further explored as a long-term treatment of serious MRSA infections due to their ability to suppress VAN resistance. This may include evaluation of mechanisms on the cell wall to determine peptidoglycan and teichoic acid composition with this combination (45), in vitro models with different media types known to affect VISA development and antibiotic activity (46, 47), and animal models of prolonged infection types. However, collateral effects of this approach on other MRSA treatment options should be investigated.
MATERIALS AND METHODS
Bacterial strains, antimicrobials, and media.
This study used two standard MRSA and two hVISA isolates to determine whether reduced susceptibility derived from passage is due to initial susceptibility differences or the hVISA phenotype. The two hVISA isolates were Mu3, the first clinically identified hVISA from Japan reported by Hiramatsu et al. with an initial VAN MIC of 1 mg/liter (2) and a clinical hVISA (confirmed by population analysis profile testing) blood isolate collected at the University of Wisconsin Hospital (W1082) with a VAN MIC of 2 mg/liter. The standard MRSA isolates consisted of a clinical MRSA blood isolate collected at the University of Wisconsin Hospital with a VAN MIC of 2 mg/liter (W217) and the ATCC strain 33591 with a VAN MIC of 1 mg/liter. VAN, SXT, and RIF were purchased from Research Products International Corp (Mount Prospect, IL). GEN, FOF, and d-glucose 6-phosphate potassium salt were obtained from Sigma-Aldrich, Inc. (St. Louis, MO). DAP was provided by Merck (Merck & Co., Inc., Kenilworth, NJ). LZD was obtained from Pfizer (Pfizer Inc., New York, NY). Allergan (Dublin, Ireland) provided CPT active powder. CFZ, MEM, and TZP were purchased from clinical stock powders. Mueller-Hinton Broth II (BD, Sparks, MD) supplemented with 25 mg/liter calcium (as CaCl2) and 12.5 mg/liter magnesium (as MgCl2) (cation-adjusted Mueller-Hinton broth [CAMHB]) was used to grow S. aureus in liquid culture.
Susceptibility testing.
MIC values of study bacteria to SXT were determined by Etest as suggested by the manufacturer (bioMérieux, Marcy l'Étoile, France). TLV (Theravance Biopharma, South San Francisco, CA) and CPT (Allergan) susceptibilities were determined by Etest. CPT susceptibilities were also confirmed by broth dilution testing. MICs of other antibiotics were determined by the broth microdilution method according to Clinical and Laboratory Standards Institute (CLSI) guidelines (48). CAMHB was adjusted to 50 mg/liter calcium for DAP testing and supplemented with d-glucose-6-phosphate for FOF testing. All samples were incubated at 37°C for 24 h. S. aureus ATCC 29213 was included simultaneously as a quality control strain. All MIC values determined were within the acceptable quality control range for this organism.
VAN exposure conditions.
All isolates were exposed in triplicate to VAN concentrations increasing from 0.5× MIC (0.5 or 1 mg/liter) to 6 mg/liter according to visual growth in vitro in CAMHB for 28 days. This passage protocol, adapted from a previously described method (25), consisted of preparing a standard inoculum of 106 in fresh CAMHB containing 0.5× VAN MIC. Cultures were grown overnight in triplicate at 37°C with shaking at 160 rpm. Cultures that showed visual turbidity were collected and diluted 1:100 into fresh CAMHB containing VAN. Cultures that did not show visual turbidity were discarded and the previous day's turbid culture was thawed from frozen stocks and used to inoculate the next day's culture. This process was repeated as a series of distinct daily passages for 28 days. The VAN concentration in the CAMHB was escalated every 7 days to 2, 4, and 6 mg/liter. When no visual growth occurred following escalated VAN concentration, cultures were recovered from the previous passage and continued in the lower VAN concentration with visual growth and continued for an additional 7 days. VAN susceptibility was determined on days 0, 7, 14, 21, and 28, whereas DAP, LZD, TLV, and CPT susceptibilities, along with the secondary antibiotics used in each treatment, were determined on days 0 and 28.
Combination exposure conditions.
For combination exposures, all four isolates were exposed to VAN and passaged for 28 days, as outlined above, except that the media contained a constant concentration of a secondary antibiotic. As the VAN concentration increased every 7 days from 0.5 or 1 mg/liter up to 6 mg/liter, the concentration of the secondary antibiotic was held constant throughout. The secondary antibiotics were added at concentrations corresponding to the value of the serum free average concentration (fCavg) obtained using standard dosing. In the case where the fCavg was above the MIC, secondary antibiotic concentrations of 1/2× MIC were used during serial passage. Antibiotics added at the fCavg included CFZ (9.5 mg/liter), FOF (145 mg/liter), GEN (2.6 mg/liter), MEM (24 mg/liter), and TZP (105 mg/liter of piperacillin and 12 mg/liter of tazobactam). Antibiotics added at 1/2× MIC included RIF (0.015 mg/liter) and SXT (0.032 mg/liter of trimethoprim and 0.776 mg/liter of sulfamethoxazole).
CWT.
Mu3 was used as the model organism to correlate cell wall thickness (CWT) to antibiotic exposure and resulting VAN MIC changes. CWT was determined by transmission electron microscopy (TEM) as described previously (49). Samples were collected from day 28 exposure cultures and compared to Mu3 prior to passage in antibiotics. Images were analyzed and measured using ImageJ 1.50b software. CWT measurements were made on a minimum of 25 cells per exposure using four separate quadrants of each cell.
Carotenoid extraction.
Mu3 was also used as the standard organism to compare carotenoid changes in the cell membrane to antibiotic exposure and resulting VAN MIC. The modified protocol of Castro et al. (50) was followed for the extraction and quantification of carotenoids. Bacteria were cultivated on brain heart infusion agar, incubated at 37°C for 24 h, and transferred to room temperature for an additional 48 h of carotenoid development. At 72 h, colonies amounting to 5 to 10 mg of wet cell weight were transferred from plates to microcentrifuge tubes, resuspended in 1 ml of methanol, and incubated at 55°C for 20 min. Clarified supernatant was assessed for carotenoid content spectrophotometrically at 450 nm using an extinction coefficient for staphyloxanthin of 250,000 M−1 cm−1. Values were normalized to nanograms of staphyloxanthin detected per milligrams of wet cell weight. The assay was repeated with a minimum of three analytical replicates, and three biological replicates were evaluated on separate days.
Cell membrane fluidity determination.
Mu3 was used to correlate membrane fluidity alterations to antibiotic exposure and resulting VAN MIC. Membrane fluidity was determined by fluorescence polarization spectrofluorimetry using 1,6-diphenyl-1,3,5-hexatriene (DPH) as previously described (51, 52) using a Synergy H1 spectrofluorometer (BioTek, Winooski, VT). The results were corrected by subtracting data from an unlabeled control reaction. The polarization index (p) was determined using the formula p = (IV − IH)/(IV + IH), where I is the fluorescence intensity, and subscripts V and H indicate vertical and horizontal orientation instrument values, respectively (52). Data represent averages and standard deviations of three experiments performed on separate days with eight readings from every sample each day. There is an inverse relationship between polarization index and the degree of membrane fluidity (the lower fluorescence polarization value, the higher degree of membrane fluidity).
Statistical analysis.
Continuous variables were summarized using the means and standard deviations and compared using an two-tailed unpaired Student t test. Comparisons of MIC values were conducted with Wilcoxon rank sum tests. P values of ≤0.05 were considered significant. The relationships among the CWT, carotenoid extraction, membrane fluidity, and the VAN MIC were compared using Spearman's rank correlation.
Supplementary Material
ACKNOWLEDGMENTS
Xuting Zheng was supported by the international scholar program from China Medical University.
This study was supported in part by NIH-1R01AI132627-01 (W.E.R.) and NIH-1R56AI102503-01A1 (A.E.R.). We thank Ben August (UW School of Medicine, Electron Microscope Facility) for technical assistance.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02100-17.
REFERENCES
- 1.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Talan DA, Chambers HF, Infectious Diseases Society of America. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 2.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 3.Singh A, Prasad KN, Misra R, Rahman M, Singh SK, Rai RP, Tripathi A, Srivastava JK. 2015. Increasing trend of heterogeneous vancomycin-intermediate Staphylococcus aureus in a tertiary care center of northern India. Microb Drug Resist 21:545–550. doi: 10.1089/mdr.2015.0004. [DOI] [PubMed] [Google Scholar]
- 4.Huang SH, Chen YC, Chuang YC, Chiu SK, Fung CP, Lu PL, Wang LS, Wu TL, Wang JT. 2016. Prevalence of vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous VISA among methicillin-resistant S. aureus with high vancomycin minimal inhibitory concentrations in Taiwan: a multicenter surveillance study, 2012–2013. J Microbiol Immunol Infect 49:701–707. doi: 10.1016/j.jmii.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Sun X, Chang W, Dai Y, Ma X. 2015. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS One 10:e0136082. doi: 10.1371/journal.pone.0136082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sader HS, Jones RN, Rossi KL, Rybak MJ. 2009. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine U S A hospitals. J Antimicrob Chemother 64:1024–1028. doi: 10.1093/jac/dkp319. [DOI] [PubMed] [Google Scholar]
- 7.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 52:3315–3320. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deresinski S. 2009. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 49:1072–1079. doi: 10.1086/605572. [DOI] [PubMed] [Google Scholar]
- 9.Singh SR, Bacon AE 3rd, Young DC, Couch KA. 2009. In vitro 24-hour time-kill studies of vancomycin and linezolid in combination versus methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 53:4495–4497. doi: 10.1128/AAC.00237-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pachon-Ibanez ME, Ribes S, Dominguez MA, Fernandez R, Tubau F, Ariza J, Gudiol F, Cabellos C. 2011. Efficacy of fosfomycin and its combination with linezolid, vancomycin and imipenem in an experimental peritonitis model caused by a Staphylococcus aureus strain with reduced susceptibility to vancomycin. Eur J Clin Microbiol Infect Dis 30:89–95. doi: 10.1007/s10096-010-1058-0. [DOI] [PubMed] [Google Scholar]
- 11.Ribes S, Pachon-Ibanez ME, Dominguez MA, Fernandez R, Tubau F, Ariza J, Gudiol F, Cabellos C. 2010. In vitro and in vivo activities of linezolid alone and combined with vancomycin and imipenem against Staphylococcus aureus with reduced susceptibility to glycopeptides. Eur J Clin Microbiol Infect Dis 29:1361–1367. doi: 10.1007/s10096-010-1007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConeghy KW, Bleasdale SC, Rodvold KA. 2013. The empirical combination of vancomycin and a beta-lactam for staphylococcal bacteremia. Clin Infect Dis 57:1760–1765. doi: 10.1093/cid/cit560. [DOI] [PubMed] [Google Scholar]
- 13.Dilworth TJ, Ibrahim O, Hall P, Sliwinski J, Walraven C, Mercier RC. 2014. β-Lactams enhance vancomycin activity against methicillin-resistant Staphylococcus aureus bacteremia compared to vancomycin alone. Antimicrob Agents Chemother 58:102–109. doi: 10.1128/AAC.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dilworth TJ, Sliwinski J, Ryan K, Dodd M, Mercier RC. 2014. Evaluation of vancomycin in combination with piperacillin-tazobactam or oxacillin against clinical methicillin-resistant Staphylococcus aureus isolates and vancomycin-intermediate S. aureus isolates in vitro. Antimicrob Agents Chemother 58:1028–1033. doi: 10.1128/AAC.01888-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werth BJ, Vidaillac C, Murray KP, Newton KL, Sakoulas G, Nonejuie P, Pogliano J, Rybak MJ. 2013. Novel combinations of vancomycin plus ceftaroline or oxacillin against methicillin-resistant vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous VISA. Antimicrob Agents Chemother 57:2376–2379. doi: 10.1128/AAC.02354-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis JS, Sud A, O'Sullivan MV, Robinson JO, Ferguson PE, Foo H, van Hal SJ, Ralph AP, Howden BP, Binks PM, Kirby A, Tong SY. 2016. Combination of vancomycin and beta-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis 62:173–180. doi: 10.1093/cid/civ808. [DOI] [PubMed] [Google Scholar]
- 17.Climo MW, Patron RL, Archer GL. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob Agents Chemother 43:1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagihara M, Wiskirchen DE, Kuti JL, Nicolau DP. 2012. In vitro pharmacodynamics of vancomycin and cefazolin alone and in combination against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 56:202–207. doi: 10.1128/AAC.05473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard SN. 2012. Synergy between vancomycin and nafcillin against Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model. PLoS One 7:e42103. doi: 10.1371/journal.pone.0042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta S, Singh C, Plata KB, Chanda PK, Paul A, Riosa S, Rosato RR, Rosato AE. 2012. beta-Lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 56:6192–6200. doi: 10.1128/AAC.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieradzki K, Roberts RB, Haber SW, Tomasz A. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med 340:517–523. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]
- 22.Sieradzki K, Tomasz A. 1999. Gradual alterations in cell wall structure and metabolism in vancomycin-resistant mutants of Staphylococcus aureus. J Bacteriol 181:7566–7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sieradzki K, Leski T, Dick J, Borio L, Tomasz A. 2003. Evolution of a vancomycin-intermediate Staphylococcus aureus strain in vivo: multiple changes in the antibiotic resistance phenotypes of a single lineage of methicillin-resistant S. aureus under the impact of antibiotics administered for chemotherapy. J Clin Microbiol 41:1687–1693. doi: 10.1128/JCM.41.4.1687-1693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob Agents Chemother 54:3161–3169. doi: 10.1128/AAC.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berti AD, Wergin JE, Girdaukas GG, Hetzel SJ, Sakoulas G, Rose WE. 2012. Altering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibiotics. Antimicrob Agents Chemother 56:5046–5053. doi: 10.1128/AAC.00502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidaillac C, Gardete S, Tewhey R, Sakoulas G, Kaatz GW, Rose WE, Tomasz A, Rybak MJ. 2013. Alternative mutational pathways to intermediate resistance to vancomycin in methicillin-resistant Staphylococcus aureus. J Infect Dis 208:67–74. doi: 10.1093/infdis/jit127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin S, Daum RS, Boyle-Vavra S. 2006. VraSR two-component regulatory system and its role in induction of pbp2 and vraSR expression by cell wall antimicrobials in Staphylococcus aureus. Antimicrob Agents Chemother 50:336–343. doi: 10.1128/AAC.50.1.336-343.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qureshi NK, Yin S, Boyle-Vavra S. 2014. The role of the Staphylococcal VraTSR regulatory system on vancomycin resistance and vanA operon expression in vancomycin-resistant Staphylococcus aureus. PLoS One 9:e85873. doi: 10.1371/journal.pone.0085873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui L, Ma XX, Sato K, Okuma K, Tenover FC, Mamizuka EM, Gemmell CG, Kim MN, Ploy MC, El Solh N, Ferraz V, Hiramatsu K. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J Clin Microbiol 41:5–14. doi: 10.1128/JCM.41.1.5-14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK. 2006. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 50:3039–3047. doi: 10.1128/AAC.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra NN, Yang SJ, Sawa A, Rubio A, Nast CC, Yeaman MR, Bayer AS. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 53:2312–2318. doi: 10.1128/AAC.01682-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra NN, Liu GY, Yeaman MR, Nast CC, Proctor RA, McKinnell J, Bayer AS. 2011. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob Agents Chemother 55:526–531. doi: 10.1128/AAC.00680-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortwine JK, Werth BJ, Sakoulas G, Rybak MJ. 2013. Reduced glycopeptide and lipopeptide susceptibility in Staphylococcus aureus and the “seesaw effect”: taking advantage of the back door left open? Drug Resist Updat 16:73–79. doi: 10.1016/j.drup.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Berti AD, Sakoulas G, Nizet V, Tewhey R, Rose WE. 2013. β-Lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:5005–5012. doi: 10.1128/AAC.00594-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berti AD, Theisen E, Sauer JD, Nonejuie P, Olson J, Pogliano J, Sakoulas G, Nizet V, Proctor RA, Rose WE. 2016. Penicillin binding protein 1 is important in the compensatory response of Staphylococcus aureus to daptomycin-induced membrane damage and is a potential target for β-lactam-daptomycin synergy. Antimicrob Agents Chemother 60:451–458. doi: 10.1128/AAC.02071-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.del Rio A, Garcia de la Maria C, Entenza JM, Gasch O, Armero Y, Soy D, Mestres CA, Pericas JM, Falces C, Ninot S, Almela M, Cervera C, Gatell JM, Moreno A, Moreillon P, Marco F, Miro JM; Hospital Clinic Experimental Endocarditis Study Group . 2016. Fosfomycin plus beta-lactams as synergistic bactericidal combinations for experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 60:478–486. doi: 10.1128/AAC.02139-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Hal SJ, Paterson DL. 2011. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother 55:405–410. doi: 10.1128/AAC.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuji BT, Rybak MJ. 2006. Etest synergy testing of clinical isolates of Staphylococcus aureus demonstrating heterogeneous resistance to vancomycin. Diagn Microbiol Infect Dis 54:73–77. doi: 10.1016/j.diagmicrobio.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Tong SY, Nelson J, Paterson DL, Fowler VG Jr, Howden BP, Cheng AC, Chatfield M, Lipman J, Van Hal S, O'Sullivan M, Robinson JO, Yahav D, Lye D, Davis JS; CAMERA2 Study Group and the Australasian Society for Infectious Diseases Clinical Research Network. 2016. CAMERA2: combination antibiotic therapy for methicillin-resistant Staphylococcus aureus infection: study protocol for a randomised controlled trial. Trials 17:170. doi: 10.1186/s13063-016-1295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hososaka Y, Hanaki H, Yanagisawa C, Yamaguchi Y, Matsui H, Nakae T, Iwata S, Hayashi I, Sunakawa K. 2006. Nosocomial infection of beta-lactam antibiotic-induced vancomycin-resistant Staphylococcus aureus (BIVR). J Infect Chemother 12:181–184. doi: 10.1007/s10156-006-0455-X. [DOI] [PubMed] [Google Scholar]
- 41.Hanaki H, Yamaguchi Y, Yanagisawa C, Uehara K, Matsui H, Yamaguchi Y, Hososaka Y, Barada K, Sakai F, Itabashi Y, Ikeda S, Atsuda K, Tanaka H, Inamatsu T, Nagayama A, Sunakawa K. 2005. Investigation of beta-lactam antibiotic-induced vancomycin-resistant MRSA (BIVR). J Infect Chemother 11:104–106. doi: 10.1007/s10156-004-0371-X. [DOI] [PubMed] [Google Scholar]
- 42.Hanaki H, Yamaguchi Y, Nomura S, Haraga I, Nagayama A, Sunakawa K. 2004. Method of detecting beta-lactam antibiotic induced vancomycin-resistant MRSA (BIVR). Int J Antimicrob Agents 23:1–5. doi: 10.1016/j.ijantimicag.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Yanagisawa C, Hanaki H, Matsui H, Ikeda S, Nakae T, Sunakawa K. 2009. Rapid depletion of free vancomycin in medium in the presence of beta-lactam antibiotics and growth restoration in Staphylococcus aureus strains with beta-lactam-induced vancomycin resistance. Antimicrob Agents Chemother 53:63–68. doi: 10.1128/AAC.00762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leonard SN, Bova S, Brebberman K, Jaros A. 2017. Several common screening methods for beta lactam induced vancomycin-resistant Staphylococcus aureus (BIVR) lack consistency in results, abstr 127-5. ASM Microbe, New Orleans, LA. [Google Scholar]
- 45.Peschel A, Vuong C, Otto M, Gotz F. 2000. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob Agents Chemother 44:2845–2847. doi: 10.1128/AAC.44.10.2845-2847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riederer K, Shemes S, Chase P, Musta A, Mar A, Khatib R. 2011. Detection of intermediately vancomycin-susceptible and heterogeneous Staphylococcus aureus isolates: comparison of Etest and agar screening methods. J Clin Microbiol 49:2147–2150. doi: 10.1128/JCM.01435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin L, Nonejuie P, Munguia J, Hollands A, Olson J, Dam Q, Kumaraswamy M, Rivera H Jr, Corriden R, Rohde M, Hensler ME, Burkart MD, Pogliano J, Sakoulas G, Nizet V. 2015. Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant gram-negative bacterial pathogens. eBioMedicine 2:690–698. doi: 10.1016/j.ebiom.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing; approved standard, 24th informational supplement M100-S24. CLSI, Wayne, PA. [Google Scholar]
- 49.Rose WE, Knier RM, Hutson PR. 2010. Pharmacodynamic effect of clinical vancomycin exposures on cell wall thickness in heterogeneous vancomycin-intermediate Staphylococcus aureus. J Antimicrob Chemother 65:2149–2154. doi: 10.1093/jac/dkq292. [DOI] [PubMed] [Google Scholar]
- 50.Castro SL, Nelman-Gonzalez M, Nickerson CA, Ott CM. 2011. Induction of attachment-independent biofilm formation and repression of hfq expression by low-fluid-shear culture of Staphylococcus aureus. Appl Environ Microbiol 77:6368–6378. doi: 10.1128/AEM.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose WE, Schulz LT, Andes D, Striker R, Berti AD, Hutson PR, Shukla SK. 2012. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother 56:5296–5302. doi: 10.1128/AAC.00797-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bayer AS, Prasad R, Chandra J, Koul A, Smriti M, Varma A, Skurray RA, Firth N, Brown MH, Koo SP, Yeaman MR. 2000. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect Immun 68:3548–3553. doi: 10.1128/IAI.68.6.3548-3553.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.