Abstract
Purpose
To report retinal findings in two patients with mucopolysaccharidosis type I (MPS I) receiving human recombinant alpha-l-iduronidase (Laronidase) as enzyme replacement therapy.
Observations
Patient 1 had visual acuity 20/20 right eye, 20/25 left eye and unremarkable anterior segment and retinal examination. Optical coherence tomography (OCT) scanning demonstrated parafoveal thinning and subfoveal hyperreflectant material. Patient 2 had visual acuity 20/20 both eyes, with dense nuclear cataract both eyes. Retinal examination demonstrated bull's eye maculopathy both eyes. OCT scanning confirmed parafoveal atrophy and demonstrated similar appearing subfoveal hyperreflectant material, more prominent than in case 1.
Conclusions and importance
These two patients with MPS I receiving Laronidase treatment have developed bull's eye maculopathy changes and subfoveal deposition of hyperreflectant material despite excellent compliance and good tolerance of the standard dose of enzyme therapy for this disorder. Further studies are required to determine the nature of the material, the incidence and the effect of enzyme replacement therapy on these findings in patients with MPS I.
Keywords: Enzyme replacement therapy, Scheie, Macula, Mucopolysaccharidosis type I, Iduronidase
1. Introduction
Mucopolysaccharidosis type I (MPS I, OMIM #607016) is an autosomal recessive lysosomal storage disorder due to deficiency of α-l-iduronidase (IDUA),1 of prevalence approximately 1 per 88,000 live births in Australia.2 Gradual lysosomal accumulation of metabolites of IDUA glycosaminoglycan (GAG) substrates heparan and dematan sulfate eventually interferes with cellular function. Although this process occurs in all cells, it is pathophysiologically most apparent in terminally differentiated, long-lived cells such as neurons, cardiomyocytes, and retinal pigment epithelium, underpinning the multisystem and progressive nature of the disorder.
Interference with normal bone development is clinically apparent in the dysostosis multiplex and typical facial dysmorphism that is characteristically developed in this disorder. Cardiorespiratory disease in the context of skeletal deformities and bronchial, pulmonary and cardiac pathology imparts significant morbidity and mortality. Interminable neurodegeneration, retinal degeneration, corneal clouding and cataracts contribute importantly to lifelong morbidity.
Phenotypic variability is wide, varying from severely affected individuals (Hurler syndrome, MPS I-H) to attenuated forms (Scheie syndrome, MPS I-S). Significant restrictive pulmonary disease and progressive neurodegeneration heralded in early childhood characteristically complicates the severe, Hurler phenotype, as opposed to the normal neurological development and sparing of cognition in the attenuated, Scheie phenotype.
Heterologous hematopoietic stem cell transplant (HSCT) has become the gold standard treatment for patients with the severe phenotype diagnosed under 2.5 years of age. Enzyme replacement therapy (ERT) with human recombinant IDUA (Laronidase, Genzyme, Cambridge MA) has demonstrated efficacy in mitigating a number of the clinical effects of the enzyme deficiency, most notably the effects on cardiopulmonary function, and is used for patients diagnosed later in life, as well as pre- and peri-HSCT.3, 4
Reported ocular features of MPS I include corneal clouding, pigmentary retinopathy, optic nerve abnormalities including glaucoma, papilledema and atrophy, ocular motility and refractive problems.5 There is one report of macular edema-like change observed on stereoscopic fundus photography.6 Between 44% and 79% of MPS I patients have visual acuity less than 20/40 in their better eye.7 Retinal involvement, when present, has been described as a progressive rod-cone retinal degeneration with attenuated electroretinographic (ERG) amplitude with relatively mild clinically apparent retinal pigment epithelial (RPE) change in the mid periphery8, 9 and typical degeneration of the outer retinal layers on histopathological examination. Patients without clinical retinal degeneration have been shown to have fine fibrillary inclusions in the RPE and retinal ganglion cells, and multimembranous inclusions in retinal ganglion cells.10 We are not aware of any published reports of macular histopathology in untreated patients with MPS I. Optical coherence tomography (OCT) studies have demonstrated thinning of the parafoveal ellipsoid line, thickening of the central foveal external limiting membrane (ELM), parafoveal retinal folds, retinal cysts and fluid in the outer nuclear layer in MPS I patients,11, 12 some of whom received ERT.13 A recent report demonstrated improved vision in 42% of patients receiving Laronidase treatment; the study recorded visual acuity and corneal clouding, but not retinal structure or function.14 There are no reports of improved retinal function in patients receiving Laronidase treatment. ERT does not cross the blood-brain barrier and presumably does not cross the blood-retina barrier either, given the ultrastructural similarity between the two.
We report macular changes in two patients with MPS I receiving ERT. Case 2 is the oldest reported patient, and the first report of a patient undergoing cataract surgery. We also report the first multifocal ERG analysis of MPS I patients. An earlier version of this work was published as a meeting abstract.15
2. Findings
2.1. Case 1
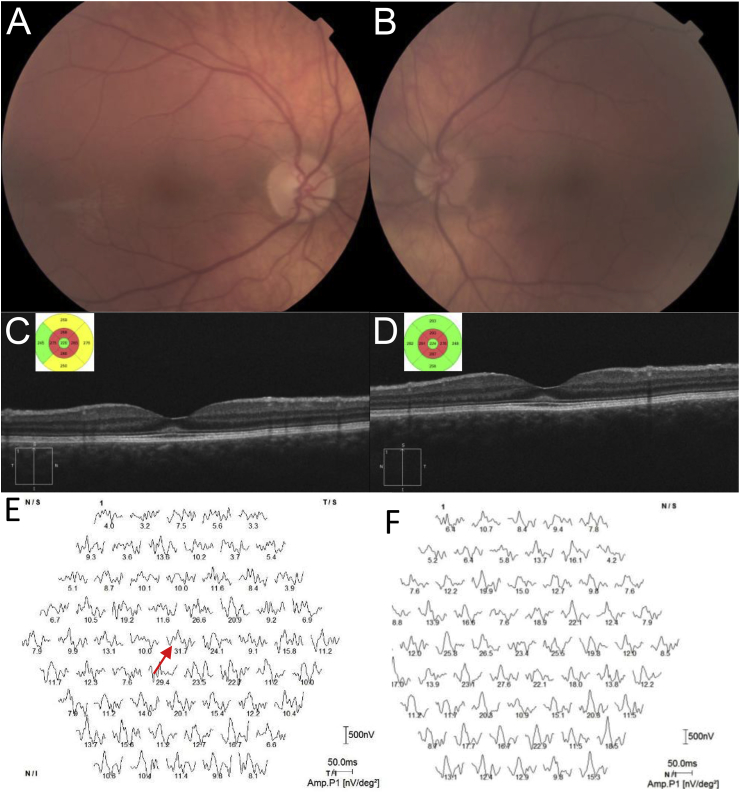
A twenty-one year old Caucasian male was referred for baseline ophthalmological monitoring during ERT. He complained of glare. He had been diagnosed with MPS I 15 years prior, at the age of 6 when his mother sought clarification for his impaired mobility. He had the typical although relatively mild facial dysmorphic features, severe obstructive pulmonary disease, mild mitral valve stenosis and trace mitral regurgitation, hepatosplenomegaly and bilateral carpal tunnel syndrome. He had received IDUA at the regular dose of 0.58 mg/kg for 8.75 years with excellent compliance and tolerance. On examination, best corrected visual acuity was right 20/20 and left 20/25. Corneae demonstrated Ashworth mild grade5 corneal haze bilaterally. The anterior chambers were deep, with clear lenses. Optic discs had cup to disc ratios of 0.1 and appeared healthy; the retinal examination was clinically normal (Fig. 1). OCT scanning (Cirrus, Zeiss Meditec, Germany) demonstrated parafoveal thinning of retinal layers and subfoveal increased hyperreflectance at the level of the external limiting membrane (ELM, Fig. 1), with outer retinal layers intact. Full-field electroretinogram (ERG, RETI-port/scan, Roland Consult, Brandenburg, Germany) was modestly reduced in amplitude, but essentially normal (Table 1); multifocal ERG showed reproducible signals in ring 1 only in the right eye, with no reproducible signals in any test sector in the left eye.
Fig. 1.
A twenty-one year old male with mucopolysaccharidosis type I undergoing Laronidase enzyme replacement therapy.
A Color fundus photographs of the right and B left eye, showing slight foveal reddening, but no bull's’ eye macular appearance. Images are blurred by corneal clouding. C Optical coherence tomography (OCT) scanning (Cirrus, Zeiss, Germany) of the right, and D left eye showing subfoveal hyperreflectant material at the level of the external limiting membrane. The key OCT features of photoreceptor structure appear intact. Inset OCT cube views demonstrate in both eyes parafoveal thinning. E Multifocal ERG responses (Roland, Brandenburg, Germany) showing reproducible response only in ring 1 in the right eye (red arrow), and F no reproducible responses in the left eye. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Clinical characteristics of two patients with mucopolysaccharidosis type I.
| Patient 1 | Patient 2 | |
|---|---|---|
| Age at case report (years) | 21 | 59 |
| Gender | Male | Female |
| Age at start of ERT (years) | 14 | 51 |
| Mucopolysaccharidosis type | I Scheie | I Scheie |
| Visual acuity right eye, left eye | 20/20, 20/25 | 20/20, 20/20 |
| Refraction right, left | +2.5–4.75 × 84; +3.0–4.25 × 82 | +8.5–0.50 × 76; +7.50–1.0 × 88 |
| ERG findings | ||
| DA 0.01 amplitude right, left (94.2–202 μV) | 119, 193 | NM, NM |
| DA 0.01 latency right, left (68.5–97.5 msec) | 77.3, 92.8 | NM, NM |
| DA 10 b-wave amplitude right, left (194.2–385.7 μV) | 111, 203 | 20.8, 48.6 |
| DA 10b-wave latency right, left (31.3–55.8 msec) | 53.2, 52.7 | 67.3, 73.3 |
| DA b-/a-wave ratio (1.2–1.9) | 1.92, 1.75 | 0.79; 3.6 |
| 30 Hz flicker amplitude right, left (46.6–130.1 μV) | 42, 53.6 | 25.6, 21.1 |
| 30 Hz flicker latency right, left (25.0–29.7 msec) | 25.8, 26.6 | 32.2, 35.7 |
| Multifocal ERG findings | Reproducible P1 responses only in ring 1 in the right eye (31.7 nV/deg2; lower limit of normal 42.5) | Reproducible responses only in ring 1 in both eyes (right 25.8, left 31.2 nV/deg2; lower limit of normal 21.6) |
DA, dark-adapted; ERG, electroretinogram; ERT, enzyme replacement therapy; NM, not measurable.
2.2. Case 2
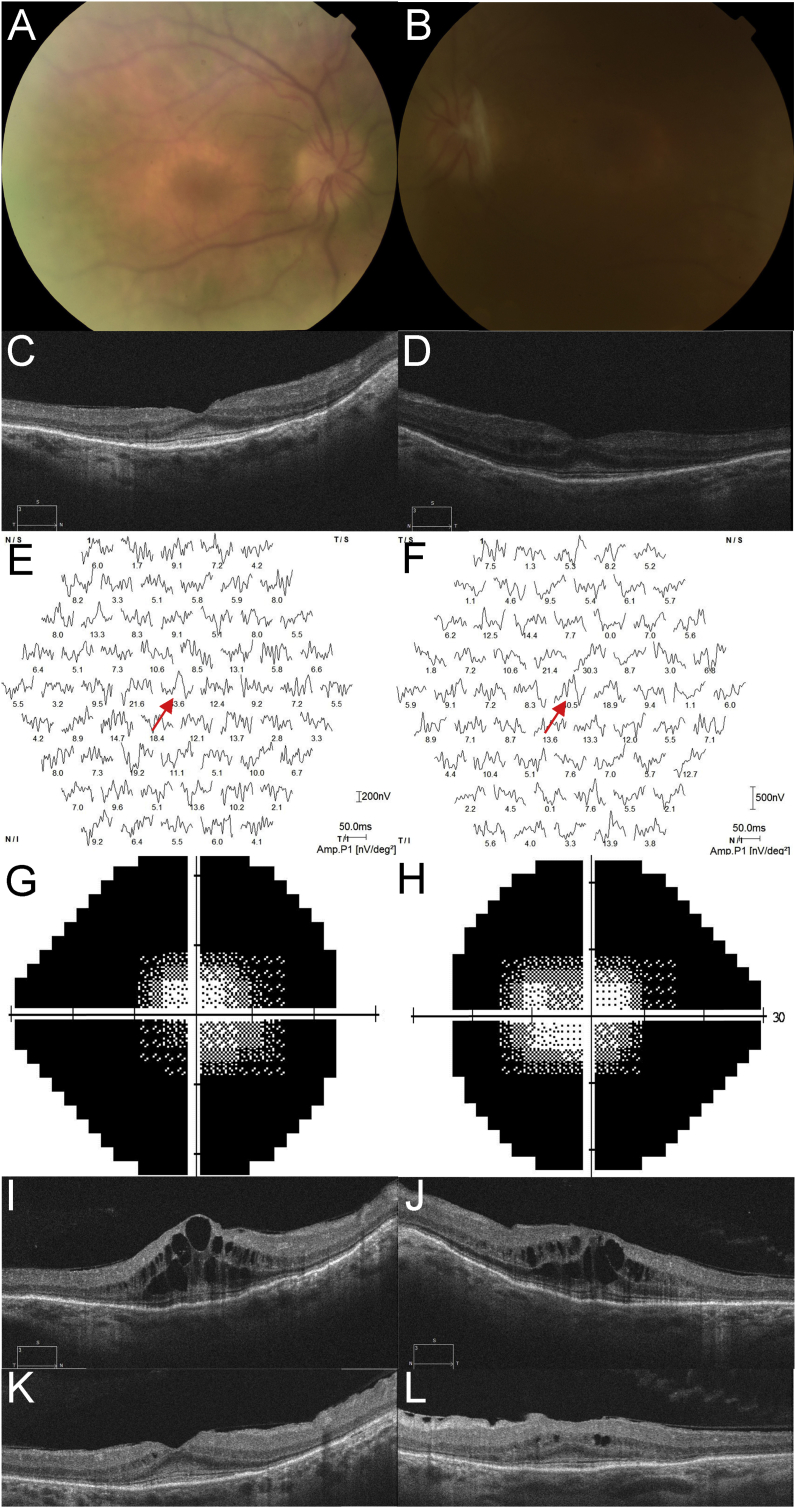
A fifty-nine year old Caucasian female was referred for ophthalmological monitoring during ERT. She complained of nyctalopia onset aged 31 years. She was diagnosed with MPS I in 2011 at age fifty-four years, and has involvement of bones and joints, with relatively mild facial dysmorphism which had been interpreted as acromegalic in years previous to the diagnosis of MPS1, obstructive pulmonary disease, aortic valve disease and bilateral carpal tunnel syndrome. In keeping with the Australian Life Saving Drugs Program guidelines, she has received IDUA at a dose of 0.58 mg/kg ideal body weight (adjusted for BMI 27) for 5 years with excellent compliance and tolerance. On examination, best visual acuity was 20/20 in both eyes. Corneae demonstrated Ashworth mild grade corneal haze bilaterally and were thin right 425 μm, left 415 μm (Pentacam, Oculus, USA). Anterior chamber depth was right 1.5 mm, left 1.46 mm. Many posterior synechiae were present, and minimal pupil dilation could be induced pharmacologically. On fundoscopy optic discs demonstrated cup to disc ratios of 0.3 and appeared healthy, a bull's eye maculopathy pattern was noted along with sparse patches of intraretinal pigment migration (Fig. 2). She readily dissociated to a large alternating exotropia. OCT scanning demonstrated parafoveal thinning of all layers, particularly the ellipsoid line and other photoreceptor components, with subfoveal increased hyperreflectance at the level of the ELM, and peripheral macular photoreceptor lines fragmented. Full-field ERG (Table 1) showed no reproducible pure rod responses, with attenuated responses, negative in waveform in the right eye, to brighter dark-adapted stimuli. Photopic responses were reduced in amplitude and prolonged in latency. Multifocal ERG showed reproducible responses only in ring 1 in both eyes.
Fig. 2.
A fifty-nine year old female with mucopolysaccharidosis type I undergoing Laronidase enzyme replacement therapy.
A Color fundus photographs of the right and B left eye, showing bilateral bull's’ eye macular appearance. Images are blurred by corneal clouding. C Optical coherence tomography (OCT) scanning (Cirrus, Zeiss, Germany) of the right, and D left eye showing subfoveal hyperreflectant material at the level of the external limiting membrane, with parafoveal thinning of the outer nuclear layers and loss of the ellipsoid lines. Multifocal ERG responses (Roland, Brandenburg, Germany) showing reproducible response only in ring 1 in the E right eye and F left eye (red arrows). Automated visual field analysis (Humphrey, Germany) of the G right and H left eye showing fields constricted to about 20° diameter. OCT scanning of the I right, and J left eye at 8 weeks post-operatively demonstrating cystoid macular edema; and K right, L left eye 8 weeks later showing response to treatment. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
She was monitored annually for three years. Nuclear sclerosis developed in both eyes, and anterior chambers progressively shallowed. She underwent uneventful bilateral YAG laser peripheral iridotomy. Her clinical examination and electrophysiology testing was otherwise stable. She developed blurred vision and visual acuity fell to right 20/32 and left 20/40 due to cataract. She underwent laser-assisted cataract surgery in both eyes, with implantation of Rayner C-Flex 970C posterior chamber lenses right eye +39D and left eye +38D. Post-operatively best visual acuity was right 20/32 and left 20/25, however cystoid macular edema (CME) developed at post-operative week 8 (Fig. 2), treated with topical prednisolone acetate 1% and ketorolac 0.5%, with slow improvement in visual acuity and OCT signs. At last review, visual acuity was right 20/32, left 20/25 and topical treatment was slowly withdrawn.
3. Discussion
In this study, we report parafoveal thinning, particularly at the level of the ellipsoid line, and thickening of the foveal ELM, similar to previous reports. Case 2 is one of the oldest patients reported to date, and the first reported patient to undergo cataract surgery. We also include the first multifocal ERG investigation of macular dysfunction. The older of the two patients (59 years) reported on here has more marked findings with a frank bull's eye maculopathy and mild CME, which worsened immediately following cataract surgery. Additionally, results consistent with a typical rod-cone dystrophy were evident on ERG testing. Both patients show preservative of function in ring 1 only on multifocal ERG (very poor function), similar to earlier reports describing the clinical appearance to be more benign than the more severe functional abnormality detected with electrophysiology testing.
The retinal changes reported here bear some similarity to changes reported in hydroxychloroquine (HCQ) macular toxicity (“flying saucer sign’ on OCT scanning).16, 17 Neither of our patients received HCQ treatment for their MPS related arthropathy. The reported changes differ from typical HCQ toxicity by being symmetrical vertically around the fovea, rather than more prominent inferiorly, and by ERG features of rod-cone dystrophy rather than affecting rods and cones to a similar degree as occurs in advanced HCQ toxicity.18
The cause of macular and retinal abnormality in patients with MPS I has not been definitively proven, but is thought to be secondary to GAG production by the RPE,19 failure of GAG metabolism leading to its accumulation, and subsequent cellular death occurring in phagocytic Müller cells and/or RPE. This is consistent with findings that gene therapy in murine models of MPS VII has been shown to reverse GAG accumulation in RPE and subsequent retinal degeneration20, 21 Bull's eye maculopathy has not been definitively demonstrated to date in untreated MPS patients or in foveated animal models, using histopathological examination or OCT scanning. Wide phenotypic variation is well recognised in patients and families with the same genetic mutation in mucopolysaccharidoses,22 similar to retinal degenerative disease, best exemplified by PRPH223 and ABCA424 and we hypothesise that the bull's eye maculopathy may be a previously unrecognised phenotypic variant of retinal degeneration occurring in MPS I patients. Our younger patient does not have rod-cone dystrophy and follow-up will be important to determine whether the maculopathy is isolated or is associated with widespread dystrophy.
The cause of increased hyperreflectance in the region of the ELM, and whether it is intracellular or extracellular, is not known. It may represent undigested abnormal photoreceptor outer segments, although this is unlikely given that it is anterior to the ellipsoid line zone, or abnormal collagen deposition, which has been recently proposed to cause corneal clouding in MPS I-H.25 Seok et al.13 proposed the material to be degraded GAG accumulating in Müller cells. The reason for deposition in the fovea with sparing of the remaining retina is not known. IDUA does not cross the blood-retina barrier. We propose that our patients with a clinically mild phenotype have some attenuated enzyme action in Müller cells allowing clearing of degraded GAG, but in the subfoveal location the increased number of photoreceptors results in a higher load of GAG and the limited enzyme capacity is overwhelmed. Also, foveal astrocytes have different structural26, 27 and presumably functional properties to Müller cells; the enzymic activity is not known, but may be less in these more primitive retinal astrocytes. It is also possible that there is an inflammatory component to maculopathy from activated Muller cells, as activated microglia have been shown to be inflammatory in the cortex in murine models of MPS I.28
4. Conclusions
We report macular changes in patients with MPS I undergoing ERT, with clinical changes less significant than functional abnormality detected with ERG. This is the first report of a patient undergoing cataract surgery and the first report of multifocal ERG assessment. Further studies of untreated patients, and patients treated with both ERT and HSCT are needed to elucidate the nature of the macular changes and their relationship to treatment.
Patient consent
Both patients gave written consent to publish case details.
Funding
No funding or grant support.
Conflicts of interest
All authors have no financial disclosures: HGM, RCAS, GdJ.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
Acknowledgements and disclosures
The abstract was presented at The Royal Australian and New Zealand College of Ophthalmologists, 48th Annual Scientific Congress, Melbourne, 19–23 November 2016, and published in Poster Abstracts, Clinical & Experimental Ophthalmology, 2016; 44: 80–140. Permission to reproduce the abstract here has been obtained from the copyright holder, the Royal Australian and New Zealand College of Ophthalmologists.
References
- 1.Beesley C.E., Meaney C.A., Greenland G. Mutational analysis of 85 mucopolysaccharidosis type I families: frequency of known mutations, identification of 17 novel mutations and in vitro expression of missense mutations. Hum Genet. 2001;109(5):503–511. doi: 10.1007/s004390100606. [DOI] [PubMed] [Google Scholar]
- 2.Meikle P.J.1, Hopwood J.J., Clague A.E., Carey W.F. Prevalence of lysosomal storage disorders. JAMA. 1999;28:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 3.de Ru M.H.1, Boelens J.J., Das A.M. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J Rare Dis. 2011;6:55. doi: 10.1186/1750-1172-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jameson E., Jones S., Remmington T. Enzyme replacement therapy with laronidase (Aldurazyme) for treating mucopolysaccharidosis type I. Cochrane Database Syst Rev. 2016;(4) doi: 10.1002/14651858.CD009354.pub4. Art. No.: CD009354. [DOI] [PubMed] [Google Scholar]
- 5.Ashworth J.L., Kruse F.E., Bachmann B. Ocular manifestations in the mucopolysaccharidoses – a review. Clin Exp Ophthal. 2010;38:12–22. [Google Scholar]
- 6.Usui T.1, Shirakashi M., Takagi M., Abe H., Iwata K. Macular edema-like change and pseudopapilledema in a case of Scheie syndrome. J Clin Neuroophthalmol. 1991;11:183–185. PMID:1836802. [PubMed] [Google Scholar]
- 7.Ashworth J.L., Biswas S., Wratih E., Lloyd I.C. The ocular features of the mucopolysaccharisoses. Eye. 2006;20:553–563. doi: 10.1038/sj.eye.6701921. [DOI] [PubMed] [Google Scholar]
- 8.Gills J.P., Hobson R., Hanley W.B., McKusick V.A. Electroretinography and fundus oculi findings in Hurler's disease and allied mucopolysaccharidoses. JAMA Ophthalmol. 1965;74:596–603. doi: 10.1001/archopht.1965.00970040598003. [DOI] [PubMed] [Google Scholar]
- 9.Caruso R.C., Kaiser-Kupfer M.I., Muenzer J. Electroretinographic findings in the mucopolysaccharidoses. Ophthalmol. 1986;93:1612–1616. doi: 10.1016/s0161-6420(86)33537-1. [DOI] [PubMed] [Google Scholar]
- 10.Chan C.C., Green W.R., Maumenee I.H., Sack G.H. Ocular ultrastructural studies of two cases of the Hurler syndrome (systemic mucopolysaccharisosis I0H) Ophthalmic Paediatr Genet. 1983;2:3–19. [Google Scholar]
- 11.Huang C.T., Chu S.Y., Lee Y.C. Optical coherence tomography of chorioretinopathy caused by mucopolysaccharidoses. Ophthalmol. 2015;122:1535–1537. doi: 10.1016/j.ophtha.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Javed A., Aslam T., Ashworth J. Use of new imaging in detecting and monitoring ocular manifestations of the mucopolysaccharidoses. Acta Ophthalmol. 2016;94:e676–e682. doi: 10.1111/aos.13098. [DOI] [PubMed] [Google Scholar]
- 13.Seok S., Lyu I.J., Park K.A., Oh S.Y. Spectral domain optical coherence tomography imaging of mucopolysaccharidoses I, II, and VI A. Graefes Arch Clin Exp Ophthalmol. 2015;253:2111–2119. doi: 10.1007/s00417-015-2953-y. [DOI] [PubMed] [Google Scholar]
- 14.Laraway S., Mercer J., Jameson E., Ashworth J., Hensman P., Jones S.A. Outcomes of long-term treatment with laronidase in patients with mucopolysaccharidosis type I. J Pediatr. 2016;178:219–226. doi: 10.1016/j.jpeds.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Mack H.G., Symons R.C.A., de Jong G. Bull's eye maculopathy and subfoveal deposition in two Mucopolysaccharidosis type I patients on long-term enzyme replacement therapy. Clin Exp Ophthalmol. 2016;44(Suppl 1):127. doi: 10.1016/j.ajoc.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen E., Brown D.M., Benz M.S. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign) Clin Ophthalmol. 2010;4:1151–1158. doi: 10.2147/OPTH.S14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmor M.F., Kellner U., Lai T.Y.Y., Melles R.B., Mieler W.F. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmology. 2016;123:1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 18.Nair A.A., Marmor M.F. ERG and other discriminators between advanced hydroxychloroquine retinopathy and retinitis pigmentosa. Doc Ophthalmol. 2017;134:175–183. doi: 10.1007/s10633-017-9588-8. [DOI] [PubMed] [Google Scholar]
- 19.deS Senanayake P., Calabro A., Nishiyama K., Hu J.G., Bok D., Hollyfield J.G. Glycosaminoglycan synthesis and secretion by the retinal pigment epithelium: polarized delivery of hyaluronan from the apical surface. J Cell Sci. 2001;114(Pt 1):199–205. doi: 10.1242/jcs.114.1.199. [DOI] [PubMed] [Google Scholar]
- 20.Li T., Davidson B.L. Phenotype correction in retinal pigment epithelium in murine mucopolysaccharidosis VIII by adenovirus-mediated gene transfer. Proc Natl Acad Sci U. S. A. 1995;92:7700–7704. doi: 10.1073/pnas.92.17.7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennig A.K., Ogilvie J.M., Ohlemiller K.K., Timmers A.M., Hauswirth W.W., Sands M.S. Aav-Mediated intravitreal gene therapy reduces lysosomal storage in the retinal pigmented epithelium and improves retinal function in adult MPS VII mice. Mol Ther. 2004;10:106–116. doi: 10.1016/j.ymthe.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Cimaz R., La Torre F. Mucopolysaccharidoses. Curr Rheumatol Rep. 2014;16:389. doi: 10.1007/s11926-013-0389-0. [DOI] [PubMed] [Google Scholar]
- 23.Apfelstedt-Sylla E., Theischen M., Rüther K., Wedemann H., Gal A., Zrenner E. Extensive intrafamilial and interfamilial phenotypic variation among patients with autosomal dominant retinal dystrophy and mutations in the human RDS/peripherin gene. Br J Ophthalmol. 1995;79:28–34. doi: 10.1136/bjo.79.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke T.R., Tsang S.H. Allelic and phenotypic heterogeneity in ABCA4 mutations. Ophthalmic Genet. 2011;32:165–174. doi: 10.3109/13816810.2011.565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan C., Bothun E.D., Hardten D.R., Tolar J., McLoon L.K. A novel explanation of corneal clouding in a bone marrow transplant-treated patient with Hurler syndrome. Exp Eye Res. 2016;148:83–89. doi: 10.1016/j.exer.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramírez J.M., Triviňo A., Ramírez A.I. Structural specializations of human retinal glial cells. Vis Res. 1996;36:2029–2036. doi: 10.1016/0042-6989(95)00322-3. [DOI] [PubMed] [Google Scholar]
- 27.Reichenbach A., Bringmann A. Müller Cells in the Healthy and Diseased Retina. Springer; New York: 2010. Müller cells in the healthy retina; pp. 35–214. [Google Scholar]
- 28.Ohmi K., Greenberg D.S., Rajavel K.S., Ryazantsev S., Li H.H., Neufeld E.F. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. PNAS. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]