Abstract
Study Objectives:
This study was conducted to validate the NoSAS score in clinical populations and to compare it with the Berlin, STOP, and STOP-Bang questionnaires, as well as the Epworth Sleepiness Scale (ESS), in screening for sleep-disordered breathing (SDB).
Methods:
A retrospective analysis was conducted of all patients aged 18 to 80 years who had completed a full-night polysomnography (PSG) at the sleep center of the First Affiliated Hospital of Guangzhou Medical University from October 2012 to November 2016. Patients who had incomplete or unanswered questionnaires were excluded. The data for the NoSAS score, ESS, STOP, STOP-Bang, and Berlin questionnaires were collected, after which the NoSAS score was compared against the other questionnaires for SDB screening.
Results:
A total of 2,208 participants were enrolled in this study. The NoSAS scores, which ranged from 0 to 17 and allocated a threshold of 8 points, identified individuals at risk of clinically significant SDB (defined as an apnea-hypopnea index [AHI] cutoff of ≥ 20 events/h), with an area under the curve (AUC) of 0.707. The NoSAS score performed significantly better than the STOP (AUC 0.655) and STOP-Bang (AUC 0.704) questionnaires and the ESS (AUC 0.642), and it was at par with the Berlin (AUC 0.697) scores for SDB screening. A significant correlation was found between the AHI and NoSAS score (r = .386, P < .001).
Conclusions:
The NoSAS score is a simple, efficient, and easy method for screening SDB in the clinical setting, especially in moderate to severe SDB.
It demonstrates a moderately high level of sensitivity for SDB.
Citation:
Hong C, Chen R, Qing S, Kuang A, Yang HJ, Su X, Zhao D, Wu K, Zhang N. Validation of the NoSAS score for the screening of sleep-disordered breathing: a hospital-based retrospective study in China. J Clin Sleep Med. 2018;14(2):191–197.
Keywords: NoSAS score, screening, sleep-disordered breathing, questionnaire
BRIEF SUMMARY
Current Knowledge/Study Rationale: The NoSAS score has been shown to be a reliable screening score for sleep-disordered breathing (SDB) in two population-based cohorts in Switzerland and Brazil. In this study, we aimed to validate the value of NoSAS scores in clinical populations and to compare it with the STOP-Bang, STOP, and Berlin questionnaires, as well as the Epworth Sleepiness Scale for SDB screening.
Study Impact: The NoSAS score is a simple, efficient, and easy method for screening SDB in the clinical setting, especially in moderate to severe SDB, making it appropriate for widespread use.
INTRODUCTION
Sleep-disordered breathing (SDB) is a highly prevalent disease that is characterized by excessive daytime sleepiness and repeated episodes of upper airway occlusion during sleep. It is a disorder that affects at least 2% of adult women and 4% of adult men worldwide.1 SDB has been identified as having a causal association with cardiovascular diseases, including coronary artery disease, hypertension, diabetes,2 metabolic syndrome,3 and cerebrovascular accidents.4 The gold standard of diagnosing SDB is a full-night polysomnography (PSG), either in a sleep laboratory or at home. Considering it is time consuming and expensive, this investigation is not recommended to be a routine screening method. It has been estimated that in approximately 80% of individuals who are suffering from SDB, varying in degree from moderate to severe, the condition may remain undiagnosed.5
As such, in recent years, different clinical scores, such as the STOP, STOP-Bang,6 Epworth Sleepiness Scale (ESS),7 and Berlin8 scores have been proposed as screening tools. Although the ESS was designed to evaluate the severity of subjective sleepiness, some authors use it for obstructive sleep apnea. The ESS questionnaire is a very subjective questionnaire that requires the subject to rate, on a scale from 0 to 3, their chances of dozing in eight different situations. These scenarios were chosen to differ in their soporific nature.7 The STOP-Bang score combines information on a patient's complaints and clinical characteristics by a self-administered questionnaire. The complaints include snoring, tiredness, observed apnea and high blood pressure,6 and the clinical characteristics taken into consideration include body mass index (BMI), age, neck circumference, and sex. The STOP-Bang questionnaire was created based on a large group of patients (2,477 patients), and 211 of them (9%) had a confirmed diagnosis by a full-channel PSG. The Berlin Questionnaire8 questions patients about snoring, daytime sleepiness, obesity, fatigue, and hypertension. It was based on a general clinical practice sample of 744 individuals, of which 100 patients (13%) received a diagnosis with a polygraphic recording in the living place. Although these instruments are currently used in clinical practice, they do not match current standards, as the Berlin Questionnaire was developed with thermistors with less sensibility, and the respiratory event scoring criteria used in the STOP-Bang is older than those in use currently. Considering that SDB diagnosis and its perceived prevalence may be influenced by these technical differences,9 Marti-Soler et al.10 developed a new screening tool for SDB using current recording standards as a reference. She used data from the HypnoLaus Sleep Cohort study 1, which included a random subset of the population-based CoLaus/Psy-CoLaus cohort,11,12 who all had a full-night PSG at their homes in Switzerland. The NoSAS score is a new screening tool that was developed in a population-based cohort of 2,121 subjects in Switzerland (HypnoLaus) and, subsequently, validated in a Brazilian cohort of 1,042 subjects (EPISONO).13 The NoSAS score ranges from 0 to 17, with a score of 8 or higher defined as having a high risk for SDB. The NoSAS score performed better than the STOP-Bang and Berlin questionnaires, as evidenced by higher area under the curve values; however, because the NoSAS score was built and validated in population-based cohorts, it may differ from one clinical population to the next. Therefore, in this study, we aimed to validate the value of NoSAS scores in clinical populations and to compare it with the STOP-Bang, STOP, and Berlin questionnaires, as well as the ESS for SDB screening.
METHODS
Study Subjects
A retrospective review of all patients at the First Affiliated Hospital of Guangzhou Medical University who underwent a full-night PSG from October 2012 to November 2016 was conducted. All patients were suspected of having and were screened for SDB. The inclusion criteria were (1) older than 18 years, (2) a total sleep time of > 4 hours and (3) completed anthropometric data and ESS, STOP, STOP-Bang, or Berlin questionnaires in the sleep laboratory. Subjects who met the following criteria were excluded from this study: (1) without or incomplete questionnaire, (2) younger than 18 years, (3) a total sleep time of < 4 hours, and (4) previous treatment or repeated examinations.
Data Collection
In our study, we collected basic demographics (eg, sex, age), BMI, neck circumference, waist circumference, blood pressure, ESS, Berlin, STOP, and STOP-Bang questionnaires, as well as the severity of SDB. Symptoms of daytime somnolence and sleepiness were measured using the ESS.7 Berlin, STOP, STOP-Bang, and ESS questionnaires were completed by all subjects, and they also underwent a full-night PSG in our sleep center. All the questionnaires were conducted at night by a physician certified in sleep medicine prior to the performance of the PSG (Alice 5 Diagnostic Device, Respironics, United States) with a standard 16-channel overnight test in our sleep laboratory. To increase robustness and hinder bias, the reports were read by another sleep medicine-certified physician, while a second sleep expert did random quality checks.
Sleep Studies
PSG included recordings of the electro-encephalogram, electromyogram, electrocardiogram, electro-occulogram, oro-nasal flow, thoracoabdominal movements, arterial oxygen saturation, body position, and snoring sounds. Breathing was recorded with nasal pressure sensors. Page-by-page analyses and scoring of the electronic raw data were performed manually. Data according to the standard criteria were analyzed manually by skilled staff. Apnea was defined as a > 90% decrease in airflow amplitude for > 10 seconds. Hypopneas were scored with use of the 2012 American Academy of Sleep Medicine Criteria13 (≥ 30% decrease of air flow lasting at least 10 seconds, associated with either an arousal or a ≥ 3% oxygen saturation decrease). The AHI was also calculated. We defined clinically significant SDB as an AHI of more than 10 events/h, according to the NoSAS score study.10 The severity of SDB was categorized as follows: mild (5 ≤ AHI < 15 events/h), moderate (15 ≤ AHI < 30 events/h), and severe (AHI ≥ 30 events/h), according to the 2012 American Academy of Sleep Medicine criteria.
Statistical Analysis
The results are presented as the mean ± standard deviation. Mean differences within and between the groups were tested with paired and unpaired t tests, respectively. The χ2 test was used to compare categorical variables; tests were considered significant at P < .05. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS version 16.0, IBM Corp, Armonk, New York, United States).
RESULTS
Baseline Characteristics
We collected data on 2,668 participants from our sleep laboratory, of which 2,208 (83%) met our inclusion criteria and were enrolled in our study. We excluded 460 patients (17%) because they had a total sleep time of < 4 hours (51, 11%), were an age younger than 18 years (154, 33%), were under treatment for SDB before the PSG (128, 28%), had no (66, 14%) or incomplete (31, 7%) questionnaires, or refused to conduct the PSG after the questionnaire (30, 7%) (Figure 1).
Figure 1. Flow diagram.
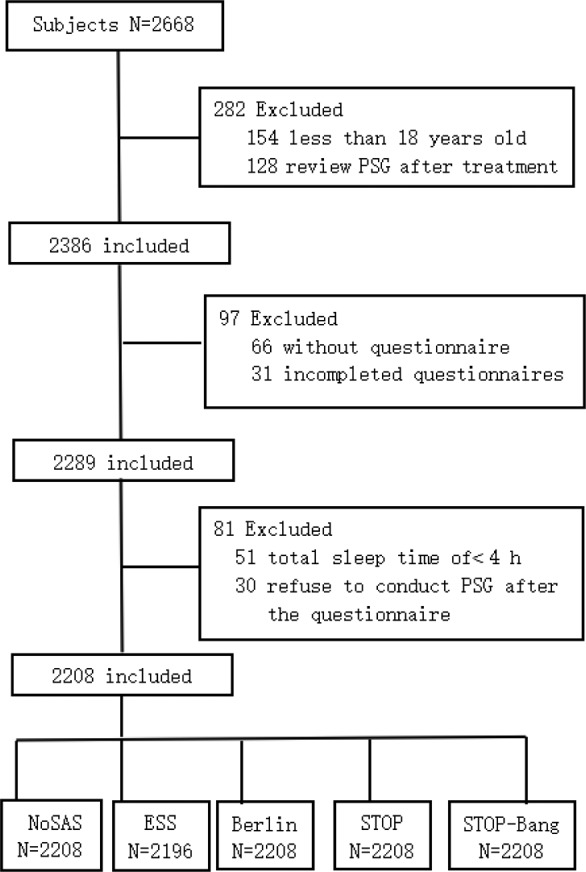
ESS = Epworth Sleepiness Scale, PSG = polysomnography.
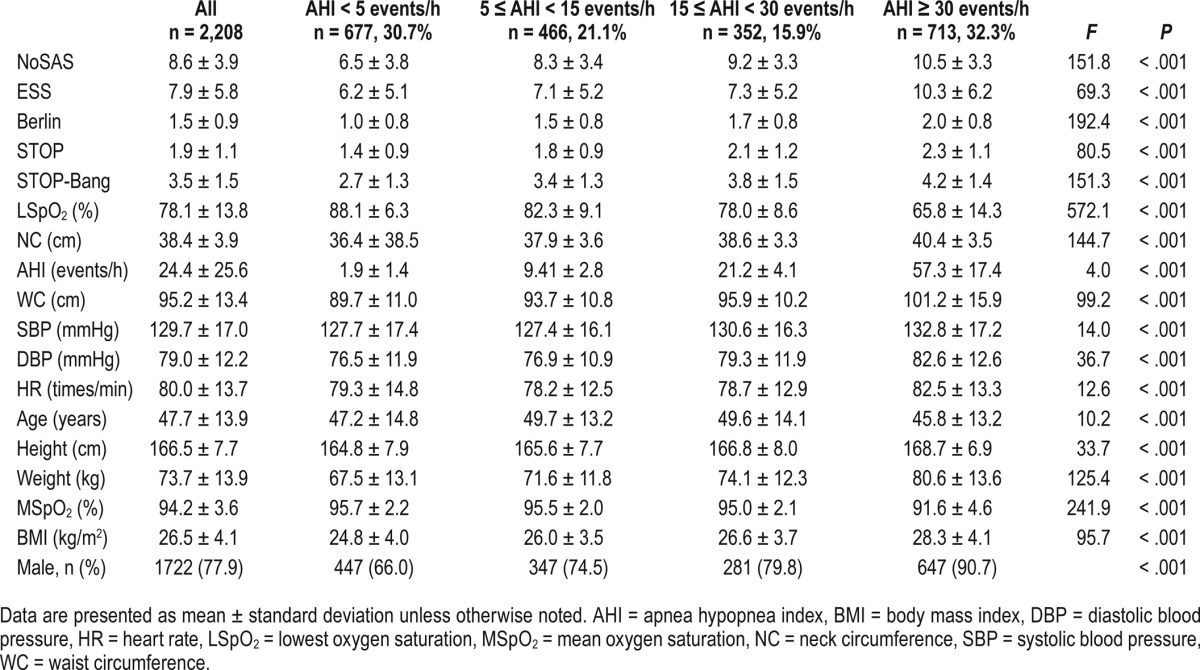
The demographic data and characteristics of the 2,208 participants in our study are shown in Table 1. We analyzed all available demographic and clinical variables classically related to SDB, such as age, sex, BMI, height, weight, neck circumference, waist circumference, systolic blood pressure, diastolic blood pressure, and ESS.
Table 1.
Baseline characteristics of study subjects.
Diagnostic Value
Similar to the study by Marti-Soler and colleagues,10 the NoSAS score was defined as positive if it was greater than or equal to 8 points and negative if it was fewer than 8 points, on the basis of its ability to discriminate participants with clinically significant SDB (ie, an AHI of ≥ 20 events/h), its positive predictive value (PPV), and negative predictive value (NPV) (Table 2). We also compared the performance of the NoSAS score with that of the ESS, STOP, STOP-Bang, and Berlin scores using AHI cutoffs of 5, 10, 15, 20, 25, and 30 events/h (Figure 2). We found that the AUC for the NoSAS score was lower than that of the Berlin score when using the AHI cutoff of ≥ 5 or 10 events/h, whereas it was higher than that of the Berlin score when using the AHI cutoffs ≥ 15, 20, 25, or 30 events/h. The diagnostic value of the NoSAS score was better than the three remaining questionnaires. The correlations between AHI and the NoSAS, ESS, Berlin, STOP, and STOP-Bang questionnaires demonstrate that a significant correlation was found between AHI and the NoSAS score (r = 0.386, P < .001).
Table 2.
Performance of NoSAS and ESS, Berlin, STOP, and STOP-Bang questionnaires at an AHI cutoff of ≥ 20 events/h.
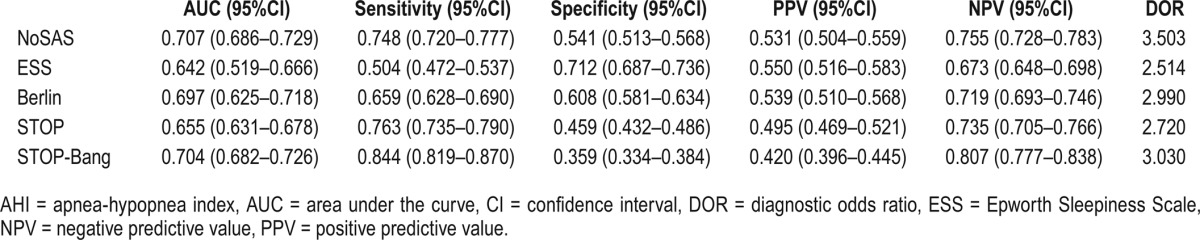
Figure 2. Performance of the NoSAS score compared with STOP-Bang, ESS, STOP, and Berlin scores.
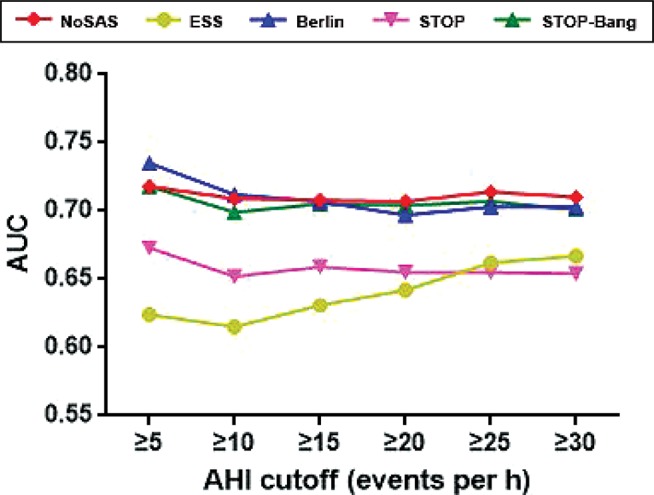
The AUC of NoSAS score was lower than that of Berlin score when using the AHI cutoff of ≥ 5 or 10 events/h, whereas it was higher than that of Berlin score when using the AHI cutoff ≥ 15, 20, 25, or 30 events/h. The diagnostic value of NoSAS score was better than the other questionnaires. AHI = apnea-hypopnea index, AUC = area under the curve, ESS = Epworth Sleepiness Scale.
Overall, the NoSAS score correctly classified 678 of 906 participants (74.8%) at an AHI cutoff of ≥ 20 events/h and detected 546 of 711 participants (76.8%) with severe SDB (an AHI cutoff of ≥ 30 events/h), with 165 of 1,497 (11.0%) having a false-negative diagnosis. The AUC curve of the five questionnaires for SDB screening of the participants with AHI of ≥ 20 events/h is shown in Figure 3. From the receiver operating characteristic curve, it appears that when the cutoff point of AHI 20 ≥ events/h in the Chinese population is used, the diagnostic performance of the NoSAS score is better than the other four questionnaires. In this study, the higher the AHI of the patient, the greater the receiver operating characteristic value of the NoSAS score, indicating that the Chinese population shows consistency with the findings of foreign scholars. In addition, this finding suggests the value of screening for SDB in patients in clinical settings.
Figure 3. ROC curve of the five screening tools at AHI cutoff of ≥ 20 events/h.
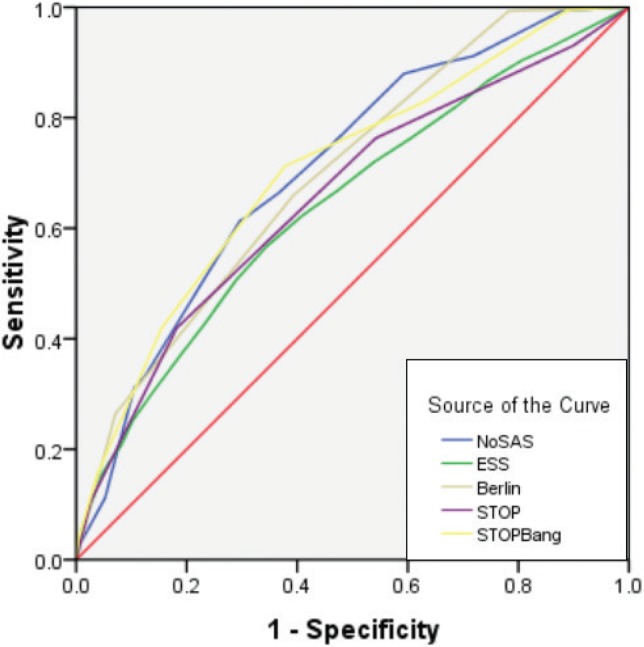
At an AHI cutoff of ≥ 20 events/h, the diagnostic performance of the NoSAS score is better than the other four questionnaires. AHI = apneahypopnea index, ESS = Epworth Sleepiness Scale, ROC = receiver operating characteristic.
DISCUSSION
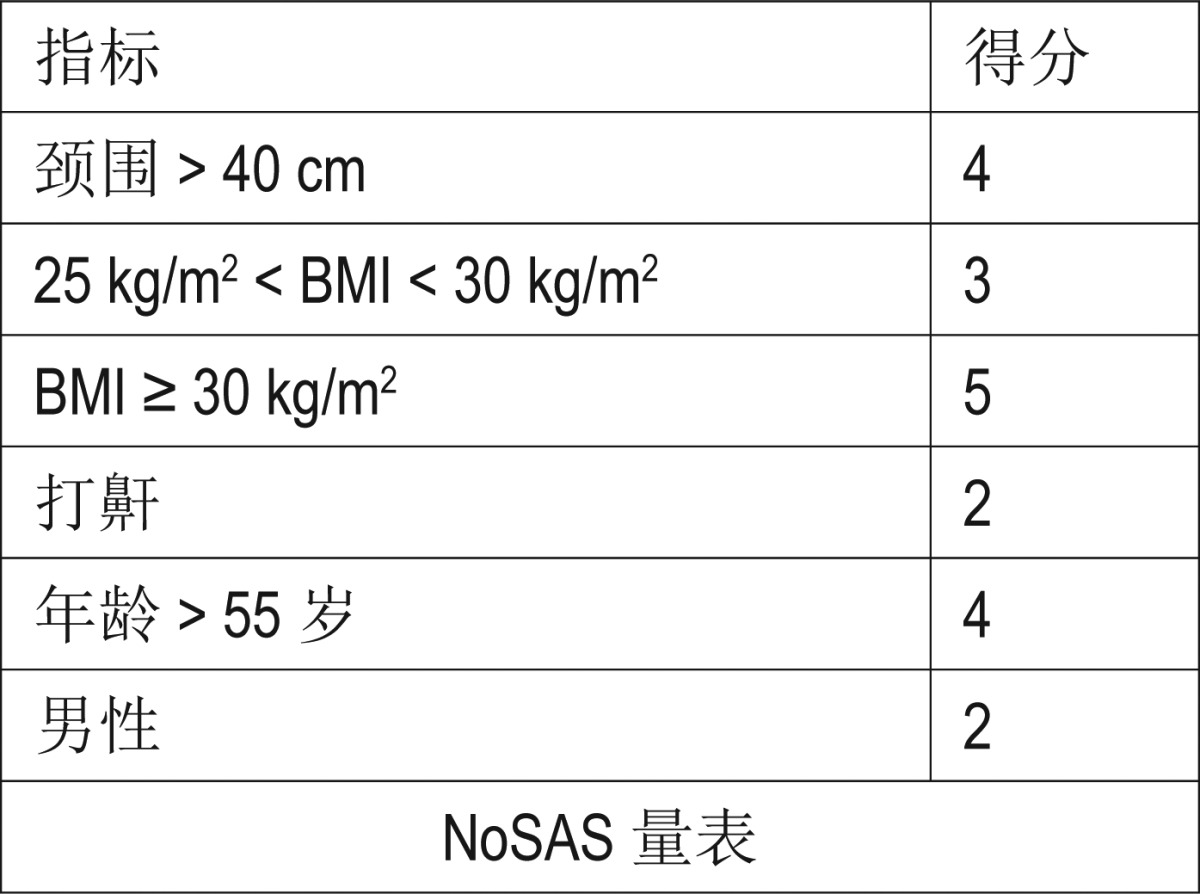
The NoSAS score (Table 3) is a simple, efficient, and easy method that can help clinicians quickly screen high-risk patients for SDB that demonstrates a moderately high level of sensitivity. A previous validation confirmed that the NoSAS score performed similarly to the STOP-Bang and Berlin questionnaires in an Asian population-based cohort.14 The NoSAS score allocates 4 points for having a neck circumference ≥ 40 cm, 3 points for having a BMI of 25–30 kg/m2, 5 points for having a BMI of 30 kg/m2 or more, 2 points for snoring, 4 points for being older than 55 years, and 2 points for being male, and it uses a threshold of 8 points or more to indicate the presence of SDB.10 Based on a large hospital-based retrospective cohort, our study confirmed the clinical utility of the NoSAS score as a screening tool for SDB. Following a high-prevalence population of patients undergoing overnight PSG at the sleep center in our hospital, we propose a new and simple clinical score to screen for clinically significant SDB. The screening tool is also easy to remember and to score, such that no computers or calculators are needed to risk-stratify a patient. Because of the simplicity of the NoSAS score and the availability of relevant data, a high proportion of our patients were able to complete the test without any help. Compared with existing screening scores, a cutoff of AHI ≥ 5 and ≥ 10 events/h resulted in an AUC of the NoSAS score that was slightly lower than the Berlin questionnaire but higher than the other screening scores. It also had high sensitivity and specificity, with higher predictive values than the other scales that can lead misdiagnoses,11 whereas the AUC of the NoSAS score was higher than that of the Berlin score when using the AHI cutoff of ≥ 15, 20, 25, and 30 events/h. The diagnostic value of the NoSAS score was better than the remaining three questionnaires. Simultaneously, the NoSAS score allowed reductions in the number of unnecessary nocturnal recordings and the number of missed diagnoses of SDB. An ideal screening tool for SDB must have high sensitivity and negative predictive values, both of which have been confirmed for the NoSAS score. With this screening tool, practitioners would be able to quickly make a reasonable decision about how likely a particular patient is to have sleep apnea and the need for a referral to a sleep physician. In other words, it is important for this tool to be able to determine patients with SDB severe enough to require treatment, so that they can be referred to specialists (high sensitivity). At the same time, it is also important that low-risk patients can be identified with this tool, so that the practitioner can be reasonably sure that the patient is unlikely to have significant SDB (high negative predictive value).
Table 3.
The Chinese translation of the NoSAS score.
As suggested by the higher AUC and proper classification than the other scores, the NoSAS score seemed to represent the best compromise between sensitivity and specificity and the significant correlation between AHI and NoSAS score (P < .001), allowing clinically significant SDB to be reliably ruled out without yielding too many unnecessary sleep investigations, especially in moderate to severe SDB. Overall, the NoSAS score had a false-negative rate of 11.0% for participants with severe SDB. The fact that the NoSAS score performed consistently well in two unrelated samples with different ethnic backgrounds, age ranges, and habits clearly supports and emphasizes its consistency and generalizability to different populations. Conversely, the STOP-Bang questionnaire has showed low specificities in predicting moderate-to-severe and severe SDB in some population samples.15,16
For ease and efficiency, a screening method should only include a small number of items that are related to easily available and objective variables. The NoSAS score uses biometric items, such as sex, age, and BMI, which are part of any standard clinical assessment, and neck circumference, which can be easily measured. Therefore, if this scale has the increased ability to predicted SDB objectivity, it is foreseeable to replace other questionnaires for the screening of SDB and to be welcomed by a wide range of clinicians. This is important because most SDB patients in all population-based studies do not receive this diagnosis from physicians.17–19 Meanwhile, the current prevalence of moderate to severe SDB (AHI ≥ 15 events/h) is approximately 10% to 20%.20 Furthermore, the prevalence of SDB is 7.2% among patients undergoing a variety of surgeries,21 8.4% in orthopedic patients,22 and 70% in patients undergoing bariatric surgery,23 suggesting a large public burden of SDB sufferers who remain to receive a diagnosis and be treated. The sensitivity, specificity, and NPV (74.8%, 54.1%, 75.5%) were slightly lower than those of HypnoLaus (79.0%, 69.0%, 90%) and EPISONO (85%, 77%, 98%). The pathogenesis of SDB in the Chinese may be related to various factors other than obesity, such as craniofacial restriction, neuromuscular control of the upper airway, or arousal threshold, and warrants further investigation.24,25 In recent years, a meta-analysis used a diagnostic odds ratio (DOR) to compare the accuracy of SDB predictive models and questionnaires.26 The results of this study showed that NoSAS scores have the highest accuracy in diagnosing SDB in screening questionnaires, with a DOR of 3.503. The NoSAS score is simple, efficient, and easy to implement, and it limits the number of subjective variables to only snoring and has the fewest number of variables out of the five questionnaires. Therefore, it should be recommended for the screening of SDB patients opposed to other questionnaires, such as the Berlin and STOP-Bang.
As the aim of this analysis was to validate the NoSAS score in specific clinical samples, we retrospectively analyzed the value of the NoSAS score in patients with suspected SDB and conducted PSG monitoring in our sleep center. To the best of our knowledge, this was the first study to use the NoSAS score in clinical populations. We believe that the NoSAS score, which includes only five items, is easier to use in clinical practice than the other four questionnaires. We, therefore, hope that the easy-to-use NoSAS score can encourage general practitioners to screen for SDB and decrease the proportion of undiagnosed and untreated patients. One of the strengths of our analysis is that it relies on a large hospital-based population, with 2,208 PSG recordings, and the use of up-to-date recording techniques and scoring criteria, which increases the relevance of the NoSAS score for clinical practice. The collection of anthropometric data and the Berlin, STOP, STOP-Bang, and ESS questionnaires were performed prior to PSG, resulting in our complete and unbiased gathering of information. Because there was a false-negative rate of 11.0% with the NoSAS score in our study, the clinical impression should obviously prevail over the results of the score, especially for sleepy patients who should require further investigations regardless.
Like most studies, ours also contains a set number of limitations. The use of a retrospective analysis to validate the predictive value of this scale is less ideal than a prospective study; however, this study was an observational study in which our center had done basic data collection before PSG monitoring, such as the Berlin, STOP, STOP-Bang, and ESS questionnaires. The NoSAS score projections could also be obtained, maintaining a high credibility for this retrospective study. In addition, this was a single-center retrospective analysis. Although the patients were just from one sleep center, the huge number of subjects may represent the Chinese population to some extent.
CONCLUSIONS
The NoSAS score is a simple, efficient, and easy method for the screening of SDB that demonstrates a moderately high level of sensitivity, especially in moderate to severe SDB. As its current clinical application is not yet mature, it is expected that future national or worldwide multicenter research will verify the No-SAS scoring method for its adoption into the clinical setting.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This work was funded by Natural Science Foundation of Guangdong Province 2014A030313501. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Yuanming Zhou and Yitao Zhang (both at The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China) for providing the questionnaire and the data.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- AUC
area under the curve
- BMI
body mass index
- ESS
Epworth Sleepiness Scale
- NPV
negative predictive value
- PPV
positive predictive value
- PSG
polysomnography
- SDB
sleep-disordered breathing
- SpO2
oxygen saturation
REFERENCES
- 1.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25(9):735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med. 2014;190(2):218–225. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- 3.Kawada T, Otsuka T, Nakamura T, Kon Y. Relationship between sleep-disordered breathing and metabolic syndrome after adjustment with cardiovascular risk factors. Diabetes Metab Syndr. 2016;10(2):92–95. doi: 10.1016/j.dsx.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Jurkovicova I, Celec P. Sleep apnea syndrome and its complications. Acta Med Austriaca. 2004;31:45–50. [PubMed] [Google Scholar]
- 5.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 6.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 7.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 8.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 9.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742–748. doi: 10.1016/S2213-2600(16)30075-3. [DOI] [PubMed] [Google Scholar]
- 11.Firmann M, Mayor V, Vidal PM, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008;8:6. doi: 10.1186/1471-2261-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preisig M, Waeber G, Vollenweider P, et al. The PsyCoLaus study:methodology and characteristics of the sample of a population-based survey on psychiatric disorders and their association with genetic and cardiovascular risk factors. BMC Psychiatry. 2009;9:9. doi: 10.1186/1471-244X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan A, Hong Y, Tan LW, et al. Validation of NoSAS score for screening of sleep-disordered breathing in a multiethnic Asian population. Sleep Breath. 2017;21(4):1033–1038. doi: 10.1007/s11325-016-1455-4. [DOI] [PubMed] [Google Scholar]
- 15.Silva GE, Vana KD, Goodwin JL, Quan SF. Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth Sleepiness Scales. J Clin Sleep Med. 2011;7(5):467–472. doi: 10.5664/JCSM.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahapetian R, Silva GE, Vana KD, Parthasarathy S, Quan SF. Weighted STOP-Bang and screening for sleep-disordered breathing. Sleep Breath. 2016;20(2):597–603. doi: 10.1007/s11325-015-1255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189(3):335–344. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10(7):753–758. doi: 10.1016/j.sleep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramachandran SK, Kheterpal S, Consens F, et al. Derivation and validation of a simple perioperative sleep apnea prediction score. Anesth Analg. 2010;110(4):1007–1015. doi: 10.1213/ANE.0b013e3181d489b0. [DOI] [PubMed] [Google Scholar]
- 22.Memtsoudis SG, Stundner O, Rasul R, et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesth Analg. 2014;118(2):407–418. doi: 10.1213/ANE.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frey WC, Pilcher J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13(5):676–683. doi: 10.1381/096089203322509228. [DOI] [PubMed] [Google Scholar]
- 24.Lee RW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in CROCasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33(8):1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li KK, Kushida C, Powell NB, et al. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110(10 Pt 1):1689–1693. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology. 2009;110(4):928–939. doi: 10.1097/ALN.0b013e31819c47b6. [DOI] [PubMed] [Google Scholar]


