Abstract
Study Objectives:
To investigate the prevalence of restless legs syndrome (RLS) in patients with type 2 diabetes mellitus (T2DM) and explore its role in quality of life (QoL) and quality of sleep of these patients.
Methods:
This is a cross-sectional study performed on 210 Iranian people with T2DM. The diagnosis of RLS was established based on the essential diagnostic criteria for RLS recommended by the National Institutes of Health. Sleep quality and QoL were assessed in all participants using Pittsburgh Sleep Quality Index and EuroQol five-dimension questionnaire, respectively. Regression models were used for final analysis of data.
Results:
The prevalence of RLS was 19.5%; of whom 38.1% had poor quality of sleep. Male sex, being single, body mass index (BMI), and RLS were associated with poor quality of sleep. Patients with RLS were almost three times as likely as the patients without RLS to have poor sleep quality. Moreover, being female, BMI value, level of glycosylated hemoglobine (HbA1C), and RLS were associated with lower QoL. RLS lowers the score of QoL even more than BMI and HbA1C. In addition, the QoL and sleep quality of this population of patients with diabetes have not been affected by the severity of RLS as well as presence or absence of neuropathy.
Conclusions:
RLS has an independent and significant role in sleep quality and QoL in the patients with diabetes. Neuropathy with RLS does not confer any additive burden on QoL and sleep quality of this population of patients with diabetes.
Citation:
Modarresnia L, Golgiri F, Madani NH, Emami Z, Tanha K. Restless legs syndrome in Iranian people with type 2 diabetes mellitus: the role in quality of life and quality of sleep. J Clin Sleep Med. 2018;14(2):223–228.
Keywords: quality of life, quality of sleep, restless legs syndrome, type 2 diabetes
BRIEF SUMMARY
Current Knowledge/Study Rationale: Restless legs syndrome (RLS) is a common disorder seen in patients with diabetes. This study aimed to determine the prevalence and severity of RSL in a population of patients with diabetes and identify its role in sleep quality and quality of life of these patients.
Study Impact: Having identified the effect of RLS on quality of life and sleep quality of patients with diabetes, we should pay more attention to this issue when determining their treatment plan.
INTRODUCTION
Restless legs syndrome (RLS) is a circadian sensorimotor disorder, manifested by an urge to move the legs, and usually associated with uncomfortable sensation in the legs.1 The urge to move or unpleasant sensation arise or worsen at nights and during the episodes of inactivity, and are partially or totally relieved by movement.1 RLS is often familial or idiopathic2 but may be associated with iron deficiency anemia,3 renal failure,4 thyroid disorders,5 neuropathy of different causes,6 pregnancy,7 and type 2 diabetes mellitus (T2DM).8
RLS imposes a significant burden not only on quality of sleep and quality of life (QoL)2,9 but also on physical health10 and health-related costs.9 Sleep-related symptoms are the most common and most destructive symptoms experienced by patients with RLS.11 Sleep is both quantitatively and qualitatively impaired by RLS.11 Furthermore, sleep disturbance is responsible for a large proportion of burden of RLS on QoL.9 The adverse effects of RLS on QoL are more severe than those imposed by hypertension, recent myocardial infarction, congestive heart failure, angina, diabetes mellitus, and osteoarthritis.12 In addition, sleep disturbance, a cardinal feature of RLS, has been shown to be a risk factor for impaired glucose tolerance,13 insulin resistance, and T2DM.14
However, diabetes, a lifelong disease of increasing prevalence, is considerably associated with sleep disturbance and subsequently poor glycemic control through different mechanisms.15 RLS, a common comorbidity in patients with diabetes,8,16 can also adversely affect the quality of sleep of these patients.16,17 However, this condition is frequently underdiag-nosed.9 Although identification of sleep abnormalities and their effects on QoL in patients with diabetes is the subject of many studies, only a few studies looked into RLS and its effect on both QoL and quality of sleep of this population. Further understanding of the link between diabetes and RLS is an important part of the efforts done to prevent and treat diabetes complications. Thus, in this study, we investigated the prevalence and severity of RLS in an Iranian population of patients with T2DM and determined the role of RLS in QoL and quality of sleep in these patients to highlight the burden of this neglected but severely disabling disorder.
METHODS
This is a cross-sectional study on patients with T2DM, done at a tertiary care center, in Iran, Tehran, in 2016. Patients with T2DM, according American Diabetic Association criteria,18 were selected using the convenience sampling method. The sample size including 210 participants was determined applying the observational study formula.16 Exclusion criteria were any known chronic kidney disease (CKD), severe heart disease, liver disease, active thyroid disorder, obstructive sleep apnea (OSA), depression, a history of trauma or hospital admission during the past 3 months, diabetic duration of less than 3 months, and age younger than 30 years or older than 70 years. An informed written consent was obtained from all the subjects eligible for including in the study.
The diagnosis of RLS was established by a physician based on the essential diagnostic criteria for RLS recommended by the National Institutes of Health,1 utilizing the validated Persian language version. The criterion excluding RLS mimics is not included in the National Institutes of Health criteria; however, we excluded RLS mimics (namely, myalgia, venous stenosis, leg edema, leg cramp, positional discomfort, habitual foot tapping) by an exact physical examination and mimics-related questions.1 Diabetic neuropathy was documented by nerve conduction study. Severity of RLS was evaluated with the International RLS Study Group Rating Scale.19 RLS severity was classified to mild (0–10), moderate (11–20), severe (21– 30), and very severe (31–40) according to total International RLS Study Group Rating Scale scores.
Sleep quality was measured in all participants using Pittsburgh Sleep Quality Index (PSQI).20,21 Sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleep medications, and daytime dysfunction were evaluated subjectively through 19 self-rating items. Each of the seven factors was scored 0 to 3. PSQI total score ranged from 0 to 21; the higher PSQI global score indicates the poorer quality of sleep. PSQI global score higher than 5 has been found to have a sensitivity of 98.7% and specificity of 84.4% in differentiating good sleepers from poor sleepers.22
QoL was assessed in all participants using EuroQol five-dimension questionnaire (EQ-5D-3L), a standardized tool for measuring general health status.23 This questionnaire consists of two components, a health state description part and a visual analog scale (VAS).24 Five dimensions of health status, including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, are measured in the description part. The respondents self-rate the level of severity of each dimension using a three-level scale. The VAS indicates health status on the day of interview on a 20-cm vertical scale with end points of 0 and 100.24 In the EQ-5D-3L, the respondent has to draw a line from the box on the questionnaire to the imagined scale.
Weight, height, and blood pressure were measured; another questionnaire including demographic information (age, sex, occupation, marital status, and smoking status), diabetes-related variables (including duration of diabetes and type of treatment), as well as physician-diagnosed comorbidities (ie, hypertension, hyperlipidemia, thyroid disorders, anemia, CKD, severe heart disease, liver disease, OSA, depression, and hospital admission during the past 3 months) were completed by the participants. In addition, in the same session a blood sample was obtained for laboratory testing of fasting blood sugar, glycated hemoglobin (HbA1C), total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglyceride.
All the questionnaires were completed via a face-to-face interview between trained physicians and participants. The questions that arose during the interview were explained and clarified by the investigators. Moreover, if the participants were unable to complete the questionnaires, the physicians asked them the questions item-by-item orally and recorded the answers truthfully.
Statistical Analysis
Data are presented as mean ± standard deviation for continuous variables with normal distribution or median and interquartile range for non-normally distributed variables. Categorical data are presented as frequency and percentage. Analysis of variance, Mann-Whitney U test, Kruskal-Wallis test, and χ2 test were used for comparison of variables between groups. Correlations were evaluated using the Spearman correlation coefficient. After performing the univariate analysis, the variables with P < .2 were included in multivariate analysis. For multivariate analysis, binary logistic regression model and linear regression analysis were used. A value of P < .05 was considered statistically significant. The Statistical Package for Social Sciences (SPSS) version 24 (SPSS Inc., Chicago, Illinois, United States) was used for statistical analysis.
RESULTS
Baseline characteristics of all participants are shown in Table 1. Two hundred ten patients with T2DM were included in the study. The mean age of participants was 54.89 ± 7.81 years and mean diabetic duration was 7.8 ± 4.89 years. The prevalence of RLS was 19.5% (n = 41) in this population of patients with diabetes, of whom 21 (51.2%) had evidence of neuropathy whereas 20 (48.8%) had no evidence of neuropathy in nerve conduction tests. Based on the International RLS Study Group Rating Scale, 29.3%, 51.2%, 14.6%, and 4.9% of the patients were affected by mild, moderate, severe, and very severe forms of RLS, respectively.
Table 1.
Baseline characteristics of the participants.
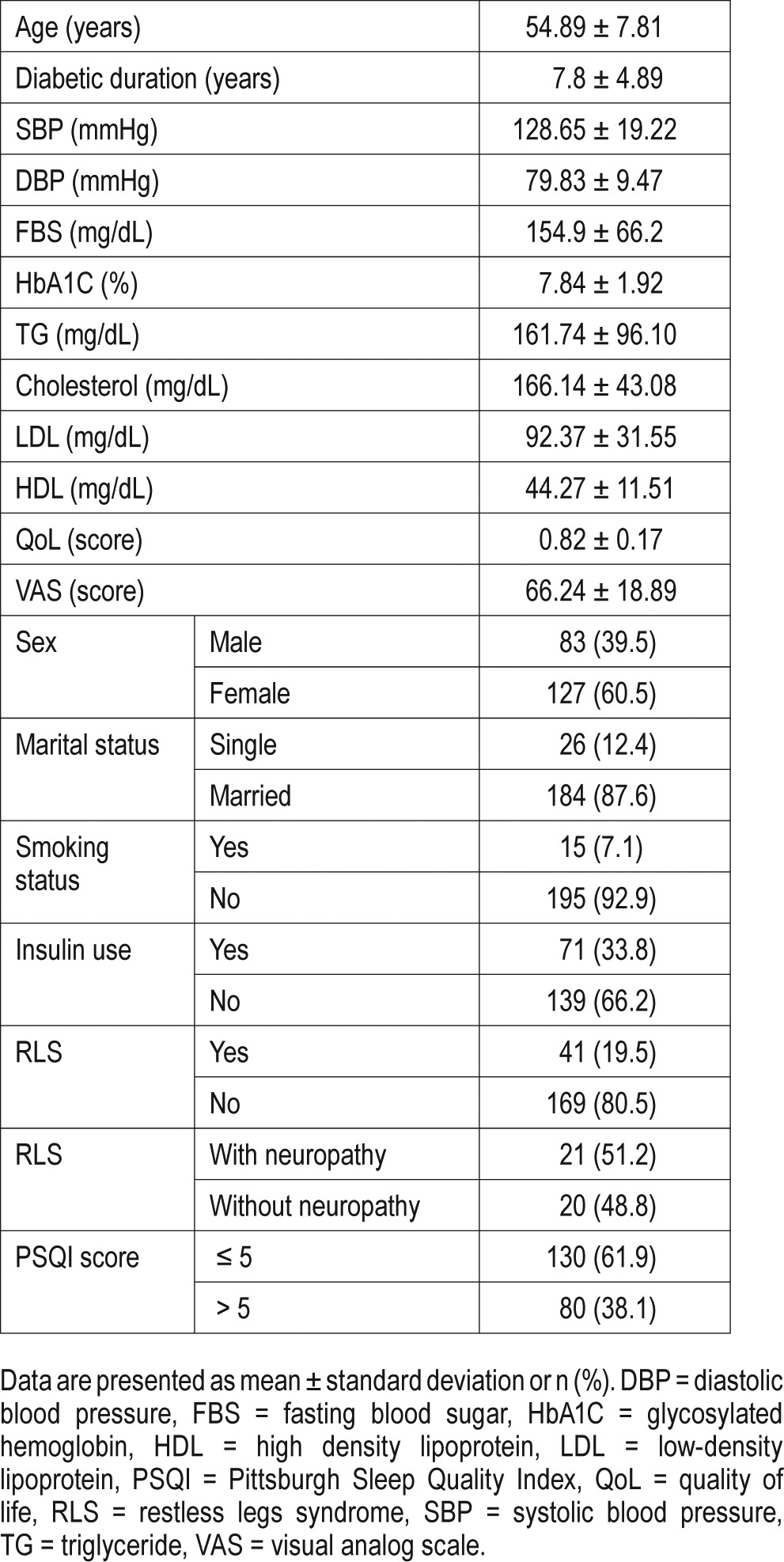
There were 38.1% of all participants and 56.1% of the participants with RLS who had poor quality of sleep. Binary logistic regression model showed poor quality of sleep is associated with male sex (odds ratio [OR] 3.17; 95% CI 1.60–6.29; P = .001), being single (OR 3.60; 95% CI 1.04–12.36; P = .04), higher body mass index (BMI) level (OR 0.93; 95% CI 0.87– 0.98; P = .014), and the presence of RLS (OR 2.79; 95% CI 1.30–5.99; P = .008), Table 2. The effect of RLS on sleep quality was independent of the presence of neuropathy.
Table 2.
Binary logistic regression analysis of factors influencing quality of sleep.
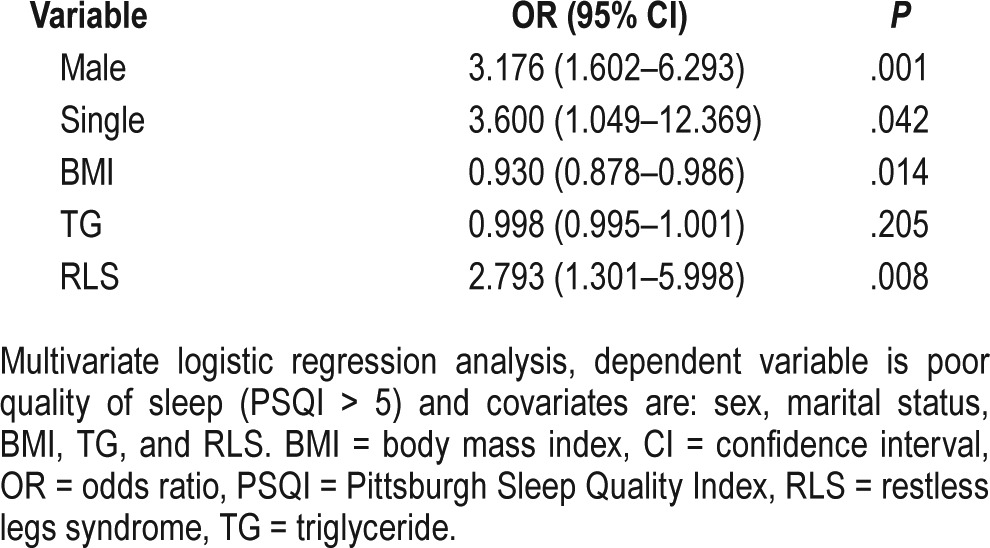
Linear regression analysis for identifying the associated factors of poor QoL demonstrated the significant effect of sex (male) (coefficient 1.50; P = .001), BMI (coefficient −0.008; P = < .001), HbA1C (coefficient −0.015; P = .02), and RLS (coefficient −0.08; P = .007) on QoL (Table 3). In the VAS part of the questionnaire, female sex (coefficient −6.04; P = .03), duration of diabetes (coefficient −0.62; P = .02), BMI (coefficient −0.5; P = .02), and being a smoker (coefficient −16.17; P = .003) all were associated with an inverse effect on QoL (Table 4). Furthermore, patients with poor quality of sleep showed significantly lower scores in both parts of the EQ-5D-3L questionnaire compared with patients with good quality of sleep (0.80 versus 0.84, P < .001, in the health state description part; and 60 versus 70, P < .001, in the VAS part). Finally, our data showed that the mobility problem and pain/discomfort aspects of the QoL are more affected by RLS. Adverse effect of RLS on QoL in patients with neuropathy was the same as those without neuropathy.
Table 3.
Linear regression analysis of factors influencing QoL.
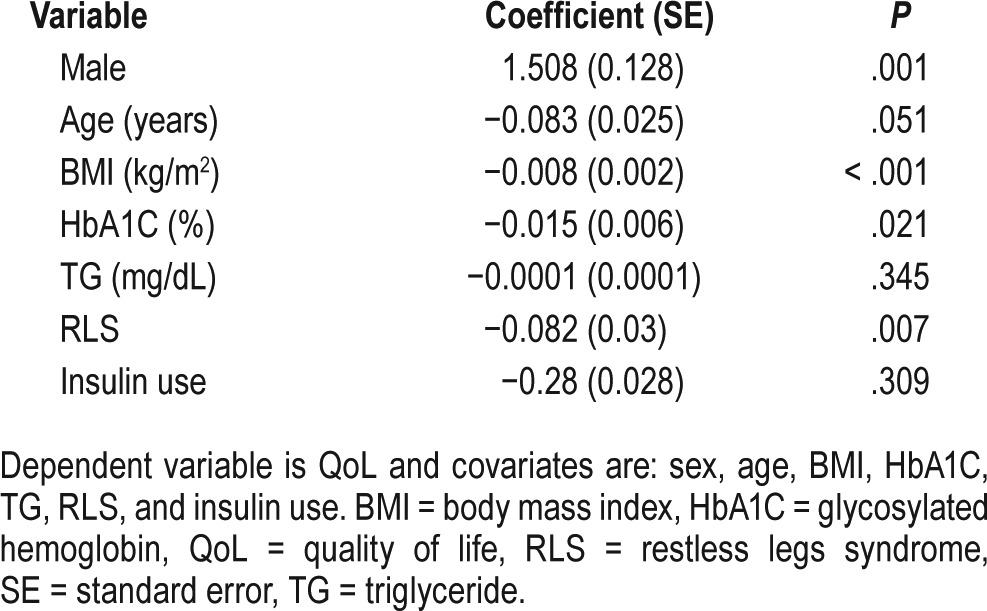
Table 4.
Linear regression analysis of factors influencing VAS.
DISCUSSION
The prevalence of RLS in this cross-sectional study, carried out in a population of Iranian people with T2DM, was 19.5%, of whom 51.2% also suffered from neuropathy. Patients with RLS were more likely to have poor quality of sleep (odds ratio 2.79; 95% confidence interval 1.30–5.99; P = .008); furthermore, they had a significantly lower QoL score compared to patients without RLS (0.83 versus 0.84, P = .009). However, there was no significant difference in QoL and sleep quality of the participants across the spectrum of RLS severity. Furthermore, RLS in the presence of neuropathy did not confer any additional burden on QoL and sleep quality compared to RLS without neuropathy.
The prevalence of RLS in our patients with diabetes (19.5%) is in line with the results of previous studies. Clinically significant RLS, although underdiagnosed,2,9 is common (2.7%) in the general population.2 However, the prevalence of any form of RLS has been reported to range from 7% to 10%.2,25 Moreover, RLS is over four times more common in patients with diabetes than in the general population,8 with a prevalence of 10% to 42% in different populations with diabetes.26,27
However, 51.2% of patients with RLS had neuropathy documented by nerve conduction studies. Peripheral neuropathy, one of the most common complications of diabetes, can mimic symptoms of RLS. Uncomfortable sensations of tingling, numbness, burning, and pain all are seen in both conditions, making it difficult to distinguish the two disorders.28 However, some aspects of history and physical examination, as well as the nerve conduction test, can be used to differentiate neuropathy and RLS.28 The fact that not all patients with diabetes and RLS suffered from neuropathy supports the results of previous studies in that neuropathy is not the only leading cause of RLS.29
Poor sleep quality was seen in 38.1% of this population of patients with diabetes, and was associated with male sex, single status, high BMI, and presence of RLS. A large proportion of our patients with diabetes were found to have poor sleep quality, consistent with the previous studies in patients with diabetes.16 However, the relationship between diabetes and sleep disorders is a complex bidirectional relationship.30 Marriage was a protective factor on quality of sleep among our studied population, similar to the results of another study that showed an elderly population who had ever married or lived with others had a better quality of sleep.31 However, in contrast to the results of previous studies,32 we could not find the protective effect of male sex on quality of sleep. This difference may be due to the difference in studied populations because the study of Denic-Roberts et al.32 was conducted among an adult population with long-standing type 1 diabetes in whom non– sex-specific prevalence of sleep disturbance did not differ from that of the general population. Like other studies, poor quality of sleep was also associated with high BMI.33 Moreover, we found that patients with RLS were almost three times as likely as those without RLS to have poor quality of sleep. This finding strongly supports the results of previous studies regarding the role of RLS in sleep disturbance.17 Nevertheless, we found no association between some other variables, such as age, diabetic duration, smoking status, blood pressure, fasting blood sugar, HbA1C, total cholesterol, triglyceride, HDL, LDL, and poor quality of sleep. However, previous studies in this respect yielded conflicting results.34–36 These controversies may be due to the difference in studied populations and applied methods. Moreover, there is a complex interrelationship between these demographic and clinical variables and sleep-related components that requires more comprehensive investigations for better understanding.
We also explored the QoL of our participants through EQ-5D-3L questionnaire. Advanced age, high BMI values, high HbA1C level, and being female were associated with lower QoL. Moreover, RLS lowers the score of QoL by 0.08. Considering the VAS part of the questionnaire, which indicates health status on the day of interview, we found lower scores for females, smokers, and patients with longer duration of diabetes and higher BMI. Furthermore, the mobility problem and pain/ discomfort dimensions of the questionnaire were more affected by the presence of RLS. Patients with diabetes are known to have worse QoL than individuals without diabetes.37 Our study supports the results of previous studies regarding the fact that being female,38 advanced age,39 higher BMI,39 and poorer glycemic control40 all are associated with lower QoL in patients with diabetes. In addition, we did not find any negative effect of duration of diabetes and insulin use on QoL, like some other studies.41,42 However, some controversies still exist about these findings.39,43,44 These controversies may be due to the influence of several factors such as the existence of other health problems, the presence of diabetes-related complications, living status (with family or alone), social relationship, and the level of education that obviously are not considered in all studies and may confound the results. Among the factors that affect QoL of patients with diabetes, RLS is more consistently associated with low QoL.2,12,45 It can adversely affect both physical and mental aspects of QoL.10,45 A study by Kushida et al. showed that RLS is associated with a unique burden on both mental and physical aspects of QoL in the general population, and this burden is even greater than that imposed by T2DM.10 Moreover, our study showed that RLS lowers QoL of the patients with diabetes, even more than obesity and high HbA1C level. Thus, physicians should pay as much attention on the evaluation and treatment of RLS as they do on the other predictors of poor QoL in patients with diabetes.
In addition, both RLS and neuropathy can interfere with sleep.28 Furthermore, it is well established that RLS and neuropathy are independent predictors of poor QoL in patients with diabetes.45,46 Our study showed that although RLS impairs QoL of patients with diabetes, neuropathic RLS does not confer additive burden on QoL compared to RLS without neuropathy.
To our knowledge, this was the first study that addressed the prevalence and severity of RLS in Iranian people with T2DM and examined the role of RLS across the spectrum of its severity on both QoL and sleep quality of these patients. Another new aspect of our study is that we compared the effect of RLS with and without neuropathy on QoL and sleep quality of patients with diabetes. Furthermore, we excluded some other diseases such as CKD, OSA, and thyroid disorders that have the potential to affect the QoL of patients with diabetes.
However, the interpretation of the results has some limitations. First, because it is a cross-sectional study, determination of causality cannot be established. Second, evaluation of sleep was subjective and self-reported. Obviously objective measures (eg, polysomnography) would offer more valid results. And finally, there were a few confounders that were not adjusted, such as diabetic-related complications other than CKD, some other comorbidities, socioeconomic factors, and education level that may have an important role in quality of sleep and QoL.
In conclusion, the prevalence of RLS in this population of Iranians with T2DM was 19.5%. A large portion of this population (51.2%) had a moderate form of RLS whereas a small number of them (4.9%) showed a very severe form of this disorder. Poor sleep quality was associated with being male, single status, high BMI, and the presence of RLS. Patients with RLS were almost three times as likely as those without RLS to have poor quality of sleep. In addition, poor QoL in this population of patients with diabetes was associated with being female, high level of HbA1C, high BMI, and presence of RLS. RLS lowers QoL score even more than HbA1C and BMI. Moreover, RLS affects the mobility problem and pain/discomfort aspects of QoL. Furthermore, RLS impairs the QoL and sleep quality of this population of patients with diabetes independently of the presence of neuropathy.
DISCLOSURE STATEMENT
Work for this study was performed at Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences (IUMS), Tehran, Iran. The authors report no conflicts of interest. All authors have seen and approved the manuscript.
ABBREVIATIONS
- BMI
body mass index
- CKD
chronic kidney disease
- EQ-5D-3L
EuroQol five-dimension questionnaire
- FBS
fasting blood sugar
- HbA1C
glycated hemoglobin
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- PSQI
Pittsburgh Sleep Quality Index
- QoL
quality of life
- RLS
restless legs syndrome
- T2DM
type 2 diabetes mellitus
- TG
triglyceride
- VAS
visual analog scale
REFERENCES
- 1.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria-history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 3.O'Keeffe ST, Gavin K, Lavan JN. Iron status and restless legs syndrome in the elderly. Age Ageing. 1994;23(3):200–203. doi: 10.1093/ageing/23.3.200. [DOI] [PubMed] [Google Scholar]
- 4.Gigli GL, Adorati M, Dolso P, et al. Restless legs syndrome in end-stage renal disease. Sleep Med. 2004;5(3):309–315. doi: 10.1016/j.sleep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Pereira JC, Jr, Pradella-Hallinan M, Pessoa HL. Imbalance between thyroid hormones and the dopaminergic system might be central to the pathophysiology of restless legs syndrome: a hypothesis. Clinics. 2010;65(5):547–554. doi: 10.1590/S1807-59322010000500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastia JK, Bhoi SK, Kalita J, Misra UK. Neuropathy in a cohort of restless leg syndrome patients. J Clin Neurosci. 2015;22(8):1314–1318. doi: 10.1016/j.jocn.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Sarberg M, Josefsson A, Wiréhn AB, Svanborg E. Restless legs syndrome during and after pregnancy and its relation to snoring. Acta Obstet Gynecol Scand. 2012;91(7):850–855. doi: 10.1111/j.1600-0412.2012.01404.x. [DOI] [PubMed] [Google Scholar]
- 8.Merlino G, Fratticci L, Valente M, et al. Association of restless legs syndrome in type 2 diabetes: a case-control study. Sleep. 2007;30(7):866–871. doi: 10.1093/sleep/30.7.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen RP, Bharmal M, Calloway M. Prevalence and disease burden of primary restless legs syndrome: results of a general population survey in the United States. Mov Disord. 2011;26(1):114–120. doi: 10.1002/mds.23430. [DOI] [PubMed] [Google Scholar]
- 10.Kushida C, Martin M, Nikam P, et al. Burden of restless legs syndrome on health-related quality of life. Qual Life Res. 2007;16(4):617–624. doi: 10.1007/s11136-006-9142-8. [DOI] [PubMed] [Google Scholar]
- 11.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespérance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12(1):61–65. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 12.Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26(6):925–935. doi: 10.1016/s0149-2918(04)90136-1. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 15.Shaw JE, Punjabi NM, Wilding JP, et al. Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract. 2008;81(1):2–12. doi: 10.1016/j.diabres.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Lopes LA, Lins Cde M, Adeodato VG, et al. Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care. 2005;28(11):2633–2636. doi: 10.2337/diacare.28.11.2633. [DOI] [PubMed] [Google Scholar]
- 17.Cuellar NG, Ratcliffe SJ. A comparison of glycemic control, sleep, fatigue, and depression in type 2 diabetes with and without restless legs syndrome. J Clin Sleep Med. 2008;4(1):50–56. [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39(Suppl 1):S13–S22. doi: 10.2337/dc16-er09. [DOI] [PubMed] [Google Scholar]
- 19.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P) Sleep Breath. 2012;16(1):79–82. doi: 10.1007/s11325-010-0478-5. [DOI] [PubMed] [Google Scholar]
- 22.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 23.Obradovic M, Lal A, Liedgens H. Validity and responsiveness of EuroQol-5 dimension (EQ-5D) versus Short Form-6 dimension (SF-6D) questionnaire in chronic pain. Health Qual Life Outcomes. 2013;11:110. doi: 10.1186/1477-7525-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whynes DK. Correspondence between EQ-5D health state classifications and EQ VAS scores. Health Qual Life Outcomes. 2008;6:94. doi: 10.1186/1477-7525-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129(1):76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 26.Mirghani HO, Amirthalingam P, Mohammed OS. The effect of restless leg syndrome on diabetes control among type-2 diabetic patients in the Northwest region of Saudi Arabia. J Diabetol. 2016;7(3):1–6. [Google Scholar]
- 27.Earley CJ, Silber MH. Restless legs syndrome: understanding its consequences and the need for better treatment. Sleep Med. 2010;11(9):807–815. doi: 10.1016/j.sleep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Cuellar NG, Dorn JM. Peripheral diabetic neuropathy or restless legs syndrome in persons with type 2 diabetes mellitus: differentiating diagnosis in practice. J Am Assoc Nurse Pract. 2015;27(12):671–675. doi: 10.1002/2327-6924.12311. [DOI] [PubMed] [Google Scholar]
- 29.Bastia JK, Bhoi SK, Kalita J, Misra UK. Neuropathy in a cohort of restless leg syndrome patients. J Clin Neurosci. 2015;22(8):1314–1318. doi: 10.1016/j.jocn.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 30.Barone MT, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91(2):129–137. doi: 10.1016/j.diabres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Zeng J, Liu M, Wu L, et al. A systematic review and meta-analysis on influencing factors related to the quality of sleep among community population aged 60 and older, in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37(12):1670–1677. doi: 10.3760/cma.j.issn.0254-6450.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Denic-Roberts H, Costacou T, Orchard TJ. Subjective sleep disturbances and glycemic control in adults with long-standing type 1 diabetes: the Pittsburgh's Epidemiology of Diabetes Complications study. Diabetes Res Clin Pract. 2016;119:1–12. doi: 10.1016/j.diabres.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arora T, Chen MZ, Omar OM, Cooper AR, Andrews RC, Taheri S. An investigation of the associations among sleep duration and quality, body mass index and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. Ther Adv Endocrinol Metab. 2016;7(1):3–11. doi: 10.1177/2042018815616549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung HC, Yang YC, Ou HY, Wu JS, Lu FH, Chang CJ. The association between self-reported sleep quality and metabolic syndrome. PLoS One. 2013;8(1):e54304. doi: 10.1371/journal.pone.0054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu BQ, Li XM, Wang D, Yu XF. Sleep quality and its impact on glycaemic control in patients with type 2 diabetes mellitus. Int J Nurs Sci. 2014;1(3):260–265. [Google Scholar]
- 36.Wan Mahmood WA, Draman Yusoff MS, Behan LA, et al. Association between sleep disruption and levels of lipids in caucasians with type 2 diabetes. Int J Endocrinol. 2013:1–7. doi: 10.1155/2013/341506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manuel DG, Schultz SE. Health-related quality of life and health-adjusted life expectancy of people with diabetes in Ontario, Canada, 1996-1997. Diabetes Care. 2004;27(2):407–414. doi: 10.2337/diacare.27.2.407. [DOI] [PubMed] [Google Scholar]
- 38.Cho YW, Kim DH, Allen RP, Earley CJ. Assessing health-related quality of life in patients with restless legs syndrome in Korea: comparison with other chronic medical diseases. Sleep Med. 2012;13(9):1158–1163. doi: 10.1016/j.sleep.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Kalda R, Rätsep A, Lember M. Predictors of quality of life of patients with type 2 diabetes. Patient Prefer Adherence. 2008;2:21–26. [PMC free article] [PubMed] [Google Scholar]
- 40.Kamarul Imran M, Ismail AA, Naing L, Wan Mohamad WB. Type 2 diabetes mellitus patients with poor glycaemic control have lower quality of life scores as measured by the Short Form-36. Singapore Med J. 2010;51(2):157–162. [PubMed] [Google Scholar]
- 41.Aalto AM, Uutela A, Aro AR. Health related quality of life among insulin-dependent diabetics: disease-related and psychosocial correlates. Patient Educ Couns. 1997;30(3):215–225. doi: 10.1016/s0738-3991(96)00963-9. [DOI] [PubMed] [Google Scholar]
- 42.Mayou R, Bryant B, Turner R. Quality of life in non-insulin-dependent diabetes and a comparison with insulin-dependent diabetes. J Psychosom Res. 1990;34(1):1–11. doi: 10.1016/0022-3999(90)90002-l. [DOI] [PubMed] [Google Scholar]
- 43.Weinberger M, Kirkman MS, Samsa GP, et al. The relationship between glycemic control and health-related quality of life in patients with non-insulin-dependent diabetes mellitus. Med Care. 1994;32(12):1173–1181. doi: 10.1097/00005650-199412000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Wubben DP, Porterfield D. Health-related quality of life among North Carolina adults with diabetes mellitus. N C Med J. 2005;66(3):179–185. [PubMed] [Google Scholar]
- 45.Merlino G, Valente M, Serafini A, et al. Effects of restless legs syndrome on quality of life and psychological status in patients with type 2 diabetes. Diabetes Educ. 2010;36(1):79–87. doi: 10.1177/0145721709351252. [DOI] [PubMed] [Google Scholar]
- 46.Benbow SJ, Wallymahmed ME, MacFarlane IA. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91(11):733–737. doi: 10.1093/qjmed/91.11.733. [DOI] [PubMed] [Google Scholar]


