Abstract
Study Objectives:
Both asthma and obstructive sleep apnea (OSA) are strongly associated with poor sleep. Asthma and OSA also have several features in common, including airway obstruction, systemic inflammation, and an association with obesity. The aim was to analyze the effect of asthma, OSA, and the combination of asthma and OSA on objectively measured sleep quality and systemic inflammation.
Methods:
Sleep and health in women is an ongoing community-based study in Uppsala, Sweden. Three hundred eighty-four women ages 20 to 70 years underwent overnight polysomnography and completed questionnaires on airway diseases and sleep complaints. C-reactive protein (CRP), interleukin 6 (IL-6), and tumor necrosis factor α were analyzed.
Results:
The group with both asthma and OSA had higher CRP, higher IL-6, a longer sleeping time in stage N1 sleep and stage N2 sleep, and less time in stage R sleep than the control group with no asthma or OSA. The group with both asthma and OSA had lower mean oxygen saturation (93.4% versus 94.7%, P = .04) than the group with OSA alone. The results were consistent after adjusting for age, body mass index, and smoking status. Asthma was independently associated with lower oxygen saturation, whereas OSA was not.
Conclusions:
Our data indicate that coexisting asthma and OSA are associated with poorer sleep quality and more profound nocturnal hypoxemia than either of the conditions alone. The results are similar to earlier findings related to OSA and chronic obstructive pulmonary disease, but they have not previously been described for asthma.
Citation:
Sundbom F, Janson C, Malinovschi A, Lindberg E. Effects of coexisting asthma and obstructive sleep apnea on sleep architecture, oxygen saturation, and systemic inflammation in women. J Clin Sleep Med. 2018;14(2):253–259.
Keywords: asthma, inflammation, OSA, polysomnography
BRIEF SUMMARY
Current Knowledge/Study Rationale: Both asthma and obstructive sleep apnea (OSA) are strongly associated with poor sleep. The aim was to analyze the effect of asthma, OSA, and the combination of asthma and OSA on objectively measured sleep quality and systemic inflammation.
Study Impact: Coexisting asthma and OSA is associated with poorer sleep quality and more profound nocturnal hypoxemia than either of the conditions alone. Asthma was independently associated with lower oxygen saturation, whereas OSA was not.
INTRODUCTION
Asthma is strongly associated with poor sleep quality.1 This can be explained in part as a result of nocturnal asthma symptoms and frequent coexisting rhinitis,2,3 but these factors may not fully explain the increased prevalence of insomnia in asthma. Associations between asthma and obstructive sleep apnea (OSA) have been described in recent years and several studies have shown a considerably higher prevalence of OSA in individuals with compared with individuals without asthma.4,5 Although OSA and asthma are conditions with a different pathophysiological background, they have several features in common. First, obesity, which is strongly associated with OSA, has also been identified as a major risk factor for asthma,6 in particular, nonallergic, difficult-to-treat asthma.7 Second, both asthma and OSA are associated with local inflammation in the upper airway, nasal obstruction, and snoring.8 Third, there are similarities in the systemic inflammatory patterns in OSA and certain asthma phenotypes. For example, inflammatory markers, such as C-reactive protein (CRP), tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6), are elevated in nonallergic, neutrophilic asthma phenotypes and also in obesity and OSA.9–13
An overlap syndrome between chronic obstructive pulmonary disease (COPD) and OSA has previously been described14 and it is known that coexisting COPD and OSA result in more profound nocturnal hypoxemia than either of the conditions alone.15 Further, treatment with continuous positive airway pressure (CPAP) has been shown to reduce airway reactivity among individuals with asthma without OSA16 and to improve asthma-related quality of life among individuals with asthma and OSA.17 However, corresponding community-based polysomnography studies of asthma and OSA have not been performed to the same extent and our knowledge of objectively measured sleep in patients with asthma is limited.
The aim of the current study was to analyze the effect of asthma and OSA and the combination of asthma and OSA on objectively measured sleep quality and systemic inflammation in a community-based sample of women.
METHODS
Study Population
Sleep and health in women is an ongoing, population-based study in Uppsala, Sweden. From a total study population of 7,051 women who had responded to a postal questionnaire, 400 participants ages 20 to 70 years underwent full-night polysomnography in their homes.18,19 Subjects were randomly selected from the study population, with oversampling of habitual snorers (230 snorers, 170 nonsnorers) to obtain a wider range of sleep-disordered breathing. Preceding the polysomnography, all participants completed new questionnaires that included questions on airway diseases, allergies, sleep disturbances, comorbidity in other diseases, and current medication. In the morning after the polysomnography, fasting blood samples were obtained. Waist circumference, weight, and height were measured and body mass index (BMI) was calculated. Complete data were available for 384 women. Informed consent was obtained from all participants and the study was approved by the ethics committee at the medical faculty at Uppsala University (Dnr 01-238).
Definitions
Polysomnography was performed and scored and the apneahypopnea index (AHI) was defined as previously described in detail.18 The oxygen desaturation index (ODI) was defined as the mean number of desaturations of ≥ 4% per hour of sleep. The percentage of total sleep time spent with oxyhemoglobin saturation below 90% (TST90) was calculated. OSA was considered relevant at an AHI of ≥ 15 events/h.20
Asthma was defined as a positive answer to either the question “Have you had an attack of asthma in the last 12 months?” and/or the question “Are you currently taking any medicine, including inhalers, aerosols or tablets, for asthma?”1
Depending on the presence of asthma and/or OSA, the population was divided into four groups; (1) the control group that had no asthma or OSA (n = 224), (2) subjects with asthma but no OSA (n = 36), (3) subjects with OSA but not asthma (n = 109), and (4) subjects with both asthma and OSA (n = 15) (Figure 1).
Figure 1. The study population.
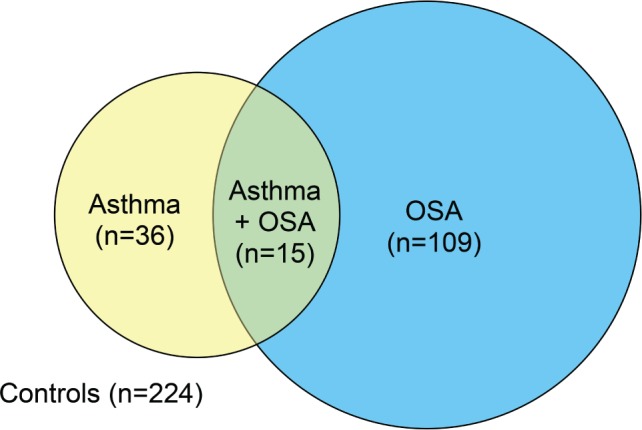
OSA = obstructive sleep apnea.
Allergic rhinitis was defined as a positive answer to the question “Do you suffer from hay fever or any other nasal allergy?”1
Daytime sleepiness was evaluated with the Epworth Sleepiness Scale and a score of ≥ 10 was considered significant.21
Subjective insomnia problems (difficulty initiating sleep [DIS], difficulty maintaining sleep [DMS] and early morning awakenings [EMA]) were evaluated with questions asking “How severe are your problems with…” The answers were given using a five-point scale and were considered positive if they were score 4 “severe” or 5 “very severe.”2
Comorbidity in hypertension or diabetes was defined as ongoing pharmacological treatment or regular check-ups due to either condition.1
Smoking habits were assessed using six questions.22 Based on the responses, the women were categorized into current smokers or ex-smokers and pack-years were calculated.
Chemical Analysis
Plasma C-reactive protein (CRP), interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) were assayed at the Department of Clinical Chemistry, Uppsala University.20 The minimum detectable levels were < 0.2 mg/L for CRP and < 0.5 ng/L for IL-6. In the statistical analyses, a value of 0.1 mg/L was used for all CRP results of < 0.2 mg/L and a value of 0.25 ng/L was used for all IL-6 results of < 0.5 ng/L.20
Statistics
Univariate analyses of categorical variables were performed using the χ2 test. Arithmetic mean values were calculated for normally distributed variables and geometric mean values were calculated for non-normally distributed variables (AHI, ODI, and TST90, CRP, IL-6, and TNF-α). χ2 test and analysis of variance with Bonferroni correction were calculated for P values. Bonferroni correction was made for all pairwise comparisons. Separate multiple linear regression analyses with objective sleep data and inflammatory markers as dependent variables were used to analyze the associations with asthma and/or OSA in models adjusted for age, BMI, and smoking status. For the regression analyses, non-normally distributed variables were log-transformed using the base-10 log.19,20 All statistical analyses were performed using STATA IC 12 (Stata Corp, College Station, Texas, United States).
RESULTS
The characteristics of the participants are presented in Table 1. In both groups with asthma, the prevalence of rhinitis and chronic bronchitis was significantly higher than in the control group. Subjects belonging to either of the groups with OSA were older and had a higher prevalence of hypertension than subjects without OSA. Participants with both asthma and OSA had a higher BMI than those with asthma (P < .001) or OSA alone (P = .001). Women with asthma alone had the highest prevalence of DMS and EMA, but there were no significant differences in self-reported sleep impairment or daytime sleepiness between any of the groups.
Table 1.
Characteristics and self-reported sleep complaints of the subgroups.
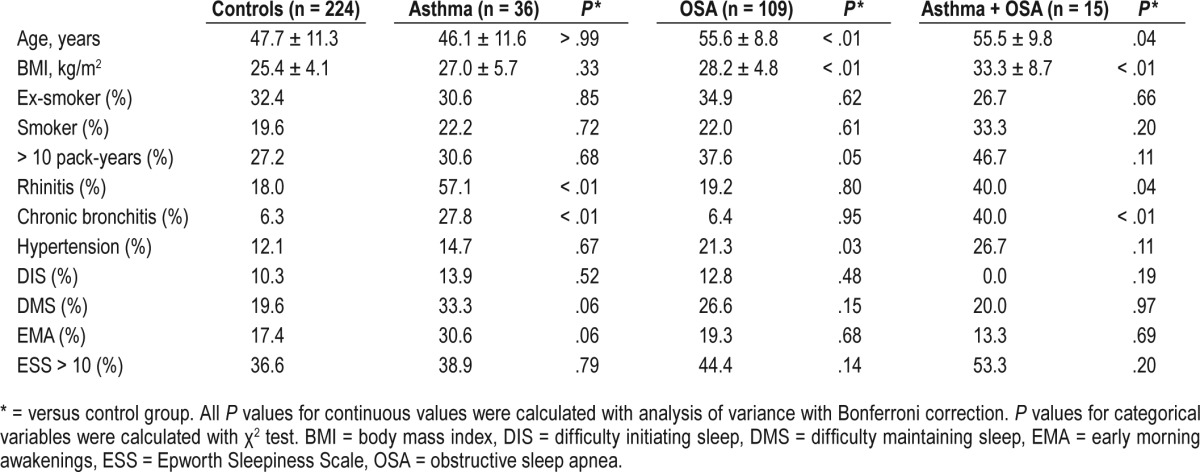
Of the women with asthma without OSA, 56% (n = 20) had ongoing medication with inhaled corticosteroids and 33% (n = 12) were regularly using long-acting beta-adrenoceptor agonists. In the group with both asthma and OSA, 73% (n = 11) used inhaled corticosteroids and 53% (n = 8) used long-acting beta-adrenoceptor agonists. One subject in each group used anticholinergic bronchodilators and one subject with only asthma used montelukast.
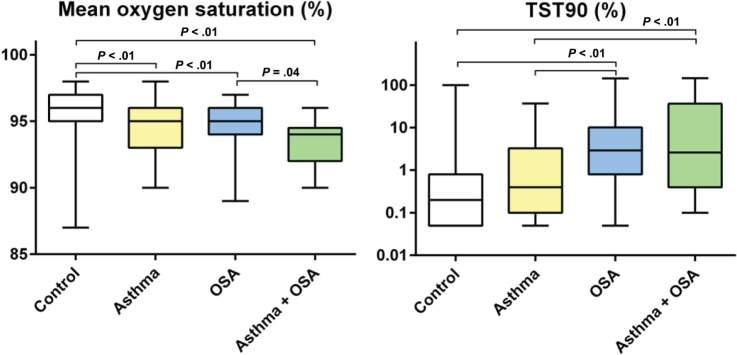
As expected, the AHI and ODI were higher in the groups with OSA (Table 2). There was no difference in the AHI or ODI between subjects with only OSA and those with asthma and OSA (P > .9). The mean oxygen saturation during sleep was highest in the control group and belonging to any other group was associated with a lower mean saturation (Figure 2). The group with both asthma and OSA had a lower mean saturation than the group with only OSA (P = .04), whereas the difference compared with the group with only asthma did not reach significance (P = .054). Both groups with OSA had longer sleeping times with oxygen saturation below 90% than the control group and the group with only asthma (P < .01), but the difference between the group with the combination of asthma and OSA and the group with only OSA did not reach statistical significance (P = .2).
Table 2.
Polysomnography data and inflammatory markers.
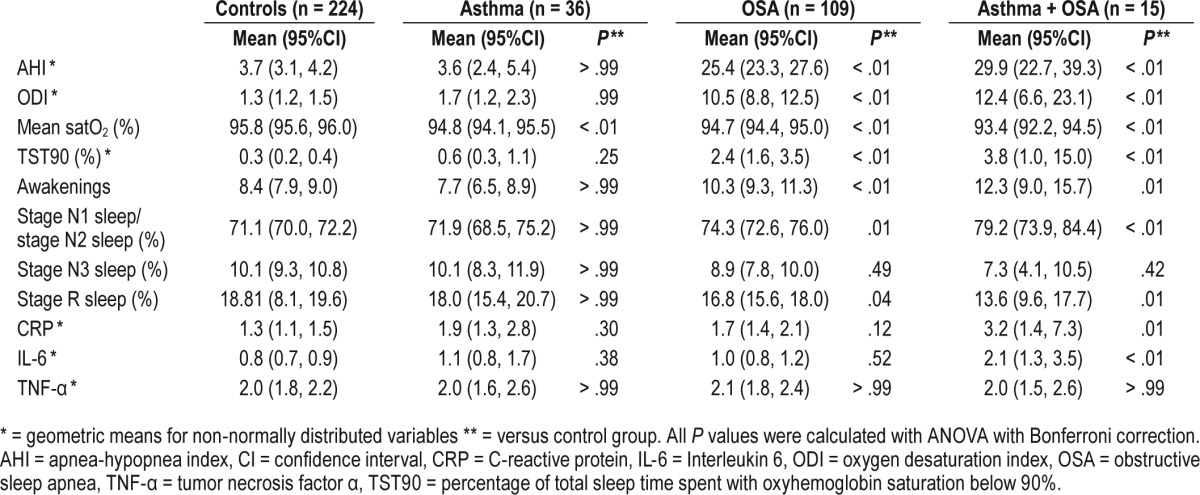
Figure 2. Mean oxygen saturation and TST90.
Analysis of variance with Bonferroni correction was calculated for P values and all significant P values are presented in the figure. OSA = obstructive sleep apnea, TST90 = percentage of total sleep time spent with oxyhemoglobin saturation below 90%.
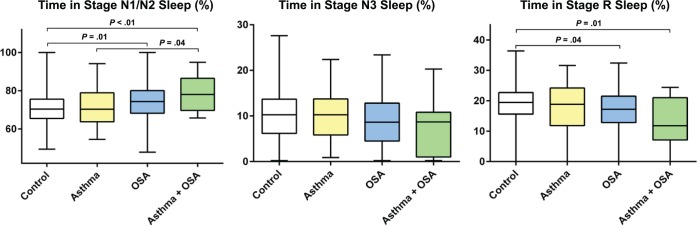
Women with both asthma and OSA had more awakenings (P = .01), a longer sleeping time in stage N1 sleep and stage N2 sleep (P = .004) and less time in stage R sleep (P = .01) than the control group (Table 2, Figure 3). The larger number of awakenings was also significant compared with the groups with only asthma or OSA (P = .03 and P = .008 respectively) and the sleeping time in stage N1 sleep and stage N2 sleep was significantly longer than in the group with only asthma (P = .04).
Figure 3. Sleep architecture.
Analysis of variance with Bonferroni correction was calculated for P values and all significant P values are presented in the figure. OSA = obstructive sleep apnea.
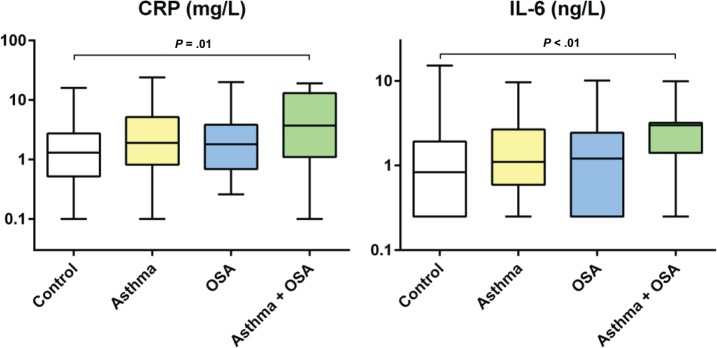
CRP and IL-6 were higher in the group with asthma and OSA than in the control group (CRP: P = .01, IL-6: P = .006) (Table 2, Figure 4).
Figure 4. Inflammation markers.
Analysis of variance with Bonferroni correction was calculated for P values and all significant P values are presented in the figure. CRP = C-reactive protein, IL-6 = interleukin 6, OSA = obstructive sleep apnea.
Prior to adjustments for multiple comparisons, there were also significant associations between asthma and longer sleeping time with oxygen saturation below 90% (P = .03), asthma and higher CRP (P = .046), and OSA and higher CRP (P = .02), in comparison to the control group.
The effect of asthma, OSA, and the combination of asthma and OSA on sleep architecture and systemic inflammation was further analyzed in a multiple linear regression model (Table 3). The combination of asthma and OSA was associated with lower mean oxygen saturation during sleep (P = .02). Moreover, asthma without OSA was associated with lower mean oxygen saturation (P = .001), whereas OSA without asthma was not (P = .09). Both OSA with and without asthma were associated with higher ODI, longer total sleeping time with saturation below 90%, and more frequent awakenings. The combination of asthma and OSA was also associated with longer sleep time in stage N1 sleep and stage N2 sleep and a tendency toward less sleep time in stage N3 sleep and less stage R sleep. The higher IL-6 levels in the group with both asthma and OSA were consistent in the adjusted model (P = .02), whereas the higher CRP did not reach statistical significance (P = .19).
Table 3.
Associations between polysomnography data, inflammation markers, and the different asthma and OSA groups.
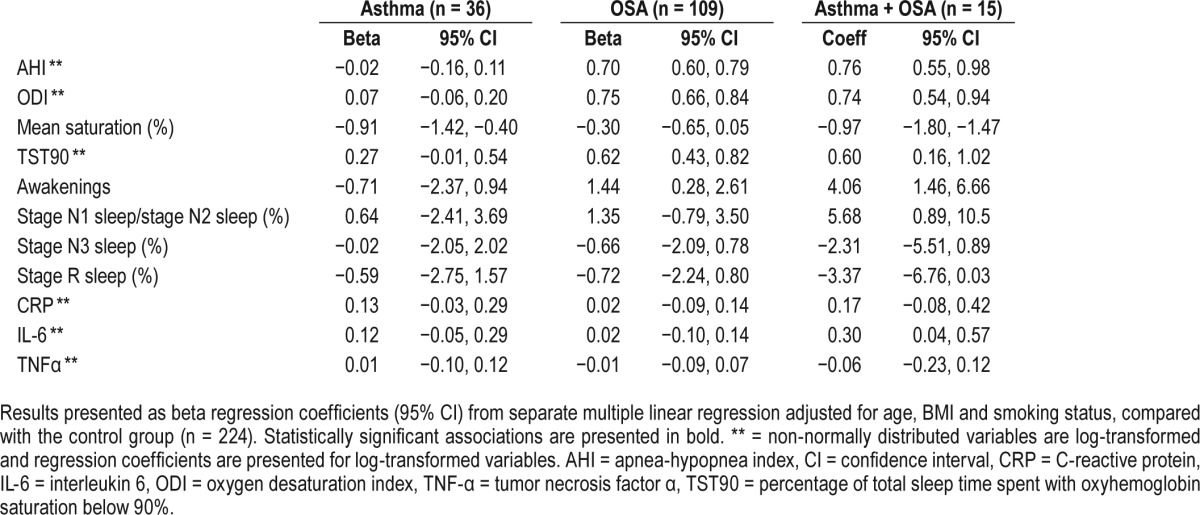
Because CRP and IL-6 are associated both with the severity of OSA and with central obesity,11 the multiple linear regression analysis was recalculated using waist circumference instead of BMI. In this analysis, the combination of asthma and OSA was significantly associated with both higher CRP (P = .008) and higher IL-6 (P = .001), and there was also an independent association between asthma and higher CRP (P = .04).
DISCUSSION
Asthma and OSA had a significant effect on nocturnal oxygen saturation and several objectively measured sleep variables, respectively. However, the effect on sleep quality was most pronounced when both asthma and OSA were present. Furthermore, the combination of asthma and OSA was associated with lower mean oxygen saturation, a longer period of sleep with oxygen saturation below 90%, higher IL-6, and impaired sleep architecture. It was thus demonstrated that asthma and OSA have additive adverse effects, in agreement with the previously described COPD-OSA overlap syndrome.15,23
The finding of a lower mean oxygen saturation, longer periods of time in stage N1 sleep and stage N2 sleep, and less stage R sleep in the group with both asthma and OSA than in the group with asthma alone is in accordance with previous data on OSA and difficult-to-treat asthma.5 Lower oxygen saturation in patients with asthma was described in a small study in 1982,24 but polysomnography studies of asthma using modern equipment and definitions are sparse and impaired oxygen saturation during sleep has previously only been described for severe asthma.4 The finding of lower mean oxygen saturation with asthma was also consistent when only never-smokers were included (data not shown). In contrast to OSA, isolated asthma did not have any effect on the period of sleep with oxygen saturation below 90%. This implies that asthma in itself is independently associated with mild nocturnal hypoxemia, which is aggravated in the presence of concomitant OSA. Subjects with OSA were older than those without OSA. However, there was no difference in age between the group with only OSA and the group with both asthma and OSA. The results were also consistent after adjusting for age. Hence, the changes in sleep pattern seen with increasing age cannot explain the polysomnography results.
The self-reported sleep complaints did not correspond to the objectively measured sleep variables. This is in accordance with previous studies, showing a higher prevalence of self-reported insomnia with asthma but no significant differences in sleep efficiency or sleep architecture in polysomnography.25 Nor did self-reported sleep complaints correspond to the level of hypoxemia, which is consistent with previous findings on COPD.26
The underlying mechanisms of the effects seen with coexisting asthma and OSA are complex and both physiological and inflammatory pathways are likely to be involved. Busk et al. showed that airway reactivity, assessed with bronchial challenge, could be reduced by applying chronic mechanical strain with CPAP treatment in individuals with asthma without OSA.16 CPAP treatment has also been shown to improve asthma control among individuals with asthma and OSA and nocturnal asthma symptoms despite optimal medical treatment.27 It has previously been reported that deep breathing has a bronchoprotective effect, which is reduced in asthma,28 and that lung volumes during sleep are reduced by obesity. Accordingly, obese individuals with asthma have increased upper and lower airway resistance and it has been demonstrated that upper airway resistance can be reduced by increasing lung volumes via bilevel positive airway pressure.29 Taken together, these findings may explain the impaired ventilation and hypoxemia seen with asthma and also illustrate some of the physiological connections between asthma and OSA. The overlap syndrome of asthma and OSA is probably common and a questionnaire-based study by Teodorescu et al. showed a significant association between high OSA risk and poor asthma control despite optimal therapy.30
The levels of CRP and IL-6 were highest in the group with both asthma and OSA and the association remained significant for IL-6 after adjusting for age, BMI, and smoking status. In addition, the prevalence of allergic rhinitis was lower among individuals with asthma and OSA than among those with asthma without OSA. This indicates that the asthma pheno-type seen with OSA is nonallergic and neutrophilic rather than allergic. Another explanation could be the overrepresentation of the “obese asthma phenotype,” which is characterized by neutrophilic inflammation and is more common in women than in men.31,32 However, defining asthma is always complicated in epidemiology, because asthma is a heterogeneous condition. This is also the main limitation of the study. Without spirometry data and bronchial challenges, it is difficult to distinguish asthma from COPD or obesity-related wheeze and distinguishing between asthma phenotypes is accordingly even more complicated. The asthma definition used here is common and it is based on having an asthma diagnosis and current medication.1,2 The results were also consistent after adjusting for smoking and the use of anticholinergic bronchodilators was low, indicating a low prevalence of misclassified patients with COPD. However, it is still possible that patients who are obese with nonasthmatic wheezing, for example, may be classified as having asthma. The misclassification of wheezing subjects as having asthma would not, however, change the outcome of the study but only reduce its statistical power. For this reason, the results can be considered reliable.
The study benefits from a large community-based sample, standardized polysomnography procedures, blood samples, and well-validated questionnaires. However, the fact that the study exclusively comprised females may be regarded as a limitation, because the results cannot be directly generalized to apply to men. In the selection of participants for polysomnography there was an oversampling of habitual snorers to yield a sample with adequate variance of the AHI. The prevalence of asthma, OSA, and the combination of asthma and OSA thereby does not reflect the prevalence in the population but would not interfere with the results on differences between groups or associations.
Our findings on asthma, OSA, and objectively measured sleep are new and could potentially have high clinical relevance. First, it was demonstrated that asthma is associated with lower nocturnal oxygen saturation and this association was also consistent in adjusted multiple regression analyses. Second, the severe additive adverse effects of asthma and OSA were highlighted. This indicates that it may be important to pay attention to asthma symptoms in clinical evaluations of patients with OSA and, conversely, pay attention to OSA symptoms in patients with asthma.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Fredrik Sundbom has received grants from Bror Hjerpstedt Foundation and the Uppsala County Association against Heart and Lung Diseases. Dr Janson has not received any financial support for this study. Dr Malinovschi has not received any financial support for this study. Dr Lindberg has not received any financial support for this study. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure
- CRP
C-reactive protein
- DIS
difficulty initiating sleep
- DMS
difficulty maintaining sleep
- EMA
early morning awakenings
- ESS
Epworth Sleepiness Scale
- IL-6
interleukin 6
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- TNF-α
tumor necrosis factor α
- TST90
percentage of total sleep time spent with oxyhemoglobin saturation below 90%
REFERENCES
- 1.Sundbom F, Lindberg E, Bjerg A, et al. Asthma symptoms and nasal congestion as independent risk factors for insomnia in a general population: results from the GA(2)LEN survey. Allergy. 2013;68(2):213–219. doi: 10.1111/all.12079. [DOI] [PubMed] [Google Scholar]
- 2.Bengtsson C, Jonsson L, Holmström M, Svensson M, Theorell-Haglöw J, Lindberg E. Impact of nasal obstruction on sleep quality: a community-based study of women. Eur Arch Otorhinolaryngol. 2015;272(1):97–103. doi: 10.1007/s00405-014-3067-6. [DOI] [PubMed] [Google Scholar]
- 3.Janson C, De Backer W, Gislason T, et al. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 1996;9(10):2132–2138. doi: 10.1183/09031936.96.09102132. [DOI] [PubMed] [Google Scholar]
- 4.Julien JY, Martin JG, Ernst P, et al. Prevalence of obstructive sleep apneahypopnea in severe versus moderate asthma. J Allergy Clin Immunol. 2009;124(2):371–376. doi: 10.1016/j.jaci.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Guven SF, Dursun AB, Ciftci B, Erkekol FO, Kurt OK. The prevalence of obstructive sleep apnea in patients with difficult-to-treat asthma. Asian Pac J Allergy Immunol. 2014;32(2):153–159. doi: 10.12932/AP0360.32.2.2013. [DOI] [PubMed] [Google Scholar]
- 6.Dixon AE, Holguin F, Sood A, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7(5):325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 7.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpagnano GE, Lacedonia D, Foschino-Barbaro MP. Non-invasive study of airways inflammation in sleep apnea patients. Sleep Med Rev. 2011;15(5):317–326. doi: 10.1016/j.smrv.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. J Investig Med. 2014;62(4):665–675. doi: 10.2310/JIM.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 10.Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8(9):1281–1290. doi: 10.7150/ijbs.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 12.Olafsdottir IS, Gislason T, Thjodleifsson B, et al. C reactive protein levels are increased in non-allergic but not allergic asthma: a multicentre epidemiological study. Thorax. 2005;60(6):451–454. doi: 10.1136/thx.2004.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu WT, Tsai SS, Shih TS, et al. The impact of obstructive sleep apnea on high-sensitivity C-reactive protein in subjects with or without metabolic syndrome. Sleep Breath. 2015;19(4):1449–1457. doi: 10.1007/s11325-015-1166-2. [DOI] [PubMed] [Google Scholar]
- 14.Lee R, McNicholas WT. Obstructive sleep apnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2011;17(2):79–83. doi: 10.1097/MCP.0b013e32834317bb. [DOI] [PubMed] [Google Scholar]
- 15.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151(1):82–86. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 16.Busk M, Busk N, Puntenney P, et al. Use of continuous positive airway pressure reduces airway reactivity in adults with asthma. Eur Respir J. 2013;41(2):317–322. doi: 10.1183/09031936.00059712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafond C, Sériès F, Lemière C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J. 2007;29(2):307–311. doi: 10.1183/09031936.00059706. [DOI] [PubMed] [Google Scholar]
- 18.Franklin KA, Sahlin C, Stenlund H, Lindberg E. Sleep apnoea is a common occurrence in females. Eur Respir J. 2013;41(3):610–615. doi: 10.1183/09031936.00212711. [DOI] [PubMed] [Google Scholar]
- 19.Ljunggren M, Lindahl B, Theorell-Haglöw J, Lindberg E. Association between obstructive sleep apnea and elevated levels of type B natriuretic peptide in a community-based sample of women. Sleep. 2012;35(11):1521–1527. doi: 10.5665/sleep.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svensson M, Venge P, Janson C, Lindberg E. Relationship between sleep-disordered breathing and markers of systemic inflammation in women from the general population. J Sleep Res. 2012;21(2):147–154. doi: 10.1111/j.1365-2869.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Svensson M, Lindberg E, Naessen T, Janson C. Risk factors associated with snoring in women with special emphasis on body mass index: a population-based study. Chest. 2006;129(4):933–941. doi: 10.1378/chest.129.4.933. [DOI] [PubMed] [Google Scholar]
- 23.Resta O, Foschino Barbaro MP, Brindicci C, Nocerino MC, Caratozzolo G, Carbonara M. Hypercapnia in overlap syndrome: possible determinant factors. Sleep Breath. 2002;6(1):11–18. doi: 10.1007/s11325-002-0011-6. [DOI] [PubMed] [Google Scholar]
- 24.Catterall JR, Douglas NJ, Calverley PM, et al. Irregular breathing and hypoxaemia during sleep in chronic stable asthma. Lancet. 1982;1(8267):301–304. doi: 10.1016/s0140-6736(82)91567-7. [DOI] [PubMed] [Google Scholar]
- 25.Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34(7):859–867. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan S, Doherty LS, Rock C, Nolan GM, McNicholas WT. Effects of salmeterol on sleeping oxygen saturation in chronic obstructive pulmonary disease. Respiration. 2010;79(6):475–481. doi: 10.1159/000235619. [DOI] [PubMed] [Google Scholar]
- 27.Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 2005;99(5):529–534. doi: 10.1016/j.rmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Kapsali T, Permutt S, Laube B, Scichilone N, Togias A. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol (1985) 2000;89(2):711–720. doi: 10.1152/jappl.2000.89.2.711. [DOI] [PubMed] [Google Scholar]
- 29.Campana LM, Malhotra A, Suki B, et al. The effect of lung stretch during sleep on airway mechanics in overweight and obese asthma. Respir Physiol Neurobiol. 2013;185(2):304–312. doi: 10.1016/j.resp.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teodorescu M, Polomis DA, Hall SV, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 2010;138(3):543–550. doi: 10.1378/chest.09-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott HA, Gibson PG, Garg ML, Wood LG. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38(3):594–602. doi: 10.1183/09031936.00139810. [DOI] [PubMed] [Google Scholar]
- 32.Hancox RJ, Milne BJ, Poulton R, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med. 2005;171(5):440–445. doi: 10.1164/rccm.200405-623OC. [DOI] [PubMed] [Google Scholar]