Abstract
This is a case report describing a 53-year-old woman with Charcot-Marie-Tooth disease, obstructive sleep apnea, and a 6-year history of numbness in bilateral upper extremities, feet, and in the trunk that resolved with initiation of continuous positive airway pressure for her obstructive sleep apnea.
Citation:
Polat P, Pham H, Li J, Bagai K. Improvement of neuropathy symptoms with treatment of obstructive sleep apnea in a patient with Charcot-Marie-Tooth disease. J Clin Sleep Med. 2018;14(2):289–291.
Keywords: Charcot-Marie-Tooth, CMT, neuropathy, obstructive sleep apnea
INTRODUCTION
We describe the case of a 53-year-old woman with Charcot-Marie-Tooth disease (CMT), obstructive sleep apnea (OSA), and a 6-year history of numbness in bilateral upper extremities, feet, and in the trunk that resolved with initiation of continuous positive airway pressure (CPAP) for her OSA.
REPORT OF CASE
A 53-year-old woman with CMT type 1A (CMT1A) was referred to our sleep clinic with several years' history of difficulty with sleep onset and sleep maintenance, as well as numbness and cramps in her hands and legs that were worse at night.
The patient had a history of foot deformities (high arches and hammer toes) and clumsiness since childhood, with progressive weakness and numbness in both upper and lower extremities. She received a diagnosis of CMT1A 10 years prior after genetic testing revealed a duplication of the peripheral myelin protein (PMP-22) gene. The patient's medical history was otherwise unremarkable.
She experienced numbness in her hands and arms daily for the past 6 years that was worse at night, resulting in nighttime and early morning awakenings. She also had intermittent numbness and cramps in her legs and trunk that occurred only at night causing her to awaken, especially in the early morning hours. A recent nerve conduction study to evaluate for carpal tunnel syndrome was inconclusive. She wore hand splints at night that provided occasional relief for her upper extremity symptoms. The numbness in her hands and legs sometimes improved with activity. In addition, she had symptoms of sharp pains in her feet for which she was taking pregabalin 150 mg daily for the past 3 years with some alleviation of pain, but no improvement of numbness. Her other medications included an over-the-counter estrogen supplement and a daily multivitamin; she was not taking any other sleep medications. Given her symptoms of frequent night awakenings, she was referred to the sleep clinic for further evaluation.
During her sleep clinic evaluation, in addition to the aforementioned symptoms, she reported restlessness in her legs that was worse at night that improved with movement and massage. Her husband reported occasional snoring when sleeping supine. She had a consistent sleep-wake schedule and followed a consistent bedtime routine with adequate sleep hygiene. She did not complain of symptoms of anxiety or depression. She reported non-restorative sleep with fatigue and excessive daytime sleepiness. However, despite complaints of sleepiness, her Epworth Sleepiness Scale score was 2. Her physical examination was remarkable for a body mass index of 34.8 kg/m2 and crowded oropharynx with a Freidman palate score of 4, without tonsillar hypertrophy. Her baseline neurological examination was remarkable for decreased sensation to light touch and pinprick below the knee bilaterally, decreased vibration sensation in bilateral toes, and decreased pinprick sensation over the medial aspect of the right hand; absent deep tendon reflexes throughout; and, slightly wide based gait.
The differential diagnosis for chronic insomnia considered in this patient included:
Insomnia secondary to neuropathy symptoms
Sleep-related breathing disorder
Restless legs symptoms, with possible periodic leg movements of sleep.
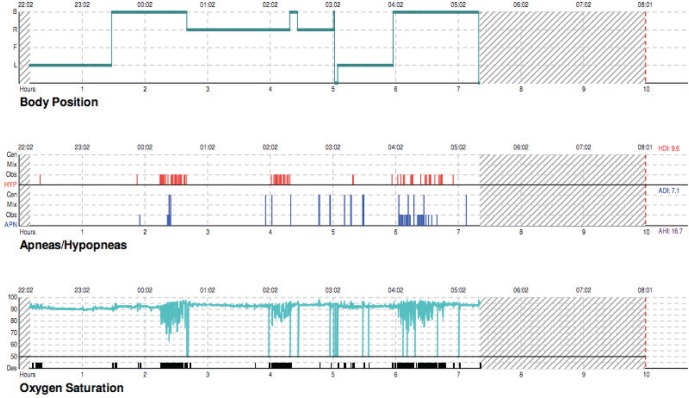
Evaluation with an in-laboratory overnight polysomnogram was discussed with patient; however, the patient preferred to have a portable home overnight polysomnogram. A portable overnight polysomnogram revealed OSA with an apnea-hypopnea index (AHI) of 16.5 events/h and a minimum oxygen desaturation to 63% that was associated with a respiratory event. The mean oxygen saturation during the baseline study was 90%. The duration with SaO2 levels less than 88% was 32 minutes; however, each of these was associated with obstructive respiratory events resulting in a sawtooth pattern of desaturations as seen in OSA (see Figure 1). Because this study was conducted as a portable study, further information regarding non-rapid eye movement and rapid eye movement AHI was not available. Figure 1 shows the sawtooth pattern of desaturations associated with obstructive apneas that were not suggestive of a hypoventilation pattern where a more sustained hypoxemia (in the absence of respiratory events) would be seen.
Figure 1. Baseline portable sleep study hypnogram.
Top panel reflects body position; middle panel reflects obstructive hypopneas in red, obstructive apneas in blue small bars, and central apneas in blue long bars; bottom panel reflects oxygen saturations. Significant oxygen desaturations were noted in association with the respiratory events.
A subsequent in-laboratory CPAP titration was performed that showed improvement in her OSA and associated oxygen desaturations as the CPAP settings were increased. She reported improved sleep with CPAP. The residual obstructive AHI was 1.6 events/h at the final CPAP pressure of 8 cm H2O with improvement of oxygen desaturation to a minimum of 91%. A baseline ferritin level was checked for her restless legs syndrome symptoms and was 28 (normal range, 15–204). The patient was recommended to start iron supplementation with vitamin C as ferritin levels of less than 50 are considered low in patients with restless legs syndrome. However, she did not start iron supplementation and continued taking the multivitamin tablet that she had been taking for several years, which contained 8 mg of elemental iron.
Approximately 8 weeks after initiating treatment of her OSA with CPAP, she had significant improvement of fatigue, daytime sleepiness, and night awakenings. Her Epworth Sleepiness Scale score remained unchanged at 2. In addition, she reported complete resolution of numbness and cramps in her legs, and she no longer had awakenings related to these symptoms. The nighttime numbness in her hands also improved significantly. Furthermore, she reported decreased numbness in her hands during the day. At her initial evaluation, she had complained of symptoms of restlessness in her legs, which she felt had improved since she started using CPAP, despite no changes in her medications.
At her 3-year follow-up appointment, the patient remained adherent with CPAP with an adherence rate of 97% with average usage of 6 hours, 57 minutes and no significant residual events or mask leaks. Her neurological examination was unchanged. She reported persistent improvement of symptoms of sleep maintenance insomnia and daytime sleepiness, continued remission of symptoms of numbness/cramps in her legs, and minimal numbness in her hands suggestive of a beneficial effect of CPAP on her symptoms. A repeat assessment of ferritin level showed that it was still low at 41. Thus, improvement of her symptoms was not attributed to an improved ferritin level.
DISCUSSION
CMT1A is a demyelinating sensory and motor neuropathy inherited in an autosomal dominant manner. Symptoms are characterized by distal muscle weakness and atrophy, sensory loss, and slow nerve conduction velocities that typically begin in adolescence or early adulthood and are slowly progressive. There is great variability in the severity of symptoms among individuals and even among family members with the disease.
Nerve conduction velocities show progressive slowing over the first 2 to 6 years of life, then stable levels remaining throughout adulthood.1 A prospective, longitudinal study of adult patients with CMT1A and age-matched controls demonstrated a similar decline in strength and compound muscle action potentials on nerve conduction study. The study findings suggest that the decline observed in adults affected with CMT1A may reflect a process of normal aging rather than progressive disease process.2
Previous studies have shown an increased prevalence of sleep apnea in patients with CMT1A.3 A more recent study by Boentert et al. showed a higher prevalence of multiple sleep disorders including OSA, restless legs syndrome, and periodic limb movements in sleep in patients with CMT type 1.4 Patients with CMT1A had both a higher prevalence of OSA and more severe OSA compared to patients with CMT type 1B and CMT type X. A study by Dziewas et al. evaluating sleep apnea in patients with CMT found no correlation between sleep apnea and body mass index or age.3 Based on these findings, a possible association between CMT and risk for developing OSA is likely. Although the mechanism of the increased risk of OSA in patients with CMT is not entirely clear, both studies propose pharyngeal neuropathy due to CMT as the most likely risk factor. However, further research is needed to more clearly elucidate the nature of the association between CMT and OSA. Additionally, other studies have shown a higher prevalence of axonal sensory polyneuropathy in patients with OSA, as compared to controls without OSA that was partially reversible with treatment of OSA with CPAP.5,6
Our patient with CMT1A had chronic insomnia that was likely multifactorial: moderately severe OSA, restless legs/ neuropathy overlap symptoms that improved dramatically with the initiation of CPAP therapy. In general, negative neuropathic signs, such as numbness, are more difficult to treat compared to positive neuropathic signs such as tingling. Improvement of this patient's numbness makes this case more unique in the setting of CMT type 1 neuropathy. In addition, her daytime alertness and fatigue improved with CPAP treatment.
Recent studies have shown that both OSA and restless legs syndrome are highly prevalent in patients with CMT type 1 and the presence of these sleep-related disorders can affect the quality of life of patients with CMT.3,4 However, the mechanisms for improvement of neuropathy symptoms with CPAP in our patient are not entirely clear. One possible mechanism could be related to the improvement of nocturnal hypoxemia. Mayer et al. showed that patients with OSA have peripheral nerve dysfunction, the severity of which may be partly related to the level of nocturnal hypoxemia.7 Our patient did have minimum oxygen desaturation to 63% during her baseline study, with improvement of all the desaturations with CPAP titration. Our patient's neuropathy symptoms improved with CPAP initiation. Patients with underlying CMT type 1 and OSA may be more susceptible to the effects of hypoxemia. It is possible that nerves already affected by CMT changes are more vulnerable to the effects of severe hypoxemia, with CPAP therapy resulting in improvement of the hypoxia associated with sleep apnea. Further work needs to be done to evaluate this in patients with hereditary neuropathies.
Our case report adds to the growing evidence that attention needs to be given to sleep-related complaints including insomnia, daytime sleepiness, and restless sleep in patients with CMT. It also demonstrates the importance of screening for sleep disorders in patients with CMT. An overnight sleep study should be considered if OSA is suspected. Treatment with CPAP may be a viable treatment for sleep-related disturbances in patients with OSA and CMT.
DISCLOSURE STATEMENT
None of the authors has any conflicts of interest to disclose. None of the authors has relevant financial support, or off-label or investigational use to disclose.
REFERENCES
- 1.Bird TD. Charcot-Marie-Tooth Neuropathy Type 1. In: Adam MP, editor. GeneReviews. Seattle, WA: University of Washington, Seattle; 2011. [Google Scholar]
- 2.Verhamme C, van Schaik IN, Koelman JH, de Haan RJ, de Visser M. The natural history of Charcot-Marie-Tooth type 1A in adults: a 5-year follow-up study. Brain. 2009;132(Pt 12):3252–3262. doi: 10.1093/brain/awp251. [DOI] [PubMed] [Google Scholar]
- 3.Dziewas R, Waldmann N, Boentert M, et al. Increased prevalence of obstructive sleep apnoea in patients with Charcot-Marie-Tooth disease: a case control study. J Neurol Neurosurg Psychiatry. 2008;79(7):829–831. doi: 10.1136/jnnp.2007.137679. [DOI] [PubMed] [Google Scholar]
- 4.Boentert M, Knop K, Schumacher C, Gess B, Okegwo A, Young P. Sleep disorders in Charcot-Marie-Tooth disease type 1. J Neurol Neurosurg Psychiatry. 2014;85(3):319–325. doi: 10.1136/jnnp-2013-305296. [DOI] [PubMed] [Google Scholar]
- 5.Dziewas R, Schilling M, Engel P, et al. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatry. 2007;78(3):295–297. doi: 10.1136/jnnp.2006.102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lüdeman P, Dziewas R, Sörös P, Happe S, Frese A. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatry. 2001;70(5):685–687. doi: 10.1136/jnnp.70.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer P, Dematteis M, Pépin JL, et al. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med. 1999;159(1):213–219. doi: 10.1164/ajrccm.159.1.9709051. [DOI] [PubMed] [Google Scholar]