Abstract
Purpose
To present a report of longitudinal changes in radial peripapillary capillaries (RPC) and changes in retinal full thickness (RFT) and peripapillary retinal nerve fiber layer (RNFL) in a patient with Leber hereditary optic neuropathy (LHON).
Observations
A 42-year-old man presented with acute- and presymptomatic-stage LHON in the left (OS) and right (OD) eyes, respectively, at the initial visit. Onset of LHON in the OD was observed 2 months after the initial visit. Once the temporal RNFL started to decrease in thickness, the areas of temporal RPC defects and RFT thinning gradually increased, indicating that these factors might be correlated.
Conclusions and importance
Optical coherence tomography angiography showed LHON from the presymptomatic stage. The results indicate that temporal RPC defects and RFT thinning start to spread once the pseudoedema begins to resolve.
Keywords: Leber hereditary optic neuropathy, Optical coherence tomography angiography, Optical coherence tomography, Radial peripapillary capillary, Retinal full thickness, Peripapillary retinal nerve fiber layer
1. Introduction
Leber hereditary optic neuropathy (LHON) is a mitochondrial DNA (mtDNA)-related disease. Recent studies have shown that radial peripapillary capillary (RPC) network defects in patients with LHON can be observed by optical coherence tomography angiography (OCT-A).1,2 However, the time course of changes in the RPC network is unknown. Here, we present a report on changes in the RPC network, retinal full thickness (RFT), and peripapillary retinal nerve fiber layer (RNFL) in a patient with LHON, imaged by spectral-domain (SD) OCT-A (RT-Vue, Optovue XR-100 with Angio Vue, CA) and SD-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany) over a 5-month longitudinal course.
2. Case report
A 42-year-old Japanese man without any relevant medical history was referred to the Kobe City Medical Center General Hospital in July 2016 with a complaint of progressive blurring of vision in the left eye (OS) for a month. A review of family history revealed that his nephew was undergoing treatment for LHON.
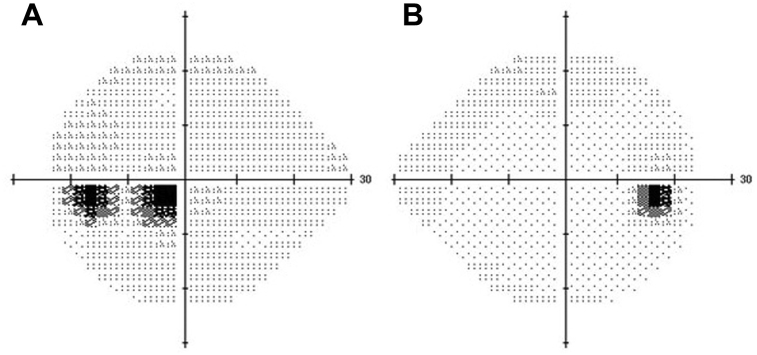
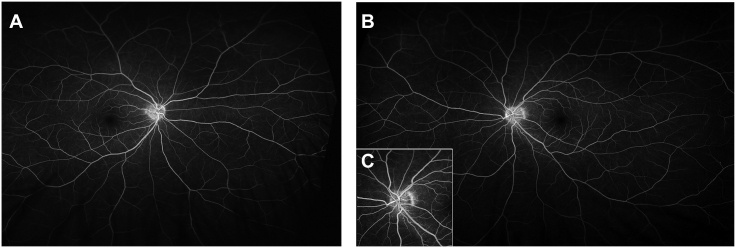
The patient exhibited best-corrected visual acuities (VAs) of 20/13 and 20/33 in the right (OD) and left eyes, respectively. Intraocular pressure was within the normal range in both eyes. Critical fusion frequencies in the OD and OS were 42 and 16 Hz, respectively. There were no relative afferent pupillary defects in either eye. While slit-lamp biomicroscopy findings of the external eye and anterior segment appeared to be normal, the findings of Humphrey field analysis (HFA, 24-2) were within normal limits in the OD and revealed central scotoma in the OS (Fig. 1). Fluorescein angiography findings revealed dilated capillary vessels at the temporal margin of the optic disc, but no staining or leakage in the late phase (Fig. 2). Upon mtDNA examination, a point mutation was observed at nucleotide position 11778; thus, the diagnosis of LHON was confirmed. We recommended lifestyle modifications, such as cessation of smoking, and performed follow-up examinations. The time course of decline in VA in the OS was as follows: 20/60 at 1 month, 20/100 at 3 months, and 20/1000 at 5 months after the initial visit. In the OD, VA started to decrease at 2 months and reached 20/100 at 3 months and 20/666 at 5 months after the initial visit. The OD VA declined to the same level as that of the OS at 5 months from the initial visit.
Fig. 1.
Visual field analysis findings. Humphrey field analysis (24-2) findings at the initial visit revealed central scotoma in the OS (A) and values within normal limits in the OD (B). The mean deviations in the OD and OS were −0.06 and −4.69 dB, respectively.
Fig. 2.
Wide-field fluorescein angiography findings in the late phase in the OS (A) and OD (B), and in the early phase in the OS (C).
2.1. Time course of changes in the OS optic disc
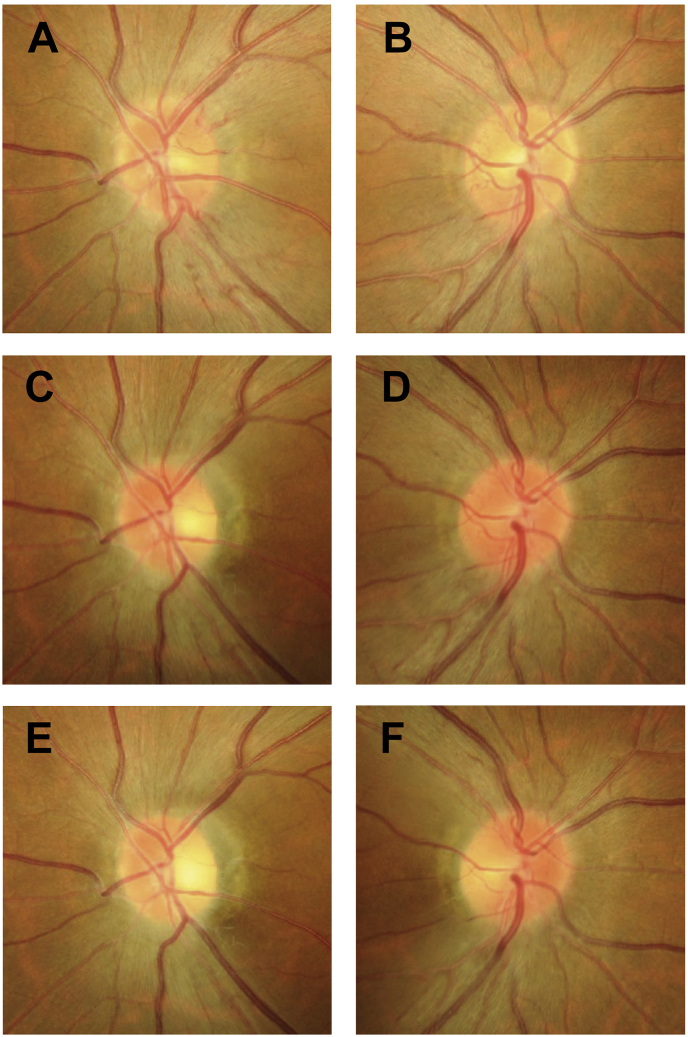
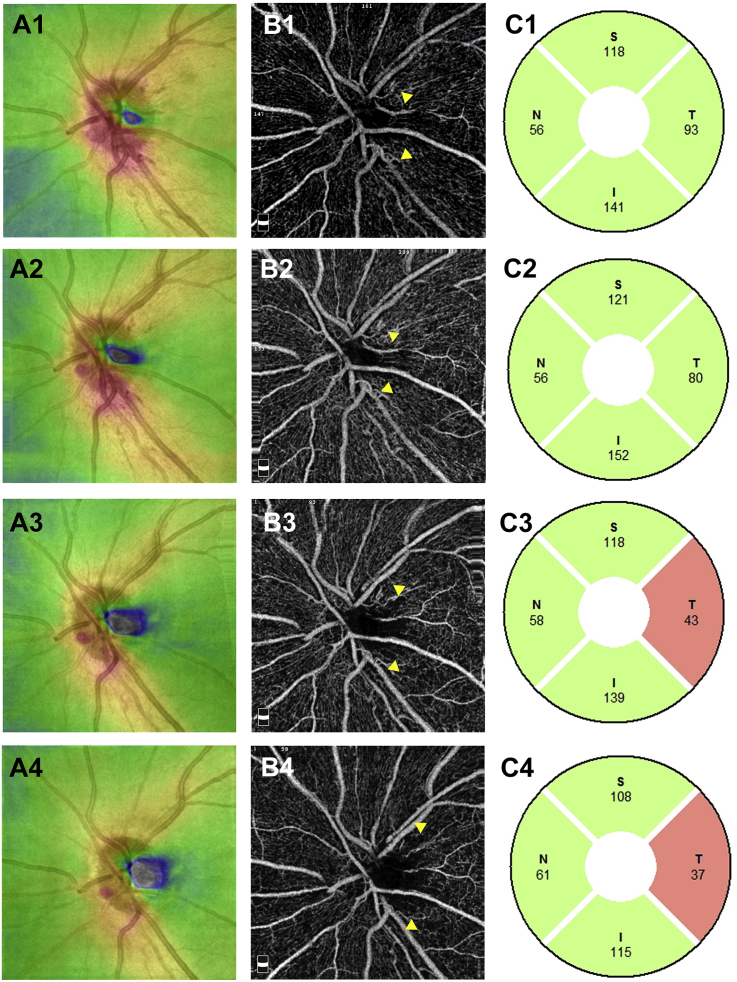
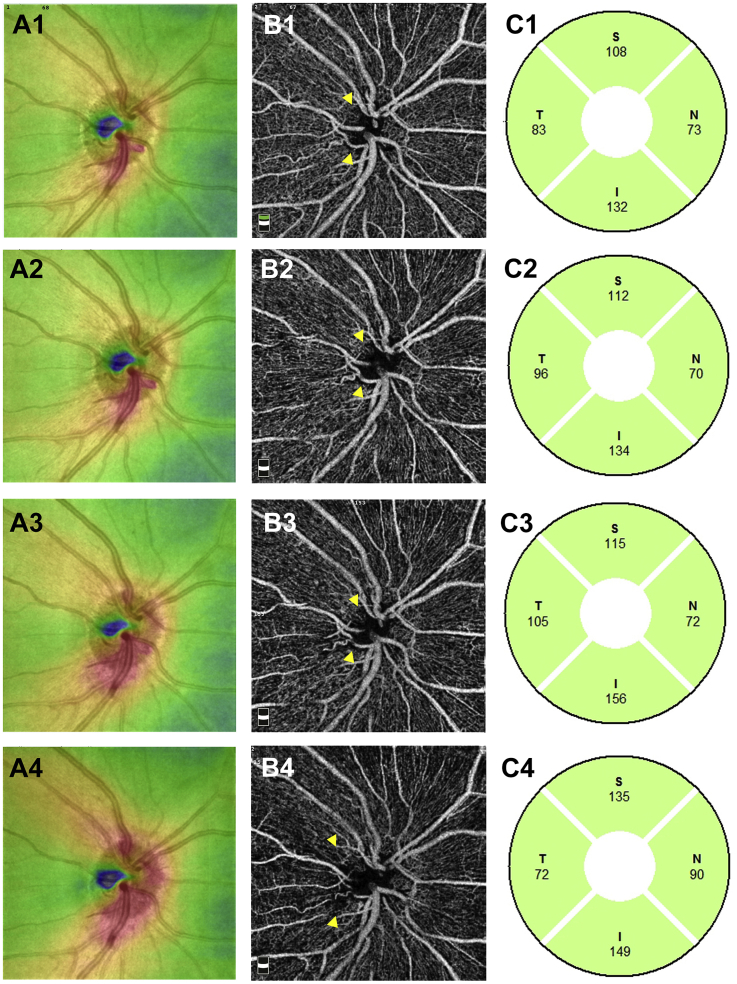
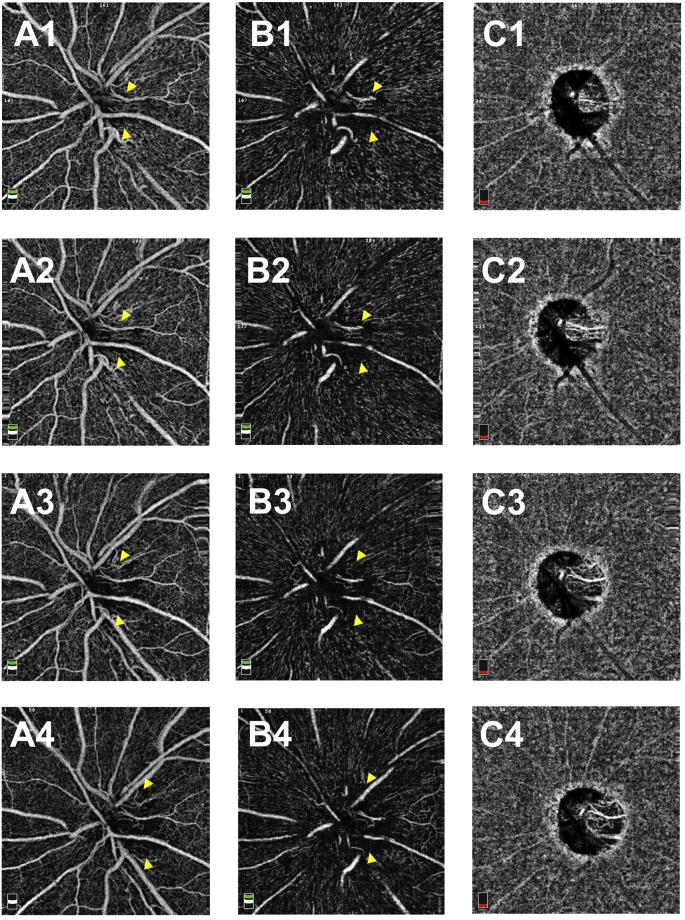
At the initial visit, the findings of disc photography (Fig. 3A) and RFT analysis of the retina surrounding the optic disc (Fig. 4A1) revealed optic disc swelling in the OS. The thickness of the temporal RNFL of the OS was within the normal range (Fig. 4C1). The findings of disc photography (Fig. 3A) and OCT-A (Fig. 4B1) revealed telangiectatic vessels in the peripapillary region. Between the first and 5-month visits, the OS optic disc developed pallor (Fig. 3A, C, E), and the temporal RNFL thickness gradually decreased (Fig. 4C1–4). A gradual decrease in RFT in the temporal section was observed upon RFT analysis (Fig. 4A1–4); OCT-A findings in the same section revealed a gradual spread of the area of RPC network defects in the RPC layer (Fig. 4B1–4, arrowhead). OCT-A revealed that the capillary defects in the nerve head (NH) and vitreous layers had also spread in the RPC layer (S-Fig. 1 A1-4 B1-4, arrowhead).
Fig. 3.
Disc photography findings of the OS and OD optic discs. Disc photography findings of the OS optic disc at the initial visit (A) and at 3 (C) and 5 (E) months from the initial visit revealed a gradual decrease in swelling. The findings of the OD optic disc at the initial visit (B) and at 3 (D) and 5 (F) months from the initial visit revealed that swelling began between the first and 3-month visits and that the temporal region of the disc had atrophied at 5 months from the initial visit.
Fig. 4.
Retinal full thickness, optic disc optical coherence tomography angiography images, and retinal nerve fiber layer in the OS. A1–A4: Retinal full thickness in the OS at the initial visit (A1) and at 1 (A2), 3 (A3), and 5 (A4) months from the initial visit. B1–B4: 4.5-mm x 4.5-mm optic disc optical coherence tomography angiography images of the radial peripapillary capillary network in the OS at the initial visit (B1) and at 1 (B2), 3 (B3), and 5 (B4) months from the initial visit. C1–4: Retinal nerve fiber layer in the OS at the initial visit (C1) and at 1 (C2), 3 (C3), and 5 (C4) months from the initial visit. S: superior, N: nasal, T: temporal, I: inferior.
2.2. Time course of changes in the OD optic disc
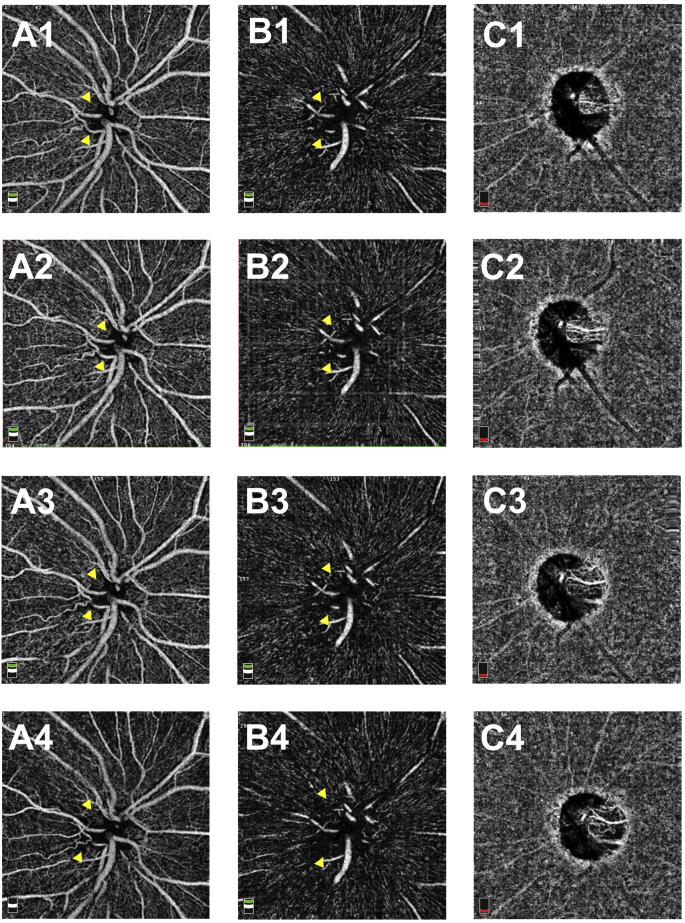
In the OD, the optic disc swelling (Fig. 3B, D) and temporal RNFL thickness (Fig. 5C1–3) gradually increased from the initial visit to the 3-month visit. At 5 months from the initial visit, the temporal region of the optic disc had atrophied (Fig. 3F), and the RNFL thickness had started to decrease (Fig. 5C4). Between the first and 3-month visits, OCT-A findings showed the areas of the RPC network defects in the RPC layer (Fig. 5B1–3, arrowhead) and the RFT in the temporal area to be almost stable (Fig. 5A1–3). However, the areas of RPC network defects in the RPC layer (Fig. 5B4) and RFT thinning in the temporal area (Fig. 5A4) began to spread between 3 and 5 months from the initial visit. Capillary defects in the NH and vitreous layers on OCT-A were as noted earlier (S-Fig. 2 A1-4, B1-4, arrowhead).
Fig. 5.
Retinal full thickness, optic disc optical coherence tomography angiography images, and retinal nerve fiber layer in the OD. A1–A4: Retinal full thickness in the OD at the initial visit (A1) and at 1 (A2), 3 (A3), and 5 (A4) months from the initial visit. B1–B4: 4.5-mm x 4.5-mm optic disc optical coherence tomography angiography images of the radial peripapillary capillary network in the OD at the initial visit (B1) and at 1 (B2), 3 (B3), and 5 (B4) months from the initial visit. C1–4: Retinal nerve fiber layer in the OD at the initial visit (C1) and at 1 (C2), 3 (C3), and 5 (C4) months from the initial visit. S: superior, N: nasal, T: temporal, I: inferior.
3. Discussion
In this case, the patient presented with acute-stage LHON in the OS at the initial visit. The OS OCT-A findings revealed telangiectatic vessels and RPC defects in the temporal section. These findings are similar to those presented in previous reports.1, 2, 3
We also found that the spread of areas with RPC defects and RFT thinning were correlated with time. In a previous study, histological findings revealed the nerve fiber count and diameter in a patient with LHON to be significantly lower than those in a healthy control subject.4 We speculate that RPC defects, which are consistent with RFT thinning area, are involved in nerve fiber atrophy.
In the present case, the OD was in the presymptomatic stage of LHON at the initial visit. Judging from the complaint of decreased vision in the OD, we speculated that LHON onset in the OD occurred at 2 months after the initial visit. Given that the 5-month time point after the initial visit corresponds to 3 months after LHON onset, the time course of changes in OD RNFL thickness in the present case are consistent with those reported in previous studies, where RNFL thickness increased from the presymptomatic stage of LHON and began to decrease at approximately 3 months after LHON onset (pseudoedema).5
Between the presymptomatic stage and a month after onset (3 months after the initial visit), the OD RNFL had increased in thickness; however, we could not find any specific change in the temporal RPC or RFT of the OD. At 3 months after onset (5 months after the initial visit), we observed that the OD temporal RNFL thickness had started to decease, and the areas of temporal RPC defects and RFT thinning in the OD had begun to spread. These findings suggested that the spread of temporal RPC defects and RFT thinning started between the time of disease onset and the follow-up 3 months later or when the temporal disc swelling and pseudoedema began to resolve.
A recent case report has highlighted that subclinical changes, such as decreased VA, visual field defect, and increased RNFL, precede the onset of symptomatic vision loss.6 In the present case, an important OCT-A finding is that the spread of areas with RPC defects did not precede the symptomatic vison loss. Thus, it is reasonable to assume that the spreading of RPC defects was a secondary association of RNFL thinning.
Optical coherence tomography angiography is a useful tool for observing the capillaries around the optic disc. In the present case, follow-up evaluations were performed on a monthly basis. Shorter intervals of OCT-A evaluation around the time of disease onset might help understand the pathogenic mechanism of LHON.
4. Conclusions
In the present case, the spread of areas with RPC defects in a patient with LHON started with the decrease in temporal RNFL thickness and was correlated with the spread of areas of RFT thinning.
Patient consent
Written informed consent was obtained from the patient for publishing personal identifying information and case details.
Acknowledgments and disclosures
Funding
No funding or grant support.
Conflicts of interest
None.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Acknowledgements
We would like to thank Dr. Seiji Takagi for his insightful comments. We also thank Editage (www.editage.jp) for English language editing.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajoc.2018.01.003.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1.
4.5-mm x 4.5-mm optic disc optical coherence tomography angiography (OCT-A) images of the OS. A1–A4: OCT-A images of the optic nerve head layer in the OS at the initial visit (A1) and at 1 (A2), 3 (A3), and 5 (A4) months from the initial visit. B1—B4: OCT-A images of the vitreous layer in the OS at the initial visit (B1) and at 1 (B2), 3 (B3), and 5 (B4) months from the initial visit. C1–4: OCT-A images of the choroid layer in the OS at the initial visit (C1) and at 1 (C2), 3 (C3), and 5 (C4) months from the initial visit.
Fig. S2.
4.5-mm x 4.5-mm optic disc optical coherence tomography angiography images (OCT-A) in the OD. A1–A4: OCT-A images of the optic nerve head layer in the OD at the initial visit (A1) and at 1 (A2), 3 (A3), and 5 (A4) months from the initial visit. B1—B4: OCT-A images of the vitreous layer in the OD at the initial visit (B1) and at 1 (B2), 3 (B3), and 5 (B4) months from the initial visit. C1–4: OCT-A images of the choroid layer in the OD at the initial visit (C1) and at 1 (C2), 3 (C3), and 5 (C4) months from the initial visit.
References
- 1.De Rojas J.O., Rasool N., Chen R.W., Horowitz J., Odel J.G. Optical coherence tomography angiography in Leber hereditary optic neuropathy. Neurology. 2016;87(19):2065–2066. doi: 10.1212/WNL.0000000000003313. [DOI] [PubMed] [Google Scholar]
- 2.Ghasemi Falavarjani K., Tian J.J., Akil H., Garcia G.A., Sadda S.R., Sadun A.A. Swept-source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina. 2016;36(Suppl 1):S168–S177. doi: 10.1097/IAE.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 3.Gaier E.D., Gittinger J.W., Cestari D.M., Miller J.B. Peripapillary capillary dilation in Leber hereditary optic neuropathy revealed by optical coherence tomographic angiography. JAMA Ophthalmol. 2016;134(11):1332–1334. doi: 10.1001/jamaophthalmol.2016.3593. [DOI] [PubMed] [Google Scholar]
- 4.Saadati H.G., Hsu H.Y., Heller K.B., Sadun A.A. A histopathologic and morphometric differentiation of nerves in optic nerve hypoplasia and Leber hereditary optic neuropathy. Arch Ophthalmol. 1998;116(7):911–916. doi: 10.1001/archopht.116.7.911. [DOI] [PubMed] [Google Scholar]
- 5.Barboni P., Carbonelli M., Savini G. Natural history of Leber's hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117(3):623–627. doi: 10.1016/j.ophtha.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Hwang T.J., Karanjia R., Moraes-Filho M.N. Natural history of conversion of Leber's hereditary optic neuropathy: a prospective case series. Ophthalmology. 2017;124(6):843–850. doi: 10.1016/j.ophtha.2017.01.002. [DOI] [PubMed] [Google Scholar]