Abstract
Background
Congenital single-side deafness (SSD) affects sound localization even after cochlear implantation (CI) in some conditions. The medial nucleus of the trapezoid body (MNTB) plays an important role in binaural benefit and sound localization, but little is known about intrinsic molecular changes in MNTB with SSD. We aimed to observe changes in MNTB in early-developmental SSD rats, including the key neurotransmitters (GABA, Gly, Glu) and major receptors (GABAa-R/GABAb-R for GABA, Gly-R for Gly, and AMPA/NMDA for Glu).
Material/Methods
The model of early-developmental SSD was acquired by right cochlear ablation at P12 and confirmed by ABR. High-performance liquid chromatography fluorescence detection (HPLC-FLD) was performed to measure the levels of neurotransmitters in MNTB. The relative expression of neurotransmitter receptors was tested by quantitative real-time PCR analysis.
Results
(1) The right MNTB of experimental rats had an increase in GABA, Gly, and Glu at 4 weeks after right cochlear ablation (P<0.05). (2) At 2 weeks, the left MNTB of experimental rats showed increases in GABAa-R, GABAb-R, Gly-R, and AMPA, while the right MNTB showed lower expression of NMDA (P<0.05). The higher receptors in left MNTB decreased to a level at which we found no difference at 1 week for GABAa-R and GABAb-R (P>0.05), and was even reversed for Gly-R and AMPA (P<0.05). (3) Gly level was significantly increased at 2 weeks bilaterally and continued to 4 weeks in the left MNTB (P<0.05).
Conclusions
Early-developmental SSD can lead to asymmetric distribution of neurotransmitters and receptors in MNTB, which can be the fundamental cause of defective sound localization after cochlear implantation.
MeSH Keywords: Hearing Loss, Unilateral; Neurotransmitter Agents; Receptors, Neurotransmitter; Sound Localization
Background
Individuals with unilateral severe-to-profound hearing loss or single-sided deafness (SSD) are reported to have difficulty hearing in many everyday situations despite the well-preserved acoustic hearing in 1 ear. For patients with SSD, hearing disabilities are particularly associated with the lack of binaural benefits [1,2].
Binaural hearing provides the benefit of sound localization in the normal auditory system and allows better understanding of speech in noisy situations when the source of speech and the noise are presented from different locations in the horizontal plane [3]. Mutations in mtDNA and other causes resulted in a large number of deaf patients with different manifestations [4], in which a considerable number received unilateral cochlear implantation. However, children with 1 cochlear implant (CI) have similar hearing problems as do children with SSD; both groups have difficulties in hearing speech in noisy environments and difficulties in localizing sound [5].
In recent years, many studies have shown that sound localization for SSD patients with a CI was significantly improved compared to that without a CI [1,2], which further suggests that CI can re-establish the benefits of binaural hearing in acquired SSD. However, research also shows that children who receive sequential CI with long inter-implant delays (>2 years) have compromised bilateral benefits for speech perception and have inaccurate sound localization [6,7]. However, the effect of early unilateral CI is similar to that of congenital SSD.
Sound localization in the horizontal plane and binaural benefit rely on the interaural level difference (ILD) and interaural time difference (ITD), which help to localize high-frequency and low-frequency sounds, respectively [3]. Binaural cues are first formed and processed at the brainstem level, in which the medial nucleus of the trapezoid body (MNTB) plays an important role [8]. MNTB receives temporally precise excitatory input from globular bushy cells of the contralateral cochlear nucleus (CN) via the large calyces of Held [9], and sends inhibitory projections to the ipsilateral lateral (LSO) and medial superior olives (MSO) for preliminary calculation [10,11]. There have been some electrophysiology and imaging studies of SSD [12–14], but little is known about the effect of SSD on intrinsic molecular changes in MNTB. In the present study, we aimed to observe the molecular changes in MNTB in early-developmental SSD rats, including the key neurotransmitters and major receptors.
The main neurotransmitters in the central nervous system are glutamate (Glu), glycine (Gly), and gamma aminobutyric acid (GABA) [15, 16]; the first is exciting and the second and third are inhibitory. Among all the receptors of Glu, AMPA and NMDA have been confirmed to be important in the central nervous system [17,18]. GABA has 3 receptors: GABAa-R, GABAb-R, and GABAc-R. However, GABAc-R is found mainly in the retina and cerebellum and is rare in the brain [19]. Therefore, in the present study we focused on 3 neurotransmitters (GABA, Gly, and Glu) and 5 receptors (GABAa-R/GABAb-R for GABA, Gly-R for Gly, and AMPA/NMDA for Glu).
Material and Methods
Animals and tissue preparations
Pregnant female Sprague-Dawley rats were purchased from WeiTongLiHua, Beijing, China and their pups were used in the study. The pups were treated with cochlear ablation at P12 to form a model of early-developmental SSD. The animals were maintained in the Animal Facility of Capital Medical University. All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Animal Care Committee of Capital Medical University.
Both normal control and experimental rats were randomly separated into 3 groups – the 1-week group, the 2-week group, and the 4-week group – after P12, and each group contained 6 rats. After the rats received deep anesthesia with 6% chloral hydrate (intraperitoneal injection, 0.6 ml/100 g), the brains were removed and MNTB tissues were obtained according to the Rat Brain in Stereotaxic Coordinates system [20]. All tissues were stored at −80°C.
Cochlear ablation
To produce the early-developmental SSD model, right cochlear ablation is performed at P12 for the critical period of auditory development, which is from P13 in rats [21]. We used 6% chloral hydrate (intraperitoneal injection, 0.6 ml/100 g) to induce and maintain anesthesia in rat pups. A small incision was made behind the pinna to expose the facial nerve and we removed the otocyst bone, which is under the muscles behind the facial nerve. Cochlear ablation was done when cochlear contents exuded after breaking the thin bone covering the cochlea. After the incision was sutured, the rats were kept warm using a thermostatically-controlled warming blanket. After recovery, the rats were returned to their home cages. The cochlea damage was reconfirmed using auditory brainstem-evoked response (ABR) before harvesting the brains.
Auditory brainstem-evoked response testing
ABR testing was performed using an AEP system (Bio-logic, USA). Rats were anesthetized with 6% chloral hydrate (intraperitoneal injection, 0.6 ml/100 g) and placed on a blanket linked to a heat pump to maintain normal body temperature. Platinum subdermal needle electrodes were positioned at the ventral surface of the tympanic bulla (recording on one side, ground on the other) referenced to an electrode placed at the skull vertex [22]. Pure-tone stimuli (2-ms duration) of 8 kHz ranging from 30 to 80 dB nHL in 10-dB steps were delivered directly to the ear through plastic tubing. Two thousand ABR responses were sampled and averaged at each frequency. The appearance of wave III means the ABR waveform can be elicited.
High-performance liquid chromatography fluorescence detection (HPLC-FLD)
Amino acid transmitters were extracted using brain tissue extract liquid (water: acetonitrile=2: 3); we added 100 μl/mg into MNTB tissues, fully ground the tissues, and ultrasonically extracted the amino acid transmitters in an ice bath for 20 min. The supernatant was obtained by 15 000 rpm centrifugation at 4°C for 10 min, followed by centrifugation at 4°C 15 000 r/min for 5 min; the supernatant containing all amino acid transmitters was then diluted to 1: 50 for use. HPLC-FLD was conducted in a High-Performance Liquid Chromatography device (Sykam, Germany) and a Fluorescence Detector device (Shimadzu RF-20A, Japan), using an amino acid analysis column (Eclipse AAA 4.6×150 mm, 5 μm) and Phthalic dicarboxaldehyde (OPA) pre-column derivatization. The mobile phase was: A: buffer solution: methanol: tetrahydrofuran (V: V: V)=400: 95: 5; and B: buffer solution: methanol (V: V)=120: 380, gradient elution. The buffer solution was 20 mM of sodium acetate solution (pH=7.2). The flow rate was 0.8 ml/min, using the fluorescence detector to detect the amino acids-derived compound after separation, with λex=340 nm and λem=455 nm.
Quantitative real-time PCR analysis
Total RNA of MNTB was extracted using the RNeasy Lipid Tissue Mini Kit (Qiagen, Germany) following the manufacturer’s instructions. Total RNA was dissolved in 30 μl of RNase-free water provided in the kit. RNA concentrations were measured using NanoDrop (Thermo Scientific, Wilmington, DE, USA) with 260 nm/280 nm ratios between 1.9 and 2.1. RNA (1 μg) was reverse-transcribed into cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions. Quantitative PCR (Q-PCR) was performed after the reverse-transcription. The total volume of Q-PCR reactions was 20 μl containing10 μl SYBR Green Mix (Applied Biosystems, UK), 8 μl distilled water, 1 μl cDNA, and 1 μl primers [23]. The sequences of the primers used in this study were: GABAa-R-specific primers were forward: CAAGAGAGGGTATGCGTGGG, reverse: CAATAGTTGCCAA GCCGGGG. GABAa-R PCR product: 155bp; GABAb-R-specific primers were forward: GAGAAGCCA GTTCCCGTTTG, reverse: TGGCATAGAGTTTCCAGGGC. GABAb-R PCR product: 173bp; Gly-R-specific primers were forward: CGGACAACAAGCTGCTGAGA, reverse: GTCTGTACGTCCATCGGGAA. Gly-R PCR product: 115bp. AMPA-specific primers were forward: GCCAGAGTCCGGAAATCCAA, reverse: TCCTTTAGGTG TGGCGATGC, AMPA PCR product: 147bp. NMDA-specific primers were forward: CAATGACCCCAGGCTCAGAAA, reverse: TAAAG GCGTGCAGCTTGTTG, NMDA PCR product: 188bp. Gapdh-specific primers were forward: GACCACCCAGCCCAGCAAGG, reverse: TCCCCAGGCCCCTCCTGTTG, Gapdh PCR product: 144bp. The amplification reaction protocol consisted of 2 min at 60°C and 10 min at 95°C, followed by 40 cycles of reaction: 15 s of denaturing at 95°C and 1 min of annealing at 60°C. Samples were held at 10°C at the end of each amplification reaction. Gapdh was used as the internal reference for each sample [23].
Statistical analysis
Data are presented as mean ±SEM (standard error of measurements). Statistical analysis was carried out using SPSS16.0. Relative mRNA level of GABAa-R/GABAb-R/Gly-R/AMPA/NMDA and concentration of GABA/Gly/Glu in MNTB between the same side of SSD and normal rats were analyzed using the independent-samples t test. Relative mRNA level of GABAa-R/GABAb-R/Gly-R/AMPA/NMDA and concentration of GABA/Gly/Glu in MNTB between the left and right side of SSD rats were analyzed using the paired t test. Neurotransmitters levels and relative receptors mRNA expression among different time points were compared with one-way analysis of variance (ANOVA) and the follow-up post hoc Tukey HSD multiple comparison tests. P<0.05 was considered to be statistically significant.
Results
Threshold of auditory brainstem-evoked response testing
None of the ABR waveforms were elicited for the cochlear ablation ears (right ear) in experimental rats by an 80-dB nHL pure tone; however, for both ears of normal rats and left ears of experimental rats, normal ABR waveforms were elicited by a 30-dB nHL pure tone (Figure 1).
Figure 1.
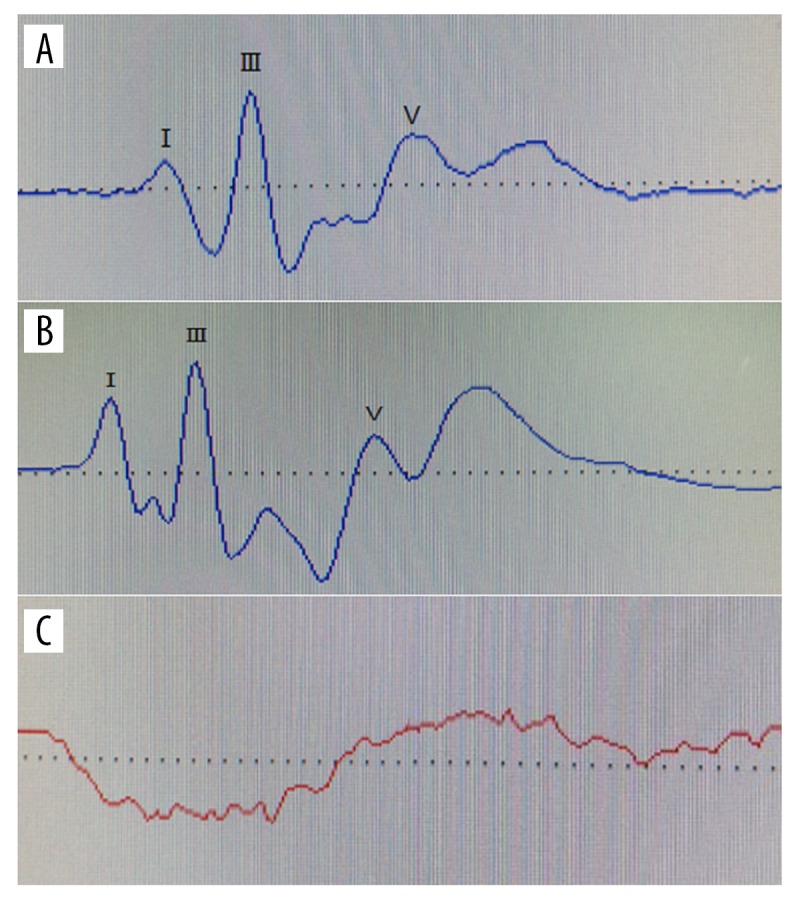
ABR waveforms. (A) ABR waveform of normal ear elicited by 30 dB nHL pure tone. (B) ABR waveform of normal ear elicited by 80 dB nHL pure tone. (C) ABR waveform of cochlear ablation ear elicited by 80 dB nHL pure tone. ABR was not elicited for the cochlear ablation ears (right ear) in experimental rats by 80 dB nHL pure tone.
Early-developmental SSD induced asymmetric neurotransmitter changes in MNTB
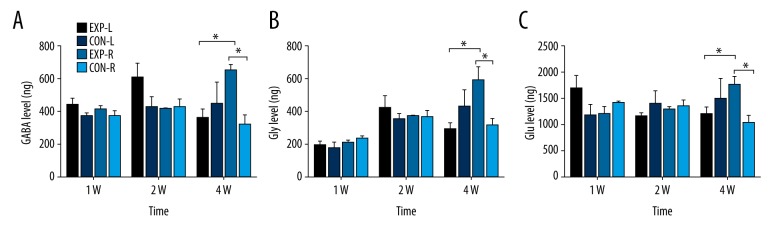
In normal control rats, the number of neurotransmitters seemed to be equal between left and right MNTB. But at 4 weeks after cochlear ablation, the right MNTB of experimental rats had an increase in GABA (Figure 2A), Gly (Figure 2B), and Glu (Figure 2C) compared to normal controls. However, there was no significant difference in left MNTB between experimental and normal control rats. Thus, significant increases in GABA, Gly, and Glu were available in the right MNTB compared to the left MNTB in experimental rats at 4 weeks after right cochlear ablation. No significant difference was seen at 1 week or 2 weeks. Therefore, early-developmental SSD induced asymmetric neurotransmitter changes in MNTB at 4 weeks after cochlear ablation.
Figure 2.
Neurotransmitter level in MNTB. (A) GABA level in bilateral MNTB of normal or experimental rats 1w/2w/4w after cochlear ablation (P12). (B) Gly level in bilateral MNTB of normal or experimental rats at 1, 2, and 4 weeks after cochlear ablation (P12). (C) Glu level in bilateral MNTB of normal or experimental rats at 1, 2, and 4 weeks after cochlear ablation (P12). * P<0.05. At 4 weeks after cochlear ablation, the right MNTB of experimental rats had increased GABA (P<0.05), Gly (P<0.05), and Glu (P<0.05) compared to normal controls.
Early-developmental SSD induced asymmetric neurotransmitter receptors changes in MNTB
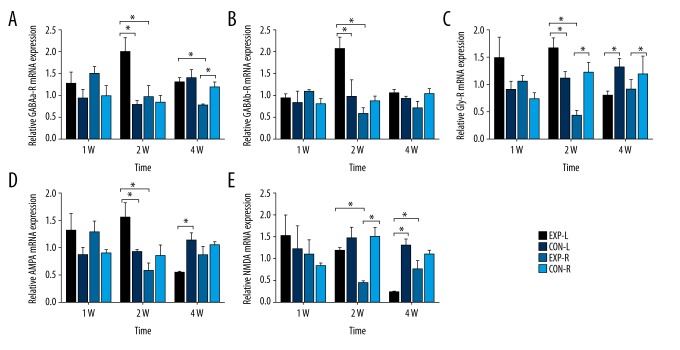
At 2 weeks after right cochlear ablation, the left MNTB of experimental rats showed significant increases in GABAa-R (Figure 3A), GABAb-R (Figure 3B), and AMPA (Figure 3D) compared to normal controls. However, no significant difference was found in right MNTB between experimental and normal control rats. On the contrary, NMDA (Figure 3E) showed lower expression in the right MNTB of experimental rats compared to normal controls, but we found no difference in left MNTB between experimental and normal control rats. For Gly-R (Figure 3C), we found an increase in left MNTB and decrease in right MNTB. All these changes finally induced the higher expression of these receptors in left compared to right MNTB in experimental rats at 2 weeks after cochlear ablation. Interestingly, at 4 weeks after right cochlear ablation, the higher number of neurotransmitter receptors expression in the left MNTB of experimental rats decreased until there was no difference between GABAa-R and GABAb-R (Figure 3A, 3B), or was even reversed to be lower than normal controls for Gly-R and AMPA (Figure 3C, 3D). Reduction of expression of left MNTB was also be found in NMDA (Figure 3E).
Figure 3.
Relative expression of neurotransmitter receptors mRNA in MNTB. (A) Relative GABAa-R mRNA expression in bilateral MNTB of normal or experimental rats 1w/2w/4w after cochlear ablation (P12). (B) Relative GABAb-R mRNA expression in bilateral MNTB of normal or experimental rats at 1, 2, and 4 weeks after cochlear ablation (P12). (C) Relative Gly-R mRNA expression in bilateral MNTB of normal or experimental rats 1w/2w/4w after cochlear ablation (P12). (D) Relative AMPA mRNA expression in bilateral MNTB of normal or experimental rats at 1, 2, and 4 weeks after cochlear ablation (P12). (E) Relative NMDA mRNA expression in bilateral MNTB of normal or experimental rats at 1, 2, and 4 weeks after cochlear ablation (P12). * P<0.05. There were higher expressions of all 5 receptors in left than right MNTB in experimental rats at 2 weeks (P<0.05), which were decreased at 4 weeks; however, the expression of Gly-R, AMPA, and NMDA were decreased more than the same side of normal rats (P<0.05).
Neurotransmitters and their receptors change over time
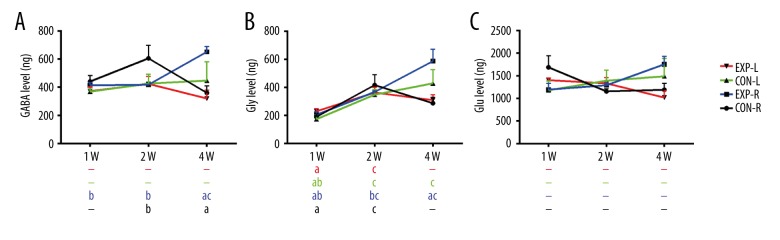
In the bilateral MNTB of normal rats, no significant difference was found in GABA or Glu levels at 1 week, 2 weeks, or 4 weeks (Figure 4A, 4C). However, the Gly level showed a significant increase at 2 weeks bilaterally and even continued to 4 weeks in the left MNTB (Figure 4B). In the right MNTB, the Gly level was also higher at 4 weeks than at 1 week, but no significant difference was found. Gly level increased significantly from 1 week to 2 weeks after P12, while GABA and Glu remained stable. In experimental rats, GABA and Gly levels showed significant increases at 4 weeks (Figure 4A, 4B), which is consistent with the results shown in Figure 2A, 2B.
Figure 4.
Neurotransmitter level in MNTB changes with time. (A) GABA level in bilateral MNTB of normal or experimental rats changes with time. (B) Glycine level in bilateral MNTB of normal or experimental rats changes with time. (C) Glutamate level in bilateral MNTB of normal or experimental rats changes with time. a P<0.05 compared to 2 weeks, b P<0.05 compared to 4 weeks, c P<0.05 compared to 1 week. ‘−’ Means no significant difference compared to the other 2 groups. Gly level showed significant increase at 2 weeks bilaterally and even continued to 4 weeks in left MNTB of normal rats (P<0.05). Gly level increased significantly from 1 week to 2 weeks after P12, while GABA and Glu remained stable.
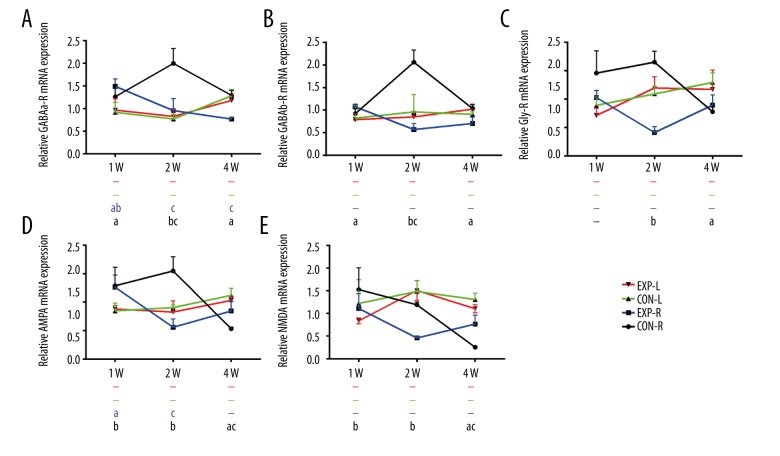
For all 5 neurotransmitter receptors, no significant changes of relative expression were found over time in normal control rats. However, in experimental rats, they decreased consistently at 4 weeks compared to 2 weeks in the left MNTB (Figure 5A–5E). Increased GABAa-R and GABAb-R were also available at 2 weeks compared to 1 week (Figure 5A, 5B), which is consistent with results shown in Figure 3A, 3B. In the right MNTB, relative expression of GABAa-R and AMPA decreased significantly at 2 weeks (Figure 5A, 5D), but no significant difference was found in GABAb-R, Gly-R, or NMDA, although they also appeared to decrease (Figure 5B, 5C, 5E).
Figure 5.
Relative expression of neurotransmitter receptors mRNA in MNTB changes with time. (A) Relative GABAa-R mRNA expression in bilateral MNTB of normal or experimental rats changes with time. (B) Relative GABAb-R mRNA expression in bilateral MNTB of normal or experimental rats changes with time. (C) Relative Gly-R mRNA expression in bilateral MNTB of normal or experimental rats changes with time. (D) Relative AMPA mRNA expression in bilateral MNTB of normal or experimental rats changes with time. (E) Relative NMDA mRNA expression in bilateral MNTB of normal or experimental rats changes with time. a P<0.05 compared to 2 weeks, b P<0.05 compared to 4 weeks, c P<0.05 compared to 1 week. ‘−’ Means no significant difference compared to the other 2 groups. For all 5 neurotransmitter receptors, no significant changes of relative expression were found over time in normal control rats. However, in experimental rats, they decreased consistently at 4 weeks compared to 2 weeks in left MNTB (P<0.05).
Discussion
Asymmetric neurotransmitters and receptors induced by early-developmental SSD
In the present study, the experimental rats showed significant increases in all 5 neurotransmitter receptors in left MNTB at 2 weeks and all 3 neurotransmitters in right MNTB at 4 weeks. It is well known that the main input of MNTB is glutamatergic excitation from globular bushy cells located in the contralateral antero-ventral cochlear nucleus (AVCN) [24,25]. There are also GABAergic and glycinergic inputs from the ventral nucleus of the trapezoid body (VNTB) and MNTB itself [24,26]. In the critical period of auditory development, the auditory center is more likely to change along with the external environment. When the right cochlea is damaged at P12, few auditory stimuli can be received and transferred to the right cochlear nuclear (CN) and then output to the left MNTB by the calyx of Held, which means the cochlear inputs to the contralateral side of ablation are weakened [27]. The increasing number of neurotransmitter receptors at 2 weeks might be a compensatory response of the auditory center to enlarge the reduced input signal. However, increasing levels of neurotransmitters in the left MNTB can lead to a new imbalance between the bilateral MNTB, which needs more modification by the central auditory system, and this might be why GABA, Gly, and Glu increased in the right MNTB at 4 weeks. This can also be the result of central decompensation after long-term SSD. GABA and Gly increased in the right MNTB but decreased in the left at 4 weeks compared to 2 weeks, although there was no significant difference for Gly in left MNTB, which reflected enhanced inhibitory input from the normal ear and weakened inhibitory input from the deaf ear. The decompensated asymmetric inhibitory input from bilateral ear was also found at the cortex [6], which might be the fundamental cause of poor sound localization in SSD, even after CI. In addition, we speculate that in central nervous system plasticity, the regulation of neurotransmitter receptors takes precedence over neurotransmitters.
Glycine level increased over time
Early in development of the MNTB-LSO circuit, postsynaptic LSO neurons receive both GABAergic and glycinergic inputs from the ipsilateral MNTB [28–30], and with development, the Gly current increases to become dominant [31,32]. We also found that the Gly level increased over time in normal rats, but no difference was found in Gly-R. In the MNTB-LSO circuit, afferent neuronal soma were located in the MNTB, while receptors were located in postsynaptic LSO neurons [28], so if there is an increase in Gly-R, it should be found in LSO but not MNTB. In addition, as with the other inhibitory neurotransmitter, the level of GABA was not changed in the present study, although Gly was dominant. This is in agreement with a mouse study, which reported that after postnatal day 12, the absolute strength of glycinergic transmission increased markedly, while GABAergic signaling remained constant [33].
Neurotransmitter receptors reduction at 4 weeks after cochlear ablation
Development of the auditory system depends on the acquisition of auditory experience, and studies have shown that aural deprivation leads to numerous changes in the central nervous system, including decreasing volume and number of neurons in the spiral ganglion and in the central auditory nucleus [34]. After right cochlear ablation, auditory signals transferred contralaterally to the left MNTB were obviously decayed, and neuronal degeneration and apoptosis subsequently appeared. It is well known that neurotransmitter receptors are mainly expressed on the cell membrane and in the cytoplasm, which can be degraded with the apoptosis of neurons.
Effect of early-developmental SSD on sound localization
During sound localization, ILD is computed in the LSO and the contralateral precisely-timed inhibitory input from the MNTB is compared to the similarly precisely-timed excitatory input from the ipsilateral AVCN [35]. ITD is computed in the MSO, which is critically dependent on precisely-timed inhibitory inputs arising from the contralateral MNTB and the ipsilateral lateral nucleus of the trapezoid body (LNTB) [36–39]. Therefore, MNTB plays a vital role in calculation of both ILD and ITD [8]. Neurotransmitters and their receptors are the basis of the electrical signals of the nervous system. Asymmetric neurotransmitters and receptors induced by early-developmental SSD disturbed the central computation of binaural signals, which might be the fundamental cause of defective sound localization, even after CI.
Conclusions
Early-developmental SSD can lead to significant increases in neurotransmitter receptors of contralateral MNTB at 2 weeks and in neurotransmitters of ipsilateral MNTB at 4 weeks. However, expression of neurotransmitter receptors was obviously reduced at 4 weeks. Asymmetric neurotransmitters and receptors induced by early-developmental SSD might be the fundamental cause of poor sound localization, even after CI.
Footnotes
Source of support: The general work was supported by the Beijing Natural Science Foundation (7162072, 7162066) and the National Natural Science Foundation of China (81770993, 81641032)
References
- 1.Távora-Vieira D, Boisvert I, McMahon CM, et al. Successful outcomes of cochlear implantation in long-term unilateral deafness: Brain plasticity? Neuroreport. 2013;24(13):724–29. doi: 10.1097/WNR.0b013e3283642a93. [DOI] [PubMed] [Google Scholar]
- 2.Távora-Vieira D, De Ceulaer G, Govaerts PJ, Rajan GP. Cochlear implantation improves localization ability in patients with unilateral deafness. Ear Hear. 2015;36(3):e93–98. doi: 10.1097/AUD.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 3.Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev. 2010;90(3):983–1012. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- 4.Iwanicka-Pronicaka K, Pollak A, Skórka A, et al. Audio profiles in mitochondrial deafness m.1555A>G and m.3243A>G show distinct differences. Med Sci Monit. 2015;21:694–700. doi: 10.12659/MSM.890965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litovsky RY, Gordon K. Bilateral cochlear implants in children: Effects of auditory experience and deprivation on auditory perception. Hear Res. 2016;338:76–87. doi: 10.1016/j.heares.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon KA, Wong DD, Papsin BC. Bilateral input protects the cortex from unilaterally-driven reorganization in children who are deaf. Brain. 2013;136:1609–25. doi: 10.1093/brain/awt052. [DOI] [PubMed] [Google Scholar]
- 7.Chadha NK, Papsin BC, Jiwani S, Gordon KA. Speech detection in noise and spatial unmasking in children with simultaneous versus sequential bilateral cochlear implants. Otol Neurotol. 2011;32:1057–64. doi: 10.1097/MAO.0b013e3182267de7. [DOI] [PubMed] [Google Scholar]
- 8.Fei G, Berrebi AS. Forward masking in the medial nucleus of the trapezoid body of the rat. Brain Struct Funct. 2016;221(4):2303–17. doi: 10.1007/s00429-015-1044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sätzler K, Söhl LF, Bollmann JH, et al. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci. 2002;22(24):10567–79. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–54. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- 11.Smith PH, Joris PX, Yin TC. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J Neurophysiol. 1998;79:3127–42. doi: 10.1152/jn.1998.79.6.3127. [DOI] [PubMed] [Google Scholar]
- 12.Schmithorst VJ, Plante E, Holland S. Unilateral deafness in children affects development of multi-modal modulation and default mode networks. Front Hum Neurosci. 2014;8(5):164. doi: 10.3389/fnhum.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis D, Schmid K, O’Leary S, et al. Effects of noise on speech recognition and listening effort in children with normal hearing and children with mild bilateral or unilateral hearing loss. J Speech Lang Hear Res. 2016;59(5):1218–32. doi: 10.1044/2016_JSLHR-H-15-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kral A, Heid S, Hubka P, Tillein J. Unilateral hearing during development: Hemispheric specificity in plastic reorganizations. Front Syst Neurosci. 2013;7:93. doi: 10.3389/fnsys.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusu SI, Borst JG. Developmental changes in intrinsic excitability of principal neurons in the rat medial nucleus of the trapezoid body. Dev Neurobiol. 2011;71(4):284–95. doi: 10.1002/dneu.20856. [DOI] [PubMed] [Google Scholar]
- 16.Szpetnar M, Luchowska-Kocot D, Boguszewska-Czubara A, Kurzepa J. The influence of manganese and glutamine intake on antioxidants and neurotransmitter amino acids levels in rats’ brain. Neurochem Res. 2016;41(8):2129–39. doi: 10.1007/s11064-016-1928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22(8):3005–15. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monyer H, Burnashev N, Laurie DJ, et al. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 19.Cutting GR, Lu L, O’Hara BF, et al. Cloning of the γ-aminobutyric acid (GABA) ρ1cDNA: A GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci USA. 1991;88(7):2673–77. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 7th ed. Academic Press; 2011. p. 11. [DOI] [PubMed] [Google Scholar]
- 21.Crins TT, Rusu SI, Rodríguez-Contreras A, Borst JG. Developmental changes in short-term plasticity at the rat calyx of Held synapse. J Neurosci. 2011;31:11706–17. doi: 10.1523/JNEUROSCI.1995-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalabi W, Kopp-Scheinpflug C, Allen PD, et al. Sound localization ability and glycinergic innervation of the superior olivary complex persist after genetic deletion of the medial nucleus of the trapezoid body. J Neurosci. 2013;33(38):15044–49. doi: 10.1523/JNEUROSCI.2604-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Shi C, Li X, et al. Injection of oxytocin into paraventricular nucleus reverses depressive-like behaviors in the postpartum depression rat model. Behav Brain Res. 2017;336:236–43. doi: 10.1016/j.bbr.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht O, Dondzillo A, Mayer F, et al. Inhibitory projections from the ventral nucleus of the trapezoid body to the medial nucleus of the trapezoid body in the mouse. Front Neural Circuits. 2014;8(13):83. doi: 10.3389/fncir.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenn NJ, Reese TS. The fine structure of nerve endings in the nucleus of the trapezoid body and the ventral cochlear nucleus. Am J Anat. 1966;118(2):375–89. doi: 10.1002/aja.1001180205. [DOI] [PubMed] [Google Scholar]
- 26.Weisz CJ, Rubio ME, Givens RS, Kandler K. Excitation by axon terminal GABA spillover in a sound localization circuit. J Neurosci. 2016;36(3):911–25. doi: 10.1523/JNEUROSCI.1132-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Pradhan J, Maskey D, et al. Glutamate co-transmission from developing medial nucleus of the trapezoid body – lateral superior olive synapses is cochlear dependent in kanamycin-treated rats. Biochem Biophys Res Commun. 2011;405(2):162–67. doi: 10.1016/j.bbrc.2010.12.129. [DOI] [PubMed] [Google Scholar]
- 28.Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci. 1995;15(10):6890–904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrlich I, Lohrke S, Friauf E. Shift from depolarizing to hyperpolarizing glycine action in rat auditory neurones is due to age-dependent Cl- regulation. J Physiol. 1999;520:121–37. doi: 10.1111/j.1469-7793.1999.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullmann PH, Kandler K. Glycinergic/GABAergic synapses in the lateral superior olive are excitatory in neonatal C57BL/6J mice. Brain Res Dev Brain Res. 2001;131(1–2):143–47. doi: 10.1016/s0165-3806(01)00271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18(12):4646–55. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim G, Kandler K. Synaptic changes underlying the strengthening of GABA/glycinergic connections in the developing lateral superior olive. Neuroscience. 2010;171(3):924–33. doi: 10.1016/j.neuroscience.2010.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyes-Haro D, Rosas-Arellano A, González-González MA, et al. GABAρ expression in the medial nucleus of the trapezoid body. Neurosci Lett. 2013;532(1):23–28. doi: 10.1016/j.neulet.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Yuan W. Central plasticity and dysfunction elicited by aural deprivation in the critical period. Front Neural Circuits. 2015;9:26. doi: 10.3389/fncir.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tollin DJ. The lateral superior olive: A functional role in sound source localization. Neuroscientist. 2003;9:127–43. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- 36.Brand A, Behrend O, Marquardt T, et al. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417(6888):543–47. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- 37.Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: The role of glycinergic inhibition. J Neurosci. 2008;28(27):6914–25. doi: 10.1523/JNEUROSCI.1660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashida G, Carr CE. Sound localization: Jeffress and beyond. Curr Opin Neurobiol. 2011;21(5):745–51. doi: 10.1016/j.conb.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts MT, Seeman SC, Golding NL. The relative contributions of MNTB and LNTB neurons to inhibition in the medial superior olive assessed through single and paired recordings. Front Neural Circuits. 2014;8:49. doi: 10.3389/fncir.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]