Abstract
Background
Acute rejection is a common predisposing cause of allograft dysfunction in kidney transplantation. Recently, the B and T lymphocyte attenuator (BTLA)/herpes virus entry mediator (HVEM)/lymphotoxin (LIGHT)/CD160 pathway was found to be potentially involved in the regulation of T cell activation. This could mean that this pathway is involved in graft rejection in kidney transplantation; the present study aimed to explore this possibility.
Material/Methods
The expression of BTLA, HVEM, LIGHT and CD160 on peripheral CD4+, CD8+ and CD19+ lymphocytes were analyzed by flow cytometry in recipients with biopsy-proven acute rejection (BPAR) or stable allograft function, as well as in healthy volunteers. Moreover, we performed HE staining and immunohistochemical staining to assess the expression of BTLA and HVEM in kidney samples from recipients with BPAR and patients who underwent the surgery of radical nephrectomy.
Results
We observed the significantly lower expression of BTLA on CD4+ T cells in recipients from the BPAR group than in recipients from the stable group. The expression of BTLA on CD8+ T cells among recipients both from the BPAR and stable group was statistically increased than that in the healthy volunteers. A significant difference in the expression of CD160 in the stable group was found when compared with the BPAR group or control group. Moreover, there was no significance in the expression of HVEM, LIGHT or CD160 on other subtypes of T cells between the 3 groups or in the expression of BTLA on CD4+ T cells between the BPAR and control group.
Conclusions
The findings indicate that the BTLA/HVEM pathway does be involved in pathogenesis of acute rejection following kidney transplantation, as well as the induction of transplant tolerance. This pathway may therefore be a useful target for therapy against acute rejection after kidney transplantation.
MeSH Keywords: Antigens, CD4; Kidney Transplantation; Rejection (Psychology)
Background
Kidney transplantation remains to be one of the optimal therapies for patients with end-stage renal disease [1]. With the introduction of novel surgical techniques and potent immunosuppressive agents that target T lymphocytes, short-term allograft and recipient survival has dramatically increased [2,3]. However, long-term allograft survival after kidney transplantation is still disappointingly poor on account of various immunological and non-immunological factors [4,5]. Among these factors, acute rejection is a crucial one that contributes to the progression of long-term allograft dysfunction [6,7]. However, the molecular and cellular mechanisms of acute rejection in renal transplant patients are unclear and need to be further investigated.
The activation of T cells located in allografts is acknowledged as an important mechanism underlying acute graft rejection. As important factors associated with T lymphocyte activation and proliferation, co-stimulatory molecules are indicated to be crucial in regulating immune response. Further, the strategies for inhibiting positive molecules or promoting negative molecules appear to be beneficial for the prevention and treatment of acute rejection following kidney transplantation, and more importantly, for the induction and maintenance of immune tolerance in solid organ transplant recipients. Therefore, it is possible that co-stimulatory molecules which regulate T lymphocyte activation and tolerance play a crucial role in the occurrence and progression of acute rejection following kidney transplantation [8]. B- and T- lymphocyte attenuator (BTLA), which is expressed on the majority of lymphocytes, is an novel inhibitory co-stimulatory molecule that comes from the CD28 super family and negatively regulates immune responses [9,10]. Another important molecule is herpes virus entry mediator (HVEM), also known as TNFRSF14, serves as a shared ligand for co-stimulatory receptors including LIGHT/lymphotoxin, and co-inhibitory receptors, including BTLA and CD160 [11,12]. In the HVEM-mediated signaling network, engagement of HVEM by LIGHT induces co-stimulatory signals that lead to activation of T lymphocytes and enhancement of T cell-mediated immune response. On the other hand, when HVEM interacts with BTLA and CD160, it inhibits T cell activation [13–15]. Numerous studies have demonstrated the role of HVEM as a ligand that induces co-inhibitory signals by engaging with BTLA and CD160, and its inhibitory function appears to be dominant over its stimulatory role via LIGHT ligation [16–19]. With regard to BTLA, its role in modulating the immune response has been described, but little is known with respect to its function in acute rejection after renal transplant [20,21]. Considering the crucial role of the HVEM co-stimulatory molecule system in acute rejection episodes, the BTLA/HVEM pathway warrants more attention as a suitable molecular target for the prevention and treatment of acute rejection and the induction of immune tolerance among renal transplant recipients.
In this study, we aimed to examine the role of BTLA/HVEM pathway in the pathogenesis of acute rejection among allograft recipients; to this end, we examined the expression of BTLA and its specific receptor HVEM, as well as LIGHT and CD160 on peripheral blood mononuclear cells (PBMCs) of renal transplant recipients with stable allograft function and biopsy-proven acute rejection (BPAR), as well as the distribution and expression of BTLA and HVEM in renal samples from recipients with BPAR.
Material and Methods
Ethics statement
The study protocol was in accordance with the ethical standards of the Declarations of Helsinki and Istanbul. As the procedures were limited to living-related transplantation of kidney tissues to lineal or collateral relatives not beyond the third degree of kinship or cadaveric allograft donors of cardiac death, the protocol of this study was approved by the local ethics committee of the First Affiliated Hospital with Nanjing Medical University. Written informed consent was obtained from all recipients and healthy volunteers. None of the transplant donors were from a vulnerable population, and all donors or next of kin freely provided their written informed consent for participation.
Reagents
Antibodies against BTLA and HVEM were purchased from Abcam (Cambridge, MA, USA). FITC-labeled anti-human CD4, FITC-labeled anti-human CD8 and APC-labeled anti-human CD19 were purchased from BD Pharmingen (Franklin Lake, NJ, USA). PE-labeled anti-human BTLA, PE-labeled anti-human HVEM, PE-labeled anti-human CD160 and PE-labeled anti-human LIGHT were purchased from Biolegend (San Diego, CA, USA).
Sample collection
A total of 10 allograft segments and blood samples, which were obtained from recipients who received the surgery of kidney transplantation from December 1st, 2013 to December 1st, 2015 at our center were collected in the BPAR group of our study. The inclusion criteria were as follows: (1). Patients were diagnosed with BPAR, which were confirmed by the HE staining and immunohistological staining according to the Banff07 criteria; (2). Patients did not experience the episodes of delayed graft dysfunction (DGF), opportunistic infections or post-transplant malignancy; (3). Patients did not suffer from the chronic virus infections, such as AIDS, chronic viral hepatitis B and C; (4). Patients were older than 18 years old and younger than 60 years old. The exclusive criteria contain: (1). Patients who did not fulfill the inclusive criteria; (2). Patients with the existing infections; (3). Pregnant. Moreover, we collected 10 cases of blood samples from renal transplant recipients who went through kidney transplantation between January 1st, 2012 and December 1st, 2014 at our center and had stable renal function (stable group). Patients were considered to have stable renal function if their serum creatinine (Scr) level was less than 120 μmol/L for at least 3 months after kidney transplantation. In addition, 10 normal kidney samples were obtained from patients who underwent the surgery of radical nephrectomy; each sample was collected under the requirements of more than 5 cm away from the tumor tissue. Blood samples were also gathered from 10 healthy volunteers (considered as control group). Peripheral blood samples were kept in BD Vacutainer tubes which contained sodium heparin. In the case of renal recipients with BPAR, blood samples were obtained immediately when the clinical symptoms occurred and before the allograft biopsy and administration of high-dose chemotherapy with methylprednisolone.
Flow cytometry analysis
For analysis of BTLA, HVEM, LIGHT and CD160 expression on CD4+, CD8+ and CD19+ lymphocytes in the PBMCs of patients from the BPAR, stable and control group, we incubated 100 μl of whole blood with FITC-labeled antibodies against CD4 or CD8 or APC-labeled antibodies against CD19, and PE-labeled antibodies against BTLA, HVEM, LIGHT or CD160 at 4°C for 30 min under a dark surrounding. Then, we added streptavidin-PE antibodies, and incubated with the cells at 4°C for another 30 min under a dark surrounding. Then, we washed the cells with phosphate buffer saline (PBS) after hemolysis.
To enable correct compensation and to confirm the specificity of the antibodies, isotype controls were used. We assessed the stained cells on the EP-ICS2XL flow cytometer (Beckman Coulter, Brea, CA, USA) and analyzed using the FlowJo software (Treestar, Ashland, OR, USA).
Hematoxylin-eosin staining
To detect morphological changes in kidney tissues from healthy volunteers and renal transplant recipients, HE staining was performed. Paraffin were embedded in arterial sections and immersed with 1% silver nitrate for 60 min, 5% sodium thiosulfate for 2 min and hematoxylin for 1 min. Images of HE-stained slides were captured, and the Banff score of the 2 groups was determined by 2 independent authors (WL Zhou and RY Tan) under microscope with a digital camera (Nikon, ECLIPSE 80i, Japan). To accurate diagnosis of acute rejection among renal recipients, the criteria of Banff 2007 were applied.
Immunohistochemical staining
Immunohistochemical (IHC) staining assays were conducted to determine the distribution and degree of expression of BTLA and HVEM in kidney tissues from transplant recipients with BPAR and the control group. Five-micrometer sections were deparaffinized in xylene and rehydrated in a graded series of alcohol. Block of non-specific epitopes with 5% normal goat serum were performed for 30 min and then incubated with anti-BTLA (1: 100) and anti-HVEM (1: 100) primary antibodies at 4°C overnight. Incubation with biotinylated goat anti-mouse/rabbit IgG (5.0 μg/ml; Abcam, USA) were carried out for 1 h. Images of immunohistochemically stained slides were captured, and analyzed with TissueQuest software (TissueGnostics), in which the hematoxylin for nuclei, BTLA and HVEM were considered as markers. To evaluate positive staining, scattergrams were created allowing the visualization of corresponding positive cells in the source region of interest using the real-time back-gating features by 2 independent authors (HW Yang and RY Tan). The number of BTLA/HVEM-positive cells in the kidney samples was determined from 3 randomly selected HPF (0.2 μm2/each).
Statistical analysis
All data were expressed as the mean ±SD values. We used the unpaired Student’s t test to analyze differences between 2 groups. Comparisons between multiple treatment groups and the control group were made using a one-way analysis of variance (ANOVA) according to Dunnett’s post hoc test. P values less than 0.05 were considered to indicate statistical significance. All statistical analysis was conducted by the STATA 12.0 Version software (College Station, TX, USA).
Results
Demographic and clinical profiles of patients
The clinical profiles of the patients included within our study are presented in Table 1. No significant difference was observed in age, gender or transplant duration between the stable group and BPAR group. Importantly, the Scr and serum blood urea nitrogen (BUN) levels in the BPAR group were significantly higher when compared to those in the stable group (369.90±125.1 μmol/L vs. 85.35±4.17 μmol/L, P<0.01 [Scr]; 16.85±2.14 mmol/L vs. 5.70±0.44 mmol/L, P<0.001 [BUN]).
Table 1.
Basic characteristics of patients involved in our study.
| Control | Stable | BPAR | |
|---|---|---|---|
| n | 10 | 10 | 10 |
| Age (years; mean ±SD) | 42.50±3.89 | 32.70±3.30 | 36.40±3.59 |
| Gender (Male/Female) | 5/5 | 8/2 | 9/1 |
| Transplant duration (months; range) | – | 1.5 (0.8–3.2) | 0.8 (0.1–3.6) |
| Primary/secondary kidney transplant | – | 10/0 | 10/0 |
| Donor source | |||
| Living-related | – | 2 | 1 |
| Cadaveric | – | 8 | 9 |
| Immunosuppressive regimen | |||
| Prednisone + MMF + CsA | – | 5 | 5 |
| Prednisone + MMF + Tac | – | 5 | 5 |
| Biochemical parameters | |||
| Serum creatinine (μmol/L; mean ±SD) | 74.81±2.98 | 85.35±4.17 | 369.90±125.1# |
| BUN (mmol/L; mean ±SD) | 4.54±0.34 | 5.70±0.44 | 16.85±2.14* |
P<0.01 vs. stable group;
P<0.001 vs. stable group.
BPAR – biopsy-proven acute rejection; MMF – mycophenolate mofetil; CsA – cyclosporine A; Tac – tacrolimus; BUN – blood urea nitrogen; SD – standard deviation; NS – no significance.
Expression of BTLA, HVEM, LIGHT and CD160 on CD4+, CD8+ and CD19+ lymphocytes in renal transplant recipients with BPAR
Expression of co-stimulatory molecules on peripheral CD4+ T cells
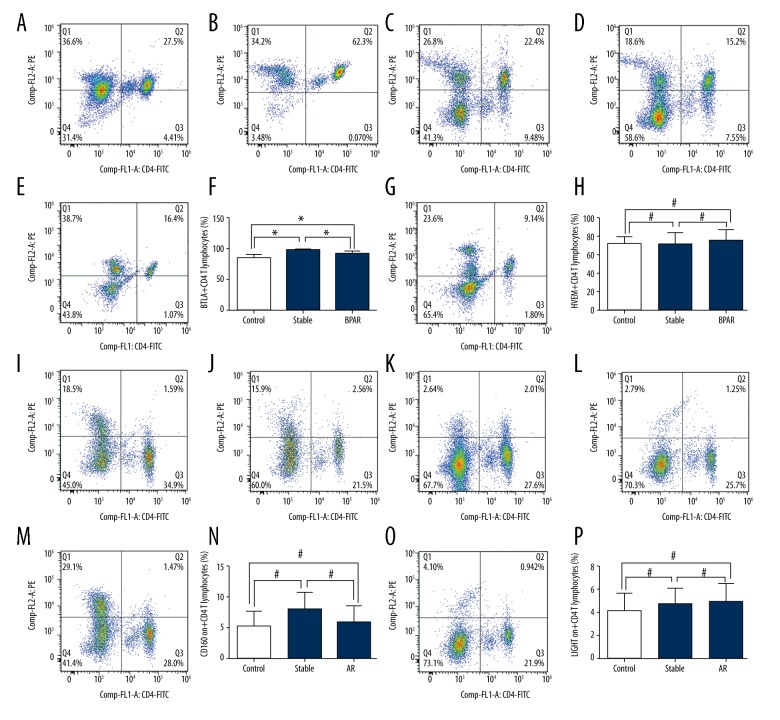
In peripheral CD4+ T cells, the expression of BTLA in recipients with BPAR was statistically decreased than that in recipients with stable allograft function (91.41±1.40% vs. 98.19±0.72%, P<0.001; Figure 1A–1D). Moreover, BTLA expression in recipients from the stable group was remarkably higher than that in healthy volunteers (98.19±0.72% vs. 90.06±1.48%, P<0.001; Figure 1A–1D). We did not observe any significant difference in the expression of BTLA between recipients with BPAR and healthy volunteers (BPAR: 91.41±1.40%, control: 90.06±1.48%, P>0.05; Figure 1A–1D). In addition, no significant difference was found in the expression of HVEM, LIGHT or CD160 between the BPAR group, stable group or control group (Figure 1E–1P). The overall expressions of BTLA/HVEM/LIGHT/CD160 molecules on the CD4+, CD8+ T cells and CD19+ B cells are presented in the Table 2.
Figure 1.
(A–P) Expression of BTLA, HVEM, LIGHT and CD160 on peripheral CD4+ T cells of healthy volunteers and renal transplant recipients with biopsy-proven acute rejection and stable allograft function.
Table 2.
Summary of the expressions of BTLA/HVEM/LIGHT/CD160 on CD4+, CD8+ and CD19+ lymphocytes.
| BTLA (%) | HVEM (%) | LIGHT (%) | CD160 (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | CD19+ | CD4+ | CD8+ | CD19+ | CD4+ | CD8+ | CD19+ | CD4+ | CD8+ | CD19+ | |
| BPAR | 91.41 ± 1.40** | 94.60 ± 1.63 | 82.58 ± 2.34 | 75.37 ± 3.72 | 69.50 ± 4.14 | 63.67 ± 3.40 | 4.90 ± 0.56 | 8.08 ± 3.22 | 1.91 ± 1.49 | 5.77 ± 1.06 | 38.53 ± 3.81* | 10.78 ± 1.32 |
| Stable | 98.19 ± 0.72 | 98.10 ± 0.46 | 83.90 ± 1.79 | 70.64 ± 4.86 | 62.90 ± 6.69 | 62.70 ± 5.49 | 4.71 ± 0.51 | 4.15 ± 1.13 | 3.059 ± 2.29 | 7.87 ± 1.05 | 55.84 ± 5.10 | 13.55 ± 1.54 |
| Healthy volunteers | 84.66 ± 1.76## | 85.00 ± 2.39## | 72.04 ± 3.87# | 71.58 ± 2.53 | 55.96 ± 6.09 | 67.33 ± 6.63 | 4.15 ± 0.48 | 5.15 ± 1.19 | 2.22 ± 0.30 | 5.26 ± 0.75 | 36.38 ± 4.97# | 9.74 ± 1.59 |
P<0.05;
P<0.001;
P<0.05;
P<0.0001.
BTLA – B- and T-lymphocyte attenuator; HVEM – herpes virus entry mediator; LIGHT – lymphotoxin; BPAR – biopsy-proven acute rejection.
Expression of co-stimulatory molecules on peripheral CD8+ T cells
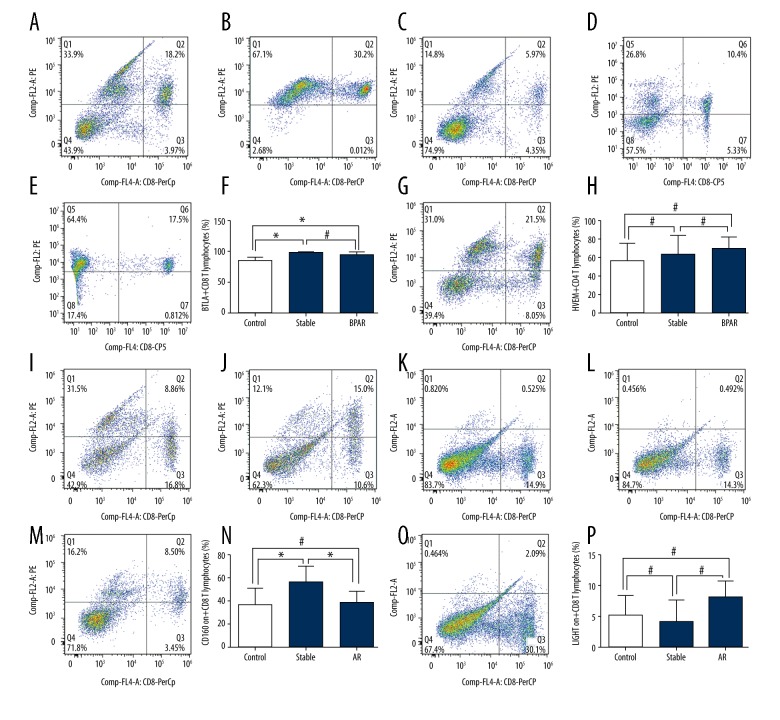
In peripheral CD8+ T cells, recipients from both the BPAR and stable groups showed significantly higher expression of BTLA than those in the control group (BPAR: 94.60±1.63%, stable: 98.10±0.46%, control: 85.00±2.39%, P<0.01 for control group vs. the BPAR group or stable group; Figure 2A–2D). Moreover, there was no significant difference in the expression of BTLA between the BPAR and stable group (BPAR: 94.60±1.63%, stable: 98.10±0.46%, P>0.05; Figure 2A–2D). Similarly, we did not observe any significant difference in the expression of HVEM and LIGHT between patients from the BPAR, stable or control groups (Figure 2E–2H, 2M–2P). In addition, we found a remarkable significance in the expression of CD160 in the stable group compared to the BPAR group and control group (stable: 55.84±5.10%, BPAR: 38.53±3.81%, control: 36.38±4.97%, P<0.05, for stable group vs. the BPAR group or control group; Figure 2I–2L).
Figure 2.
(A–P) Expression of BTLA, HVEM, LIGHT and CD160 on peripheral CD8+ T cells of healthy volunteers and renal transplant recipients with biopsy-proven acute rejection and stable allograft function.
Expression of co-stimulatory molecules on peripheral CD19+ cells
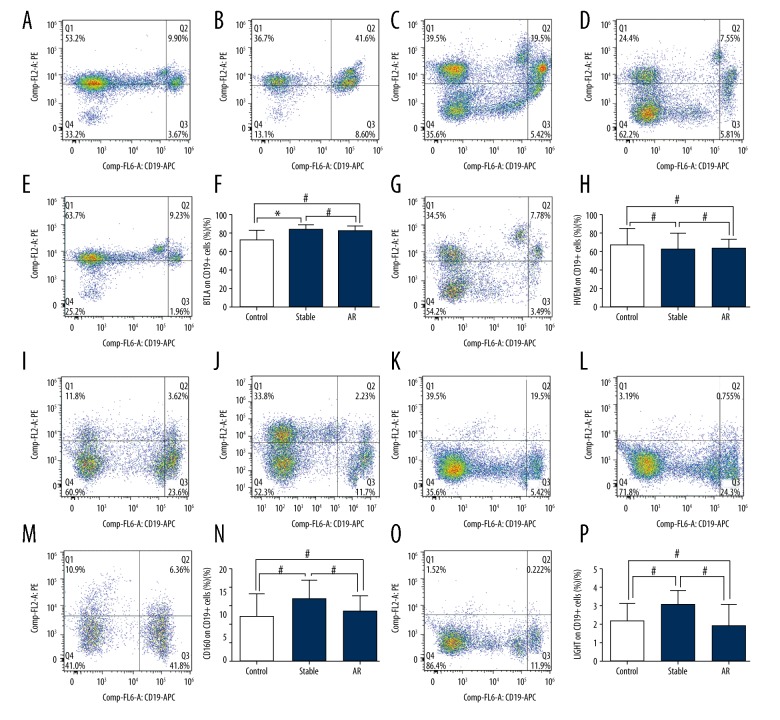
In peripheral CD19+ cells, BTLA expression was statistically increased in the stable group than in the control group (83.90±1.79% vs. 72.04±3.89%, P<0.05; Figure 3A–3D). However, no remarkable significance was observed between the BPAR group and stable group or between the BPAR and control group (BPAR: 82.58±2.34%, stable: 83.90±1.79%, P>0.05; BPAR: 82.58±2.34%, control: 72.04±3.89%, P>0.05; Figure 3A–3D). In addition, no significant difference was found in the expression of HVEM, LIGHT or CD160 between the 3 groups (Figure 3E–3P).
Figure 3.
(A–P) Expression of BTLA, HVEM, LIGHT and CD160 on peripheral CD19+ cells of healthy volunteers and renal transplant recipients with biopsy-proven acute rejection and stable allograft function.
Expression of BTLA and HVEM in allograft samples from renal recipients with BPAR
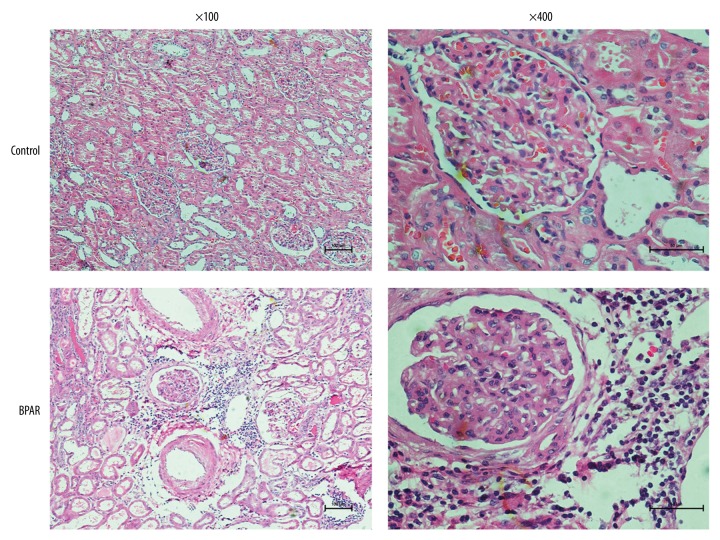
Figure 4 shows the histological features of renal samples obtained from renal recipients with BPAR and healthy volunteers after HE staining. Compared to renal samples from the control group, significant mononuclear cell infiltration and edema were observed in the allograft interstitial tissue sections from the BPAR group; this was in accordance with the diagnosis of BPAR based on the biopsy sample.
Figure 4.
Histological features of renal tissue obtained from renal transplant recipients with biopsy-proven acute rejection and healthy volunteers.
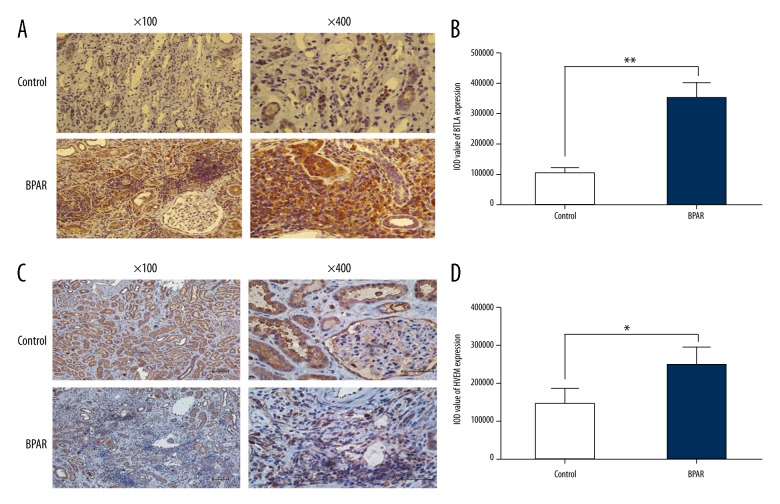
IHC staining assays were performed to analyze the expression of BTLA and HVEM in renal samples of the control group and BPAR group. The results showed significant overexpression of BTLA and HVEM in allograft samples from the BPAR group when compared with the control group (Figure 5A–5D).
Figure 5.
(A–D) Expression of BTLA and HVEM in kidney samples of healthy volunteers and renal transplant recipients with biopsy-proven acute rejection.
Discussion
In this research, we focused on the expression and potential mechanisms of a novel co-stimulatory network involving BTLA, LIGHT, and CD160 and their specific receptor HVEM in renal transplant recipients with BPAR.
BTLA, a newly identified negative co-stimulatory molecule, is structurally and functionally similar to the T cell inhibitory receptors cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) [9,22,23]. However, unlike CTLA-4 and PD-1, which combine with specific receptors from B7 family members, BTLA combines with its specific receptor HVEM [11,16]. In our study, we found out that expression of the inhibitory co-stimulatory molecule BTLA on CD4+ T cells dramatically declined among recipients with BPAR in comparison of those with stable allograft function. In the case of T lymphocytes, it has been reported that BTLA is quickly distributed across activated human Th1- and Th2-polarized CD4 T cells, and is selectively induced in Th1 cells but not in Th2 cells after tertiary activation [24]. Consequently, the significant results of BTLA on CD4+ T cells observed in our study indicates that the activation of CD4+ T cells is remarkably higher during acute rejection after kidney transplantation and contributes to the progression of mononuclear cell infiltration in allograft interstitial tissue. Unfortunately, we did not analyze BTLA expression on subgroups of CD4+ T cells, including Th1, Th2 and Treg cells.
The role of BTLA in the field of transplantation has been explored thoroughly in models of transplantation. On initiation of allogeneic hematopoietic stem cell transplantation, targeting BTLA with monoclonal therapy has been proven to be effective in the treatment of graft-versus-host disease [25]. Moreover, in the cardiac transplantation model, in vivo administration of antagonistic anti-BTLA mAb (Clone 6A6) led to accelerated rejection; more importantly, MHC class II-mismatched cardiac allografts showed acute rejection faster in PD-1/BTLA double-deficient recipients than in PD-1- or BTLA-deficient mice [26]. In the present study, BTLA/HVEM was found to be crucial in the pathogenesis of acute rejection among renal transplant recipients. Similar to our study, a sirolimus-based study observed the beneficial effects of sirolimus in the induction of transplant tolerance by targeting the BTLA/HVEM inhibitory pathway [27]. Furthermore, in an original clinical study, the total mRNA and protein expression of BTLA/HVEM in the serum significantly increased during episodes of acute rejection, which could be considered to be an early indicator of acute rejection following kidney transplantation [28]. In our study, the total protein expression of BTLA/HVEM observed in the BPAR and control groups was consistent with our IHC staining assay results. These results in combination with our results show that the BTLA/HVEM pathway may be involved in the negative modulation of T cell activation and the induction of transplant tolerance.
There are some limitations to our study. Due to the difficulty involved in the collection of fresh blood samples from patients with BPAR, the number of recipients enrolled in this study was low. Moreover, more subtypes of immune cells, such as Treg cells, natural kill cells and dendritic cells, should be further investigated based on the current outcomes. Therefore, a large-scale, well-designed, comprehensive research study is required to confirm and further explore the present findings.
Conclusions
In conclusion, our study indicated that the expression of BTLA on CD4+ T cells from recipients with BPAR was significantly lower than that in recipients with stable allograft function. In CD8+ T cells, significantly higher expression of BTLA was observed among the BPAR and stable group when compared with the control group. Our findings provide novel insight into the BTLA/HVEM signaling pathway, with regard to its role in acute rejection after kidney transplantation, and the induction of transplant tolerance.
Acknowledgments
We would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Footnotes
Conflict of interests
None.
Source of support: This work was supported the National Natural Science Foundation of China [grant numbers 81570676, 81100532, 81470981], the Science and Education Health Project of Jiangsu Province for Important Talent [grant number RC2011055], the “333 High Level Talents Project” in Jiangsu Province (2011 and 2013) [grant number BRA2015469], the Jiangsu Province Six Talents Peak from Department of Human Resources, Social Security Office of Jiangsu Province of China [grant numbers 2010WSN-56, 2011-WS-033], the General Program of Department of Health of Jiangsu Province of China [grant number H2009907], and the Priority Academic Program Development of Jiangsu Higher Education Institutions [grant number JX10231801]
References
- 1.Wang Z, Han Z, Tao J, et al. Clinical efficacy and safety of pamidronate therapy on bone mass density in early post-renal transplant period: A meta-analysis of randomized controlled trials. PloS One. 2014;9(9):e108106. doi: 10.1371/journal.pone.0108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nickel P, Bestard O, Volk HD, Reinke P. Diagnostic value of T-cell monitoring assays in kidney transplantation. Curr Opin Organ Transplant. 2009;14(4):426–31. doi: 10.1097/MOT.0b013e32832c5999. [DOI] [PubMed] [Google Scholar]
- 3.Snijder PM, van den Berg E, Whiteman M, et al. Emerging role of gasotransmitters in renal transplantation. Am J Transplant. 2013;13(12):3067–75. doi: 10.1111/ajt.12483. [DOI] [PubMed] [Google Scholar]
- 4.Maamoun HA, Soliman AR, Fathy A, et al. Diabetes mellitus as predictor of patient and graft survival after kidney transplantation. Transplant Proc. 2013;45(9):3245–48. doi: 10.1016/j.transproceed.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Gilmore D, Dib M, Evenson A, et al. Endovascular management of critical limb ischemia in renal transplant patients. Ann Vasc Surg. 2014;28(1):159–63. doi: 10.1016/j.avsg.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Lebranchu Y, Baan C, Biancone L, et al. Pretransplant identification of acute rejection risk following kidney transplantation. Transpl Int. 2014;27(2):129–38. doi: 10.1111/tri.12205. [DOI] [PubMed] [Google Scholar]
- 7.Cicora F, Mos F, Petroni J, et al. Belatacept-based, ATG-Fresenius-induction regimen for kidney transplant recipients: A proof-of-concept study. Transpl Immunol. 2015;32(1):35–39. doi: 10.1016/j.trim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Dooms H, Abbas AK. Control of CD4+ T-cell memory by cytokines and costimulators. Immunol Rev. 2006;211:23–38. doi: 10.1111/j.0105-2896.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–79. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez LC, Loyet KM, Calemine-Fenaux J, et al. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci USA. 2005;102(4):1116–21. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: A bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229(1):244–58. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg MW, Cheung TC, Ware CF. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev. 2011;244(1):169–87. doi: 10.1111/j.1600-065X.2011.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrop JA, Reddy M, Dede K, et al. Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J Immunol. 1998;161(4):1786–94. [PubMed] [Google Scholar]
- 14.Wang J, Lo JC, Foster A, et al. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest. 2001;108(12):1771–80. doi: 10.1172/JCI13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granger SW, Rickert S. LIGHT-HVEM signaling and the regulation of T cell-mediated immunity. Cytokine Growth Factor Rev. 2003;14(3–4):289–96. doi: 10.1016/s1359-6101(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 16.del Rio ML, Lucas CL, Buhler L, et al. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol. 2010;87(2):223–35. doi: 10.1189/jlb.0809590. [DOI] [PubMed] [Google Scholar]
- 17.Adams AB, Larsen CP, Pearson TC, Newell KA. The role of TNF receptor and TNF superfamily molecules in organ transplantation. Am J Transplant. 2002;2(1):12–18. doi: 10.1034/j.1600-6143.2002.020104.x. [DOI] [PubMed] [Google Scholar]
- 18.Harrop JA, McDonnell PC, Brigham-Burke M, et al. Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998;273(42):27548–56. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- 19.Sedy JR, Gavrieli M, Potter KG, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6(1):90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 20.Pasero C, Truneh A, Olive D. Cosignaling molecules around LIGHT-HVEM-BTLA: From immune activation to therapeutic targeting. Curr Mol Med. 2009;9(7):911–27. doi: 10.2174/156652409789105589. [DOI] [PubMed] [Google Scholar]
- 21.Saito T, Yamasaki S. Negative feedback of T cell activation through inhibitory adapters and costimulatory receptors. Immunol Rev. 2003;192:143–60. doi: 10.1034/j.1600-065x.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 22.Veillette A, Latour S, Davidson D. Negative regulation of immunoreceptor signaling. Ann Rev Immunol. 2002;20:669–707. doi: 10.1146/annurev.immunol.20.081501.130710. [DOI] [PubMed] [Google Scholar]
- 23.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Ann Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 24.Hurchla MA, Sedy JR, Gavrieli M, et al. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174(6):3377–85. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 25.Albring JC, Sandau MM, Rapaport AS, et al. Targeting of B and T lymphocyte associated (BTLA) prevents graft-versus-host disease without global immunosuppression. J Exp Med. 2010;207(12):2551–59. doi: 10.1084/jem.20102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao R, Wang L, Han R, et al. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J Immunol. 2005;175(9):5774–82. doi: 10.4049/jimmunol.175.9.5774. [DOI] [PubMed] [Google Scholar]
- 27.Bai Y, Shi Y, Li Y, et al. Sirolimus-based regimen promotes inhibitory costimulatory signal of HVEM/BTLA/CD160/LIGHT pathway in allo-renal recipients. Transpl Immunol. 2013;28(1):38–47. doi: 10.1016/j.trim.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Tian C, Liu YG, Yan JK, et al. B- and T-lymphocyte attenuator/herpes virus entry mediator as early indicators for acute rejection following kidney transplantation. Transplant Proc. 2013;45(1):157–62. doi: 10.1016/j.transproceed.2012.10.019. [DOI] [PubMed] [Google Scholar]