Abstract
Introduction
Chronic obstructive pulmonary disease (COPD) is a major and growing cause of morbidity and mortality worldwide. The global prevalence of COPD is growing faster in women than in men. Women are often exposed to indoor pollutants produced by biomass fuels burning during household activities.
Methods
We conducted a meta-analysis to establish the association between COPD and exposure to biomass smoke in women.
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, we searched MEDLINE and Scopus databases in 31December 2016, with the terms: “wood”, “charcoal”, “biomass”, “solid fuels”, “organic fuel”, “biofuel”, “female”, “women”, “COPD”, “chronic bronchitis”, “emphysema”, “chronic obstructive pulmonary disease”. Studies were eligible if they were case–control or cross-sectional studies involving exposure to indoor biomass smoke, conducted at any time and in any geographic location. Fixed-effects or random-effects meta-analysis was used to generate pooled OR.
Results
24 studies were included: 5 case–control studies and 19 cross-sectional studies. Biomass-exposed individuals were 1.38 times more likely to be diagnosed with COPD than non-exposed (OR 1.38, 95% CI 1.28 to 1.57).
Spirometry-diagnosed COPD studies failed to show a significant association (OR 1.20, 95% CI 0.99 to 1.40). Nevertheless, the summary estimate of OR for chronic bronchitis (CB) was significant (OR 2.11, 95% CI 1.70 to 2.52). The pooled OR for cross-sectional studies and case–control studies were respectively 1.82 (95% CI 1.54 to 2.10) and 1.05 (95% CI 0.81 to 1.30). Significant association was found between COPD and biomass smoke exposure for women living as well in rural as in urban areas.
Conclusions
This study showed that biomass smoke exposure is associated with COPD in rural and urban women.
In many developing countries, modern fuels are more and more used alongside traditional ones, mainly in urban area. Data are needed to further explore the benefit of the use of mixed fuels for cooking on respiratory health, particularly on COPD reduction.
Keywords: copd epidemiology, occupational lung disease, emphysema, clinical epidemiology
Key messages.
What is the relation between chronic obstructive pulmonary disease (COPD) and biomass smoke exposure for women living in rural and in urban areas?
Significant association was found between COPD and biomass smoke exposure for women living as well in rural as in urban areas.
This study provides detailed analysis of the association between COPD and biomass smoke exposure in women. This study shows that energy poverty continues to be a public health problem both in rural and in urban areas. Designing policies to reduce energy poverty would alleviate the burden of chronic respiratory diseases of women and improve their living conditions.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major and growing cause of morbidity and mortality worldwide.1 WHO estimates COPD to be the tenth leading cause of disability-adjusted life-years in all countries.2 According to WHO, 65 million people suffer from moderate to severe COPD and >3 million people died of COPD in 2015, among which 90% death occurs in low-income and middle-income countries.3 COPD deaths have been increasing these past years, and projections suggest it could become the third leading cause of death by 2030.4 More common in men once upon a time, COPD now affects almost equally men and women.3
Among the various risk factors, the most important is tobacco smoking as it happens in men and women in high-income and middle-income countries.1 5 However, in low-income, middle-income countries, 35% of patients with COPD have developed the disorder after a chronic exposure to indoor smoke from biomass fuels burning.2 6
One-third of the world’s population use biomass fuel, like wood, crop residues such as straw and sticks, dried leaves, twigs, wild grass, animal dung or charcoal, for cooking and/or heating.7 The smoke from these organic fuels increases the incidence of respiratory illness such as COPD.8 WHO has estimated that indoor air pollution from solid fuel use is responsible for 2.6% of the total global burden of disease.9 The use of biomass has contributed to >577 000 premature deaths in Africa and 74 000 in Latin Americas, in 2012.9 Exposure to indoor pollutants produced by biomass fuels burning is particularly high among women and young children,10 leading to 2 million deaths each year.11 Many of these deaths occur either in children under 5 years of age, primarily due to acute lower respiratory infection such pneumonia or in adult women due to COPD.9
The prevalence of COPD is two to three times higher in rural women exposed to biomass smoke compared with urban women who are considerably less exposed.11 12
The worldwide prevalence of COPD is growing faster in women than in men.13 Over the past two decades, COPD-related mortality rates have also increased faster for women, and since the year 2000 more women than men have died from COPD.13 Many individual studies have been conducted worldwide to evaluate the relation between energy choice for cooking and COPD, and these studies lead to a wide variation in findings. Notwithstanding, controversies remain concerning the link between biomass fuel use and COPD in women. The meta-analyses previously conducted have either analysed the link between COPD and solid fuel14 or the association between the disease and biomass exposure in adults. The one meta-analysis that focuses on women only analysed studies in a rural environment and considered so many respiratory diseases that did not allow an in-depth assessment of the relation between COPD and indoor biomass smoke exposure in women. It remains a field that still requires considerable attention. Thus, we conducted a systematic review and meta-analysis to highlight the relationship between COPD and domestic biomass fuel use in women.
Methodology
Search strategy
Using MEDLINE and Scopus database, we did a systematic literature search according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines up to 31 December 2016, with keywords including “wood”, “charcoal”, “biomass”, “solid fuels”, “organic fuel”, “biofuel”, “female”, “women”, “COPD”, “chronic bronchitis”, “emphysema”, “chronic obstructive pulmonary disease”.
The same search terms were used for both databases.
The search was restricted to English and French languages. No limitations were set for participants’ ages. Studies were considered if they estimate the association between COPD and biomass smoke exposure.
To better fulfil our objectives, studies searching was based on the participants, interventions, comparators, outcomes and study design approach. Participants were women, cooking or cooking and heating with biomass fuels represented the exposure (intervention), cooking or cooking and heating with non-biomass fuel or clean fuel were the comparator and the outcomes were COPD phenotypes (COPD, chronic bronchitis (CB), emphysema). We considered case–control or cross-sectional studies.
So, studies were eligible if comparing exposure to biomass smoke to exposure to other fuels, conducted at any time and in any geographic location. They had to use case–control or cross-sectional designs. Papers had to provide calculable or reported ORs to estimate the association between COPD and biomass smoke with corresponding 95% CI in female; and they had to be based on an independent set of data from other studies. References in each of the identified papers were screened for any additional article that had not been identified in the original search. Articles were excluded when they could not distinguish statistical association between exposure to biomass smoke and respiratory diseases found in women from men. Studies without a proper comparator were also discarded.
Studies were included in the final analysis when they had considered COPD as an airflow limitation that is not fully reversible (assessed by postbronchodilator spirometry), either according to the American Thoracic Society/European Respiratory Society criteria15 (postbronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio ≤ 0.70) or the Global Initiative for Obstructive Lung Disease criteria16 (presence of a postbronchodilator FEV1/FVC ratio <0.70) or the method of FEV1/FVC below the lower limit of normal value. Case–control studies based on medical confirmed case of COPD are also analysed as well as studies that had considered CB according to the British Medical Research Council criteria ‘daily productive cough for at least 3 consecutive months for more than 2 successive years’.17
Studies selection and data extraction were performed by two of the authors (AS and SMAS), following the established protocol and the consensual data extraction table. Disagreements were resolved by consensus, but sometimes the issue was discussed with CB.
Data management
We screened titles, abstracts and full texts according to study eligibility (inclusion criteria), and data were extracted using an internally validated data extraction form. When required, additional information was obtained from authors.
Data analysis
The ORs and their CI were extracted from the publications or calculated when the paper did not report ORs but provided sufficient information for its calculation. The ORs of COPD associated with biomass smoke were estimated using no exposure to biomass smoke as the reference. In this work, only a few studies provided adjusted estimates of OR. In addition, those which provided adjusted OR did not consider confounders in the same way. To avoid heterogeneity due to adjustment for confounding factors, pooling the mix of adjusted ORs and unadjusted ORs is not appropriate in this case.18 19 Then we preferred analysing using only the unadjusted ORs.19 Meta-analysis was performed using Stata software V.13. The ORs and the 95% CI were used to estimate the pooled effect size of all the studies. The homogeneity Q statistic and the I² index were computed. A random-effects model was used when the heterogeneity was high (I²>50%), given that the Cochran Q statistic is known to be anticonservative.20 When the heterogeneity was low (I²<50%), a fixed-effects model was used. The variance of the fixed-effects model was estimated with the Mantel and Haenszel method,21 and one of the random-effects model was obtained using the DerSimonian and Laird method.22
Subgroup analyses were performed with stratifications by study design (case–control and cross-sectional), geographic location (rural, including semirural; urban, including semiurban; and rural/urban location), phenotypes (COPD, emphysema or CB) and smoking status. The significance of pooled ORs was determined by z test. Two-tailed P values<0.05 were considered statistically significant. Publication bias was assessed through the Egger test and funnel plots.23
The study is reported in accordance with the Meta-analysis Of Observational Studies in Epidemiology guidelines for meta-analysis and systematic reviews of Observational Studies in Epidemiology.
Results
Characteristics of the articles
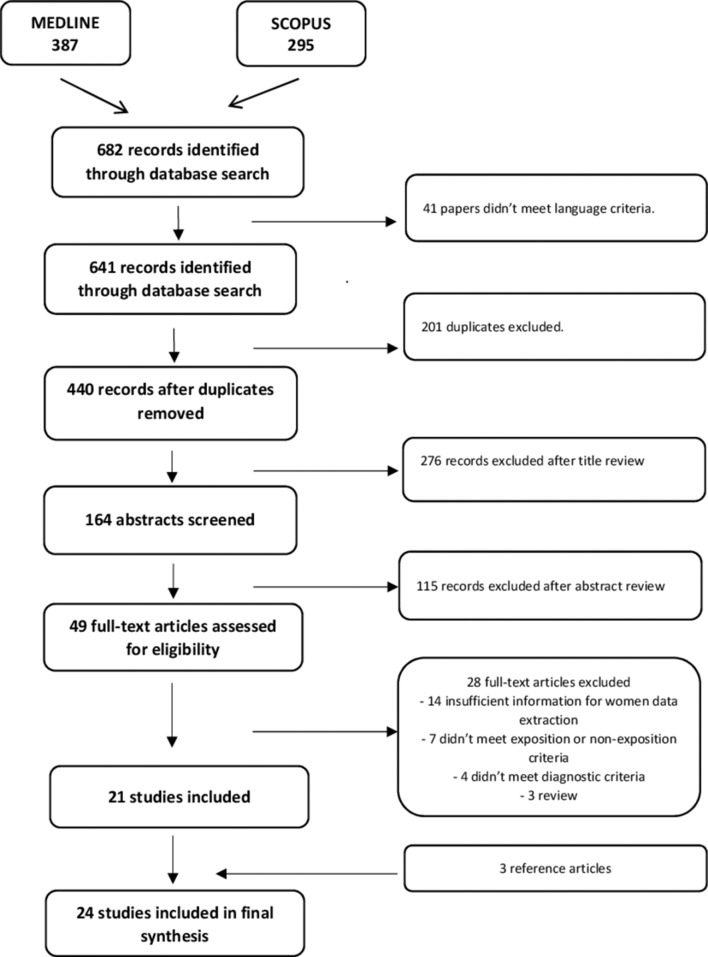
Up to 31 December 2016, our search identified 641 citations. After title review and duplicates removed, 164 abstracts were reviewed and 49 full-text articles were kept. We excluded 28 full-text papers as they failed to meet inclusion criteria or had insufficient information for data extraction. Twenty-one papers were eligible, and we found three more papers in references of full-text articles screened. The detailed selection process is shown in figure 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses study selection flow chart.
The meta-analysis includes 24 articles divides into 5 case–control studies and 19 cross-sectional studies.
Concerning location, 11 studies were based on rural populations, 3 on urban, 4 on urban hospital, 5 on mixed rural/urban populations and 1 on mixed rural/semiurban/urban populations. Concerning gender, six mixed-gender studies had sufficient information to allow women data extraction; the remaining 18 studied only women. About smoking status of the subjects, 12 studies were conducted on non-smoking women and 12 on both smoking and non-smoking women with only one study providing data for non-smokers group. Considering phenotypes, 13 articles studied COPD, 10 solely CB and 1 studied both COPD and CB.
In total, the selected papers accounted for 19 099 subjects, of whom 669 suffered of CB, 1594 of COPD and the remaining 16 836 subjects were healthy participants.
Several biomass fuels were studied, including various combinations of biomass kind, or wood only.24–27 Comparator fuels included liquefied petroleum gas (LPG), gas or gas/electricity and kerosene. Two studies were conducted in Africa.28 29 The others were distributed between Asia, South America, Middle East and Europe.
The details of the included studies in the meta-analysis are shown in table 111 12 24–27 29–46 and (online supplementary table S1) (spirometry lung function test results). No unpublished or ongoing studies were retrieved.
Table 1.
Studies included in the meta-analysis investigating the relation between COPD and biomass fuel exposure
| Authors | Location | Study design | Population | Sample size | Definition of exposure | Definition of non-exposure | Phenotypes | OR (95% CI) | Smoking status | PBD FEV1% mean±SD (case/exposed) |
| Van Gemert et al30 | Uganda Rural |
CS | ≥30 years | 297 | Indoor biomass fuel exposure | Non-exposed to biomass fuel | COPD | 1.7 (0.53 to 5.51) | B | 91.8±20.3 (COPD)* |
| Örnek et al31 | Turkey Urban |
CS | ≥10 years | 351 | Biomass for cooking or heating | No biomass use | COPD | 0.97 (0.42 to 2.22) | B | N/A |
| Jaganath et al32 | Peru Rural/urban |
CS | ≥35 years | 1487 | Biomass fuel for daily cooking | Non-biomass fuel | COPD | 2.22 (1.02 to 4.81) | B | N/A |
| Mukherjee et al33 | India Rural |
CS | 23–43 years | 1119 | Biomass (dung, wood, dried leaves, jute stick, hay) | LPG | COPD | 5.18 (1.81 to 20.3) | N | 71.8±6.82 (biomass) |
| Dutta et al34 | India Rural |
CS | 22–41 years | 480 | Cooking with biomass | Cooking with LPG | COPD | 4.07 (1.34 to 12.36) | N | 69.8±26.6 (biomass) |
| Alim et al35 | Bangladesh Rural/urban |
CS | ≥15 years | 420 | Biomass | Gas | CB | 4.62 (1.32 to 16.2) | N | N/A |
| Sukhsohale et al36 | India Rural |
CS | ≥15 years | 760 | Biomass | LPG | CB | 1.40 (0.82 to 2.41) | N | N/A |
| Laniado-Laborin et al37 | Mexico Urban |
CS | ≥40 years | 1380 | Exposure to biomass smoke | Not exposed to biomass smoke | COPD | 1.65 (1.24 to 2.2) | B | 60.6±21.8 (COPD) |
| Johnson et al38 | India Rural |
CS | ≥30 years | 900 | Biomass | Clean fuel (kerosene, LPG) | COPD | 1.24 (0.36 to 4.25) | N | N/A |
| Desalu et al29 | Nigeria Rural |
CS | ≥35 years | 269 | Biomass fuel | Non-biomass fuel | CB | 3.75 (1.07 to 13.16) | N | 70.8±9.50 (biomass) |
| Akhtar et al39 | Pakistan Rural |
CS | ≥10 years | 2557 | Solid biomass fuels for cooking | LPG for cooking | CB | 2.51 (1.65 to 3.83) | N | N/A |
| Liu et al40 | China Rural/urban |
CS | ≥40 years | 1719 | Biomass (wood, crop residues) | LPG | COPD | 3.11 (1.63 to 5.94) | N | N/A |
| Xu et al41 | China Rural/urban |
CC | ≥35 years | 1396 | Firewood/straw | Electricity/gas | COPD | 0.98 (0.76 to 1.27) | B | N/A |
| Orozco-Levi et al12 | Spain Urban |
CC | >50 years | 120 | Exposure to wood, charcoal or both smoke | Not exposed to wood, charcoal | COPD | 2.24 (0.84 to 6.28) | B | 51 (33–75) (COPD) |
| Regalado et al42 | Mexico Rural |
CS | ≥38 years | 845 | Using biomass as cooking fuel | Using gas as cooking fuel | COPD | 1.5 (0.5 to 4.3) | N | 97.8±13.7(biomass) |
| Sezer et al43 | Turkey Rural/urban |
CC | ≥38 years | 148 | Biomass use (wood, dung)≥10 years | No exposure to biomass | COPD | 1.32 (0.63 to 2.73) | N | N/A |
| Ekici et al11 | Turkey Rural |
CS | >40 years | 596 | Biomass (Wood, grass, crop, dung) | LPG | CB | 1.4 (1.2 to 1.7) | N | Exposition index (biomass) 96.2±17.4 (<68.8 hour-years) 95.8±21.5 (68.8–152.4 hour-years) 87.8±26.2 (>152.4 hour-years) |
| Kiraz et al44 | Turkey Rural/urban |
CS | ≥25 years | 344 | Biomass for cooking and heating (dung, wood, sticks) | LPG | COPD CB |
3.47 (1.19 to 10.11) 2.15 (1.07 to 4.33) |
B | Study group (rural/biomass) FEV1 %: 80.96±12.61 (smokers) FEV1 %: 82.36±11.48 (non-smokers) |
| Uzun et al27 | Turkey Rural/urban |
CS | 17–75 years | 177 | Biomass fuel use (wood) | Non-biomass fuel use | CB | 3.36 (1.8 to 6.26) | B | N/A |
| Golshan et al25 | Iran Rural |
CS | 1 month to 81 years (27.62±16.6) | 561 | Using wood fuel in the past | Using gas fuel | CB | 2.91 (2.08 to 4.4) | B | N/A |
| Pérez-Padilla et al26 | Mexico Urban |
CC | >40 years | 438 | Wood smoke exposure while cooking | No exposure to wood smoke | CB | 3.9 (2.0 to 7.6) N 7.2 (2.8 to 20) |
B N |
N/A |
| Dennis et al24 | Colombia Urban |
CC | ≥35 years | 208 | Using wood as cooking fuel | Not using wood as cooking fuel | COPD | 3.43 (1.69 to 7.05) | B | FEV1 % 57.60±13 (COPD) |
| Dutt et al45 | India Urban |
CS | 15–60 years | 195 | Biomass | LPG, kerosene | CB | 4.17 (0.46 to 38.02) | B | N/A |
| Behera et al46 | India Rural |
CS | N/A | 3608 | Cooking with ‘chulla’ in which biomass fuels are used | Cooking with LPG stove | CB | 1.75 (1.03 to 2.98) | N | FEV1 %: 83.21±1.89 (symptomatic) |
*A ll the sample (including men and women).
B, both smokers and non-smokers; CB: chronic bronchitis; CC, case–control; COPD, chronic obstructive pulmonary disease; CS, cross-sectional; FEV1, forced expiratory volume for 1 s; LPG, liquefied petroleum gas; N, non-smokers only; N/A, not available; PBD, postbronchodilator.
bmjresp-2017-000246supp003.pdf (352.5KB, pdf)
Publication bias
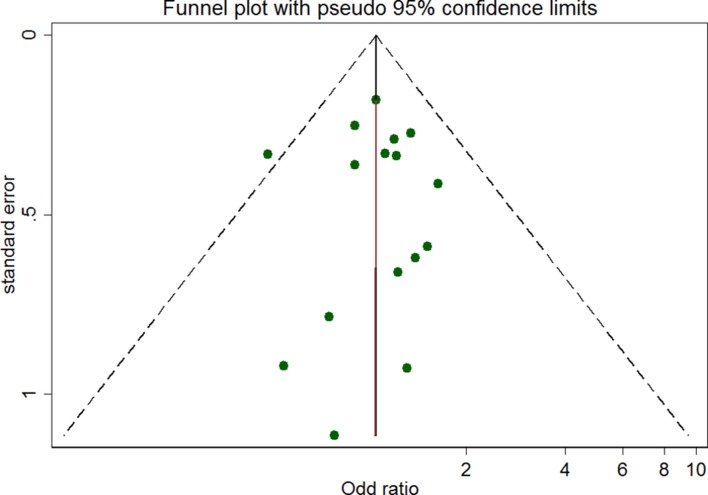
Begg’s funnel plot visualisation indicated no publication bias in the studies included in the meta-analysis (see figure 2).
Figure 2.
Funnel plot for studies included in the meta-analysis.
Exposure to biomass smoke and COPD
From the raw data, we observe that COPD, all phenotypes included, represents 11.85% of the total studied population (2263/19 099).
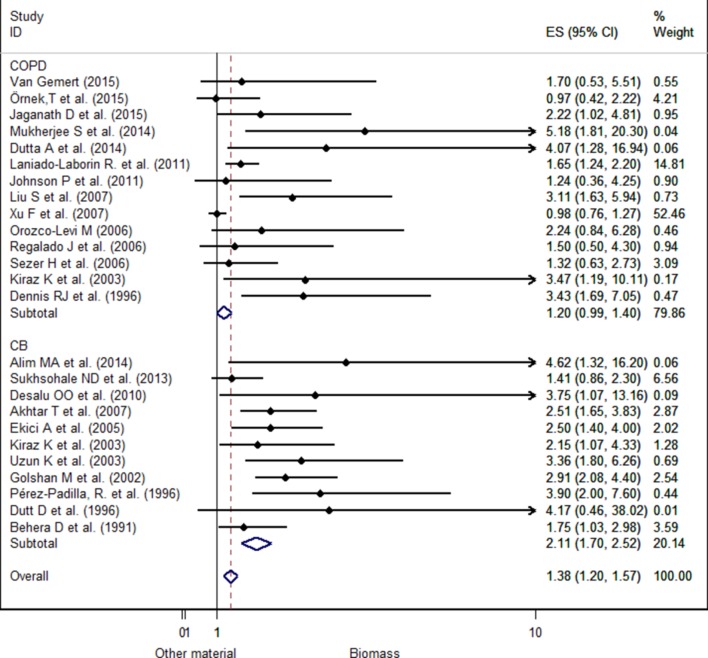
A fixed-effects model was used for the analysis because no heterogeneity had been found among the selected studies. Considering all COPD phenotypes OR, the pooled analysis shows that individuals exposed to biomass are 1.38 times (OR 1.38, 95% CI 1.28 to 1.57) more likely to be diagnosed with COPD than those not exposed.
The pooled analysis of only the COPD phenotype failed to show a significant association (OR 1.20, 95% CI 0.99 to 1.40). Nevertheless, we observe that ORs are significantly higher for CB (OR 2.11, 95% CI 1.70 to 2.52) than for COPD (figure 3).
Figure 3.
Forest plot for chronic obstructive pulmonary disease (COPD) comparing biomass smoke exposed with non-exposed to biomass smoke separated by COPD phenotypes in women. CB, chronic bronchitis; ES, effect size.
The studies were then stratified between non-cigarette smokers’ participants (n=13) and both cigarette smokers and non-cigarette smokers’ participants (n=12). The analysis showed heterogeneity. However, a significant effect was highlighted as well in studies on both smokers and non-smokers (OR 1.90, 95% CI 1.35 to 2.45) and in studies on only non-smokers (OR 1.80, 95% CI 1.40 to 2.20) (see online supplementary figure S1).
bmjresp-2017-000246supp002.jpg (158.4KB, jpg)
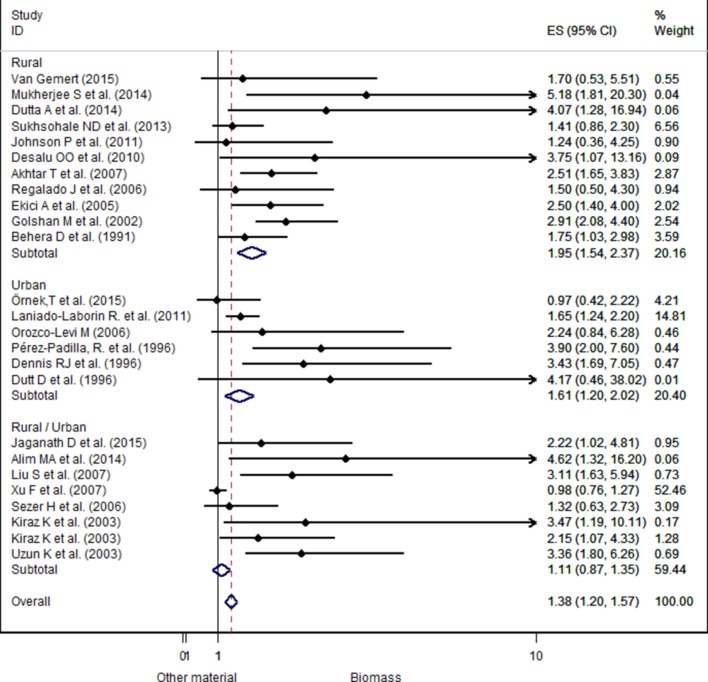
The analyses were also stratified by location (urban/rural). The OR was very strong in studies conducted in rural area (pooled OR 1.95, 95% CI 1.54 to 2.37) compared with the pooled ORs of studies conducted in urban area (pooled OR 1.61, 95% CI 1.20 to 2.02) or both locations (rural/urban) (pooled OR 1.11, 95% CI 0.87 to 1.35) (see figure 4). However, studies using both locations did not present any significant difference.
Figure 4.
Forest plot showing the effect size of chronic obstructive pulmonary disease due to exposure to biomass fuels compared with other fuels, separated by location.
When stratifying by study design, the pooled ORs for cross-sectional and case–control studies were respectively 1.82 (95% CI 1.54 to 2.10) and 1.05 (95% CI 0.81 to 1.30). Association is found between COPD and biomass smoke exposure in both case–control and cross-sectional groups even if this association is not statistically significant in case–control studies group.
Discussion
The present systematic literature review and meta-analysis re-examines existing research findings about the association between domestic biomass smoke exposure and COPD in women.
A total of 24 individual studies that evaluated COPD or CB as a health outcome in women, in the context of biomass fuel exposure compared with other fuels, were analysed.
Based on 19 cross-sectional and 5 case–control studies, the meta-analysis provides confirmation that exposure to indoor biomass fuel smoke is associated with an increased risk of COPD. Women exposed to biomass fuel smoke were more at risk of developing COPD or CB than those exposed to other fuels with a reported OR of 1.38.
In our analyses of location/geographical subgroups, we found a significant association between biomass smoke exposure and COPD for women living in rural as well as urban areas. In rural environments, women exposed to biomass smoke were more at risk of developing COPD than non-exposed women. The same pattern of association has been found in urban areas. Even if the crude ORs were different, one could not conclude that the risk was higher in the rural areas, as in rural areas OR was estimated to be 1.95 (95% CI 1.54 to 2.37) and in urban areas OR was estimated to be 1.61 (95% CI 1.20 to 2.02).
Although there was no significant heterogeneity among the studies included in the meta-analysis, we stratified their results by cigarette smoking status of participants included in each study and found a significant heterogeneity among studies involving both cigarette smokers and non-smokers and those involving only non-cigarette-smoking women. These subgroup analyses showed that associations did not differ between both groups of studies. The pooled OR increased to 1.90 (95% CI 1.35 to 2.45) for those including cigarette smokers and non-smokers and to 2.55 (95% CI 2.06 to 3.05) for those including only cigarette non-smokers. Some studies suggested that biomass smoke may interact with cigarette smoking in the pathogenesis of COPD.47
When considering COPD and CB separately, women were more at risk of developing COPD and CB if exposed to biomass fuel smoke compared with not-exposed women. The relationship between biomass smoke exposure and spirometry-defined and/or hospital-diagnosed COPD was not significant in studies, with OR values ranging from 0.97 to 5.18. Among the 14 publications evaluating the relationship between biomass exposure and the COPD phenotype, half of them did not show statistically significant association. This difference between studies may be due to difference in terms of design, difference in the way of conceiving exposed and non-exposed groups. We pooled only crude OR estimates, without adjustment for potential confounders. Consequently, potential confounding factors such as age, smoking status, body mass index (BMI), kitchen ventilation, socioeconomic status, history of tuberculosis and women educational attainment, which may have influenced the individual study’s result,40 48 49 are not being taken into account. To deal with that, we stratified studies by smoking status (studies involving both smokers and non-smokers and studies involving only non-smokers); the association remains not statistically significant in the two groups (see online supplementary figure S2). Concerning the link between BMI and lung function, the authors did not reach a consensus yet, for some there is an effect,50 leading to an eventual underestimation of COPD among those who are overweight and obese51; for others there is none.52 In addition, several studies have shown that low BMI is an important risk factor for COPD.49 53
bmjresp-2017-000246supp001.jpg (94.7KB, jpg)
The stratification of the publications by study design showed that exposure to biomass fuel smoke is associated with COPD (COPD and CB phenotypes) regardless of the study design with pooled OR of 1.82 (95% CI 1.54 to 2.10) for cross-sectional studies and 1.05 (95% CI 0.81 to 1.30) for case–control studies (see online supplementary figure S3). Although the association between COPD and biomass smoke exposure is not statistically significant in case–control studies group. Our finding can also be explained by the effect of hospital-diagnosed studies. In fact, Kurmi and colleagues14 have found in previous meta-analysis a non-significant association between solid fuel and hospital-diagnosed COPD (OR 2.29, 95% CI 0.70 to 7.52) comparatively with their pooled effect size for lung function-defined COPD which was largely significant (OR 2.96, 95% CI 2.01 to 4.37).
bmjresp-2017-000246supp004.jpg (128.7KB, jpg)
Among case–control studies, only one concerned CB diagnosis.26 Among the four others that studied COPD, two revealed non-significant association between biomass exposure and COPD,12 43 and one did not find any association.41 Case–control design is prone to bias, especially when hospital-based,24 when regarding selection bias. By selecting cases from the hospital population, selected individuals would not be representative of all possible cases happening within the population (severe cases might be more present). Less severe cases (that can be biomass smoke-exposed COPD), asymptomatic or never-smoking patients with COPD could have been overlooked, resulting in an underestimation of the association’s strength between COPD and biomass smoke. Xu et al41 reported an OR of 0.98 (95% CI 0.76 to 1.27), incoherent with most of the results reported in the literature on the relationship between exposure to biomass smoke and COPD. The author explains this difference by the fact that, in households using biomass or traditional fuels, kitchens were usually large and doors and windows were usually opened during cooking, and in urban areas kitchens were often properly ventilated.41 Consequently, air quality in these households is less affected by the use of biomass fuel, hence the absence of statistical difference between biomass users and electricity/gas users concerning COPD. Another explanation of the absence of difference could be the small sample size of some studies.12 43 45
No significant publication bias was found in the studies. Additionally, no significant heterogeneity was found for the group of studies included within the meta-analysis. However, heterogeneity was found after stratification due to the small number of studies corresponding to each subgroup. At this case, random-effect analysis was performed. Most of the studies were cross-sectional, decreasing the level of evidence of the meta-analysis. However, rigorous statistical methods were used to get accurate results.
This paper gathers 25 different results from 24 different publications. The publication period ranged from 1991 to 2015, representing almost 25 years. In comparison with the previous meta-analysis, additional studies were found. The large studies set of this systematic literature review enables us to better evaluate the association between biomass smoke exposure and COPD diagnosis in women compared with previous the meta-analysis based on nearly a dozen studies.14 54 The fact that our study focused especially on women allows us a more detailed analysis concerning this group of subjects than general studies involving all adult subjects.
Although our estimation of the OR was slightly lower than the OR found in the previous meta-analysis, the trend of association between domestic biomass exposure and COPD/CB was consistent with these studies.14 47 54 The findings confirm that use of clean fuels such as LPG, gas or electricity can reduce the risk of COPD in women. However, some studies highlight that clean cooking fuel choice was significantly associated with household socioeconomic status (such as income and education) and location (urban vs rural).55 To be effective, interventions aimed at reducing impact of biomass fuel on COPD must take this into account. Therefore, in poor or rural communities, improving the efficiency of current fuel stoves and energy user behaviours (fuel drying, avoiding smoke exposure as much as possible during cooking, improved kitchen ventilation, properly used and maintained stoves, promoting outdoor cooking) will be more effective in reducing smoke emission and exposure rather than by attempting to replace the solid fuel stoves with any clean fuel stoves.56 57 However, ‘for communities benefiting from a cheaper and more reliable access to clean fuels, then strategies to support a switch to LPG or other liquid or gaseous fuels have a higher chance of success’.56 A 9-year prospective cohort study performed in southern China revealed that improving kitchen ventilation and biomass stoves was associated with a reduced decline in FEV1 by 13 mL/year (95% CI 4 to 23 mL/year) compared with those who took up neither intervention. According to the same study, the use of clean fuels (biogas) instead of biomass for cooking reduced the FEV1 decline by 12 mL/year (95% CI 4 to 20 mL/year).58 ‘Compared with participants without improved ventilation for cooking, those with improvement for 5–9 y had a lower risk of COPD, with an adjusted OR of 0.39 (95% CI, 0.15 to 0.99)’, and there was no significant difference between the clean fuel and ventilation interventions.58 Aunan’s study highlights that a significant difference in COPD prevalence was observed between women who used stoves without chimney compared with those who used improved stoves (with a chimney), after adjustment for age, socioeconomic status and ventilation (OR 3.48, 95% CI 1.02 to 11.90).59 Chapman et al found that installation of a chimney was associated with a reduction in the incidence of COPD among women compared with people who did not have chimneys (relative risk 0.75 (0.62 to 0.92, P=0.005)).60
These results suggested that attention must be paid to the burden faced primarily by women in relation to traditional fuel like biomass fuels and traditional stoves use, particularly in rural areas. Cleaner energy for cooking is women’s and children’s respiratory health improvement, but not only! Improving traditional cooking stoves could be a more accepted and less cost-effective alternative, especially in the context of poverty. Healthier cooking means also environment protection and economic empowerment.
Conclusion
This study confirms that biomass smoke exposure is associated with COPD in women. An increased attention must be paid to cooking energy and cooking stoves improvement in view of the burden primarily faced by women in relation to traditional fuels like biomass and traditional stoves use, particularly in rural areas.
Additional studies with well-designed lung function measurement methods are needed to further highlight the causal link between lung function-diagnosed COPD and indoor exposure to biomass.
Many low-income, middle-income countries face an important energy transition geared towards energy substitution. Modern fuels such as LPG are more and more used but mostly used alongside traditional ones, mainly in urban areas. Additional data are needed to further explore the benefit of the usage of mixed fuel for cooking or heating on respiratory health, particularly on COPD reduction, in a context of energy transition, as seen more and more in many low-income, middle-income countries.
Acknowledgments
The authors acknowledge ULB and Health Inter Network Access to Research Initiative (HINARI) team for providing opportunities to access many scientific publications. They are grateful to Dimitri Krasucki for all his help and availability in finding full text papers.
Footnotes
Contributors: AS contributed to the study protocol conception, articles searching and studies selection, data extraction, statistical analyses and the writing of all the versions of the manuscript. SMAS contributed to the studies selection, data extraction, statistical analyses, conception of the first draft of the manuscript and the final version of the manuscript. NM contributed to revising the manuscript (first draft and the final version). CB was involved in revising the study protocol and all the versions of this manuscript.
Funding: AS was supported by a PhD studentship granted by the International Development Research Centre (IRDC) via the project ’Chairepol' (Project IDRC 107347) of the Community of Practice in EcoHealth—West and Central African (CoPEH-WCA) and the ULB (’Université Libre de Bruxelles') cooperation funds.
Competing interests: None declared.
Provenance and peer review: Open peer review.
Data sharing statement: Not applicable
References
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765–73. doi:10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- 2.Fullerton DG, Suseno A, Semple S, et al. . Wood smoke exposure, poverty and impaired lung function in Malawian adults. Int J Tuberc Lung Dis 2011;15:391–8. [PubMed] [Google Scholar]
- 3.World Health Organisation. Chronic obstructive pulmonary disease (COPD). 2016. http://www.who.int/mediacentre/factsheets/fs315/en/ (accessed 15 Jun 2017).
- 4.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442 doi:10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis 2008;12:703–8. [PubMed] [Google Scholar]
- 6.Lopez AD, Mathers CD, Ezzati M, et al. . Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747–57. doi:10.1016/S0140-6736(06)68770-9 [DOI] [PubMed] [Google Scholar]
- 7.Organisation mondiale de la santé. Lignes directrices OMS relatives à la qualité de l’air intérieur: consommation domestique de combustibles. 2014. http://www.who.int/indoorair/guidelines/hhfc/ExecSumm_Fr.pdf?ua=1.
- 8.Raj T JB. Altered lung function test in asymptomatic women using biomass fuel for cooking. J Clin Diagn Res 2014;8:1–3. doi:10.7860/JCDR/2014/7253.4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balmes JR. When smoke gets in your lungs. Proc Am Thorac Soc 2010;7:98–101. doi:10.1513/pats.200907-081RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon SB, Bruce NG, Grigg J, et al. . Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med 2014;2:823–60. doi:10.1016/S2213-2600(14)70168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekici A, Ekici M, Kurtipek E, et al. . Obstructive airway diseases in women exposed to biomass smoke. Environ Res 2005;99:93–8. doi:10.1016/j.envres.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Orozco-Levi M, Garcia-Aymerich J, Villar J, et al. . Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J 2006;27:542–6. doi:10.1183/09031936.06.00052705 [DOI] [PubMed] [Google Scholar]
- 13.Cote CG, Chapman KR. Diagnosis and treatment considerations for women with COPD. Int J Clin Pract 2009;63:486–93. doi:10.1111/j.1742-1241.2008.01987.x [DOI] [PubMed] [Google Scholar]
- 14.Kurmi OP, Semple S, Simkhada P, et al. . COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax 2010;65:221–8. doi:10.1136/thx.2009.124644 [DOI] [PubMed] [Google Scholar]
- 15.Celli BR, Decramer M, Wedzicha JA, et al. . An Official American Thoracic Society/European Respiratory Society Statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;191:e4–e27. doi:10.1164/rccm.201501-0044ST [DOI] [PubMed] [Google Scholar]
- 16.GIfCOL D. Pocket guide to COPD diagnosis, management, and prevention. A Guide for Health Care Professionals. 2017:37 http://goldcopd.org/
- 17.Anon. Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet 1965;1:775–9. [PubMed] [Google Scholar]
- 18.Jacobsen KH. Tertiary studies: systematic reviews and meta-analyses. Introduction to Health Research Methods a practical guide. USA: Jones & Bartlett Learning, 2012:287. [Google Scholar]
- 19.Voils CI, Crandell JL, Chang Y, et al. . Combining adjusted and unadjusted findings in mixed research synthesis. J Eval Clin Pract 2011;17:429–34. doi:10.1111/j.1365-2753.2010.01444.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med 1998;17:841–56. doi:10.1002/(SICI)1097-0258(19980430)17:8<841::AID-SIM781>3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. JNCI 1959;22:719–48. [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. doi:10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. doi:10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis RJ, Maldonado D, Norman S, et al. . Woodsmoke exposure and risk for obstructive airways disease among women. Chest 1996;109:115–9. doi:10.1378/chest.109.1.115 [DOI] [PubMed] [Google Scholar]
- 25.Golshan M, Faghihi M, Marandi MM. Indoor women jobs and pulmonary risks in rural areas of Isfahan, Iran, 2000. Respir Med 2002;96:382–8. doi:10.1053/rmed.2002.1288 [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Padilla R, Regalado J, Vedal S, et al. . Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. Am J Respir Crit Care Med 1996;154:701–6. doi:10.1164/ajrccm.154.3.8810608 [DOI] [PubMed] [Google Scholar]
- 27.Uzun K, Ozbay B, Ceylan E, et al. . Prevalence of chronic bronchitis-asthma symptoms in biomass fuel exposed females. Environ Health Prev Med 2003;8:13–17. doi:10.1007/BF02897938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Gemert F, Chavannes N, Kirenga B, et al. . Socio-economic factors, gender and smoking as determinants of COPD in a low-income country of sub-Saharan Africa: fresh air Uganda. NPJ Prim Care Respir Med 2016;26:16050 doi:10.1038/npjpcrm.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desalu OO, Adekoya AO, Ampitan BA. Increased risk of respiratory symptoms and chronic bronchitis in women using biomass fuels in Nigeria. J Bras Pneumol 2010;36:441–6. [DOI] [PubMed] [Google Scholar]
- 30.van Gemert F, Kirenga B, Chavannes N, et al. . Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health 2015;3:e44–e51. doi:10.1016/S2214-109X(14)70337-7 [DOI] [PubMed] [Google Scholar]
- 31.Örnek T, Tor M, Kıran S, et al. . Prevalence of chronic obstructive pulmonary disease in Zonguldak province of Turkey. Tuberk Toraks 2015;63:170–7. [DOI] [PubMed] [Google Scholar]
- 32.Jaganath D, Miranda JJ, Gilman RH, et al. . Prevalence of chronic obstructive pulmonary disease and variation in risk factors across four geographically diverse resource-limited settings in Peru. Respir Res 2015;16:40 doi:10.1186/s12931-015-0198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee S, Roychoudhury S, Siddique S, et al. . Respiratory symptoms, lung function decrement and chronic obstructive pulmonary disease in pre-menopausal Indian women exposed to biomass smoke. Inhal Toxicol 2014;26:866–72. doi:10.3109/08958378.2014.965560 [DOI] [PubMed] [Google Scholar]
- 34.Dutta A, Ray MR. Hypertension and respiratory health in biomass smoke-exposed premenopausal Indian women. Air Qual Atmos Health 2014;7:229–38. doi:10.1007/s11869-013-0228-5 [Google Scholar]
- 35.Alim MA, Sarker MA, Selim S, et al. . Respiratory involvements among women exposed to the smoke of traditional biomass fuel and gas fuel in a district of Bangladesh. Environ Health Prev Med 2014;19:126–34. doi:10.1007/s12199-013-0364-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sukhsohale ND, Narlawar UW, Phatak MS. Indoor air pollution from biomass combustion and its adverse health effects in central India: an exposure-response study. Indian J Community Med 2013;38:162–7. doi:10.4103/0970-0218.116353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laniado-Laborin R, Rendón A, Bauerle O. Chronic obstructive pulmonary disease case finding in Mexico in an at-risk population. Int J Tuberc Lung Dis 2011;15:818–23. doi:10.5588/ijtld.10.0546 [DOI] [PubMed] [Google Scholar]
- 38.Johnson P, Balakrishnan K, Ramaswamy P, et al. . Prevalence of chronic obstructive pulmonary disease in rural women of Tamilnadu: implications for refining disease burden assessments attributable to household biomass combustion. Glob Health Action 2011;4:7226 doi:10.3402/gha.v4i0.7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akhtar T, Ullah Z, Khan MH, et al. . Chronic bronchitis in women using solid biomass fuel in rural Peshawar, Pakistan. Chest 2007;132:1472–5. doi:10.1378/chest.06-2529 [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Zhou Y, Wang X, et al. . Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax 2007;62:889–97. doi:10.1136/thx.2006.061457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu F, Yin X, Shen H, et al. . Better understanding the influence of cigarette smoking and indoor air pollution on chronic obstructive pulmonary disease: a case-control study in Mainland China. Respirology 2007;12:891–7. doi:10.1111/j.1440-1843.2007.01178.x [DOI] [PubMed] [Google Scholar]
- 42.Regalado J, Pérez-Padilla R, Sansores R, et al. . The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am J Respir Crit Care Med 2006;174:901–5. doi:10.1164/rccm.200503-479OC [DOI] [PubMed] [Google Scholar]
- 43.Sezer H, Akkurt I, Guler N, et al. . A case-control study on the effect of exposure to different substances on the development of COPD. Ann Epidemiol 2006;16:59–62. doi:10.1016/j.annepidem.2004.12.014 [DOI] [PubMed] [Google Scholar]
- 44.Kiraz K, Kart L, Demir R, et al. . Chronic pulmonary disease in rural women exposed to biomass fumes. Clin Invest Med 2003;26:243–8. [PubMed] [Google Scholar]
- 45.Dutt D, Srinivasa D, Rotti S, et al. . Effect of indoor air pollution on the respiratory system of women using different fuels for cooking in an urban slum of Pondicherry. Natl Med J India 1996;9:5. [PubMed] [Google Scholar]
- 46.Behera D, Jindal SK. Respiratory symptoms in Indian women using domestic cooking fuels. Chest 1991;100:385–8. doi:10.1378/chest.100.2.385 [DOI] [PubMed] [Google Scholar]
- 47.Hu G, Zhou Y, Tian J, et al. . Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest 2010;138:20–31. doi:10.1378/chest.08-2114 [DOI] [PubMed] [Google Scholar]
- 48.Smith M, Li L, Augustyn M, et al. . Prevalence and correlates of airflow obstruction in ∼317,000 never-smokers in China. Eur Respir J 2014;44:66–77. doi:10.1183/09031936.00152413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding Y, Xu J, Yao J, et al. . The analyses of risk factors for COPD in the Li ethnic group in Hainan, People’s Republic of China. Int J Chron Obstruct Pulmon Dis 2015;10:2593–600. doi:10.2147/COPD.S86402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melo LC, Silva MA, Calles AC. Obesity and lung function: a systematic review. Einstein 2014;12:120–5. doi:10.1590/S1679-45082014RW2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Çolak Y, Marott JL, Vestbo J, et al. . Overweight and obesity may lead to under-diagnosis of airflow limitation: findings from the Copenhagen City Heart Study. COPD 2015;12:5–13. doi:10.3109/15412555.2014.933955 [DOI] [PubMed] [Google Scholar]
- 52.Al Ghobain M. The effect of obesity on spirometry tests among healthy non-smoking adults. BMC Pulm Med 2012;12:10 doi:10.1186/1471-2466-12-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, Wang D, Liu S, et al. . The association between BMI and COPD: the results of two population-based studies in Guangzhou, China. COPD 2013;10:567–72. doi:10.3109/15412555.2013.781579 [DOI] [PubMed] [Google Scholar]
- 54.Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax 2011;66:232–9. doi:10.1136/thx.2010.147884 [DOI] [PubMed] [Google Scholar]
- 55.Lewis JJ, Pattanayak SK. Who adopts improved fuels and cookstoves? A systematic review. Environ Health Perspect 2012;120:637–45. doi:10.1289/ehp.1104194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg 2008;102:843–51. doi:10.1016/j.trstmh.2008.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langbein J, Firewood LJ. Firewood, smoke and respiratory diseases in developing countries-The neglected role of outdoor cooking. PLoS One 2017;12:e0178631 doi:10.1371/journal.pone.0178631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y, Zou Y, Li X, et al. . Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med 2014;11:e1001621 doi:10.1371/journal.pmed.1001621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aunan K, Alnes LWH, Berger J, et al. . Upgrading to cleaner household stoves and reducing chronic obstructive pulmonary disease among women in rural China — A cost-benefit analysis. Energy for Sustainable Development 2013;17:489–96. doi:10.1016/j.esd.2013.06.002 [Google Scholar]
- 60.Chapman RS, He X, Blair AE, et al. . Improvement in household stoves and risk of chronic obstructive pulmonary disease in Xuanwei, China: retrospective cohort study. BMJ 2005;331:1050 doi:10.1136/bmj.38628.676088.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2017-000246supp003.pdf (352.5KB, pdf)
bmjresp-2017-000246supp002.jpg (158.4KB, jpg)
bmjresp-2017-000246supp001.jpg (94.7KB, jpg)
bmjresp-2017-000246supp004.jpg (128.7KB, jpg)