Abstract
Background
Surgical resection remains the best chance at long-term survival in pancreatic cancer, though margin-positive resections are associated with diminished survival. We examined the effect of margin-positive resection on survival, as well as the role and timing of additional therapies through the National Cancer Database (NCDB).
Patients and methods
Patients with stage IIA–III pancreatic adenocarcinoma diagnosed from 2004 to 2013 were identified in NCDB. Survival was compared using univariate and multivariate Cox proportional hazards modelling for patients who underwent surgery with negative (R0), microscopically positive (R1) and macroscopically positive (R2) margins or non-surgical treatment. We further analysed patients by margin status, timing of additional therapy (neoadjuvant therapy (NAT) vs adjuvant therapy (AT) vs none) and clinical stage.
Results
We analysed 44 852 patients. Median survival (MS) for patients who did not undergo surgery was 10.3 months, compared with 19.7 months for R0 (P<0.001), 14.3 months for R1 (P<0.001) and 9.8 months (P=0.07) for R2 resections. NAT (MS 23.2 months) was associated with improved survival compared with AT (MS 21.5 months) in negative-margin patients and equivalent (MS 17.6 months) to AT (MS 16.8 months) in positive-margin patients. Survival for stage III NAT positive-margin patients (MS 19.8 months) was equivalent to AT after negative margins (MS 18.4 months, P=1.00). Improved R0 rates were seen with NAT (88% vs 81%, P<0.001), especially in stage III patients (85% vs 59%, P<0.001).
Conclusion
R1 resections portend poorer survival than R0 but do not negate the benefit of surgery when additional therapy is given. NAT was associated with improved R0 rates and improved survival for stage III positive-margin patients.
Keywords: pancreatic cancer, surgical margins, NCDB
Key questions.
What is already known about this subject?
It is known that R1 resections portend worse prognosis than R0 resections in pancreas cancer, but the magnitude of this detriment and the effect of neoadjuvant or adjuvant therapy on margin positive resections is unknown.
What does this study add?
In an aggregate dataset of 44 852 patients, we found that median survival after R1 resection was 14.3 months compared with 19.7 months for R0 resection. Median survival for margin positive patients was further improved by neoadjuvant therapy (17.6 months) or adjuvant therapy (16.8 months). Furthermore, R0 rates were improved after neoadjuvant therapy.
How might this impact on clinical practice?
These data may encourage resection in more patients who receive appropriate neoadjuvant treatment even if the risk of R1 resection is significant.
Introduction
Pancreatic cancer is a deadly disease; even patients who present with localised tumours have a median survival (MS) of fewer than 3 years.1–8 Most patients present with metastatic disease or advanced tumours that abut or encase the vasculature and thus are difficult to resect with negative margins. Both prospective8 9 and retrospective series10–16 have found that survival is reduced if surgical margins are positive, even microscopically. There is uncertainty over the acceptable rate of margin-positive resections with older data suggesting that a margin-positive resection portends survival similar to no resection at all.17 Only more recently have randomised clinical trials included patients with R1 resections in order to examine the most appropriate care in the adjuvant setting.3 4 8 18
Whether a margin-positive resection is equivalent to best non-surgical therapy has not been examined thoroughly in the era of neoadjuvant therapy (NAT). NAT has theoretical advantages in this setting, including a reduced rate of positive margins, a potential to downstage the primary tumour, and the ability to address microscopic distant disease earlier than with adjuvant therapy (AT).19–22 Additionally, given the propensity of pancreatic cancer to progress distantly, patients who progress through NAT may be spared the morbidity of a surgery that would not have resulted in a survival benefit.19–22 A common clinical question is how to proceed with patients who do not progress through NAT but remain at risk of positive margins based on imaging evaluation after NAT.
In order to better quantify the effects of surgical margins on survival, we sought to determine the benefit of surgical resection in patients who underwent surgery with positive and negative margins to those who underwent non-surgical treatment (chemotherapy or chemoradiotherapy (CRT)). We further sought to quantify the effect of a margin-positive resection on survival and whether the presence and timing of additional therapy impacted that survival. To our knowledge, no properly powered study has examined the role of margin status, timing of therapy and benefit of surgical resection.
Methods and materials
Patient data were obtained from the National Cancer Database (NCDB), a joint programme of the Commission on Cancer and the American Cancer Society, which includes data from approximately 1500 hospitals and clinics in the USA and its territories. This database captures nearly 70% of new cancer diagnoses made in the USA.23 All NCDB data are deidentified and therefore exempt from review by our institutional review board.
The initial query included all patients with pancreatic cancer diagnosed between the years of 2004 and 2013, which yielded 309 709 patients (online supplementary figure 1). Patients with clinical stage II or III disease were selected for analysis (44 852). Stage refers to clinical stage throughout the analysis unless otherwise specified. Patients were first separated into groups by treatment strategy: surgery alone (Surg), NAT followed by surgery, AT following surgery and no surgery (chemotherapy or CRT alone) to ascertain whether there were differences between the treatment groups that might explain potential survival differences. The primary outcome variable was survival based on surgical resection and margin status. Survival was compared between patients who underwent no surgery, surgery with negative margins (R0), surgery with microscopically positive margins (R1) and surgery with macroscopically positive margins (R2) using univariate (UVA) Cox proportional hazards modelling. A P value of 0.05 was required for significance.
esmoopen-2017-000282supp001.jpg (1.2MB, jpg)
Patients were separated into cohorts by treatment status, timing of treatment and surgical margin status. Treatment cohorts included: no surgery (NoSurg), NAT followed by surgical resection with either positive (NAT/Pos) or negative (NAT/Neg) margins, upfront surgery followed by treatment with either positive (AT/Pos) or negative margins (AT/Neg) and surgery alone with either positive (Surg/Pos) or negative margins (Surg/Neg). Univariate and multivariate (MVA) Cox proportional hazards modelling were done. Factors with a P value of 0.2 or lower were included in the MVA. A P value of 0.05 or lower was required for significance on MVA.
Patients were further broken down by clinical stages (IIA, IIB and III), and UVA Cox proportional hazards modelling was done based on resection and margin status. The margin-negative resection rates were also obtained by clinical stage. Patients who underwent surgery and had positive margins were then isolated in order to determine the effect of the presence and timing of additional therapies via UVA and MVA. Patients were separated into the following cohorts: NAT, AT and Surg (no additional therapy). Finally, patients who underwent surgical resection were isolated to identify predictors of positive margins via UVA and MVA.
All analyses were performed using the STATA V.14.0 statistical package.
Results
After all exclusions had been made, 44 852 patients were available for analysis. Patient characteristics were examined for Surg, NAT followed by surgery, surgery followed by AT and NoSurg patients (table 1). Patients who did not undergo surgery tended to have bigger tumours (18% greater than 5 cm vs 10%–13% P<0.001), be clinically node-positive (42% vs 33%–36%, P<0.001) and be clinical stage III (59% vs 6%–23%, P<0.001) than patients who did. Patients treated neoadjuvantly tended to have tumours located in the pancreatic head (77% vs 63%–74%, P<0.001) and be clinical stage III compared with patients treated with surgery upfront (23% vs 6%–8%, P<0.001). They were equally likely, however, to be stage III pathologically (5% vs 4%–5%, P<0.001) and were less likely to have positive lymph nodes at surgery (46% vs 66%–73%, P<0.001), though clinical node positivity did not vary by more than 3% between the groups. Negative margins were more likely in NAT (88% vs 81%, P<0.001) than patients treated with surgery upfront. Patients treated with NAT were more likely to be treated with CRT (69% vs 46%–50%, P<0.001) and multiagent chemotherapy (58% vs 31%–44%, P<0.001) compared with other groups. Thirty-day (2% NAT vs 3.6% AT+Surg, P<0.001) and 90-day (6% NAT vs 7% AT+Surg, P<0.001) mortality were not significantly different between NAT and surgery upfront patients (AT+Surg).
Table 1.
Patient characteristics (n=44 852)
| Variable | Surgery alone (n=5500) (n (%)) | NAT (n=3007) (n (%)) | AT (n=10 458) (n (%)) | No surgery (n=25 887) (n (%)) | P value |
| Sex | <0.001 | ||||
| Male | 2677 (49) | 1528 (51) | 5514 (53) | 12 981 (50) | |
| Female | 2823 (51) | 1479 (49) | 4944 (47) | 12 906 (50) | |
| Age group (years) | <0.001 | ||||
| 18–49 | 241 (4) | 267 (9) | 738 (7) | 1708 (7) | |
| 50–69 | 2206 (40) | 1885 (63) | 5986 (57) | 13 418 (52) | |
| 70–84 | 2765 (50) | 832 (28) | 3614 (35) | 9870 (38) | |
| 85+ | 288 (5) | 23 (1) | 120 (1) | 891 (3) | |
| Charlson | <0.001 | ||||
| 0 | 3466 (63) | 2037 (68) | 7003 (67) | 18 265 (71) | |
| 1 | 1528 (28) | 794 (26) | 2776 (27) | 6049 (23) | |
| 2+ | 506 (9) | 176 (6) | 679 (6) | 1573 (6) | |
| Tumour size | <0.001 | ||||
| 2 cm or less | 761 (14) | 382 (13) | 1585 (15) | 1466 (7) | |
| 2.1–5 cm | 3933 (73) | 2205 (77) | 7510 (73) | 16 844 (76) | |
| >5 cm | 686 (13) | 272 (10) | 1170 (11) | 3900 (18) | |
| Location | <0.001 | ||||
| Head | 3978 (72) | 2328 (77) | 7731 (74) | 16 292 (63) | |
| Body | 333 (6) | 236 (8) | 634 (6) | 3872 (15) | |
| Tail | 578 (11) | 122 (4) | 1102 (11) | 1017 (4) | |
| Duct | 47 (1) | 4 (<1) | 68 (1) | 72 (<1) | |
| Other | 564 (10) | 317 (11) | 923 (9) | 4634 (18) | |
| Clinical nodes | <0.001 | ||||
| Negative | 3543 (64) | 1856 (64) | 6705 (67) | 13 533 (58) | |
| Positive | 1702 (36) | 1052 (36) | 3339 (33) | 9655 (42) | |
| Grade | <0.001 | ||||
| 1 | 465 (9) | 233 (11) | 840 (8) | 1195 (18) | |
| 2 | 2703 (52) | 1102 (54) | 5264 (53) | 2789 (41) | |
| 3 | 1946 (38) | 688 (34) | 3709 (37) | 2733 (40) | |
| 4 | 51 (1) | 26 (1) | 92 (1) | 68 (1) | |
| Clinical stage | <0.001 | ||||
| IIA (T3N0) | 6977 (41) | 1441 (48) | 6462 (62) | 5858 (23) | |
| IIB (T1-3N1) | 3886 (23) | 887 (29) | 3360 (32) | 4865 (19) | |
| III (T4N0-1) | 5960 (35) | 679 (23) | 636 (6) | 15 164 (59) | |
| Chemoradiation | <0.001 | ||||
| Chemo only | N/A | 935 (31) | 5643 (54) | 12 966 (50) | |
| Chemoradiation | N/A | 2072 (69) | 4815 (46) | 12 921 (50) | |
| Chemo number | <0.001 | ||||
| Single agent | N/A | 1183 (42) | 6769 (69) | 13 505 (56) | |
| Multiagent | N/A | 1652 (58) | 2974 (31) | 10 501 (44) | |
| Path stage | <0.001 | ||||
| IA | 2 (<1) | 18 (1) | 7 (<1) | N/A | |
| IB | 9 (<1) | 13 (1) | 10 (<1) | N/A | |
| IIA | 70 (1) | 55 (2) | 125 (1) | N/A | |
| IIB | 4595 (91) | 2353 (91) | 9245 (93) | N/A | |
| III | 251 (5) | 126 (5) | 385 (4) | N/A | |
| IV | 106 (2) | 33 (1) | 166 (2) | N/A | |
| Path nodes | <0.001 | ||||
| Negative | 1843 (34) | 1537 (54) | 2733 (27) | N/A | |
| Positive | 3549 (66) | 1293 (46) | 7553 (73) | N/A | |
| Margins | <0.001 | ||||
| No surgery | N/A | N/A | N/A | N/A | |
| R0 | 4481 (81) | 2638 (88) | 8472 (81) | N/A | |
| R1 | 938 (17) | 352 (12) | 1857 (18) | N/A | |
| R2 | 81 (1) | 17 (<1) | 129 (1) | N/A | |
| 30day mortality | <0.001 | ||||
| By group | 544 (10) | 70 (2) | 1 (<1) | N/A | |
| NAT | 70 (2) | ||||
| Upfront surgery | 546 (3.6) | ||||
| 90-day mortality | <0.001 | ||||
| By group | 1033 (19) | 167 (6) | 67 (1) | N/A | |
| NAT | 167 (6) | ||||
| Upfront surgery | 1100 (7) |
AT, adjuvant therapy; chemo, chemotherapy; NAT, neoadjuvant therapy; path, pathology.
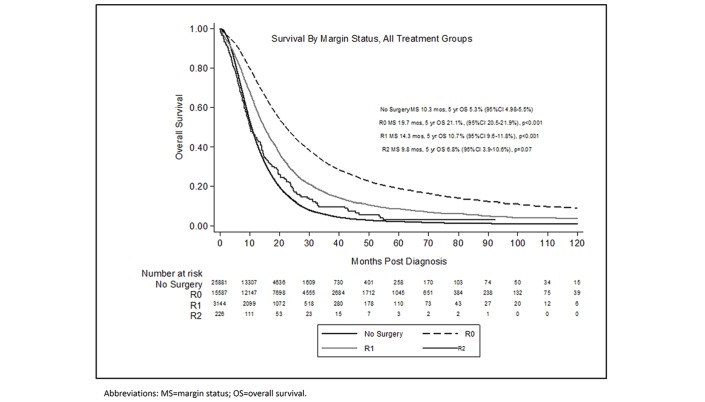
Patients who underwent R0 (MS 19.7 months, P<0.001) or R1 (MS 14.3 months, P<0.001) resections experienced a survival benefit compared with NoSurg patients (MS 10.3 months) (figure 1). Patients who underwent R2 resections experienced poorer survival (MS 9.5 months, P=0.07) that was not significantly different than that of NoSurg patients.
Figure 1.
Overall survival by margin status and surgical resection.
When survival was compared by surgical resection status, margin status and presence/timing of additional therapy, all groups experienced improved survival outcomes over NoSurg (MS 10.3 months), except for the Surg/Pos patients (MS 6.8 months, P<0.001) (see table 2, online supplementary figure 2). This finding remained significant on MVA (see table 3). The highest survival was achieved by NAT/Neg patients compared with all other cohorts (table 2). There was no difference in survival between NAT/Pos and AT/Pos patients, but both groups experienced improved survival compared with Surg/Pos patients (tables 2 and 3). Both NAT/Neg and AT/Neg patients achieved superior survival compared with the Surg/Neg patients (see table 2).
Table 2.
Median and 5-year overall survival by treatment, timing of treatment and stage
| Timing | Margin status | Median survival (months) | Five-year overall survival (%), 95% CI | HR | P value | Margin negative resection rate (%), P value |
| NAT | Neg | 23.2 | 26.5 (24.7 to 28.4) | 0.28 | <0.001 | 88, <0.001 |
| NAT | Pos | 17.6 | 14.5 (10.97 to 18.5) | 0.43 | <0.001 | |
| AT | Neg | 21.5 | 22.7 (21.7 to 23.6) | 0.32 | <0.001 | 81, <0.001 |
| AT | Pos | 16.8 | 11.9 (10.5 to 13.5) | 0.47 | <0.001 | |
| Surg | Neg | 13.1 | 16.1 (15 to 17.2) | 0.50 | <0.001 | 81, <0.001 |
| Surg | Pos | 6.8 | 6.7 (5.2 to 8.4) | 0.96 | 0.25 | |
| No surgery | N/A | 10.3 | 5.3 (4.98 to 5.5) | Reference | Reference | N/A |
| Timing/ stage |
Margin status | Median urvival (monthss) | Five-year overall survival (%), 95% CI | P value | Margin negative resection rate (%), P value | |
| Stage IIA | ||||||
| NAT | Neg | 23.6 | 28.2 (25.2 to 31.0) | <0.001 | 88, <0.001 | |
| NAT | Pos | 16.9 | 14.3 (9.3 to 20.4) | Reference | ||
| AT | Neg | 22.3 | 25.1 (23.8 to 26.3) | <0.001 | 83, reference | |
| AT | Pos | 18.0 | 13.8 (11.8 to 15.9) | 0.56 | 83, reference | |
| Surg | Neg | 14.8 | 18.2 (16.7 to 20) | 0.84 | ||
| Surg | Pos | 7.7 | 8.5 (6.3 to 11.1) | <0.001 | ||
| NoSurg | 10.4 | 5.6 (5.3 to 6.5) | <0.001 | |||
| Stage IIB | ||||||
| NAT | Neg | 22.4 | 25.4 (22.2 to 28.8) | <0.001 | 89, <0.001 | |
| NAT | Pos | 17.4 | 13.3 (7.3 to 21.2) | Reference | ||
| AT | Neg | 20.3 | 18.7 (17.1 to 20.3) | 0.03 | 83, reference | |
| AT | Pos | 16.5 | 11.3 (8.8 to 14.2) | 0.61 | 83, reference | |
| Surg | Neg | 11.6 | 13.7 (11.1 to 15.6) | 0.03 | ||
| Surg | Pos | 6.8 | 5.2 (3.1 to 8.2) | <0.001 | ||
| NoSurg | 9.7 | 5.5 (4.9 to 6.2) | <0.001 | |||
| Stage III | ||||||
| NAT | Neg | 23.4 | 24.4 (20.7 to 28.3) | 0.015 | 85, <0.001 | |
| NAT | Pos | 19.8 | 16.0 (9.4 to 24) | Reference | 59, reference | |
| AT | Neg | 18.4 | 19.2 (15.2 to 23.6) | 1.00 | ||
| AT | Pos | 13.6 | 5.6 (3.3 to 8.9) | <0.001 | 59, reference | |
| Surg | Neg | 8.2 | 7.7 (4.9 to 11.3) | <0.001 | ||
| Surg | Pos | 6.1 | 3.5 (1.4 to 7.2) | <0.001 | ||
| NoSurg | 10.4 | 4.9 (4.6 to 5.3) | <0.001 | |||
AT, adjuvant therapy; NAT, neoadjuvant therapy; Neg, negative; Pos, positive; Surg, surgery.
Table 3.
Multivariate analysis for survival in all patients/margin positive patients
| All patients Variable | HR | P value | Margin positive HR | P value |
| Tx sequence | ||||
| No surgery | Reference | Reference | N/A | N/A |
| NAT/NEG | 0.48 | <0.001 | N/A | N/A |
| NAT/POS | 0.71 | <0.001 | Reference | Reference |
| AT/NEG | 0.54 | <0.001 | N/A | N/A |
| AT/POS | 0.77 | <0.001 | 1.03 | 0.65 |
| SURG/NEG | 0.88 | <0.001 | N/A | N/A |
| SURG/POS | 1.71 | <0.001 | 2.44 | <0.001 |
| Sex | ||||
| Male | N/A (UVA) | N/A (UVA) | Reference | Reference |
| Female | N/A (UVA) | N/A (UVA) | 0.97 | 0.57 |
| Age group (years) | ||||
| 18–49 | Reference | Reference | N/A (UVA) | N/A (UVA) |
| 50–69 | 1.06 | 0.16 | N/A (UVA) | N/A (UVA) |
| 70–84 | 1.21 | <0.001 | N/A (UVA) | N/A (UVA) |
| 85+ | 1.31 | <0.001 | N/A (UVA) | N/A (UVA) |
| Charlson | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1 | 1.09 | <0.001 | 1.19 | <0.001 |
| 2+ | 1.32 | <0.001 | 1.46 | <0.001 |
| Tumour size | ||||
| 2 cm or less | Reference | Reference | Reference | Reference |
| 2.1–5 cm | 1.41 | <0.001 | 1.26 | <0.001 |
| >5 cm | 1.57 | <0.001 | 1.40 | <0.001 |
| Tumour ocation | ||||
| Head | Reference | Reference | Reference | Reference |
| Body | 0.97 | 0.44 | 1.08 | 0.38 |
| Tail | 0.91 | 0.007 | 0.94 | 0.27 |
| Duct | 0.64 | 0.001 | 0.82 | 0.34 |
| Other | 0.93 | 0.035 | 0.93 | 0.14 |
| Clinical nodes | ||||
| Negative | Reference | Reference | Reference | Reference |
| Positive | 1.05 | 0.35 | 1.01 | 0.84 |
| Grade | ||||
| 1 | Reference | Reference | Reference | Reference |
| 2 | 1.30 | <0.001 | 1.25 | 0.005 |
| 3 | 1.74 | <0.001 | 1.55 | <0.001 |
| 4 | 1.56 | <0.001 | 1.08 | 0.77 |
| Clinical stage | ||||
| IIA (T3N0) | Reference | Reference | Reference | Reference |
| IIB (T1-3N1) | 1.21 | 0.001 | 1.11 | 0.34 |
| III (T4N0-1) | 1.26 | <0.001 | 1.47 | <0.001 |
| Path stage group | ||||
| IA | Reference | Reference | Reference | Reference |
| IB | 0.85 | 0.68 | N/A (UVA) | N/A (UVA) |
| IIA | 1.38 | 0.32 | N/A (UVA) | N/A (UVA) |
| IIB | 1.09 | 0.79 | N/A (UVA) | N/A (UVA) |
| III | 1.29 | 0.44 | N/A (UVA) | N/A (UVA) |
| IV | 2.16 | 0.017 | N/A (UVA) | N/A (UVA) |
| Chemoradiation | ||||
| Chemo only | Reference | Reference | Reference | Reference |
| Chemoradiation | 0.89 | <0.001 | 0.78 | <0.001 |
| Chemo number | ||||
| Single agent | Reference | Reference | Reference | Reference |
| Multiagent | 0.92 | <0.001 | 0.93 | 0.199 |
AT, adjuvant therapy; chemo, chemotherapy; N/A (UVA), did not meet requirement for significance on UVA; N/A (UVA), did not meet requirement for significance on UVA; NAT, neoadjuvant therapy; NEG, negative; path, pathology; POS, positive; Surg, surgery; Tx, treatment.
esmoopen-2017-000282supp002.jpg (1.1MB, jpg)
When positive-margin patients were isolated as a group on MVA, AT was equal to NAT (HR 1.11, P=0.17) (table 3). Treatment with CRT (HR 0.78, P<0.001) but not multiagent chemotherapy (HR 0.93, P=0.199) was associated with a decreased risk of death. When broken down by clinical stage, NAT/Pos and AT/Pos yielded equivalent survival for stage IIA and IIB patients (see table 2). For stage III patients, NAT/Pos patients experienced improved survival compared with AT/Pos and Surg/Neg patients and similar to AT/Neg patients. When NAT was given, the margin-positive rate decreased by approximately 5% for stage II patients and 26% for stage III patients (table 2).
Finally, predictors of positive margins included Charlson score, tumour size, clinical stage, pathological stage and timing of therapy. Tumours greater than 2 cm (OR 1.59, P<0.001), greater than 5 cm (OR 2.03, P<0.001), grade 3 tumours (OR 1.19, P=0.03), clinical stage III (OR 1.65, P<0.001), pathological stage III (OR 2.46, P<0.001) and stage IV (OR 1.86, P=0.009) were all associated with positive margins. Upfront surgery was associated with increased risk of positive margins compared with NAT (OR 1.69, P<0.001).
Discussion
In patients with stage II and III pancreas cancer, surgical resection was associated with a survival benefit compared with patients who did not undergo surgery. This benefit was maximised in patients able to undergo R0 resection, though patients who underwent an R1 resection still experienced a statistically and clinically significant survival benefit compared with patients who did not undergo surgery, adjusting for available cofactors. While we could not control for every difference that may exist between the surgical and non-surgical patients, the observation of improved outcomes even for R1 resection represents a possible imbalance in the current practice patterns. There was no survival benefit after an R2 resection. The timing of additional therapy also affected the relevance of marginal status in patients who underwent surgical resection. Neoadjuvant treatment was associated with improved survival compared with AT if margins were negative, and there was no difference in survival if margins were positive. Surgical resection alone with positive margins was no better than no surgery. For stage III patients, NAT/Pos was associated with improved survival compared with AT/Pos with improved margin-negative resection rate (85% vs 59%).
In this study, patients who underwent an R1 resection were half as likely to be alive at 5 years as patients who underwent R0 resection, though they were twice as likely to be alive at 5 years when compared with patients who did not undergo surgery. Perioperative deaths were not censored from this analysis. Thus, it does appear that surgical resection is associated with a survival benefit compared with no surgery, and an R1 resection, while detrimental, does not negate that benefit. R2 resections, however, offer no survival benefit. This finding was confirmed when patients were stratified by stage. This is concordant with a previous multicentre randomised study in resectable pancreatic cancer that compared definitive chemoradiotherapy to surgical resection alone: MS was almost 1 year longer for the surgical group, with a 3-year survival rate gain of 20% compared with definitive chemoradiotherapy.24 In terms of surgical margins, these data are difficult to compare with other published series, as (1) these series do not compare their survival results to non-surgical results,1 8 9 11–18 25 (2) these series are mostly in the setting of AT1 8 9 11–18 25 and (3) R2 resections are often excluded1 8 18 25 (table 4). No matter the initial extent of disease, R0 resections trend towards improved survival in the majority of retrospective series and in the most recent prospective adjuvant study.8 9 11–17 25 Some aggregated prospective data, however, show no effect of margin status on survival.3 4 It is interesting that NAT was associated with a survival benefit in margin-negative patients but was no different than AT for margin-positive patients. This finding may be secondary to attrition in the NAT group, as a certain percentage of patients may not proceed to surgery due to disease progression, decompensation or other reasons. In patients with negative margins, this may be the driver of the perceived survival benefit. In the positive-margin patients, survival may have been affected by margin status such that any perceived benefit for the NAT group was offset by the survival detriment of a margin-positive resection.
Table 4.
Selected publications about the value of neoadjuvant or adjuvant therapy in relation to margin status
| Number of patients | R0 resection rate (%) | R0 definition | Neoadjuvant therapy | Median survival in months based on resection status | Adjuvant therapy | Notes | ||
| Resectable, no neoadjuvant treatment | R0 | R1/R2* | ||||||
| Fatima et al (Mayo)13 | 617 | 76 | 0 mm | 3.6% C 3.9% RT |
19 (en bloc) 18 (non-en bloc) |
15 (R1) 10 (R2) |
C or CRT in 80% | |
| Butturini et al (meta-analysis of four randomised trials: Norway, EORTC, Japan, ESPAC-1)4 | 875 | 68 | Variable | No | 15.9 | 14.1 | All/any | No difference in survival based on margin status |
| 53 | Variable | No | 15.9 | 14.7 | CRT | CRT only beneficial (NS) in R1 | ||
| 53 | Variable | No | 15.8 | 11.2 | No CRT | |||
| 109 | Variable | No | 20.8 | 15.0 | C | C beneficial (SS) in R0 | ||
| 114 | Variable | No | 13.4 | 13.2 | No C | |||
| Van den Broeck et al (Belgium)14 | 144 | 67 | <1 mm | No | 24 | 12.4 | CRT in 38, C in 31 | SS |
| Menon et al (Leeds)36 | 27 | 18 | 0 mm | No | >55 | 14 | Adjuvant NOS in 8/27 (30%) | Rigorous pathology protocol → higher R1 rate. All pts pT3 |
| Westgaard et al (Oslo)15 | 40 | 55 | <1 mm | No | 15 | 11 | No | |
| Herman et al (Johns Hopkins†)16 | 616 | 45 | <1 mm | No | 20 | 14 | CRT in 45% | Mean tumour size 3.2 cm |
| Oettle et al (CONKO-001)3 | 354 | 79 | 0 mm | No | 20 | No | Effect of margin status not significant; survival by margin status not given | |
| No | 23 | Gem | ||||||
| Richter et al (Germany)37 | 194 | 61 | ND | No | 12‡ | 24‡ | No | |
| Sperti et al (Italy)38 | 113 | 83 | ND | No | 14 | 7 | No | |
| Yeo et al (Johns Hopkins†)39 | 201 | 71 | ND | No | 18 | 10 | CRT in 56/201 (28%; MS 20 months for those pts) | |
| Willett et al (MGH)17 | 72 | 49 | ND | No | 19‡ | 11‡ | Any | |
| No | 20‡ | 12‡ | RT | |||||
| No | 18‡ | 9‡ | No RT | |||||
| Resectable, with neoadjuvant treatment | R0 | R1/2 | ||||||
| Golcher et al (Germany Phase II Trial)40 | 42 | 48 | ND | CRT | 25 | Phase II trial of upfront surgery versus neoadjuvant CRT. Results NS. | ||
| No | 19 | |||||||
| Breslin et al (MDACC)41 | 132 | 88 | ND | CRT; some IORT | 25 | 20 | No | ‘Potentially resectable’; margin status did not affect survival |
| Mixed stage, some neoadjuvant treatment | R0 | R1/2 | ||||||
| Raut et al (MDA)42 | 360 | 83 | 0 mm | Yes but % and details not recorded | 28 | 22 | Mix of resectable and BRPC; R0 did not predict survival on MVA | |
| Nishimura et al (Kyoto)43 | 157 | 55 | ND | Some received RT and/or IORT | 12 | 6 | Some CRT | Mixed stages |
| Borderline resectable or locally advanced | R0 | R1/R2 | ||||||
| Torgeson et al (NCDB)44 | 3077 | 88 | ND | C or CRT | 23 | 17 (R1) 12 (R2) |
Variable | |
| Ashman et al (Mayo Arizona)45 | 31 | 65 | ND | CRT and IORT | 23 | 17 | Some CT | BRPC (11) or LAPC (20) |
| Denost et al (Bordeaux)46 | 39 | 85 | ND | CRT | 33 | No | 97% T3/4 | |
| 72 | 75 | No | 24 | Some | 13% T3/4 | |||
*The majority of patients with positive resections in these studies had only microscopic disease.
†The Johns Hopkins report by Herman et al covers all pancreatic subsites, while the Johns Hopkins report by Yeo et al covers only head of pancreas.
‡Median survival not specifically reported; value estimated from printed survival curves.
BRPC, borderline resectable pancreas cancer; C, chemotherapy; CRT, chemoradiation therapy; Gen, gemcitabine; IORT, intraoperative radiation therapy; LAPC, locally advanced pancreas cancer; MDACC, MD Anderson Cancer Center; MGH, Massachusetts General Hospital; NCDB, National Cancer Database; ND, not defined; NS, not significant; pts, patients; SBRT, stereotactic body radiation therapy; SS, statistically significant.
The exception to this may be in patients at highest risk for positive margins (stage III). In these patients, NAT may allow for more successful surgeries. In our series, the overall cohort experienced an improved R0 rate with NAT (88% vs 81%, P<0.001), and stage III patients experienced a significant improvement (85% vs 59%, P<0.001). These data are concordant with other series demonstrating significantly reduced margin-positive resection rates with NAT (12.3% vs 19%–51%).4 17 18 26–28 In stage III patients, NAT/Pos was associated with a survival benefit compared with AT/Pos and equivalent survival to AT/Neg. These data suggest that NAT should be considered for all patients with borderline or locally advanced disease, which is concordant with current National Comprehensive Cancer Network (NCCN) guidelines and the subject of two ongoing clinical trials.29 30 For resectable disease, NAT should be considered based on the improvement in survival seen in margin-negative patients as well as the improved margin-negative rate.
Unfortunately, in the entire cohort of patients treated with CT or CRT upfront, only 11% of patients underwent resection, which is consistent with available literature including the recently reported results from LAP-07, where only 4% of patients with locally advanced pancreas cancer (LAPC) (stage III) underwent surgery after treatment.31 Some series using NAT in borderline and LAPC report higher resection rates ranging from 31% to 67%, with R0 resection rates ranging from 86% to 100%.26 28 32 Although the number of patients able to undergo resection was fewer in this study, the percentage of R0 resections was consistent (88%). MS for patients treated with NAT who underwent resection in these series ranged from 21.7 to 32 months, also consistent with our data (23 months with R0 resection). Ferrone et al 33, reported on 40 patients with LAPC treated with FOLFIRINOX and radiotherapy followed by resection. Although nearly half (19) of patients were deemed unresectable by imaging after NAT, the R0 resection rate was 90%.
The major weakness of this study is that it is difficult to compare surgical versus non-surgical patients as we do not have access to detailed reasons why surgery was or was not part of the planned course of treatment. This would be especially helpful in stage IIA-B ‘resectable’ patients who did not undergo surgery and for stage III ‘borderline/locally advanced’ patients who did, as these patients may be representative of extenuating circumstances that may create bias. Though guidelines for resectability exist, significant heterogeneity likely remains. Even with heterogeneity (which ideally would decrease selection bias), it is possible that the healthiest patients were consistently more likely to receive the most aggressive treatments (ie, multiagent chemotherapy, CRT and surgery). Thus, the perceived survival benefit in these patients could have been enhanced by a disproportionate inclusion of patients who were likely to live longer regardless of treatment. Also, patients who began treatment with localised disease may have developed distant or otherwise unresectable disease during the course of treatment and thus may not have been candidates for aggressive local treatment. Patients in the no surgery group may also have had findings suspicious for metastatic disease at presentation or were otherwise deemed medically ineligible for surgery (ie, elevated CA-19–9 and severe weight loss). The presence of these patients in high numbers could certainly affect the perceived survival benefit. Finally, the definition of an R1 resection can vary between studies, and more strict standardisation of pathology processing can increase the margin positivity rate substantially.34 It is further suggested that survival is affected by distance of tumour to the margin even over the range of 0–1.5 mm, which is not standardly reported.35
Though these weaknesses are significant and a source of potential selection bias, there are also important strengths in this study. Over 44 000 patients were examined, which lends significant power. Additionally, these patients were treated by physicians from different institutional settings whose practice patterns and expertise likely varied, giving an accurate national picture of practice patterns and the current state of the field, which adds to the external validity of these results. There will likely never be a randomised study addressing the value of surgery in these high-risk patients, so large retrospective studies can add value by providing hypothesis-generating data to guide future prospective studies.
Conclusion
An overall survival benefit to surgical resection, even in microscopically positive-margin patients, is demonstrated in this large aggregated dataset for patients with clinical stage IIA–III pancreatic cancer. Patients who underwent R1 resections were twice as likely to be alive at 5 years compared with patients who did not undergo resection, though only half as likely as patients who underwent R0 resections. The timing of additional therapies affects the significance of margin status on survival. NAT was associated with improved R0 resection rate and improved survival in the R0 setting. NAT in R1 resections was equivalent to AT for stage IIA-B patients but superior for stage III patients. Thus, NAT should be favoured in borderline and LAPC. Given the improved outcomes seen in patients with an R1 resection compared with patients who do not undergo surgery, these data suggest surgical resection should be heavily considered even if the significant possibility of an R1 resection exists.
Acknowledgments
The authors would like to acknowledge Michelle Denney for her assistance in copyediting and manuscript preparation.
Footnotes
Contributors: Concept and design: SL and AT. Statistical analysis: SL and AT. Manuscript writing, editing and final version: all authors.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Neoptolemos JP, Stocken DD, Friess H, et al. . A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200–10. 10.1056/NEJMoa032295 [DOI] [PubMed] [Google Scholar]
- 2. Hsu CC, Herman JM, Corsini MM, et al. . Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol 2010;17:981–90. 10.1245/s10434-009-0743-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oettle H, Neuhaus P, Hochhaus A, et al. . Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473–81. 10.1001/jama.2013.279201 [DOI] [PubMed] [Google Scholar]
- 4. Butturini G, Stocken DD, Wente MN, et al. . Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75–83. 10.1001/archsurg.2007.17 [DOI] [PubMed] [Google Scholar]
- 5. Stocken DD, Büchler MW, Dervenis C, et al. . Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer 2005;92:1372–81. 10.1038/sj.bjc.6602513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899–903. [DOI] [PubMed] [Google Scholar]
- 7. Regine WF, Winter KA, Abrams RA, et al. . Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019–26. 10.1001/jama.299.9.1019 [DOI] [PubMed] [Google Scholar]
- 8. Neoptolemos JP, Palmer DH, Ghaneh P, et al. . Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011–24. 10.1016/S0140-6736(16)32409-6 [DOI] [PubMed] [Google Scholar]
- 9. Yeo CJ, Abrams RA, Grochow LB, et al. . Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997;225:621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allema JH, Reinders ME, van Gulik TM, et al. . Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer 1995;75:2069–76. [DOI] [PubMed] [Google Scholar]
- 11. Wenger FA, Peter F, Zieren J, et al. . Prognosis factors in carcinoma of the head of the pancreas. Dig Surg 2000;17:29–35. 10.1159/000018797 [DOI] [PubMed] [Google Scholar]
- 12. Millikan KW, Deziel DJ, Silverstein JC, et al. . Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg 1999;65:618–23. [PubMed] [Google Scholar]
- 13. Fatima J, Schnelldorfer T, Barton J, et al. . Pancreatoduodenectomy for ductal adenocarcinoma: implications of positive margin on survival. Arch Surg 2010;145:167–72. 10.1001/archsurg.2009.282 [DOI] [PubMed] [Google Scholar]
- 14. Van den Broeck A, Sergeant G, Ectors N, et al. . Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2009;35:600–4. 10.1016/j.ejso.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 15. Westgaard A, Tafjord S, Farstad IN, et al. . Resectable adenocarcinomas in the pancreatic head: the retroperitoneal resection margin is an independent prognostic factor. BMC Cancer 2008;8:5 10.1186/1471-2407-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herman JM, Swartz MJ, Hsu CC, et al. . Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503–10. 10.1200/JCO.2007.15.8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willett CG, Lewandrowski K, Warshaw AL, et al. . Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg 1993;217:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neoptolemos JP, Stocken DD, Dunn JA, et al. . Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 2001;234:758–68. 10.1097/00000658-200112000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christians K, Evans DB. Pancreaticoduodenectomy and vascular resection: persistent controversy and current recommendations. Ann Surg Oncol 2009;16:789–91. 10.1245/s10434-009-0322-y [DOI] [PubMed] [Google Scholar]
- 20. Christians KK, Tsai S, Mahmoud A, et al. . Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist 2014;19:266–74. 10.1634/theoncologist.2013-0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evans DB, Varadhachary GR, Crane CH, et al. . Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496–502. 10.1200/JCO.2007.15.8634 [DOI] [PubMed] [Google Scholar]
- 22. Varadhachary GR, Wolff RA, Crane CH, et al. . Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487–95. 10.1200/JCO.2007.15.8642 [DOI] [PubMed] [Google Scholar]
- 23. Bilimoria KY, Stewart AK, Winchester DP, et al. . The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683–90. 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doi R, Imamura M, Hosotani R, et al. . Surgery versus radiochemotherapy for resectable locally invasive pancreatic cancer: final results of a randomized multi-institutional trial. Surg Today 2008;38:1021–8. 10.1007/s00595-007-3745-8 [DOI] [PubMed] [Google Scholar]
- 25. Nitecki SS, Sarr MG, Colby TV, et al. . Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995;221:59–66. 10.1097/00000658-199501000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. . Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267 10.1371/journal.pmed.1000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cloyd JM, Katz MHG, Prakash L, et al. . Preoperative Therapy and Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma: a 25-Year Single-Institution Experience. J Gastrointest Surg 2017;21:164–74. 10.1007/s11605-016-3265-1 [DOI] [PubMed] [Google Scholar]
- 28. Spitz FR, Abbruzzese JL, Lee JE, et al. . Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997;15:928–37. 10.1200/JCO.1997.15.3.928 [DOI] [PubMed] [Google Scholar]
- 29. Sohal D, McDonough SL, Ahmad SA, et al. . SWOG S1505: A randomized phase II study of perioperative mFOLFIRINOX vs. gemcitabine/nab-paclitaxel as therapy for resectable pancreatic adenocarcinom. J Clini Oncol 2017;35:TPS508 10.1200/JCO.2017.35.4_suppl.TPS508 [DOI] [Google Scholar]
- 30. Katz MHG, Ou FS, Herman JM, et al. . Alliance for clinical trials in oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer 2017;17:505 10.1186/s12885-017-3441-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hammel P, Huguet F, van Laethem JL, et al. . Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 2016;315:1844–53. 10.1001/jama.2016.4324 [DOI] [PubMed] [Google Scholar]
- 32. Cloyd JM, Katz MH, Prakash L, et al. . Preoperative therapy and pancreatoduodenectomy for pancreatic ductal adenocarcinoma: a 25-year single-institution experience. J Gastrointest Surg 2017;21 10.1007/s11605-016-3265-1 [DOI] [PubMed] [Google Scholar]
- 33. Ferrone CR, Marchegiani G, Hong TS, et al. . Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12–17. 10.1097/SLA.0000000000000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esposito I, Kleeff J, Bergmann F, et al. . Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008;15:1651–60. 10.1245/s10434-008-9839-8 [DOI] [PubMed] [Google Scholar]
- 35. Chang DK, Johns AL, Merrett ND, et al. . Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol 2009;27:2855–62. 10.1200/JCO.2008.20.5104 [DOI] [PubMed] [Google Scholar]
- 36. Menon KV, Gomez D, Smith AM, et al. . Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB 2009;11:18–24. 10.1111/j.1477-2574.2008.00013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richter A, Niedergethmann M, Sturm JW, et al. . Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg 2003;27:324–9. 10.1007/s00268-002-6659-z [DOI] [PubMed] [Google Scholar]
- 38. Sperti C, Pasquali C, Piccoli A, et al. . Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg 1996;83:625–31. 10.1002/bjs.1800830512 [DOI] [PubMed] [Google Scholar]
- 39. Yeo CJ, Cameron JL, Lillemoe KD, et al. . Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221:721–33. 10.1097/00000658-199506000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Golcher H, Brunner TB, Witzigmann H, et al. . Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer. Strahlentherapie und Onkologie 2015;191:7–16. 10.1007/s00066-014-0737-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Breslin TM, Hess KR, Harbison DB, et al. . Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 2001;8:123–32. 10.1007/s10434-001-0123-4 [DOI] [PubMed] [Google Scholar]
- 42. Raut CP, Tseng JF, Sun CC, et al. . Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007;246:52–60. 10.1097/01.sla.0000259391.84304.2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nishimura Y, Hosotani R, Shibamoto Y, et al. . External and intraoperative radiotherapy for resectable and unresectable pancreatic cancer: analysis of survival rates and complications. Int J Radiat Oncol Biol Phys 1997;39:39–49. 10.1016/S0360-3016(97)00295-2 [DOI] [PubMed] [Google Scholar]
- 44. Torgeson A, Lloyd S, Boothe D, et al. . Multiagent induction chemotherapy followed by chemoradiation is associated with improved survival in locally advanced pancreatic cancer. Cancer 2017;123:3816–24. 10.1002/cncr.30780 [DOI] [PubMed] [Google Scholar]
- 45. Ashman JB, Moss AA, Rule WG, et al. . Preoperative chemoradiation and IOERT for unresectable or borderline resectable pancreas cancer. J Gastrointest Oncol 2013;4:352–60. 10.3978/j.issn.2078-6891.2013.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Denost Q, Laurent C, Adam JP, et al. . Pancreaticoduodenectomy following chemoradiotherapy for locally advanced adenocarcinoma of the pancreatic head. HPB 2013;15:716–23. 10.1111/hpb.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000282supp001.jpg (1.2MB, jpg)
esmoopen-2017-000282supp002.jpg (1.1MB, jpg)