Abstract
Background
Immune checkpoint inhibitors are successfully introduced as anticancer treatment. However, they may induce severe immune-related adverse events (irAEs). One of the most frequent irAEs is diarrhoea. The main objective of this study was to analyse symptoms (ie, grade of diarrhoea), endoscopic and histological features and response to management in immune checkpoint inhibition-related colitis (IRC).
Patients and methods
We retrospectively analysed patients who developed diarrhoea on checkpoint inhibition and therefore underwent an endoscopy and/or were treated with corticosteroids. Patients were treated between August 2010 and March 2016 for metastatic melanoma or non-small cell lung cancer. Severity of IRC was scored using the endoscopic Mayo score and the van der Heide score.
Results
Out of a cohort of 781 patients, 92 patients were identified who developed diarrhoea and therefore underwent an endoscopy and/or were treated with corticosteroids. Patients were treated with monotherapy anticytotoxic T-lymphocyte antigen-4, antiprogrammed death receptor-1 or a combination of both. All patients had symptoms of diarrhoea (grade 1: 16%; grade 2: 39% and grade 3: 44%). A complete colonoscopy was performed in 62 (67%) patients, of whom 42 (68%) had a pancolitis (≥3 affected segments). Ulcers were seen in 32% of endoscopies. There was no significant correlation between the grade of diarrhoea at presentation and endoscopic severity scores, the presence of ulcers or histological features. In 54 episodes of diarrhoea (56%), patients received one or more cycles infliximab for steroid-refractory colitis. Patients with higher endoscopic severity scores, ulcers and/or a pancolitis needed infliximab more often.
Conclusions
The correlation between grade of diarrhoea and endoscopic or histological features for severity of colitis is poor. Patients with higher endoscopic severity scores, ulcers or a pancolitis needed the addition of infliximab more often. Therefore, endoscopy may have value in the evaluation of the severity of IRC and may help in decision making for optimal management.
Keywords: immunotherapy, colitis, infliximab, endoscopy
Key questions.
What is already known about this subject?
Immunotherapy can induce adverse events, which are predominantly immune related.
One of the most common and severe immune-related adverse events is diarrhoea.
Diarrhoea is seen in 35% of patients treated with anticytotoxic T-lymphocyte antigen-4, 20% in patients treated with antiprogrammed death receptor-1 and even 44% in patients treated with the combination therapy.
What does this study add?
Patients in which ulcers were seen during endoscopy required significantly more often the addition of infliximab for steroid-refractory colitis compared with patients in which no ulcers were seen.
Patients with a high van der Heide score, a high Mayo score or a pancolitis required significantly more often the addition of infliximab for steroid-refractory colitis compared with patients with a low van der Heide score, low Mayo score or no pancolitis.
There was no significant correlation between the grade of diarrhoea at presentation and endoscopic Mayo score, van der Heide score or presence of ulcers.
There was no correlation between the presence of abdominal pain and any endoscopic feature.
The most common histopathological feature was an increase in lamina propria cellularity, primarily consisting of mononuclear cells. The second most common histopathological feature was neutrophilic infiltration, either intraepithelial or as crypt abscesses.
Key questions.
How might this impact on clinical practice?
Algorithms to guide management of immune-related diarrhoea should not be based on the grade of diarrhoea.
Endoscopic features, such as the presence of ulcers or a pancolitis, can help clinicians to intensify immune suppression more rapidly.
Histopathology does not seem to have an added value to guide therapy beyond what is found endoscopically. Mucosal biopsies appear to mainly serve to confirm diagnosis.
Introduction
The introduction of immune checkpoint inhibitors has changed treatment options and improved survival of patients with advanced cancer. Ipilimumab, a monoclonal antibody blocking cytotoxic T-lymphocyte antigen-4 (CTLA-4) on T cells, showed an overall survival benefit in patients with advanced melanoma.1 Nivolumab and pembrolizumab, both antibodies blocking programmed death receptor-1 (PD-1), improved survival compared with chemotherapy and ipilimumab.2 3 The combination of ipilimumab with an anti-PD-1 antibody improves overall response rate and progression free survival even further compared with single agent therapy.4 Checkpoint inhibitors also show activity in several other types of cancer, such as metastatic non-small cell lung cancer (NSCLC) and bladder cancer.5–7 Although efficacy and durability of response with checkpoint inhibitors has been well established, one of the major concerns is the high rate of adverse events that are predominantly immune related.
Diarrhoea
One of the most common and severe immune-related adverse events is diarrhoea, with an incidence of 35% for anti-CTLA-4, 20% for anti-PD-1 and even 44% for the combination therapy.4 8 The median time to onset of diarrhoea is 7–8 weeks after start for ipilimumab (or combinations with ipilimumab), compared with 3–6 months for anti-PD-1.9–12 The Common Terminology Criteria for Adverse Events (CTCAE version 4.03) are often used to define grades of diarrhoea in patients treated in clinical trials. Grade 1 diarrhoea is defined as an increase of <4 stools over baseline, grade 2 as between 4 and 6 stools over baseline, grade 3 as ≥7, grade 4 as life-threatening consequences and grade 5 as death.
Treatment algorithms
Current treatment algorithms for immune checkpoint inhibition-related colitis (IRC) are based on symptoms of diarrhoea graded according to CTCAE.10–13 For patients with grade 2 diarrhoea, delay of immunotherapy and start of symptomatic treatment with loperamide are considered. If symptoms persist for >3 days, oral corticosteroids in a dose of 0.5–1.0 mg/kg are recommended. For patients with grade 3 or 4 diarrhoea, discontinuation of immunotherapy (IT) and treatment with 1.0–2.0 mg/kg prednisone is advised. Steroid-refractory colitis is defined as the persistence of symptoms within 3 days of high-dose corticosteroids. These patients could be treated with the addition of 5 mg/kg infliximab. The implementation of these treatment algorithms has resulted in a decrease of serious complications such as perforation and colectomy.14 According to these algorithms, a lower endoscopy is advised for patients with grade 3 or 4 symptoms of diarrhoea, but no recommendations are provided on differential treatment based on endoscopic findings. The aim of this study was to try to correlate symptoms, endoscopic features, histology and response to management in patients who developed diarrhoea on immune checkpoint inhibition.
Methods
Patients
Patients who developed diarrhoea on immunotherapy and therefore underwent an endoscopy and/or were treated with corticosteroids were retrospectively identified. All patients were treated for melanoma or NSCLC, between August 2010 and March 2016. Patients were treated with monotherapy anti-CTLA-4, anti-PD-1, a combination of both or the combination of anti-CTLA-4 and radiofrequency ablation (RFA). Diarrhoea was scored according to CTCAE version 4.03. All patient characteristics were derived from the electronic patient records. Routinely, stools were tested for microorganisms, including SSYC, Clostridium difficile and viral pathogens. Severity of IRC on endoscopy was scored retrospectively using two different scoring systems (online supplementary table 1). Endoscopic characteristics of IRC are very diverse, and there are no available validated scoring systems. Often, a diffuse component of inflammation was present, and therefore we used the Mayo score, which is validated for scoring diffuse inflammation seen in ulcerative colitis (UC).15 However, this score is not ideal in patients with ulcers among a normal or slightly friable mucosa. When ulcers were present in a further normal mucosa, a Mayo score of 0 with a positive ulcer score was given in our study. We also used the van der Heide score, as it is more descriptive and therefore potentially more useful for the diverse characteristics seen in IRC. This score has been used previously for this purpose.16 17 However, the van der Heide score does not take into account the extensiveness of inflammation. Therefore, numbers of affected segments of the colon (rectosigmoid, descending, transverse and ascending) were scored separately. Involvement of ≥3 segments was defined as pancolitis. Scores were gathered through saved images and endoscopy reports and revised by one gastroenterologist (JvD), blinded for the grade of diarrhoea. As the scores may be influenced by subjectivity, the most objective endoscopic feature, namely the presence of ulcers, was analysed as a separate variable. An ulcer was defined as a mucosal break of ≥0.5 cm. All H&E stained slides of biopsies taken during endoscopies were reassessed by one gastrointestinal pathologist (PS).
esmoopen-2017-000278supp001.pdf (127KB, pdf)
Treatments
Patients treated with ipilimumab, nivolumab or pembrolizumab as monotherapy received standard or flat doses. Patients who received the combination of ipilimumab and RFA underwent RFA of one liver metastasis, directly followed by four cycles of ipilimumab (depending on the cohort, either 3 mg/kg or 10 mg/kg every 3 weeks). Patients received either the standard combination of ipilimumab (3 mg/kg) and nivolumab (1 mg/kg) or a sequential but overlapping scheme of two cycles ipilimumab 3 mg/kg on days 1 and 22 followed directly by nivolumab (3 mg/kg) or pembrolizumab (2 mg/kg) from day 23 and onwards every 2 weeks or every 3 weeks, respectively.
Statistical analysis
For continuous variables, data are presented as median with IQR and categorical variables as a number (%). Correlations between clinical symptoms and the endoscopic features were assessed using Spearman rank correlation coefficient. Associations between clinical symptoms, endoscopic features, histology and outcome of management were analysed by χ2 tests. A P value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS V.22.
Results
Patient characteristics
Out of a cohort of 781 patients, 92 patients were identified who developed diarrhoea and therefore underwent an endoscopy and/or were treated with corticosteroids. All patient characteristics have been summarised in table 1. Four patients had two different episodes of diarrhoea (median days between episodes 318 days; range 190–632). Mean age was 58 years (range 30–88) and 54% of patients were female. Eighty patients were treated for metastatic melanoma (87%), and 12 patients (13%) were treated for metastatic NSCLC. Fifty-six per cent (54/96) of episodes were due to anti-CTLA-4 (of which 10/54 received the combination with RFA), 22% due to anti-PD-1% and 22% due to the combination of anti-CTLA-4 and anti-PD-1. In 16% of episodes patients had grade 1 diarrhoea, 39% had grade 2 diarrhoea and 44% had grade 3 diarrhoea. In 48 episodes (50%) patients also experienced abdominal pain and in 29 episodes (30%) patients had bloody stools. Infectious causes for diarrhoea were ruled out in 68 episodes (71%). Three patients had a positive stool culture for which they were treated with antibiotics. However, as symptoms did not resolve, an IRC component was present as well. The median time between the first cycle of immunotherapy and onset of diarrhoea was 38 days (IQR 23–62). For patients treated with ipilimumab, the median time to onset of diarrhoea was 33 days, for anti-PD-1 84 days and for the combination 27 days. Three patients developed a perforation of the colon, for which they underwent surgery (online supplementary table 2). No patients died due to colitis.
Table 1.
Patient characteristics
| No. (%) | |
| Age median (range) | 58 (30–88) |
| Gender | |
| Male | 42 (46) |
| Female | 50 (54) |
| Type of cancer | |
| Melanoma | 80 (87) |
| NSCLC | 12 (13) |
| Immunotherapy among 96 episodes | |
| Ipilimumab (3 mg/kg) | 44 (46) |
| Ipilimumab (10 mg/kg) | 10 (10) |
| Nivolumab | 11 (12) |
| Pembrolizumab | 10 (10) |
| Sequential ipilimumab+pembrolizumab | 7 (7) |
| Sequential ipilimumab+nivolumab | 2 (2) |
| Combined ipilimumab+nivolumab | 12 (13) |
| Diarrhoea at presentation among 96 episodes | |
| Grade 1 | 15 (16) |
| Grade 2 | 37 (39) |
| Grade 3 | 43 (44) |
| Grades 4–5 | 0 (0) |
| Unknown | 1 (1) |
| Prednisone at start of diarrhoea | |
| None | 4 (4) |
| <1 mg/kg | 32 (33) |
| 1 mg/kg | 57 (60) |
| >1 mg/kg | 3 (3) |
| Budesonide | |
| No | 84 (87) |
| Yes | 12 (13) |
| Infliximab | |
| No | 42 (44) |
| Yes | 54 (56) |
| Mycophenolic acid | |
| No | 93 (97) |
| Yes | 3 (3) |
| Tacrolimus | |
| No | 94 (98) |
| Yes | 2 (2) |
NSCLC, non-small cell lung cancer.
Endoscopic results
In all but three episodes an endoscopy was performed. Endoscopy images were not available in one episode. These four patients were excluded from endoscopic and histopathological analysis. The median time between start of diarrhoea and endoscopy was 8 days (IQR 5–14), the median endoscopic Mayo score was 1 (range 0–3) and the median van der Heide score was 6 (range 0–12). Ulcers were seen during 29 endoscopies (32%). In the majority of endoscopies (79%), a continuous pattern of inflammation was seen. A complete colonoscopy was performed in 62 (67%) patients, of whom 42 (68%) had a pancolitis (≥3 affected segments). No serious side effects of colonoscopy were seen in our patients. All endoscopic features are summarised in table 2. There was no significant correlation between grade of diarrhoea at presentation and endoscopic Mayo score (ρ 0.12; P=0.28), van der Heide score (ρ 0.13; P=0.23) or presence of ulcers (ρ 0.12; P=0.25). Also, no correlation was found between the presence of abdominal pain and any endoscopic feature. A correlation was found between the presence of bloody stools and the endoscopic scores: Mayo: ρ 0.35 (P=0.001) and van der Heide: ρ 0.43 (P<0.001). There was no difference in the presence of ulcers in patients with grade 2 (37%) or grade 3 diarrhoea (33%; P=0.73). In 15 (24%) of the complete colonoscopies, the ascending colon was more severely affected than the descending colon (an example can be seen in figure 1E,F). Moreover, in 5 out of 64 colonoscopies (8%), endoscopic signs of inflammation were only seen in the ascending colon. Endoscopic features and their association with symptoms and treatment management have been summarised in table 3.
Table 2.
van der Heide and endoscopic Mayo scores from 92 endoscopies
| Endoscopic feature according to the van der Heide classification | No. (%) |
| Color | |
| Normal | 12 (13) |
| Red | 58 (63) |
| Deeply red | 22 (24) |
| Vascular patter | |
| Normal | 18 (20) |
| Partially absent | 45 (49) |
| Completely absent | 29 (31) |
| Friability | |
| Normal | 17 (19) |
| Slightly friable | 48 (52) |
| Severely friable | 27 (29) |
| Granularity | |
| Absent | 23 (25) |
| Fine granularity | 62 (67) |
| Coarse granularity | 7 (8) |
| Rectal valves | |
| Sharp | 46 (50) |
| Swollen | 46 (50) |
| Absent | 0 (0) |
| Ulcers | |
| Absent | 63 (69) |
| Few | 18 (19) |
| Multiple | 11 (12) |
| Spontaneous bleeding | |
| Absent | 87 (95) |
| Discrete | 4 (4) |
| Severe | 1 (1) |
| Mucopurulent exudate | |
| Absent | 35 (38) |
| Little | 35 (38) |
| Much | 22 (24) |
| van der Heide score | |
| Low (0–6) | 50 (54) |
| High (7–16) | 42 (46) |
| Mayo score* | |
| 0 | 14 (16) |
| 1 | 46 (52) |
| 2 | 25 (29) |
| 3 | 3 (3) |
*The endoscopic Mayo score was available for 88 episodes (four episodes could not be classified according to the endoscopic Mayo score).
Figure 1.
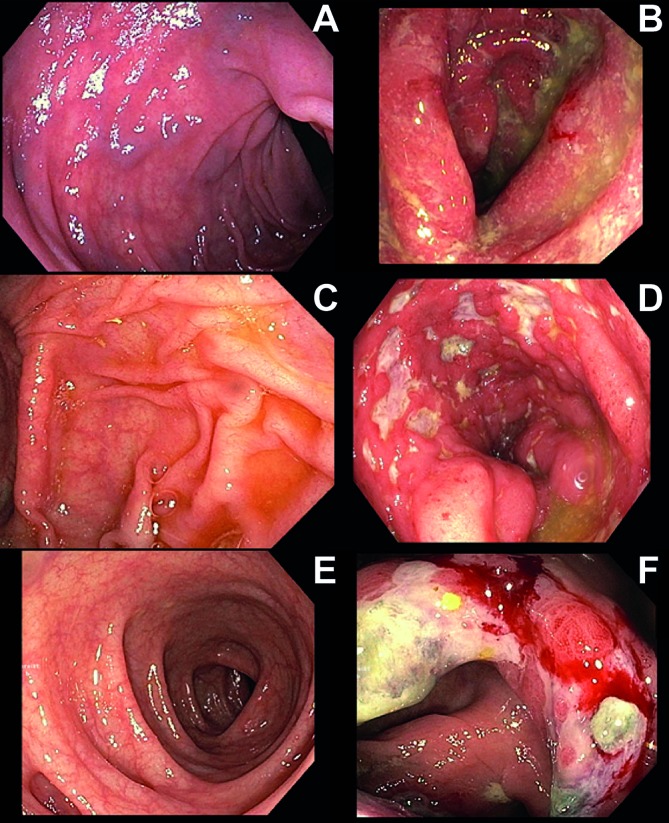
(A–F) Examples of differences in immune checkpoint inhibition-related colitis. Figure parts A and B show two different patients with grade 2 diarrhoea. Figure part A shows no abnormalities on colonoscopy. Figure part B shows a swollen, erosive and friable mucosa. Figure parts C and B show two different patients with grade 3 diarrhoea. Figure part C shows no abnormalities on colonoscopy. Figure part D shows a deeply red colon where the vascular pattern is partially absent, the mucosa appears severely friable and multiple ulcers can be seen. Figure parts E and F show a single patient with grade 1 diarrhoea. During colonoscopy, the entire descending colon (E) showed no abnormalties, while the ascending colon (F) showed a swollen, severely friable mucosa, with deep ulcers.
Table 3.
Endoscopic features and association with symptoms and treatment management in 92 episodes of diarrhoea
| Endoscopic features | Total No. (%) |
Grade of diarrhoea* G2/G3 No. (%) | P value | Bloody stools no/yes No. (%) | P value | Need for infliximab no/yes No. (%) | P value |
| Endoscopic Mayo† | |||||||
| 0–1 (low) | 60 (68) | 21 (44)/27 (56) | 0.84 | 47 (78)/13 (22) | <0.01 | 32 (53)/28 (47) | <0.01 |
| 2–3 (high) | 28 (32) | 12 (46)/14 (54) | 13 (46)/15 (54) | 6 (21)/22 (79) | |||
| Total van der Heide score | |||||||
| 0–6 (low) | 50 (54) | 20 (49)/21 (51) | 0.53 | 44 (88)/6 (12) | <0.01 | 29 (58)/21 (42) | <0.01 |
| 7–12 (high) | 42 (46) | 15 (42)/21 (58) | 19 (45)/23 (55) | 12 (29)/30 (71) | |||
| Ulcers | |||||||
| No | 63 (69) | 22 (44)/28 (56) | 0.73 | 47 (75)/16 (25) | 0.06 | 35 (56)/28 (44) | <0.01 |
| Yes | 29 (31) | 13 (48)/14 (52) | 16 (55)/13 (45) | 6 (21)/23 (79) | |||
| Pancolitis‡ | |||||||
| No | 20 (32) | 7 (50)/7 (50) | 0.36 | 17 (85)/3 (15) | 0.13 | 15 (75)/5 (25) | <0.01 |
| Yes | 42 (68) | 14 (36)/25 (64) | 28 (67)/14 (33) | 10 (24)/32 (76) | |||
Cases with missing values not included in χ2 test.
*Grade of diarrhoea only G2 versus G3.
†The endoscopic Mayo score was available for 88 episodes (four episodes could not be classified according to the endoscopic Mayo score).
‡Pancolitis only available for 62 episodes in which a full colonoscopy was performed.
Histological features
In 90 episodes (94%), biopsies were taken during endoscopy. Histopathological features have been summarised in table 4. In the majority of episodes, patients had received immunosuppressive drugs before the endoscopic procedure (52%, n=47). Median days on high-dose steroids was 4 (IQR 2–6). The most common change was an increase in lamina propria cellularity (83%, n=75), primarily consisting of mononuclear cells (online supplementary figure 1). The second most common change was neutrophilic infiltration, either intraepithelial (79%, n=71) or as crypt abscesses (62%, n=56). In both circumstances, usually mild and patchy. Mild to prominent intraepithelial lymphocytosis was present in only 10% (n=9). Small foci with minimal increase in intraepithelial lymphocytes were noted in an additional 15 cases (17%). Increased numbers of apoptotic cells were seen in the crypts in 42% (n=38), but usually this was mild (n=28). Outcomes of exploratory association analysis of histopathological features with various clinical and endoscopic features are displayed in online supplementary table 3. Analogous to endoscopic features, none of the histopathological features had an association with the grade of diarrhoea. However, multiple histopathological features were associated with endoscopic features (such as Mayo score and the presence of ulcers), bloody stools and the need for infliximab. Endoscopic and histopathological features had no association with different types of immune check point inhibitors (online supplementary table 4). Also, histopathological features did not correlate with whether immunosuppressive therapy had been administered before taking the biopsies (data not shown).
Table 4.
Histopathological features of biopsies taken in 90 endoscopies
| Histopathological feature | No. (%) |
| Lamina propria cellularity | |
| Normal | 15 (17) |
| Increased | |
| Focal | 7 (8) |
| Patchy | 24 (27) |
| Diffuse, superficial | 4 (4) |
| Diffuse, transmucosal – mild | 24 (26) |
| Diffuse, transmucosal – moderate | 16 (18) |
| Diffuse, transmucosal – severe | 0 (0) |
| Crypt architecture | |
| Normal | 58 (64) |
| Irregular – mild | 23 (26) |
| Irregular – moderate | 8 (9) |
| Irregular – severe | 1 (1) |
| Mucosal surface | |
| Flat/normal | 74 (82) |
| Irregular | 15 (17) |
| Villous | 1 (1) |
| Apoptotic cells in crypt epithelium | |
| Absent/hardly any | 52 (58) |
| Mild | 28 (31) |
| Moderate | 6 (7) |
| Severe | 4 (4) |
| Extension of chronic inflammatory infiltrate into submucosa | |
| Not present | 46 (58) |
| Present | 33 (42) |
| Location of intraepithelial neutrophilic infiltration | |
| Absent | 19 (21) |
| Present in crypt epithelium | 8 (9) |
| Present in superficial epithelium | 16 (18) |
| Present in crypt and superficial epithelium | 47 (52) |
| Grade of intraepithelial neutrophilic infiltration | |
| None | 19 (21) |
| Minimal | 15 (17) |
| Mild | 46 (51) |
| Moderate | 10 (11) |
| Severe | 0 (0) |
| Neutrophilic crypt abscesses | |
| Absent | 34 (38) |
| Mild | 46 (51) |
| Moderate | 8 (9) |
| Severe | 2 (2) |
| Location of intraepithelial lymphocytosis | |
| Absent | 66 (73) |
| Present in crypt epithelium | (5) |
| Present in superficial epithelium | 8 (9) |
| Present in crypt and superficial epithelium | 12 (13) |
| Grade of intraepithelial lymphocytosis | |
| Absent | 66 (73) |
| Minimal and patchy | 15 (17) |
| Mild | 6 (7) |
| Moderate | 2 (2) |
| Severe | 1 (1) |
| Mucin depletion of epithelial cells | |
| Not present | 46 (51) |
| Mild | 32 (36) |
| Moderate | 10 (11) |
| Severe | 2 (2) |
| Ulceration | |
| Absent | 71 (79) |
| Present | 19 (21) |
| Granuloma | |
| Absent | 85 (94) |
| Present in lamina propria | 4 (5) |
| Present in submucosa | 1 (1) |
Figure 2.
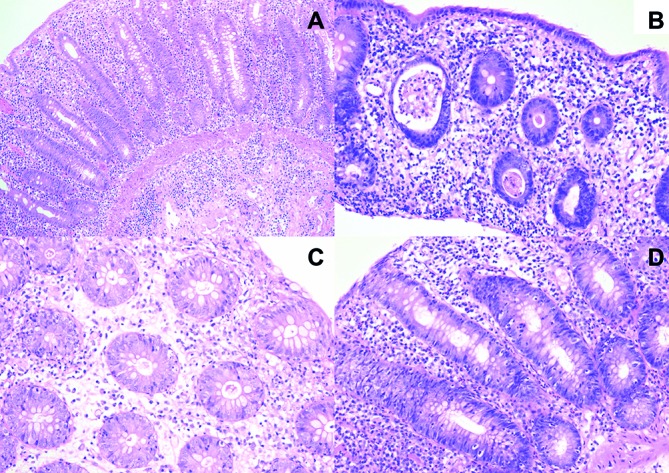
Representative hematoxylin-eosin stained (HE) sections demonstrating immune checkpoint inhibition-related colitis representative HE sections demonstrating immune checkpoint inhibition-related colitis characterised by increased lamina propria cellularity (A, B and D). (A) extension of the infiltrate into the submucosa. (B) neutrophilic inflammation with a crypt abscesses, mild cryptitis, mucin depletion of epithelial cells and small foci with minimal increase in intraepithelial lymphocytes. (C) Apoptotic cells in crypt epithelium. (D) Prominent intraepithelial lymphocytosis.
Initial management of IRC
In all but four episodes, patients received high-dose corticosteroids. Median time between onset of symptoms and start of high-dose corticosteroid therapy was 5 days (IQR 3–11). Of all 92 episodes of diarrhoea treated with high-dose corticosteroids, 32 (35%) were initially treated with a dosage of <1 mg/kg, 57 episodes (62%) with 1 mg/kg and 3 episodes (3%) with >1 mg/kg. Patients used high-dose corticosteroids for a median of 44 days (IQR 29–73). In 12 episodes (13%), patients received budesonide as local treatment.
Steroid-refractory colitis
In 54 (56%) episodes, patients required infliximab (5 mg/kg) for corticosteroid-refractory colitis, and of those patients, 50% (n=27) were given more than one cycle infliximab. Median time between start of prednisone and start of infliximab was 9 days (IQR 5–19.5), and median time to response on infliximab was 2 days (IQR 1–4). In three episodes, patients required additional immunosuppressive agents such as mycophenolic acid or tacrolimus. None of 15 patients with grade 1 diarrhoea required infliximab therapy. We did not see any difference in the requirement of infliximab for patients that presented with grade 2 (68%) or grade 3 (67%) diarrhoea. Interestingly, in 79% of episodes in which ulcers were seen during endoscopy, patients needed infliximab, while this was only the case for 44% of episodes in which no ulcers were seen (P=0.002). Patients with a van der Heide score between 7 and 12 (high score) received infliximab in 71% of episodes, while this was 42% in case of a van der Heide score of 0–6 (low score; P=0.005). Similarly, with these data, 79% of patients with a Mayo score of 2–3 (high score) received infliximab compared with 47% for patients with a Mayo score of 0–1 (low score; P=0.005). Seventy-six per cent of patients with a pancolitis (≥3 affected segments) required infliximab, while this was only the case in 25% of patients with <3 affected segments (P<0.001). In total, 6 patients (16%) had a response (complete response (CR) or partial response (PR)) in the group who did not receive infliximab versus 10 patients (22%) in the group who did receive infliximab (P=0.53). Also when looking at disease control rate (stable disease+PR+CR), there was no significant difference in response in patients who received infliximab versus those who did not. Disease control rate was 59% (22 out of 37 patients) in the group of patients who did not receive infliximab versus 44% (20 out of 46 patients) in the group of patients who did receive infliximab (P=0.15). No serious infliximab-related side effects were seen.
Colitis and best overall response
In total 60 patients with colitis and a cutaneous melanoma had an evaluable response. In the group of patients treated with anti-CTLA-4 monotherapy, the response rate was 18% (7 out of 39 patients). In the group of patients treated with anti-PD-1 monotherapy, the response rate was 44% (four out of nine patients), and it was 33% (4 out of 12) for patients treated with the combination of anti-CLTA-4 and anti-PD-1. These response rates appear not different from what has been demonstrated in phase III clinical trials (Checkmate-069 and Checkmate-067 or Keynote-006).
Discussion
In this retrospective study on IRC, we have shown that there is no significant difference between patients with grade 2 or 3 diarrhoea with regard to endoscopic severity scores, histopathological features, the requirement for infliximab or the presence of ulcers. This is important because IRC is usually managed based on the grade of diarrhoea according to the CTCAE. Instead, we have found a correlation between endoscopic features and the need for immune suppression beyond high-dose corticosteroids. In that light, our findings are relevant, as they would help the clinician to more rapidly intensify immune suppression, with the aim to reduce the time to recovery. Endoscopic characteristics in IRC are very diverse, and there are no available validated scoring systems. Of note, due to the retrospective nature of our study, the endoscopic findings may have influenced physicians in their choice for management. Based on our findings, we suggest that these variables are taken into account in future scores for IRC. Our study suggests that the presence of ulcers and pancolitis (≥3 affected colon segments) are predictors of steroid-refractory colitis, perhaps warranting immediate start of infliximab on colonoscopy.
Given the rapid improvement in symptoms after infliximab treatment (median time to response on infliximab was 2 days), we therefore strongly advise to consider the use of infliximab earlier, especially in patients with ulcerations or pancolitis. Furthermore, our study supports the findings by Schadendorf et al,18 who showed that infliximab did not seem to affect the development of a response or the durability of response. The rationale of early initiation of infliximab is based on its efficacy in patients with IBD. In IBD, treatment with infliximab resulted in more clinical responses, mucosal healing, sparing of steroids, fewer admissions to the hospital and less surgical interventions.19 20 An earlier start of infliximab—top-down approach—is now increasingly used in severe cases of IBD. Currently, a trial is being performed that investigates early treatment with infliximab in immune-checkpoint inhibition induced colitis (NCT02763761).
In our study, we have also shown that in 23% of colonoscopies, the ascending colon was more severely affected than the descending colon. In these cases, the severity of IRC would have been underestimated by sigmoidoscopy only. Therefore, performing a full colonoscopy in patients that present with grade ≥2 diarrhoea may have added value to sigmoidoscopy, as the underestimated amount and severity of colonic inflammation may results in under treatment of patients. The choice for colonoscopy, however, has to be judged in relation to other factors such as: burden for the patient and the possibility of perforation, which is about 2–4 times higher than that of sigmoidoscopy.21 Therefore, if severe ulceration is present in the left-sided colon, assessment of the right-sided colon is not necessary for decisions on further management. CT colonography could be used as an alternative to colonoscopy, as it has a slightly lower iatrogenic perforation rate. However, CT colonography has low sensitivity for correct detection of acute colitis (64%), offers no possibility to take biopsies and still is a considerable burden for patients.21–23
In this largest series to date analysing histopathology of IRC, we found that IRC is most typically described as an increase in lamina propria cellularity (83%), commonly extending slightly into the submucosa (42%), combined with patchy neutrophilic infiltrate (intraepithelial: 79% and/or crypt abscesses: 62%). In 36% of cases, there were also some irregularities in the crypt architecture present but still the overall morphology appeared different from IBD. This is in line with the results of earlier analyses.17 24 Some cases showed an increase in intraepithelial lymphocytes and/or apoptosis, but these features were inconsistent. Histopathological features neither correlate with the different types of immune checkpoint inhibitors used nor with the time point of start of immunosuppression (before or after biopsies were taken). Many of the histopathological features were correlated with endoscopic features. Therefore, histopathology did not seem to have an added value to guide therapy beyond what was found endoscopically. Mucosal biopsies appear to mainly serve to confirm diagnosis.
Despite the information that is provided by colonoscopy and mucosal biopsies, which may help guide optimal management of diarrhoea and colitis, one could argue that performing a colonoscopy will not change the management of IRC. Based on management algorithms, patients will start with high-dose steroids and in case of insufficient improvement of symptoms, the addition of infliximab within several days—all without any guidance of information from colonoscopy or mucosal biopsies. In addition, colonoscopies are cumbersome for patients, not without risk of perforation and the time benefit until infliximab treatment might be marginal with a waiting time of several days before a colonoscopy can be performed. Currently, it is not known whether omission of a colonoscopy affects the outcome of patients developing immunotherapy-induced gastrointestinal toxicity negatively, and therefore should be the subject of further studies. Nevertheless, patients with severe IRC should be treated promptly to prevent serious complications, such as perforation. However, patients with only mild or no active colitis, based on endoscopic findings, could be sufficiently treated with local steroids only.
Limitations of our study are the retrospective analysis, the fact that there might be classification bias due to the retrospective scoring of symptoms and endoscopic severity. Also, biopsies were taken in a non-standardised manner.
Conclusions
The correlation between grade of diarrhoea and endoscopic features for severity of colitis is poor. Patients with higher endoscopic severity scores, ulcers or a pancolitis needed the addition of infliximab more often. Therefore, endoscopy may have value in the evaluation of the severity of IRC and may help in decision making for optimal management.
Acknowledgments
We would like to acknowledge the NKI-AvL Core Facility Molecular Pathology and Biobanking (CFMPB) for supplying NKI-AvL Biobank material.
Footnotes
JD and JBAGH contributed equally,
MHGF and EAR contributed equally.
Contributors: Study concept and design: MHGF, EAR, JvD and JBAGH. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: MHGF, EAR, PS, JvD and JBAGH. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: MHGF, EAR and SvW. Administrative, technical or material support: SvW, CP, PS, CUB, JVvT, MEvL, MvdH, JvD and JBAGH. Study supervision: CUB, JvD and JBAGH.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 3. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 4. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol 2011;29(15 suppl):8583 10.1200/jco.2011.29.15_suppl.8583 [DOI] [Google Scholar]
- 9. Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013;119:1675–82. 10.1002/cncr.27969 [DOI] [PubMed] [Google Scholar]
- 10. Yervoy. Highlights of prescribing information, 2011. pp 1–20. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125377s0000lbl.pdf (accessed 30 May 2017).
- 11. Keytruda. Highlights of prescribing information, 2016. pp 1–26. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125514s012lbl.pdf (accessed 30 May 2017).
- 12. Opdivo. Highlights of prescribing information, 2014. pp 1–20. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125554lbl.pdf (accessed 30 May 2017).
- 13. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 14. Lin R, Yellin MJ, Lowy I, et al. An analysis of the effectiveness of specific guidelines for the management of ipilimumab-mediated diarrhea/colitis: prevention of gastrointestinal perforation and/or colectomy. J Clin Oncol 2008;26(15 suppl):9063 10.1200/jco.2008.26.15_suppl.9063 [DOI] [Google Scholar]
- 15. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. 10.1056/NEJM198712243172603 [DOI] [PubMed] [Google Scholar]
- 16. van der Heide H, van den Brandt-Gradel V, Tytgat GN, et al. Comparison of beclomethasone dipropionate and prednisolone 21-phosphate enemas in the treatment of ulcerative proctitis. J Clin Gastroenterol 1988;10:169–72. 10.1097/00004836-198804000-00013 [DOI] [PubMed] [Google Scholar]
- 17. Verschuren EC, van den Eertwegh AJ, Wonders J, et al. Clinical, Endoscopic, and Histologic Characteristics of Ipilimumab-Associated Colitis. Clin Gastroenterol Hepatol 2016;14:836–42. 10.1016/j.cgh.2015.12.028 [DOI] [PubMed] [Google Scholar]
- 18. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase ii and iii trials. J Clin Oncol 2017;35:3807–14. 10.1200/JCO.2017.73.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. 10.1016/S0140-6736(02)08512-4 [DOI] [PubMed] [Google Scholar]
- 20. Järnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805–11. 10.1053/j.gastro.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 21. Panteris V, Haringsma J, Kuipers EJ, et al. mechanisms and outcome: from diagnostic to therapeutic colonoscopy. Endoscopy 2009;41:941–51. [DOI] [PubMed] [Google Scholar]
- 22. Bellini D, Rengo M, De Cecco CN, et al. Perforation rate in CT colonography: a systematic review of the literature and meta-analysis. Eur Radiol 2014;24:1487–96. 10.1007/s00330-014-3190-1 [DOI] [PubMed] [Google Scholar]
- 23. Singh K, Narula AK, Thukral CL, et al. Role of ct colonography in colonic lesions and its correlation with conventional colonoscopic findings. J Clin Diagn Res 2015;9:TC14–18. 10.7860/JCDR/2015/12686.5853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000278supp001.pdf (127KB, pdf)


