Abstract
Concurrent Pneumocystis jirovecii (PJ) and pulmonary histoplasmosis (PHP) are rare in a single HIV individual. We present a challenging case of concomitant PJ and PHP in a young HIV individual. A 44-year-old man presented to the emergency department with progressive pulmonary symptoms. He was hypoxic with bilateral pulmonary opacities on chest radiograph. CT of the chest showed a geographical pattern of ground-glass attenuation. He started receiving intravenous antibiotics in addition to oral Bactrim for suspected PJ. He also began receiving itraconazole, given suspected PHP with recent bat-droppings exposure. HIV test was positive, though history was negative; the CD4 count was 5 cells/mm3. Later, he developed respiratory failure without clinical improvement. First bronchoalveolar lavage (BAL) failed to confirm opportunistic pathogens. Repeat BAL revealed PJ but no Histoplasma. Histoplasma antigens were positive, confirming histoplasmosis. The patient died despite aggressive treatment with intravenous Bactrim and amphotericin B.
Keywords: infections, hiv / aids, adult intensive care, medical management
Background
AIDS is a disease of diverse opportunistic infections (OIs). These OIs cause significant disease burden in HIV-positive individuals. In the modern era, HIV-related outcomes in terms of mortality and morbidity seem to be improving. However, such OIs still occur despite the widespread use of antiretroviral therapy (ART).1 Of these OIs, pathogens of pulmonary concern include Pneumocystis jirovecii (PJ), Mycobacterium tuberculosis, Histoplasma capsulatum, Blastomyces dermatitidis and Coccidioides immitis among others.2 Apart from a single OI, the concurrence of these infections is possible, especially among individuals who remain HIV undetected for a considerable amount of time and hence fail to receive ART or their respective chemoprophylaxis. As there are several OIs, their combinations could be diverse in case of multiple concurrent infections. One such example includes synchronous PJ and pulmonary histoplasmosis (PHP) in a single HIV individual. Based on the literature search and our clinical experience, these concurrent pulmonary infections are highly uncommon,3 4 portend a serious life threat and hence imply a grave prognosis. We present a challenging case of an undiagnosed HIV patient who presented with progressive pulmonary symptoms and had concurrent PJ and PHP.
Case presentation
A 44-year-old man came to the emergency department (ED) with a 2-week history of progressive fatigue, dyspnoea and generalised weakness. One week before admission, he developed intermittent high-grade fever, rigors, chills and watery diarrhoea followed by progressive dyspnoea, both at rest and exertion, and worse when supine. His diarrhoea had resolved; however, his dyspnoea was worsening. He had been checking his oxygen saturation (SpO2) at home using his mother’s pulse oximeter which revealed occasional readings as low as 86%, mainly when supine. He denied skin rash, oral thrush, lymphadenopathy (LAD), productive cough, odynophagia, headache, or visual or memory problems. He visited an urgent care clinic and was given intravenous steroids and discharged with oral levofloxacin for bacterial pneumonia (PNA); he returned after 5 days, again received intravenous steroids and was discharged on amoxicillin. However, his condition continued to deteriorate, and he came to the ED for further medical management. He denied a history of HIV, sexually transmitted disease, intravenous drug use and blood transfusions. He denied same-sex or promiscuous behaviour and had no travel to Midwestern states. He was working as a construction worker in Michigan and had no health issues or prior hospitalisations. He was a heavy drinker but reported no vomiting or possible aspirations.
On physical examination, he appeared well built without signs of distress. Vitals included blood pressure 131/85, pulse 86/min, respiratory rate 21/min, SpO2 92% on 5 L/min per nasal cannula, temp 97.7°F. Oral cavity was negative for thrush, and there was no cervical, axillary or inguinal LAD. No signs of fluid overload were present, including jugular venous distension. The skin had no rash or track marks of intravenous drug abuse. There was no hepatosplenomegaly, and cardiac examination was unremarkable. He had decreased breath sounds on lung auscultation but no crackles or rhonchi.
Investigations
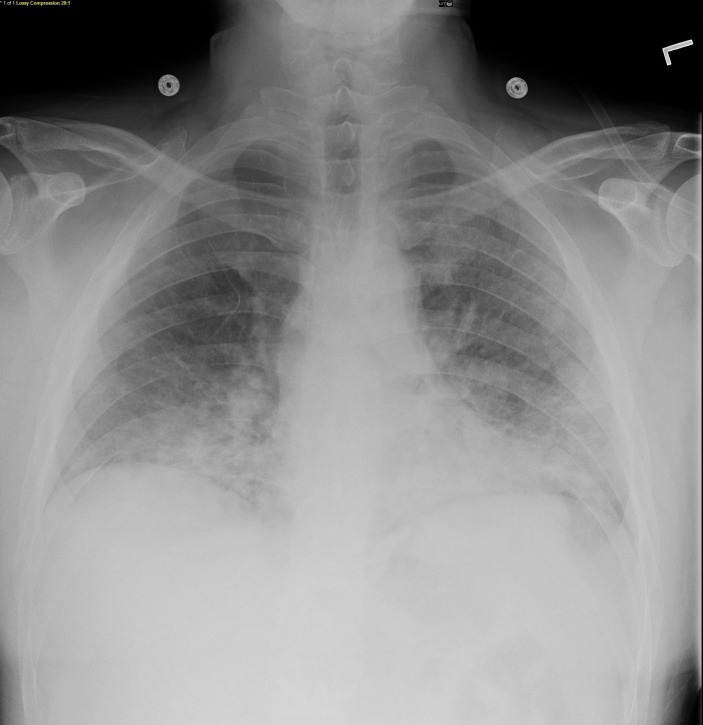
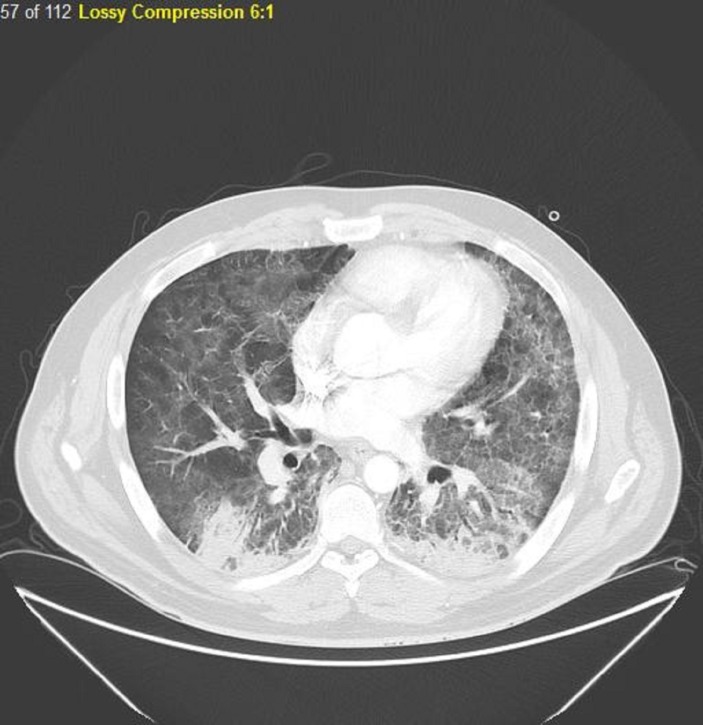
Laboratory tests showed a white cell count of 3.4×109/L (4.5–11.0), absolute lymphocyte count of 0.6×103/uL (1.2–3.4) and lactate dehydrogenase (LDH) of 117 U/L (100–125). Arterial blood gases, performed while the patient was on a non-rebreather mask, showed a pH of 7.50, pCO2 of 26.4 mm Hg, pO2 of 56.7 mm Hg and HCO3 of 18.9 mmol/L. Blood and sputum cultures were performed, and HIV test was ordered. Chest radiograph showed bilateral lower lobes hazy ground-glass opacities (figure 1). Thoracic CT with contrast (figure 2) showed a striking geographical pattern in upper and middle lung fields demonstrated by the foci of ground-glass attenuation along with well-defined foci of apparent lung sparing. Also, there were bilateral lower lobe infiltrates but no LAD.
Figure 1.
Chest radiograph showed bilateral lower lobes hazy ground-glass opacities.
Figure 2.
CT chest with contrast showing a striking geographical pattern in upper and middle lung fields demonstrated by the foci of ground-glass attenuation along with well-defined foci of apparent lung sparing. Also, there are bilateral lower lobe infiltrates but no lymphadenopathy.
Differential diagnosis
Because of no known history of HIV and the absence of high-risk sexual behaviour, the patient was started on intravenous ceftriaxone and azithromycin to cover for common respiratory pathogens, given the presumptive diagnosis of community-acquired PNA. However, there was a strong suspicion of PJ due to the presence of ground-glass pattern on patient’s chest imaging in the presence of hypoxia. In this context, the patient was started on oral Bactrim; however, intravenous Bactrim was deferred. On interval history, the patient reported visiting an old, dusty barn which had a massive pile of bat droppings, guiding to the suspicion of PHP as one of the possibilities. Therefore, he was started on oral itraconazole (ITCZ) 200 mg two times a day for 3 days followed by 200 mg once a day. Extracerebral toxoplasmosis (pulmonary), though uncommon, can also present with fever and dyspnoea in an immunocompromised individual. Furthermore, it can show reticulonodular infiltrates or nodular opacities on chest imaging, but that was not the case in our patient.5 Other important differentials included Legionnaires’ disease, mycoplasma PNA and atypical viral PNA. Other than infections, based on chest imaging, cardiac dysfunction including congestive heart failure was another differential. However, the patient had no cardiac risk factors, and the echocardiogram was normal.
Treatment
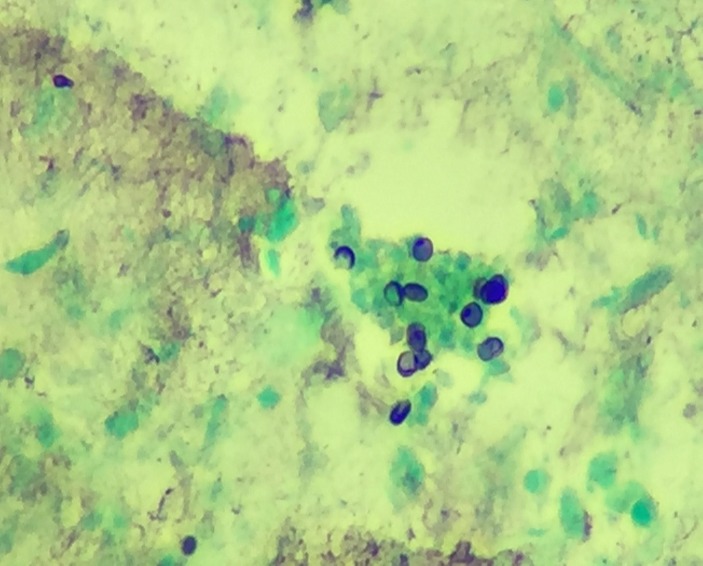
Regarding differential-based testing, workup for Legionella (urine antigen, sputum culture), Mycoplasma (IgM and IgG), Toxoplasma (IgM and IgG) was negative. As an immune deficiency workup, HIV was positive for HIVAB24 and HIV-1 Ab, and the CD4 count was extremely low, 5 (481–1793 cells/mm3). In the interim, the patient continued a downward course, making OIs higher on the differential list. For that reason, oral Bactrim was switched to intravenous Bactrim at 15 mg/kg once a day along with prednisone 40 mg two times a day (21-day oral regimen with tapering dosage6) given the possibility of PJ pneumonia (PJP). Regarding PHP, ITCZ was continued while awaiting Histoplasma workup (urine/serum antigen, antibodies). On the contrary, he continued a downhill course, prompting the need for a definite diagnosis as early as possible. Given this fact, a bronchoalveolar lavage (BAL) was performed on the third day of admission; however, it failed to yield an optimal specimen. On the other hand, the patient required intubation and mechanical ventilation on the fourth day of admission. As the microbiological evidence is the conventional method of detection in these OIs, a repeat BAL was attempted on the second day of intubation which revealed organisms, morphologically consistent with PJ (figure 3); however, H. capsulatum could not be confirmed. Transbronchial biopsy (TBB) could not be performed as there were no pulmonary masses or LAD. A viral panel PCR, performed on bronchial washings, was negative for common viral pathogens, including herpes simplex type 1 and 2, cytomegalovirus, adenovirus, enterovirus, influenza A and B, respiratory syncytial virus, rhinovirus, and parainfluenza type 1, 2 and 3. Though there is evidence regarding the usefulness of molecular testing like multiplex real-time PCR in opportunistic coinfections, the clinical experience with such testing is still limited. Moreover, unavailability of these tests in usual clinical settings is another potentially limiting factor.7 Therefore, a multiplex real-time PCR could not be performed in our patient. However, urine and serum Histoplasma antigens were positive, providing the evidence for PHP on day 7. ITCZ was switched to intravenous amphotericin B with a dose of 3 mg/kg/day as induction therapy for severe histoplasmosis (HP). Antibiotics were changed to intravenous cefepime to broaden the coverage as his condition was deteriorating; treatment with Bactrim and amphotericin B was continued.
Figure 3.
Bronchial washings smear stained with Gomori methenamine silver stain showing a small group of typical small-sized (4–6 microns) Pneumocystis organisms with rounded to cup-shaped (crushed ping-pong ball) morphology.
Outcome and follow-up
The patient continued to decline, whereas multiple blood and sputum cultures remained negative, providing no additional evidence for his profound deterioration. On day 11, he developed ventilator-associated PNA and was started on vancomycin, levofloxacin and piperacillin/tazobactam after discontinuing cefepime, whereas Bactrim and amphotericin B were continued, though the patient continued to worsen. Despite this treatment, he developed refractory illness and died on the 15th day of admission.
Discussion
Patients who are HIV seropositive can acquire various dual and triple OIs. Focusing on HIV, individuals at substantial risk of developing OIs include those who (1) have a delayed healthcare access, (2) exhibit ART resistance, (3) have a lack of prophylaxis when required and (4) show an inadequate drug adherence.1
As with a single OI, the risk of multiple OIs increases as the CD4 count decreases, as in our patient. Though the improved healthcare and availability of ART have resulted in a significant decline in mortality and morbidity, the occurrence of OIs is possible due to several individual, socioeconomic and geography-related risk factors. On the same note, the pattern of OIs may vary based on these risk factors and hence needs a due consideration. Like other OIs, the actual incidence of concurrent PHP and PJP is unknown. To our knowledge, their concurrence in HIV/AIDS is rare and offers a unique clinical challenge when encountered. The report of Almeida-Silva et al involved the concurrence of multiple OIs in a single HIV individual, with a CD4 count of 20 cells/mm3. The patient presented with tachypnoea and hypoxaemia and was concurrently diagnosed with PJP (by direct immunofluorescence of induced sputum), cryptococcosis (positive India ink preparation of cerebrospinal fluid and positive respiratory culture on Sabouraud agar) and M. tuberculosis (mycobacterial growth culture medium). After few months of treatment, he returned with intermittent fever, pancytopenia and hepatosplenomegaly. At that time, the induced sputum and histopathology of bone marrow revealed a positive H. capsulatum. Concurrent PJP and HP were further confirmed with a nested PCR.8 This case demonstrates the diagnosing of multiple OIs in a highly straightforward manner and hence offers no diagnostic challenges. Another report of Tschudy and Michail involved this rare combination of PJP and HP in a non-HIV, immunocompromised child with infliximab-treated Crohn’s disease. The presence of concurrent infections was successfully confirmed with conventional microbiological testing9 but the examples of such cases are unusual; moreover, the gravity of such illnesses might be undermined, and hence the concomitance of infections might go undetected. Apart from these sporadic cases, our literature search failed to reveal cases suffering from concomitant PJP and PHP. However, few reports may provide insight into the concurrence rate of these infections. Baughman et al performed BAL in 894 patients with HIV to establish the causes of non-specific pulmonary symptoms.3 The resulting data revealed a wide-ranging infectious aetiology, including single and multiple OIs. Consistent with other studies, PJ was the single most common infectious pathogen, accounting for 47% of cases. A concurrent fungal pathogen (in addition to PJ) existed in only 1% of these cases. Of them, concomitant HP and PJ resulted in only five pulmonary cases, spanning over a period of 7 years.3 In another report, Johnson et al studied 48 pulmonary cases with disseminated HP, as the first AIDS presentation from 1983 to 1987.4 A concurrent PJ infection was detected in two HP cases in this period.4 The findings of both Baughman et al and Johnson et al are somewhat similar, depicting 2–5 cases over 5–7 years. It should also be noted that both these reports belong to an early HIV era and therefore fail to demonstrate a current concurrence rate. To our understanding, this coinfection is not typical, so it might be overlooked or might not be treated aggressively, therefore delay in treatment and subsequent poor outcomes.
As of clinical experience with our patient, the concurrent OIs should be of the high clinical suspicion, especially among individuals with profound deterioration and risky clinical profile. In our patient, a high-risk sexual behaviour could not be ascertained, and concurrent OIs could not be anticipated until late in the disease course. Given this, individuals of special concern may include those (1) with unknown HIV status, (2) not on ART, (3) not on prophylaxis and (4) low CD4 count. For instance, in our patient, the concomitant PHP and PJP might have led to an overwhelming infection because of deficient cellular immunity, low clinical suspicion and delayed aggressive therapy. Though a portion of such cases with an inadequate treatment response could have a third or fourth concomitant pathogen,8 we retrieved no additional pathogen despite multiple cultures.
Regarding concomitant PJP and PHP, the clinical picture could be misleading as both can present with fever, dyspnoea and non-productive cough in the clinical milieu of advanced immunosuppression. Generally, PJP is of prime concern in patients with newly diagnosed HIV with CD4 <200 cells/mm3. However, such presentations in Histoplasma endemic region should trigger HP as one of the leading differentials. Another clue, in such circumstances, would be of potential Histoplasma exposure though the evidence of such exposures does not exclude the presence of concomitant PJ infection. Features suggestive of HP, different from PJP, might include LAD, hepatosplenomegaly and mucosal or oral ulcerations, again not helpful in concomitant OIs. On chest radiograph, PJP presents with diffuse pulmonary infiltrates. These findings are non-specific and bring a lengthy list of differentials, including PHP and community-acquired PNA. Though high-resolution CT reveals more specific findings of patchy or nodular ground-glass attenuation as in our patient, the differential of this pattern is broad, including hypersensitivity pneumonitis. Radiologically, PHP may present with pulmonary nodules, masses, hilar or mediastinal LAD, and cavitation.10 None of them was present in our patient. Biochemically, elevated LDH is non-specific and could be present in both PJP and HP; levels more than 600 IU/L are suggestive more of PJP than of HP.11 In case of presumptive PJP, 1–3-beta-D-glucan (fungal cell wall component) is helpful, if available.12 However, 1–3-beta-D-glucan is elevated in HP as well13 and might be of less importance in multiple coinfections. In our patient, 1–3-beta-D-glucan could not be performed due to its unavailability, however was of less significance even if performed. In cases with such a huge clinical and laboratory overlap, microbial identification testing becomes extremely important. Testing on induced sputum might be the first non-invasive approach towards this detection and can identify both HP and PJ on microscopy and cultures. Other non-invasive testing targeted at PHP alone includes Histoplasma (serum/urine) antigens and antibodies (via immunodiffusion or complement fixation testing). In few pulmonary cases, Histoplasma antigens or antibodies could be absent, and diagnosis can be missed.14 Of note, the detection rate of antigen is higher in disseminated cases than in pulmonary. Furthermore, serology might be negative at the beginning of infection and require months before becoming positive. Histoplasma yeast can be seen in peripheral smear and bone-marrow histopathology in disseminated Histoplasma infection.
When the diagnosis is crucial, and concomitant infections are suspected, BAL with or without TBB via bronchoscopy will be the most beneficial test as it can detect concomitant OIs. BAL fluid can be used for microscopy and culture in addition to Histoplasma antigens. When combined with TBB, false negative (FN) rates of BAL become fewer, and their combined sensitivity could be as high as 100%.15 One study reported seven cases (two with PJP, one with HP and four with Mycobacterium avium-intracellulare) that were not detected on the first bronchoscopic examination.15 Two PJP cases, not initially detected on BAL (first case) and TBB (second case), were detected on open lung biopsy and repeat TBB, respectively. None of the PJP cases was missed when both BAL and TBB were combined. Regarding HP, this case was missed on TBB and later detected on open lung biopsy. Thus, FN cases might require either a repeat BAL with or without TBB or even more invasive testing such as open lung biopsy. Though TBB can increase the diagnostic yield for such pathogens, the addition of TBB could be challenging in high-risk patients due to the greater chances of complications. If we had to perform TBB in our patient, there was a significant risk of complications (9% risk of pneumothorax, 5.9% risk of tube thoracostomy).15 Patients with higher than usual risk of complications are those with mechanical ventilation, acute respiratory distress syndrome (ARDS) and bleeding tendency. Though there is no easy road to definite diagnosis-making in complicated patients, the confirmation of diagnosis is important and could be life-saving. Recently, there is a proposed role of multiplex real-time PCR on biological secretions as it can detect concomitant OIs in a highly efficient manner7; however, the experience with such testing is limited.
The treatment with concomitant OIs should be the same as with individual OIs. The anti-PJ drug is warranted when PJP is suspected; the role of systemic steroids is adjuvant in moderate-to-severe cases. Mild-to-moderate cases of PHP might be managed with oral ITCZ. However, patients with hypoxaemia and ARDS require amphotericin B.
Retrospectively, our patient could have been saved by an early HIV screening and subsequent empiric therapy, including anti-PJ and antihistoplasma agents, but this had required a high clinical suspicion and a close clinical follow-up. Furthermore, the patient had not responded to the outpatient antibiotic treatment and was critically sick with hypoxia at the beginning of hospitalisation; this denotes a progressive pulmonary infection in the absence of appropriate treatment. Given this context, it can be proposed that OI-related hypoxia is of crucial concern especially among individuals (1) with absence of chronic cardiopulmonary pathology, (2) who are young and (3) fail to respond to empiric antibiotic therapy. Such patients should undergo a definite testing as soon as possible. After establishing a single cause of pulmonary infection, testing should focus on the detection of coexistent opportunistic pathogens, particularly among those with ongoing clinical deterioration. Sophisticated testing like a multiplex real-time PCR can be helpful, if available. The experience with our patient implies an aggressive approach from the beginning. However, the ultimate prognosis seems to be poor.
Learning points.
Pulmonary histoplasmosis concomitant with Pneumocystis jirovecii pneumonia is rare and offers a unique diagnostic challenge when encountered.
Concomitant opportunistic infections cause a perplexed clinical picture, warranting an urgent diagnostic testing in high-risk patients.
Poor treatment response to a single treatment agent should raise the suspicion for a possible concomitant pathogen.
Bronchoalveolar lavage and transbronchial biopsy could be the only viable solution for confirmation of such diagnoses.
Presumptive diagnosis should follow an aggressive treatment strategy in patients with a grave clinical picture.
The prognosis remains poor despite aggressive therapy.
Early diagnosis, high suspicion and a low threshold for the initiation of empiric treatment are essential to saving lives in such critically ill patients.
Footnotes
Contributors: AW has written the main manuscript of the case report and was actively involved in the literature search. SC was involved in the writing of the discussion part of the article and he also added few articles for the discussion part. MK reviewed the whole article. He was involved in the patient care. He mainly focused on the patient’s presentation and series of events occurred in intensive care unit. SJS revised the article and edited it from the English language perspective.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jones JL, Hanson DL, Dworkin MS, et al. Surveillance for AIDS-defining opportunistic illnesses, 1992-1997. MMWR CDC Surveill Summ 1999;48:1–22. [PubMed] [Google Scholar]
- 2.Beck JM, Rosen MJ, Peavy HH. Pulmonary complications of HIV infection. Report of the Fourth NHLBI Workshop. Am J Respir Crit Care Med 2001;164:2120–6. 10.1164/ajrccm.164.11.2102047 [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Dohn MN, Frame PT. The continuing utility of bronchoalveolar lavage to diagnose opportunistic infection in AIDS patients. Am J Med 1994;97:515–22. 10.1016/0002-9343(94)90346-8 [DOI] [PubMed] [Google Scholar]
- 4.Johnson PC, Khardori N, Najjar AF, et al. Progressive disseminated histoplasmosis in patients with acquired immunodeficiency syndrome. Am J Med 1988;85:152–8. 10.1016/S0002-9343(88)80334-6 [DOI] [PubMed] [Google Scholar]
- 5.Goodman PC, Schnapp LM. Pulmonary toxoplasmosis in AIDS. Radiology 1992;184:791–3. 10.1148/radiology.184.3.1509069 [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health-University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia. Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. N Engl J Med 1990;323:1500–4. 10.1056/NEJM199011223232131 [DOI] [PubMed] [Google Scholar]
- 7.Gago S, Esteban C, Valero C, et al. A multiplex real-time PCR assay for identification of pneumocystis jirovecii, histoplasma capsulatum, and cryptococcus neoformans/cryptococcus gattii in samples from AIDS patients with opportunistic pneumonia. J Clin Microbiol 2014;52:1168–76. 10.1128/JCM.02895-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida-Silva F, Damasceno LS, Serna MJ, et al. Multiple opportunistic fungal infections in an individual with severe HIV disease: a case report. Rev Iberoam Micol 2016;33:118–21. 10.1016/j.riam.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 9.Tschudy J, Michail S. Disseminated histoplasmosis and pneumocystis pneumonia in a child with Crohn disease receiving infliximab. J Pediatr Gastroenterol Nutr 2010;51:221–2. 10.1097/MPG.0b013e3181c2c10d [DOI] [PubMed] [Google Scholar]
- 10.Wheat LJ, Conces D, Allen SD, et al. Pulmonary histoplasmosis syndromes: recognition, diagnosis, and management. Semin Respir Crit Care Med 2004;25:129-44 10.1055/s-2004-824898 [DOI] [PubMed] [Google Scholar]
- 11.Butt AA, Michaels S, Greer D, et al. Serum LDH level as a clue to the diagnosis of histoplasmosis. AIDS Read 2002;12:317–21. [PubMed] [Google Scholar]
- 12.Sax PE, Komarow L, Finkelman MA, et al. Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis 2011;53:197–202. 10.1093/cid/cir335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan L, Connolly P, Wheat LJ, et al. Histoplasmosis as a cause for a positive Fungitell (1 --> 3)-beta-D-glucan test. Med Mycol 2008;46:93–5. 10.1080/13693780701642235 [DOI] [PubMed] [Google Scholar]
- 14.Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011;53:448–54. 10.1093/cid/cir435 [DOI] [PubMed] [Google Scholar]
- 15.Broaddus C, Dake MD, Stulbarg MS, et al. Bronchoalveolar lavage and transbronchial biopsy for the diagnosis of pulmonary infections in the acquired immunodeficiency syndrome. Ann Intern Med 1985;102:747–52. 10.7326/0003-4819-102-6-747 [DOI] [PubMed] [Google Scholar]