Abstract
We report a case of a healthy, right-hand dominant young male who was a volunteer for a pilot run of a functional MRI (fMRI) study. The fMRI was performed with a 3.0 Tesla MRI scanner using a finger tapping task-based activity. The subjects were instructed to perform flexion of the right thumb and left thumb consecutively (activation task) and neuronal activation in bilateral primary motor cortex (PMC) were observed during each task. One particular subject demonstrated bilateral PMC activation during the left-thumb movement task, instead of the expected activation of the contralateral PMC alone.
Keywords: radiology, disability, rehabilitation medicine, neuroimaging
Background
It has been a fundamental principle of neurology that hand and finger movements are controlled by the contralateral side of the primary motor cortex (PMC) and supplementary motor area (SMA) in the human brain. This theory has been proven inaccurate with the advent of functional neuroimaging methods that can directly assess the neuronal pathways and interactions in real time. We report an observation of bilateral PMC and SMA activation for left thumb movement in a healthy volunteer during an fMRI study.
Case presentation
A 22-year-old man had presented as a volunteer for an functional MRI (fMRI) study at a university research centre. He did not have any medical or surgical history and was noted to be right-hand dominant. He also stated that he was neither ambidextrous nor was he forced to switch from left-hand preference to right hand during childhood.
The subject stated that he was not taking any regular medication, did not have any history of head injury or cerebral ischaemic event. He also denied performing any type of strenuous activity on the day of the examination.
The subject was placed in a 3.0 Tesla fMRI scanner and a head coil was placed over his head. The head coil was used to detect brain activation signals based on blood oxygenation level-dependent fMRI imaging sequence. The concept of the scan was that during a given motor task, there will be increase in oxygen flow to the activated lobe or area in the brain. Subsequently, the differences in oxygen gradients in activated versus non-activated parts of the brain will be detected as an fMRI signal and converted into a functional brain map. These images are superimposed on a brain atlas such as the Talairach atlas which is available in postprocessing softwares such as Statistical Parametric Mapping and FMRIB Software Library.
In the fMRI scanner gantry, the subject was instructed to perform right-thumb flexion as well as dorsiflexion followed by fMRI image acquisition. Similarly, the task was repeated using the left thumb. A task-based fMRI software was used to give instructions that were projected onto a screen which could be visualised by the subject in the scanning suite. The subject was instructed to perform several repetitions of using his right thumb to press a joystick button in the fMRI scanner. Subsequently, specific time coordination was implemented to initiate and sustain right-thumb movements and the corresponding activation areas in the brain were recorded. After completion of the task using his right thumb, a similar process was repeated to assess his left-thumb movement.
During his right-thumb flexion and dorsiflexion, activation of contralateral PMC and SMA were noted. Interestingly, bilateral PMC and SMA were observed during left-thumb movement instead of the expected activation from purely contralateral side of the brain.
Investigations
A brain fMRI scan was carried out using a Magnetom PRISMA V.3.0 Tesla MRI scanner (Siemens, Erlangen, Germany). Serial echo planar images were acquired and the images were reviewed on a Siemens workstation (Syngovia). Image findings were documented. The technique used the fundamental difference in paramagnetic property of oxyhaemoglobin and deoxyhaemoglobin concentration gradients in activated parts of the brain. It relied on regional difference in cerebral blood flow to delineate regional neuronal activity. As blood flow increases in certain areas of the brain during performance of a particular task, fMRI is able to display in real time the cerebral cortical locations that are activated, either contralaterally or ipsilaterally or even bilaterally.
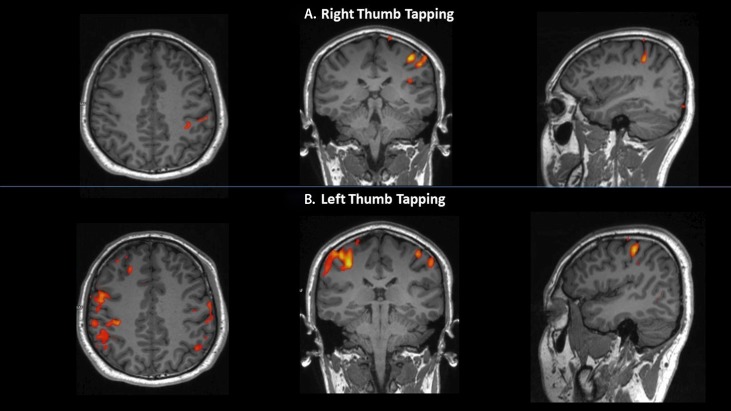
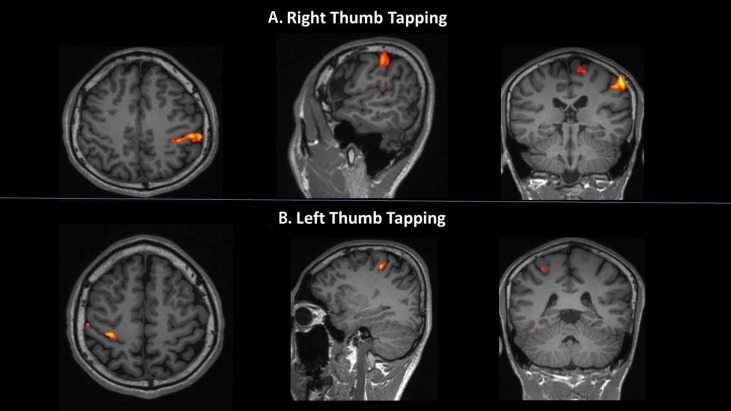
Out of the 10 subjects assessed, this particular subject had activation of the contralateral PMC during right-thumb movement (figure 1A), and interestingly, bilateral motor cortices activation of almost equal magnitude during left-thumb movement (figure 1B). Comparison with a normal subject’s brain activation seen on fMRI scans during consecutive bilateral thumb movements are shown in figure 2.
Figure 1.
Functional MRI image of the brain cortical spectral activation in the subject during (A) right-thumb movement and (B) left-thumb movement; shown in axial, coronal and sagittal planes. Bilateral primary motor cortex activation is noted during left-thumb movement.
Figure 2.
Functional MRI image of the brain cortical spectral activation in a normal subject during (A) right-thumb movement and (B) left-thumb movement; shown in axial, coronal and sagittal planes. Conventional spectral activation of the contralateral motor cortex and supplementary motor area is noted for the corresponding thumb movement.
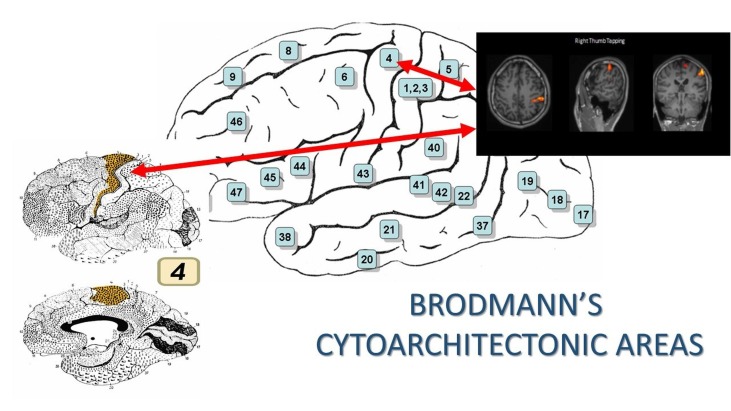
The images are superimposed on Talairach brain atlas, a software commonly used in fMRI study to localise and map specific brain activations into x, y, z coordinates that assigns the anterior commissure as its centring point of origin. Each coordinate corresponds to a specific Brodmanns’s area in the brain cortex, which in our case was the Area 4; the PMC or also known as the precentral gyrus (figure 3).1
Figure 3.
Brodmann’s Cytoarchitectonic Area 4; region of primary motor cortex control at the precentral gyrus. (Image from https://en.wikipedia.org/wiki/Brodmann_area).
Differential diagnosis
It is possible that the subject could have moved both his thumbs instead of only moving his left thumb during the task-based activity, thus leading to bilateral PMC activation on fMRI. Nevertheless, a similar fMRI activation pattern was demonstrated despite repeating the scan after reinforcing verbal instructions to the subject.
Treatment
As this is not a disease process but a normal variant, thus no specific treatment is required for this condition. However, the subject was informed about his condition and its relevance.
The main theory regarding activation of bilateral motor cortices and supplementary motor cortices with certain inhibitory transcallosal interactions to prevent redundant movements, as well as signalling from uncrossed neuronal pathways, can be explored for future clinical applications. For example, one could take advantage of this mechanism of interactions when rehabilitation is planned to strengthen the affected limb in stroke patients.
Outcome and follow-up
There were no complications arising from the neuroimaging procedure and the subject did not require follow-up.
Discussion
The PMC and SMA in the human brain control motor functions such as tongue, upper limb and lower limb movements. These regions are assigned specific locations in the cerebral cortex, known as the Brodmann’s areas as demonstrated by the homunculus of Penfield. Approximately 90% of humans are right handed and cortical activation of the thumb occurs in the contralateral motor, SMA and sensorimotor cortex as the corticospinal fibres decussate completely at the midbrain level.2 Nevertheless, as this case study has demonstrated, there can be a subgroup of people who have consecutive ipsilateral cortical activation during thumb movement. Bilateral motor cortical spectral activation demonstrated on this fMRI study is unique, whereby all the other subjects demonstrated contralateral motor cortex activation.
There are at least seven known distinct descending corticospinal tract pathways that control human skeletomotor system.3 The potential for ipsilateral activation of the motor cortex during upper limb movement can be explained by the fact that there are approximately 8%–10% of the pyramidal tract fibres that are uncrossed corticospinal fibres. Furthermore, uncrossed fibres of the corticospinal tract (right cerebral M1 fibres descend in the ipsilateral ventral white matter of the cord and tend to innervate axial muscle groups) and axons that recross (right M1 fibres that descend in the contralateral corticospinal tract, enter the ventral horn and cross under the central canal to homologous motor pools).4 Hence, partial compensation for the loss of contralateral motor pathway activations, particularly in stroke patients, can be achieved.
Furthermore, some studies have identified that there are motor evoked potentials at contralateral and ipsilateral upper limb motor movements, likely due to directly descending neural excitations arising from bilateral SMA.5 Nevertheless, due to asymmetric interhemispheric corpus callosum inhibition, the dominant hemisphere could have a more profound effect compared with the non-dominant side.6 Conversely, there has also been a hypothesis that control of active hand movements are initiated in the contralateral hemisphere and spread to the hemisphere on the same side of the active limb, with higher weightage of influence of the generally dominant left cerebral hemisphere over the non-dominant right hemisphere rather than vice versa. Changes in blood oxygenation have also noted to be similar in contralateral and ipsilateral motor cortices during unilateral motor tasks, especially for left-hand movements in right-handed subjects which may be due to suppression of inhibition of corpus callosum thus enabling defter left-hand movements orchestrated by the dominant left cerebral hemisphere.7
As a matter of fact, a study conducted for bimanual motor activity detected that there was bilateral asymmetrical activation of the SMA for improved bilateral hand coordination, which revealed complex neuronal connections that control hand and finger movements.5 Moreover, transcranial magnetic stimulation studies in stroke patients showed evidence of motor evoked potentials elicited in the recovering limb by means of recruitment of bigger cerebral motor networks arising from the same side of the body as the paralysed limb. This indicated that the transcallosal inhibition was actively inhibited to help compensate the loss of function caused by ischaemia of the cerebral hemisphere on the opposite side.8
This case report depicts a rare occurrence of bilateral, instead of contralateral, motor cortex control of unilateral left thumb movement. The clinical implication related to this is that it can help to explain that there is a minority group of people with bilateral motor cortex control for upper limb movements. In future, they might have better rehabilitative prognosis following ischaemic cerebral infarction events. In addition, clinicians should be aware that some stroke patients may not elicit the typical contralateral upper limb power loss based on the expected motor activation regions. Furthermore, this finding indicates there is potential to restore function in the hemiparetic limb by enforcing the activation of the ipsilateral motor cortex in patients suffering from stroke.
Patient’s perspective.
I feel special to know that I have both sides of my brain controlling my left thumb movement. I understand that this means that in the future if I have an unfortunate ischaemic event that affects my left brain, my right brain can be trained to take over and help me regain function of my left-sided limb movements.
Learning points.
A minority of individuals have bilateral primary motor cortex control for unilateral upper limb that is, thumb movement.
Patients with stroke may not elicit the typical contralateral upper limb power loss pertaining to the motor activation region.
In minority of individuals, there is potential to restore function in the hemiparetic limb by enforcing the activation of the ipsilateral motor cortex in patients suffering from stroke.
Footnotes
Contributors: AR and SS have performed the procedure on the subject. AR, SS and FKH have drafted the manuscript. RM has reviewed and edited the final manuscript draft.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wikipedia. Brodmann area: Wikipedia, The Free Encyclopedia, 2017. https://en.wikipedia.org/w/index.php?title=Brodmann_area&oldid=794135267 [Google Scholar]
- 2.Grabowska A, Gut M, Binder M, et al. . Switching handedness: fMRI study of hand motor control in right-handers, left-handers and converted left-handers. Acta Neurobiol Exp 2012;72:439–51. [DOI] [PubMed] [Google Scholar]
- 3.Dobkin BH. Training and exercise to drive poststroke recovery. Nat Clin Pract Neurol 2008;4:76–85. 10.1038/ncpneuro0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davare M, Duque J, Vandermeeren Y, et al. . Role of the ipsilateral primary motor cortex in controlling the timing of hand muscle recruitment. Cereb Cortex 2007;17:353–62. 10.1093/cercor/bhj152 [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi T, Matsumoto R, Mikuni N, et al. . Asymmetric bilateral effect of the supplementary motor area proper in the human motor system. Clin Neurophysiol 2012;123:324–34. 10.1016/j.clinph.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 6.Reid CS, Serrien DJ. Primary motor cortex and ipsilateral control: a TMS study. Neuroscience 2014;270:20–6. 10.1016/j.neuroscience.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 7.Shibuya K, Kuboyama N. Bilateral motor control during motor tasks involving the nondominant hand. J Physiol Anthropol 2009;28:165–71. 10.2114/jpa2.28.165 [DOI] [PubMed] [Google Scholar]
- 8.Nair DG, Hutchinson S, Fregni F, et al. . Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage 2007;34:253–63. 10.1016/j.neuroimage.2006.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]