Abstract
Juvenile dermatomyositis (JDM) is a multisystemic disorder. Vasculitic ulcers in JDM have been reported to involve axilla, elbow or extensor surfaces of other joints. We report a young boy with JDM who presented with extensive cutaneous ulcers involving scrotum, prepuce, gluteal region, neck, bilateral axilla, periumbilical area and bilateral elbows and popliteal fossa. His disease course was marked by several relapses and he required immunosuppression with prednisolone, subcutaneous methotrexate and intravenous cyclophosphamide. His muscle weakness improved and skin ulcers healed after 6 months of intensive immunosuppressive therapy. Children with JDM and ulcers often show increased resistance to immunosuppressive therapy. Extensive cutaneous ulcers in JDM, especially those involving the scrotum, have never been described. Awareness regarding the uncommon manifestations is important to guide appropriate therapy.
Keywords: musculoskeletal and joint disorders
Background
Juvenile dermatomyositis (JDM) is a multisystem disorder that predominantly affects striated muscles and skin and is the most common type of inflammatory myopathy seen in children. Characteristic clinical findings include proximal symmetrical muscle weakness and cutaneous changes, including heliotrope discoloration of eyelids, Gottron papules, rash involving malar and sun-exposed areas and cutaneous ulcers. Despite their association with significant morbidity, cutaneous ulcers in JDM have not attracted much attention in the published literature.
Case presentation
A 6-year-old boy was diagnosed as a case of JDM at 3 years of age when he presented with symmetrical proximal muscle weakness, heliotrope rash, periorbital puffiness, Gottron papules over dorsal surface of proximal interphalangeal (PIP) and metacarpophalangeal (MCP) joints and weak gag reflex. Investigations had revealed elevated lactate dehydrogenase (LDH) (1179 U/L; normal: 240–480 U/L) and N-acetyl-cysteine creatine kinase (CK-NAC) (1177 IU/L; normal: 26–308 IU/L) with diffuse hyperintensity involving all the muscles of bilateral thighs on MRI. ELISA for HIV was negative. He had received pulse methylprednisolone (30 mg/kg/day) for 5 days followed by oral prednisolone in gradually tapering doses, subcutaneous methotrexate (15 mg/m2/week) and hydroxychloroquine. However, the disease course was marked by several relapses of muscle and skin diseases. He required further immunosuppression with mycophenolate mofetil (MMF) at 800 mg/m2/day, which was gradually increased to 1200 mg/m2/day on which he reported minimal improvement in muscle weakness and skin rash.
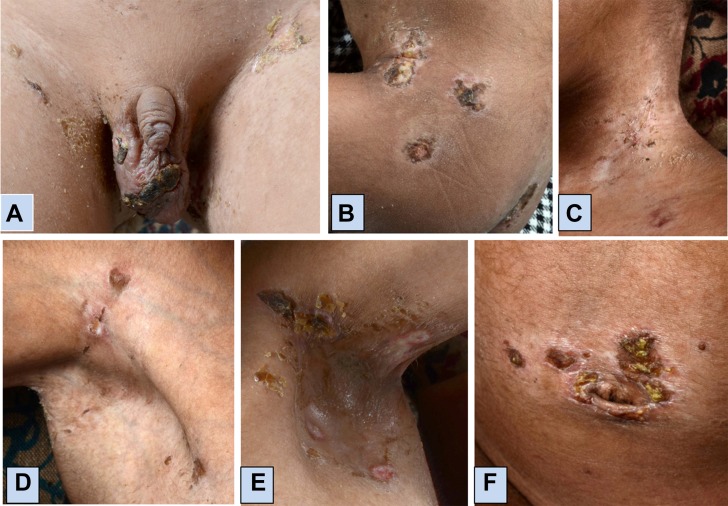
At 6 years of age, he presented with painful skin ulcers along with proximal muscle weakness. He also reported nasal regurgitation of milk and change in voice. Skin lesions began as an erythematous area over scrotum which developed into a punched out lesion with subsequent blackish discoloration followed by appearance of new lesions involving several parts of the body. On examination, extensive skin ulcers were noted over the scrotum, prepuce, gluteal region, neck, bilateral axilla, periumbilical area and bilateral elbows and popliteal fossa (figure 1). He also had heliotrope rash, healed Gottron papules over dorsal aspect of MCP and PIP joints, weakness of proximal muscles, neck flexors and poor gag reflex. There was no clinical evidence of calcinosis. Radiographs of chest and bilateral knee and elbow joints also did not show underlying calcinosis.
Figure 1.
Location of ulcers in juvenile dermatomyositis: (A) scrotum and groin; (B) gluteal region; (C) neck; (D) right axilla; (E) left axilla and (F) periumbilical area.
Investigations
Investigations revealed haemoglobin 9.1 g/dL, total leucocyte count 12 570/mm3 with differential count of 65% polymorphs, 23% lymphocytes, 10% monocytes and 2% eosinophils and platelet count 6.35×103/mm3. Erythrocyte sedimentation rate was 73 mm in the first hour and C reactive protein was 2.26 mg/L. His alanine aminotransferase was 88 U/L (normal: 2–41 U/L), aspartate aminotransferase was 85 U/L (normal: 2–40 U/L), LDH was 671 U/L (normal 240–480 U/L) and CK-NAC was 73.8 U/L (normal: 26–308 U/L). Serum aldolase level was not available. Antinuclear antibody by indirect immunofluorescence using Hep2 cell lines was negative.
Treatment
He received five doses of methylprednisolone pulse therapy at 30 mg/kg/day followed by oral prednisolone at 60 mg/day, which was gradually tapered. Intravenous cyclophosphamide was given at 500 mg/m2 and the child was continued on MMF at 1200 mg/m2/day and subcutaneous methotrexate at 15 mg/m2/week. No antimicrobials were administered for skin ulcers.
Outcome and follow-up
After 6 doses of monthly intravenous cyclophosphamide, his muscle weakness has improved and skin ulcers have healed.
Discussion
Cutaneous ulceration is seen in 5–30% of patients with JDM, more frequently in younger children.1 Cutaneous ulcers in JDM may be related to vasculitis or may occur secondary to calcinosis. Our patient had no evidence of calcinosis and ulcers were attributed to vasculitis. Vasculitic ulcers in JDM usually are circular with sharp margins and have been reported to involve axilla, elbow or extensor surfaces of other joints.1 2 Extensive cutaneous ulcers in JDM, especially those involving the scrotum, have never been described.
Cutaneous ulcers in children with JDM portend a protracted illness.1 3 Vasculitic ulcers in JDM are associated with severe disease, including both cutaneous and muscle manifestations, and thus can be considered to reflect inflammatory process in skin and muscle.1 3 However, it has not been substantiated in all reports.4 They are associated with significant pain and morbidity and can also develop secondary bacterial infections. Patients with ulcers often show increased resistance to immunosuppressive therapy, both for skin and muscle diseases. Awareness regarding the uncommon manifestation is important to guide appropriate therapy. The presence of vasculitic skin ulcers can help to prognosticate regarding the disease course and response to immunosuppressive therapy. Studies in adults have shown an association between anti-melanoma differentiation-associated gene 5 (MDA 5) antibodies and cutaneous ulcers5; however, the role of autoantibodies in children with JDM and cutaneous ulcers has not been clearly elucidated.
Learning points.
Children with juvenile dermatomyositis (JDM) can have extensive skin ulcers that are associated with significant pain and morbidity.
The presence of cutaneous ulcers in JDM is often associated with increased resistance to immunosuppressive therapy, both for skin and muscle diseases.
Awareness regarding this uncommon manifestation is important to guide appropriate therapy.
Footnotes
Contributors: AG prepared the manuscript and was involved in patient care, RKP reviewed the literature on the subject, VDP reviewed the literature on the subject and was involved in patient care and SG was involved in patient care and reviewed the literature. All authors had read and approved the final manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Rider LG, Lindsley CB, Miller FW. et al. Juvenile Dermatomyositis : Petty RE, Laxer RM, Lindsley CB, Wedderburn LR, Textbook of Pediatric Rheumatology. 7th ed Philadelphia: Elsevier Saunders, 2015:351–83. [Google Scholar]
- 2. Wakiguchi H, Takei S, Kawano Y. Axillary skin ulcers in infants with juvenile dermatomyositis. Pediatr Neonatol 2017;58:30141–3. 10.1016/j.pedneo.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 3. Bowyer SL, Blane CE, Sullivan DB, et al. Childhood dermatomyositis: factors predicting functional outcome and development of dystrophic calcification. J Pediatr 1983;103:882–8. 10.1016/S0022-3476(83)80706-9 [DOI] [PubMed] [Google Scholar]
- 4. Martin N, Krol P, Smith S, et al. Comparison of children with onset of juvenile dermatomyositis symptoms before or after their fifth birthday in a UK and Ireland juvenile dermatomyositis cohort study. Arthritis Care Res 2012;64:1665–72. 10.1002/acr.21753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Narang NS, Casciola-Rosen L, Li S, et al. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res 2015;67:667–72. 10.1002/acr.22498 [DOI] [PMC free article] [PubMed] [Google Scholar]