Erythropoietin, the major hormone produced by the kidneys in response to anaemia, stimulates the bone marrow to produce reticulocytes. As the degree of anaemia worsens, serum erythropoietin levels increase, although this response is blunted in patients with sickle cell anaemia (SCA). (Sherwood, et al 1986) Older age and renal dysfunction further impair the erythropoietin response to anaemia in SCA. (Morgan, et al 1982, Sherwood, et al 1986) Studies of erythropoiesis-stimulating agents (ESAs) to improve haemoglobin concentrations in SCA have demonstrated conflicting results. (el-Hazmi, et al 1995, Goldberg, et al 1990, Little, et al 2006, Nagel, et al 1993, Roger, et al 1991, Steinberg 1991, Tomson, et al 1992) Some studies using ESAs alone have demonstrated improvements in anaemia (Steinberg 1991) or reductions in red blood cell transfusion requirements, (Tomson, et al 1992) but other studies did not. (Goldberg, et al 1990, Roger, et al 1991) In addition, two of these studies raised concerns that ESAs may trigger vaso-occlusive crises (VOC). (Goldberg, et al 1990, Roger, et al 1991) Some studies of ESAs administered to patients on a stable hydroxycarbamide (HC) dose have demonstrated augmentation of haemoglobin F% and haemoglobin concentrations (Little, et al 2006) while others have demonstrated either variable (el-Hazmi, et al 1995) or no beneficial effects. (Goldberg, et al 1990) Limited data is available on the effects of concurrently starting an ESA with HC. (Little, et al 2006)
We conducted a retrospective analysis of adults with SCA treated with an ESA at the University of Illinois at Chicago (UIC). Thirty-two adults with a diagnosis of SCA (HbSS or HbSβ0-thalassemia) who received a minimum of 24 doses of erythropoietin or 12 doses of darbepoetin over a 12-month period between January 2001 and January 2015 were evaluated in a protocol approved by the UIC Institutional Review Board.
The SCA patients receiving ESA therapy were divided into the following groups for analysis: 1) ESA-only, defined as patients receiving an ESA and not HC; 2) newly prescribed ESA+HC, defined as patients newly prescribed both ESA and HC within three months of each other and continuing together for at least 12 months; 3) newly prescribed HC-only, defined as patients newly prescribed HC alone and were age- and gender-matched to the newly prescribed ESA+HC group, and 4) ESA+stable HC, defined as patients on a stable dose of HC at least three months prior to initiating ESA therapy and continuing HC during ESA treatment. We compared linear variables pre-ESA therapy and after 12 months of ESA therapy using the Wilcoxon Signed-Rank test. A linear regression model was used to test if ESA and stable or new HC therapy had independent effects on the change in haemoglobin concentration, adjusting for age and advanced chronic kidney disease (CKD), defined as an estimated glomerular filtration rate <60 ml/min/1.73m2, at the time of initiating therapy. Systat 13 (Systat Software Corporation, Chicago, IL, USA) and GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA) were used for the statistical analyses.
Thirty-two SCA patients, median age 43 years (range, 21–63 years) and 44% female, were prescribed ESA therapy for at least one-year duration. Baseline characteristics of the patients according to the treatment groups are provided in Supplementary Table I. We did not observe increases in the rates of VOC requiring medical attention (Figure 1A) or venous thromboembolism (VTE) during the year of ESA therapy compared to the year before ESA therapy was initiated. Two VTE were observed during the year of ESA therapy, one in the ESA-only group and one in the newly prescribed ESA+HC group, and two patients had VTE in the year preceding ESA therapy, one in the ESA-only group and one in the stable HC+ESA group.
Figure 1A. Rates of vaso-occlusive crises 1 year prior and during the 1 year of erythropoiesis-stimulating agent (ESA) therapy.
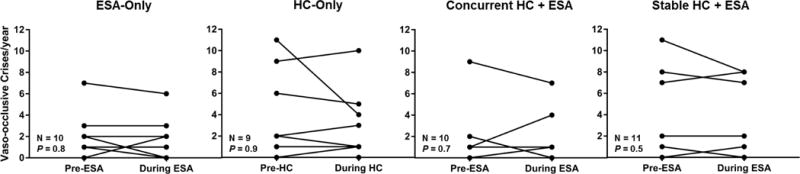
We did not observe increases in the rates of vaso-occlusive crises, given as median (range), with ESA only [2/year (0 – 7/year) vs. 2/year (0 – 6/year)], newly prescribed HC-only [2/year (0 – 11/year) vs. 2/year 0 – 10/year)], newly prescribed HC+ESA [1/year (0 – 9/year) vs. 1/year (0 – 7/year)] or stable HC+ESA [1/year (0 – 11/year) vs. 0/year (0 – 8/year)] from the year pre-ESA when compared to the year of ESA therapy, respectively.
To further evaluate the safety of ESAs in sickle cell disease, we utilized claims data from the Truven Health MarketScan® Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits databases (http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases) between January 2009 and December 2014. Patients aged over 18 years old with sickle cell disease, which included genotypes HbSS, HbSC, HbSβ+-thalassemia, and HbSβ0-thalassemia, were identified using a validated algorithm. (Michalik, et al 2017) Details on specific selection criteria and determination of adverse events are provided in Supplementary Figure 1 and Supplementary Table II, respectively. Twenty-four sickle cell disease patients had at least one year of ESA therapy based on inpatient or outpatient administration or pharmacy dispensing claims. The median age of this cohort was 55.5 years (range 20–92years) and 50% were female. Similar to the UIC cohort, the rates of VOC events were not different during the first year of ESA therapy (median 1/year, range 0–36/year) compared to the year prior to ESA therapy (median 1/year, range 0–42/year). Furthermore, no VTE events were reported during the year of ESA therapy.
After one year of ESA therapy in the UIC cohort, trends for increased haemoglobin concentrations were observed in each group that received ESAs as well as in the HC-alone group (Figure 1B). In a multiple linear regression model, ESA therapy (β=0.64, 95% confidence interval: 0.05–1.22; P=0.035) had an independent association with increase in haemoglobin concentration after adjustment for age, advanced CKD, stable HC therapy, and new HC therapy (Table I).
Figure 1B. Haemoglobin response to erythropoiesis stimulating agents (ESA).
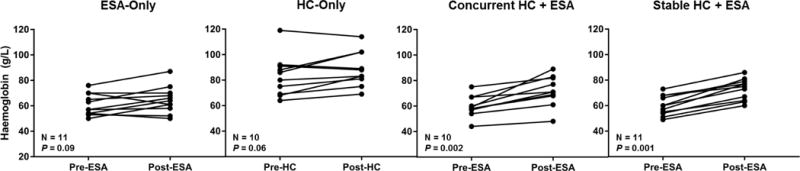
Trends for higher haemoglobin concentrations, given as median (range), with ESA-only [57 g/l (50 – 76) to 64 g/l (50 – 87 g/l)] and hydroxycarbamide (HC)-only [83 g/l (64 – 119 g/l) to 86 g/l (69 – 114 g/l)] were observed while significant improvements were observed when an ESA was started concurrently with HC [59 g/l (44 – 75g/l) to 71 g/l (48 – 89 g/l)] or when a sickle cell anaemia patient already on a stable dose of HC was started on an ESA [60 g/l (49 – 73 g/l) to 75 g/l (60 – 86 g/l)] from the year pre-ESA when compared to after one year of ESA therapy, respectively.
Table I.
Multivariate analysis for the change in haemoglobin concentration in sickle cell anaemia patients treated with erythropoiesis-stimulating agents and/or hydroxycarbamide for one year at the University of Illinois at Chicago.
| Variable | β | 95% Confidence Interval | P Value |
|---|---|---|---|
| ESA therapy | 0.64 | 0.05 – 1.22 | 0.035 |
| New HC therapy | 0.74 | 0.16 – 1.32 | 0.014 |
| Stable HC therapy | 0.95 | 0.37 – 1.54 | 0.002 |
| Age | 0.02 | 0.003 – 0.04 | 0.023 |
| Advanced CKD | −0.27 | −0.76 – 0.23 | 0.3 |
CKD, chronic kidney disease; ESA, erythropoiesis-stimulating agent; HC, hydroxycarbamide, Advanced CKD defined as estimated glomerular filtration rate < 60 ml/min/1.73m2
Our study is limited by the small sample size, variability in the patients in terms of treatment and baseline characteristics, such as age and renal function, and retrospective design. Nevertheless, we found no evidence that ESA therapy increased the rate of VOC or of VTE in the UIC SCA cohort or in the Truven database of sickle cell disease patients and, in the UIC cohort, we observed a significant increase in the haemoglobin concentration after one year of ESA therapy after adjusting for age, CKD and HC. Our findings require substantiation in prospective clinical trials to evaluate the safety and efficacy of ESAs in SCA, both alone and in combination with HC.
Supplementary Material
Acknowledgments
The project described was supported by the National Institutes of Health through grant K23HL125984 (S.L.S). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Authorship Contributions:
J.H., J.Z., V.K., X.Z., G.S.C., S.L.S. designed and performed the research, analysed the data and wrote the paper. R.E.M., M.G., J.H., S.J. performed the research and wrote the paper. V.R.G. designed the research, analysed the data and wrote the paper.
Disclosure of Conflicts of Interest: The authors declare no competing financial interests.
References
- el-Hazmi MA, al-Momen A, Kandaswamy S, Huraib S, Harakati M, al-Mohareb F, Warsy AS. On the use of hydroxyurea/erythropoietin combination therapy for sickle cell disease. Acta Haematol. 1995;94:128–134. doi: 10.1159/000203994. [DOI] [PubMed] [Google Scholar]
- Goldberg MA, Brugnara C, Dover GJ, Schapira L, Charache S, Bunn HF. Treatment of sickle cell anemia with hydroxyurea and erythropoietin. N Engl J Med. 1990;323:366–372. doi: 10.1056/NEJM199008093230602. [DOI] [PubMed] [Google Scholar]
- Little JA, McGowan VR, Kato GJ, Partovi KS, Feld JJ, Maric I, Martyr S, Taylor JGt, Machado RF, Heller T, Castro O, Gladwin MT. Combination erythropoietin-hydroxyurea therapy in sickle cell disease: experience from the National Institutes of Health and a literature review. Haematologica. 2006;91:1076–1083. [PMC free article] [PubMed] [Google Scholar]
- Michalik DE, Taylor BW, Panepinto JA. Identification and Validation of a Sickle Cell Disease Cohort Within Electronic Health Records. Acad Pediatr. 2017;17:283–287. doi: 10.1016/j.acap.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Morgan AG, Gruber CA, Serjeant GR. Erythropoietin and renal function in sickle-cell disease. Br Med J (Clin Res Ed) 1982;285:1686–1688. doi: 10.1136/bmj.285.6356.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel RL, Vichinsky E, Shah M, Johnson R, Spadacino E, Fabry ME, Mangahas L, Abel R, Stamatoyannopoulos G. F reticulocyte response in sickle cell anemia treated with recombinant human erythropoietin: a double-blind study. Blood. 1993;81:9–14. [PubMed] [Google Scholar]
- Roger SD, Macdougall IC, Thuraisingham RC, Raine AE. Erythropoietin in anemia of renal failure in sickle cell disease. N Engl J Med. 1991;325:1175–1176. doi: 10.1056/NEJM199110173251614. [DOI] [PubMed] [Google Scholar]
- Sherwood JB, Goldwasser E, Chilcote R, Carmichael LD, Nagel RL. Sickle cell anemia patients have low erythropoietin levels for their degree of anemia. Blood. 1986;67:46–49. [PubMed] [Google Scholar]
- Steinberg MH. Erythropoietin for anemia of renal failure in sickle cell disease. N Engl J Med. 1991;324:1369–1370. doi: 10.1056/NEJM199105093241916. [DOI] [PubMed] [Google Scholar]
- Tomson CR, Edmunds ME, Chambers K, Bricknell S, Feehally J, Walls J. Effect of recombinant human erythropoietin on erythropoiesis in homozygous sickle-cell anaemia and renal failure. Nephrol Dial Transplant. 1992;7:817–821. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


