Abstract
Because of the ethical and regulatory hurdles associated with human studies, much of what is known about the psychopharmacology of hallucinogens has been derived from animal models. However, developing reliable animal models has proven to be a challenging task due to the complexity and variability of hallucinogen effects in humans. This chapter focuses on three animal models that are frequently used to test the effects of hallucinogens on unconditioned behavior: head twitch response (HTR), prepulse inhibition of startle (PPI), and exploratory behavior. The HTR has demonstrated considerable utility in the neurochemical actions of hallucinogens. However, the latter two models have clearer conceptual bridges to human phenomenology. Consistent with the known mechanism of action of hallucinogens in humans, the behavioral effects of hallucinogens in rodents are mediated primarily by activation of 5-HT2A receptors. There is evidence, however, that other receptors may play secondary roles. The structure–activity relationships (SAR) of hallucinogens are reviewed in relation to each model, with a focus on the HTR in rats and mice.
Keywords: Psychedelic; Wet dog shake; 5-HT2C receptor; Lisuride; Quipazine; Lysergic acid diethylamide; LSD; DOI; SB-242, 084; M100907; Mescaline; Psilocybin; Locomotor activity; 25I-NBOMe; 25CN-NBOH; 1-methylpsilocin; Rat; Mouse
1 Introduction
Because of the difficulties associated with hallucinogen studies in human subjects, investigations of the mechanism of action of serotonergic hallucinogens have relied heavily on animal models. Most clinical work with hallucinogens ceased in the early 1970s; although human testing resumed in 1994 and has accelerated in recent years (e.g., Griffiths et al. 2006; Grob et al. 2011; Carhart-Harris et al. 2012; Schmid et al. 2015), animal behavioral models remain the primary methodology used to characterize the pharmacology of hallucinogens in vivo. In fact, much of what is known about the actions of this class has been derived from animal behavioral models. Drug discrimination studies in rats provided some of the first evidence that 5-HT2A activation is responsible for mediating the behavioral effects of hallucinogens (Glennon et al. 1983, 1984), a finding later confirmed in human volunteers (Vollenweider et al. 1998; Kometer et al. 2013). Animal models have also provided important insights into the structure–activity relationships of hallucinogens and the downstream signaling events responsible for mediating their effects. They have also helped to elucidate how the expression of hallucinogen effects is regulated by a variety of receptors and transporters (González-Maeso et al. 2007, 2008; Canal and Morgan 2012; Nichols 2012). Importantly, there is a highly significant and robust correlation between the potencies of hallucinogens in humans and in animal behavioral models (Glennon et al. 1984).
Given the complexity, variety, and variability of the effects of hallucinogens in humans, it has been difficult to define animal behavioral models of hallucinogenic activity. Few methods have satisfied the criteria for an animal model of acute hallucinogenic activity, as we have discussed elsewhere (Adams and Geyer 1985b; Segal and Geyer 1985; Geyer and Krebs 1994). Behavioral models of hallucinogen effects can be divided into two classes: (1) models based on behaviors that are analogous to the effects of hallucinogens in humans and (2) models with no human counterpart. The first type of model is often preferred because they have a conceptual link to human phenomenology, and therefore can be used to investigate the mechanisms responsible for the human response to hallucinogens. Such models may exhibit construct validity to the degree that they assess the same behavioral and physiological effects observed in humans administered hallucinogens. On the other hand, the second type of model often has considerable predictive validity but not construct validity since it is unclear how the behaviors being studied relate to the effects of hallucinogens in humans. Numerous animal models of hallucinogen effects have been proposed used over the last four decades, but most were found to lack specificity or to suffer from other drawbacks. In this chapter, we review the effects of hallucinogens on three unconditioned behaviors: HTR, prepulse inhibition (PPI) of startle, and exploratory behavior. Drug discrimination, another popular model of hallucinogen effects, will be addressed in a separate chapter.
2 Head Twitch Response
Serotonergic hallucinogens induce a variety of spontaneous unconditioned movements in laboratory animals, including ear scratching (mice), limb flicks (cats), and head bobs (rabbits). Hallucinogens also elicit the head twitch response (HTR), a paroxysmal rotational shaking of the head, in mice. The HTR is similar to the head shaking reflex induced by mechanical or chemical irritation of the pinna. The kinematics of the HTR induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine (DOI) in mice have been extensively characterized in C57BL/6J mice (Halberstadt and Geyer 2013). When mice make a head twitch, the head repeatedly twists from side-to-side, with each individual head movement lasting approximately 10 ms. Rats also display head twitches after administration of hallucinogens, but in that species the response is a mixture of head shakes and whole-body shakes similar to the behavior of dogs emerging from the water. Hence, in rats the behavior is sometimes referred to as a wet dog shake (WDS).
Although the hallucinogen-induced HTR is most commonly associated with rats and mice, hallucinogen-induced shaking has been observed in many other species. According to observational studies, Stumptail macaque monkeys (Macaca arctotdes) exhibit a WDS-like behavior after administration of serotonergic hallucinogens (Schlemmer and Davis 1986). DOI reportedly produces a robust HTR in the least shrew (Darmani et al. 1994). In addition to limb flicks, cats also make head and body shakes after administration of LSD (Jacobs et al. 1976, 1977).
In recent years, the HTR has been widely adopted as a behavioral proxy in rodents for human hallucinogen effects. There are several reasons why use of the HTR has become so common in preclinical behavioral studies of hallucinogens. Most importantly, the HTR is one of only a few behaviors that can reliably distinguish hallucinogenic and non-hallucinogenic 5-HT2A agonists (González-Maeso et al. 2007). The HTR has also proven useful for characterizing the behavioral and neurochemical interactions between the serotonergic system and a variety of other neurotransmitters and neuromodulators, including dopamine, norepinephrine, glutamate, opioids, and cannabinoids (Canal and Morgan 2012). Another reason for the widespread use of this behavior is that it can be assessed by direct observation, and therefore studies do not require specialized training or equipment. Nevertheless, although the observational methods used in HTR studies are accurate, they are also extremely labor intensive and do not allow the behavior to be analyzed in a qualitative fashion or with great temporal precision. As we have recently shown, however, a head-mounted magnet and a magnetometer coil can be used to detect head twitches with high sensitivity and specificity, providing a semi-automated assessment of the response (Halberstadt and Geyer 2013). Our studies to date have confirmed that this method markedly increases the throughput of HTR studies.
2.1 Ergolines
LSD is the first hallucinogen that was shown to induce shaking behavior in laboratory animals. In the course of a behavioral study with LSD in rats, Winter and Flataker (1956) made the following observation:
When LSD is injected intraperitoneally…the first sign can be observed within 1–2 min, and consists of hyperactivity. The animal explores its cage more busily than usual. After 2 or 3 min, this activity is interrupted momentarily while the animal violently shakes his head; this shaking may be so pronounced that it involves not only the head but the entire body.
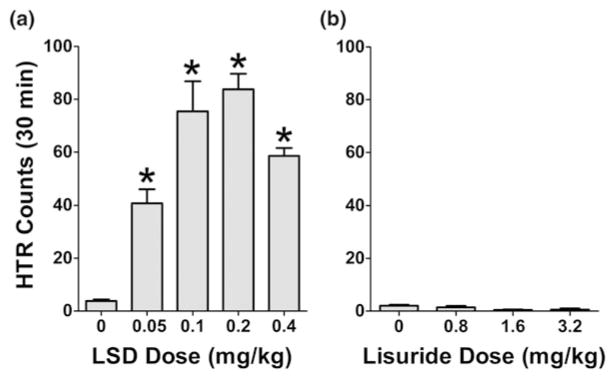
Later that year, it was reported (Keller and Umbreit 1956) that LSD induces head shaking in mice. However, it does not appear that the behavior observed by Keller and Umbreit is identical to the HTR because the response was only expressed in response to light tactile stimulation of the head and did not occur spontaneously in mice treated with LSD. Furthermore, mescaline did not elicit the same response, even though it is well known that mescaline induces the HTR in mice. Follow-up studies by other groups failed to replicate the finding that LSD facilitates shaking induced by tactile stimuli (Corne et al. 1963). Nevertheless, it was subsequently confirmed that LSD induces the HTR in mice, rats, and cats (Corne and Pickering 1967; Silva and Calil 1975; Jacobs et al. 1976; Bedard and Pycock 1977). LSD is highly potent, inducing the HTR with an ED50 of 53 μg/kg (133 nmol/kg) in mice (Halberstadt and Geyer 2013).
Very few lysergamides other than LSD have been investigated in HTR studies. Mice display head twitches in response to 1-methyl-LSD, which is reportedly about half as potent as LSD (Corne and Pickering 1967). The potency in mice is consistent with human studies indicating that 100 μg 1-methyl-LSD produces hallucinogenic effects roughly comparable to 35 μg LSD (Abramson et al. 1958). 1-Propionyl-LSD (1P-LSD) has recently been sold online as a “research chemical” and reportedly produces LSD-like hallucinogenic effects. We have confirmed that 1P-LSD induces the HTR in mice with about one-third the potency of LSD (1P-LSD: ED50 = 159 μg/kg or 350 nmol/kg; Brandt et al. 2016). Two other lysergamides sold as “research chemicals,” N6-allyl-6-nor-LSD (AL-LAD) and (2′ S,4′S)-lysergic acid 2,4-dimethylazetidide (LSZ), have also been shown to induce the HTR (Brandt et al. 2017). The potency of LSZ (ED50 = 114 nmol/kg) is approximately the same as LSD, whereas AL-LAD is slightly less potent (ED50 = 175 nmol/kg). The ergot alkaloid ergometrine (also known as ergonovine or (+)-lysergic acid β-propanolamide) is a uterotonic and is used to reduce post-partum bleeding. According to anecdotal reports, ergometrine produces minor LSD-like effects at p.o. doses of 2–10 mg (Wasson et al. 1978; Bigwood et al. 1979). Consistent with the weak hallucinogenic activity of ergometrine, two groups have observed the HTR in mice treated with high doses of ergometrine (Corne and Pickering 1967; Balsara et al. 1986). Finally, as discussed below, non-hallucinogenic lysergamides such as lisuride and ergotamine fail to induce head twitches in mice and rats.
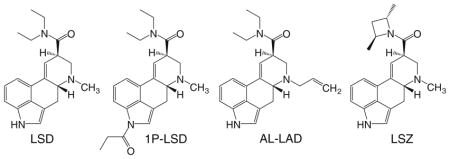
The activity of LSD is very sensitive to halogenation at the 2-position of the indole ring. 2-Bromo-LSD, also known as BOL-148, is a non-hallucinogenic derivative of LSD that acts as a 5-HT2A antagonist (Burris et al. 1991). As expected, 2-bromo-LSD does not produce the HTR in mice or cats (Corne and Pickering 1967; Jacobs et al. 1976).
2.2 Phenylisopropylamines
Many 4-substituted derivatives of 2,5-dimethoxyamphetamine (2,5-DMA) are potent hallucinogens and several are known to induce the HTR. The most important examples are the 4-methyl (DOM), 4-bromo (DOB), and 4-iodo (DOI) derivatives. DOI is widely used in HTR studies due to the fact that it produces a robust response, is commercially available, and is uncontrolled throughout most of the world (Canal and Morgan 2012). According to Schreiber et al. (1995), the ED50 for DOI in rats is 0.78 mg/kg (2.43 μmol/kg). To our knowledge, the ED50 of DOI in mice has not been reported. Most studies have shown that the response to DOI peaks at doses between 1 and 5 mg/kg (Darmani et al. 1990; Schreiber et al. 1995; Canal et al. 2010; Fantegrossi et al. 2010). DOM and DOB are essentially equipotent with DOI (Yamamoto and Ueki 1975; Wieland et al. 1990; Fantegrossi et al. 2005a). Reports also indicate that DOM, DOB, and trans-2-(2,5-dimethoxy-4-methylphenyl)-cyclopropylamine (DMCPA) produce head shakes and body shakes in cats (Nichols et al. 1978; Rusterholz et al. 1978).
Phenylisopropylamine hallucinogens are chiral molecules with one stereocenter. According to several reports, DOM, DOB, DOI, and DOET have stereoselective effects in humans, and the R-(−)-isomers are the eutomers (Shulgin et al. 1971; Shulgin 1973; Snyder et al. 1974; Shulgin and Shulgin 1991). Drug discrimination studies have confirmed that that the R-(−)-isomers of phenylisopropylamine hallucinogens are more potent than their S-(+) enantiomers (Glennon et al. 1982a, b, 1986, 1987). Several head twitch studies have compared the enantiomeric potency of the stereoisomers of phenylisopropylamine hallucinogens. For example, Glennon and colleagues reported that 2.5 mg/kg R-(−)-DOI produces almost twice as many head twitches as 2.5 mg/kg S-(+)-DOI in mice (Darmani et al. 1990). Another study compared the effects of the stereoisomers of DOM and DMCPA in cats (Nichols et al. 1978). When administered at 0.125 mg/kg, the R-(−)-isomers provoked head and body shaking, whereas their S-(+)-enantiomers failed to elicit a response. Likewise, tests of R-(−)- and S-(+)-DOB over a wide range of doses confirmed that R-(−)-DOB is more potent and effective than S-(+)-DOB (Rusterholz et al. 1978). These results clearly show that phenylisopropylamine hallucinogens produce the HTR in a stereospecific manner.
Methylenedioxy-substituted phenylisopropylamines have been assessed in head twitch studies. Racemic 3,4-methylenedioxyamphetamine (MDA) and S-(+)-MDA reportedly induce WDS in monkeys and rats, respectively (Schlemmer and Davis 1986; Hiramatsu et al. 1989). Although (±)-3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) does not induce the HTR in mice, both of the stereoisomers of MDMA have been shown to elicit the response (Fantegrossi et al. 2004, 2005b). 5-HT depletion inhibits the response to S-(+)-MDMA but does not alter the response to R-(−)-MDMA, suggesting the isomers act through different mechanisms (Fantegrossi et al. 2005b). This suggestion is consistent with the fact that S-(+)- and R-(−)-MDMA exhibit qualitatively distinct pharmacological profiles, with the S-(+)isomer working primarily as a monoamine releaser (Johnson et al. 1986; Baumann et al. 2008; Murnane et al. 2010) and the R-(−)-enantiomer acting directly through 5-HT2A receptors (Lyon et al. 1986; Nash et al. 1994). In contrast to their effects in mice, Hiramatsu reported that S-(+)- and R-(−)-MDMA fail to produce WDS in rats (Hiramatsu et al. 1989). The discrepant findings with MDMA in mice and rats may reflect species differences in sensitivity to the HTR (see below for further discussion).
2.3 Phenethylamines
There is some disagreement in the literature regarding whether phenethylamine hallucinogens induce the HTR. Mescaline produces the HTR in rats and mice (Silva and Calil 1975; Yamamoto and Ueki 1975; González-Maeso et al. 2007). Likewise, 2,5-dimethoxy-4-n-propylthiophenethylamine (2C-T-7) has been shown to induce the HTR in mice (Fantegrossi et al. 2005a; Smith et al. 2014). Mice treated with theconformationally restricted phenethylamine (4-bromo-3,6-dimethoxybenzo-cyclobuten-1-yl)methylamine (TCB-2) exhibit head twitches (Fox et al. 2010a). In contrast to those findings, Moya and colleagues reported that 2,5-dimethoxy-4-iodophenethylamine (2C–I), 2,5-dimethoxy-4-bromophenethylamine (2C-B), and 2,5-dimethoxy-4-methylphenethylamine (2C–D) do not produce the HTR in rats, even after administration of relatively high doses (Moya et al. 2007). Our experiments, however, have shown that 2C–I induces the HTR in mice with an ED50 of 0.83 mg/kg (2.42 μmol/kg; Halberstadt and Geyer 2014). It appears that mice are more sensitive than rats to the HTR induced by 5-HT2A partial agonists, which may explain why mouse and rat studies sometimes yield disparate results. For example, m-trifluoromethylphenylpiperazine (TFMPP), a weak 5-HT2A partial agonist (Grotewiel et al. 1994), produces head twitches in mice (Yarosh et al. 2007) but not in rats (Arnt and Hyttel 1989; Schreiber et al. 1995). 2C–I has relatively low intrinsic activity at 5-HT2A (Acuña-Castillo et al. 2002; Parrish et al. 2005; Moya et al. 2007), so may not have sufficient efficacy to provoke the HTR in rats.
Although N-alkyl substitution attenuates the potency and 5-HT receptor affinity of phenethylamine hallucinogens (Shulgin and Shulgin 1991; Glennon et al. 1994), the addition of an N-benzyl group results in a dramatic increase in potency (Braden et al. 2006). Potency is increased even further if a polar oxygen substituent is present at the 2-position of the N-benzyl group. For example, 2C–I is active in humans at doses ranging from 14 to 22 mg (Shulgin and Shulgin 1991), whereas the N-(2-methoxybenzyl)-substituted derivative 25I-NBOMe is active at doses of 500–800 μg. We found that 25I-NBOMe is highly potent in mice, inducing the HTR with an ED50 of 78 μg/kg (0.17 μmol/kg), making it 14-fold more potent than 2C–I (Halberstadt and Geyer 2014) and only slightly less potent than LSD. The 4-bromo analog 25B-NBOMe reportedly induces the HTR in mice at 0.5 mg/kg but not at 0.05 mg/kg (Ettrup et al. 2013). Fantegrossi et al. reported that the HTR to N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenethylamine (25CN-NBOH), another N-benzyl-substituted phenethylamine, peaks at 1 mg/kg (Fantegrossi et al. 2014). Studies in our laboratory have confirmed that 25CN-NBOH produces the HTR with an ED50 = 0.36 mg/kg (1.03 μmol/kg; Halberstadt et al. 2016a).
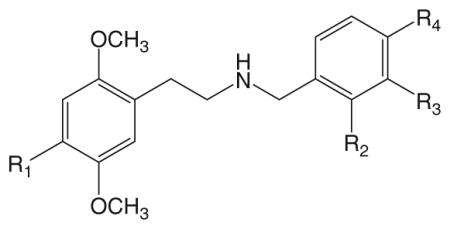
We have used the HTR to characterize a series of 25I-NBOMe analogs with varied substituents on the N-benzyl ring (Nichols et al. 2015). As shown in Table 1, the ability of 25I-NBOMe to induce the HTR is dramatically altered if the position of the methoxy group on the N-benzyl ring is altered or if the group is replaced by bromine. Compared with 25I-NBOMe, the analog with a meta-methoxy group (25I-NB3OMe) is markedly less potent, whereas the para-methoxy analog (25I-NB4OMe) is completely inactive. Replacing the ortho-methoxy moiety of 25I-NBOMe with bromine resulted in a dramatic reduction in potency. Although the ortho-bromo-substituted compound (25I-NB2B) is active, rearrangement of the bromine to either the meta- or para-position was not tolerated, and no HTR was observed with 25I-NB3B or 25I-NB4B at doses up to 30 mg/kg. The N-(2,3-methylenedioxybenzyl) analog 25I-NBMD is significantly less potent than 25I-NBOMe and shows the same potency as the non-benzylated parent compound 2C–I. Therefore, it appears that for N-benzylphenethylamines the highest potency in the HTR assay is associated with an ortho-substituent on the benzyl ring, especially if the substituent contains an oxygen atom.
Table 1.
Head twitch response induced by N-benzylphenethylamines
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | ED50 (mg/kg) | ED50 (μmol/kg) | Reference1 |
| 25I-NBOMe | –I | –OCH3 | –H | –H | 0.078 | 0.17 | A |
| 25I-NB3OMe | –I | –H | –OCH3 | –H | 4.34 | 9.36 | B |
| 25I-NB4OMe | –I | –H | –H | –OCH3 | nd (>30) | B | |
| 25I-NB2B | –I | –Br | –H | –H | 2.31 | 4.50 | B |
| 25I-NB3B | –I | –H | –Br | –H | nd (>30) | B | |
| 25I-NB4B | –I | –H | –H | –Br | nd (>30) | B | |
| 25I-NBMD | –I | –OCH2 | O– | –H | 1.13 | 2.36 | A |
| 25CN-NBOH | –CN | –OH | –H | –H | 0.36 | 1.03 | C |
nd not determined
2.4 Tryptamines
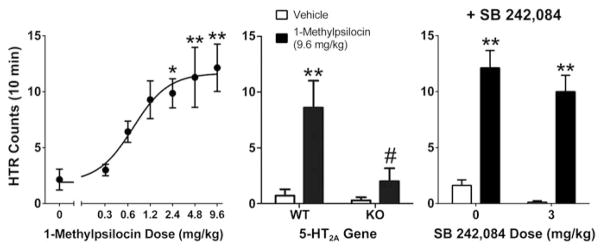
A variety of tryptamine hallucinogens have been tested for HTR activity. There have been several reports that DMT elicits head twitches in mice (Corne and Pickering 1967; González-Maeso et al. 2007; Carbonaro et al. 2015). One study found that DMT did not produce a significant HTR (Fantegrossi et al. 2006), but that may have been a consequence of using a strain of mice (NIH-Swiss) that is hypo-responsive to hallucinogen-induced HTR (Canal and Morgan 2012). The HTR has also been observed in rodents treated with N-methyl-N-ethyltryptamine (MET), N,N-diethyltryptamine (DET), N,N-dipropyltryptamine (DPT), N,N-diisopropyltryptamine (DIPT), and N,N-diallyltryptamine (DALT) (Fantegrossi et al. 2008; Smith et al. 2014; Carbonaro et al. 2015; Halberstadt and Klein, unpublished observations). Likewise, the HTR is induced by ring-substituted tryptamines, including psilocin, psilocybin, 5-MeO-DMT, and 5-MeO-DIPT (Corne and Pickering 1967; Bedard and Pycock 1977; Fantegrossi et al. 2006; González-Maeso et al. 2007; Halberstadt et al. 2011). The HTR also occurs in mice after administration of 1-methylpsilocin (Halberstadt et al. 2011). As shown in Fig. 1, 1-methylpsilocin acts with an ED50 of 0.70 mg/kg (3.22 μmol/kg).
Fig. 1.
Effect of 1-methylpsilocin on the head twitch response (HTR) in mice. Left panel Dose-response of 1-methylpsilocin in male C57BL/6J mice. Middle panel Response to 1-methylpsilocin (9.6 mg/kg) in wild-type (WT) and 5-HT2A receptor knockout (KO) mice. Right panel Effect of pretreatment with the 5-HT2C antagonist SB-242,084 (3 mg/kg) on the response to 1-methylpsilocin (9.6 mg/kg) in male C57BL/6J mice (n = 8/group, 32 total). SB-242,084 was injected SC 20 min prior to testing; 1-methylpsilocin was injected SC immediately prior to testing. Data are shown as mean ± SEM. The data in the first two panels are redrawn from: Halberstadt et al. (2011). *p < 0.05, **p < 0.01 versus the respective vehicle control group (Tukey’s test). #p < 0.01 versus 1-methylpsilocin in WT mice (Tukey’s test)
Several side-chain-substituted tryptamine hallucinogens have been assessed. α-Methyltryptamine (AMT) induces the HTR in mice but is not very potent (Corne and Pickering 1967). The 5-fluoro- and 6-fluoro- derivatives of AMT also induce head twitches (Tadano et al. 1995). The HTR has also been observed in mice treated with 5-MeO-AMT (May et al. 2006), which is known to be a potent hallucinogen with long-lasting effects (Shulgin and Nichols 1978; Kantor et al. 1980).
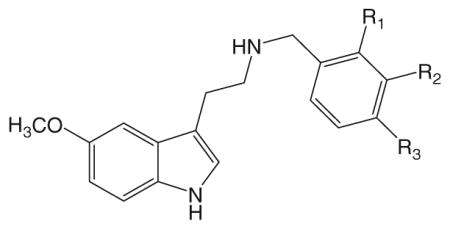
A series of N-benzyl derivatives of 5-methoxytryptamine have been characterized (Table 2). Although several N-benzyl-5-methoxytryptamines produce the HTR, none of the compounds are particularly potent (Nichols et al. 2015). In contrast to the N-benzylphenethylamines, where compounds with an ortho-substituted benzyl group were the most active, activity in the N-benzyl-5-methoxytryptamines was linked to the presence of a meta-substituent. Almost all the meta-substituted N-benzylated-5-methoxytryptamines were active, including compounds with 3-methyl, 3-methoxy, 3-fluoro, 3-chloro, 3-bromo, and 3-iodo groups (see Table 2). With the exception of the 2-methoxy-substitued compound (5MT-NB2OMe), which elicited the HTR with an ED50 of 3.15 mg/kg (9.08 μmol/kg), none of the compounds with ortho- or para-substituted benzyl groups produced a response at doses up to 30 mg/kg.
Table 2.
Head twitch response induced by N-benzyl-5-methoxytryptamines
| |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | ED50 (mg/kg) | ED50 (μmol/kg) |
| 5MT-NB2OMe | –OCH3 | –H | –H | 3.15 | 9.08 |
| 5MT-NB3OMe | –H | –OCH3 | –H | 3.28 | 7.69 |
| 5MT-NB4OMe | –H | –H | –OCH3 | nd (>30) | |
| 5MT-NB2B | –Br | –H | –H | nd (>30) | |
| 5MT-NB3B | –H | –Br | –H | 5.18 | 10.89 |
| 5MT-NB4B | –H | –H | –Br | nd (>30) | |
| 5MT-NB3F | –H | –F | –H | 3.33 | 8.04 |
| 5MT-NB3Cl | –H | –Cl | –H | 4.43 | 10.29 |
| 5MT-NB3I | –H | –I | –H | 7.77 | 14.88 |
| 5MT-NB3Me | –H | –CH3 | –H | 2.31 | 5.63 |
| 5MT-NB3TFM | –H | –CF3 | –H | nd (>30) | |
| 5MT-NB3SMe | –H | –SCH3 | –H | nd (>30) | |
nd not determined. Data from: Nichols et al. (2015)
2.5 Receptor Mechanisms for the Hallucinogen Head Twitch Response
The 5-HT2A receptor is believed to be responsible for the hallucinogen HTR. It was reported as early as 1967 that the nonselective 5-HT antagonists methysergide and cyproheptadine block the ability of LSD and other hallucinogens to induce the HTR (Corne and Pickering 1967); subsequent studies confirmed that many other 5-HT antagonists ameliorate the response. The first evidence that the 5-HT2A receptor is specifically involved appeared in 1982 when Leysen reported that the potency of 19 5-HT antagonists to block the HTR to mescaline is highly correlated (r = 0.88) with their 5-HT2A binding affinity (Leysen et al. 1982). Subsequently, a similar correlation (r = 0.83) was shown to exist for blockade of the HTR to DOI (Schreiber et al. 1995; Dursun and Handley 1996). The ability of hallucinogens to elicit the HTR is blocked by the highly selective 5-HT2A antagonists M100907 and MDL 11,939 (Vickers et al. 2001; Fantegrossi et al. 2006, 2008, 2010; Fox et al. 2010a; Halberstadt and Geyer 2014; Carbonaro et al. 2015). Several studies have also reported that hallucinogens do not produce head twitches in knockout mice lacking the 5-HT2A receptor gene (González-Maeso et al. 2007; Halberstadt et al. 2011; Fig. 1). Importantly, however, the ability of hallucinogens to elicit the HTR can be rescued in 5-HT2A knockout mice by selective restoration of 5-HT2A receptors in forebrain regions (González-Maeso et al. 2007).
5-HT2C activation is not required for hallucinogens to induce head twitches. DOI produces head twitches in 5-HT2C knockout mice, although the response is somewhat blunted compared to wild-type mice (Canal et al. 2010). Furthermore, extensive testing has confirmed that the response to DOI is not blocked by the 5-HT2C/2B antagonist SB 200,646A (Kennett et al. 1994; Schreiber et al. 1995; Wettstein et al. 1999) or by the selective 5-HT2C antagonists SB-242,084 and RS102221 (Vickers et al. 2001; Fantegrossi et al. 2010). It is also important to note that 25CN-NBOH, a 5-HT2A-selective agonist (Hansen et al. 2014), induces the HTR in mice (Fantegrossi et al. 2014; Halberstadt et al. 2016a). The fact that a selective 5-HT2A agonist produces head twitches indicates that 5-HT2C activation is not required to elicit shaking behavior. Indeed, the response to 25CN-NBOH is completely ameliorated by pretreatment with M100907 but not by RS102221 (Fantegrossi et al. 2014).
Evidence demonstrates that the 5-HT2C receptor modulates expression of head twitch behavior. There is disagreement in the literature, however, regarding whether the response is inhibited or augmented by 5-HT2C activation. According to Vickers and colleagues (Vickers et al. 2001), the nonselective 5-HT2C agonists Ro 60-0175, MK-212, and mCPP do not induce the HTR when administered alone, but they do provoke the behavior if they are administered in combination with a 5-HT2C antagonist. Several studies have also reported that 5-HT2C agonists attenuate the HTR produced by treatment with DOI (Berendsen and Broekkamp 1990; Schreiber et al. 1995; Siuciak et al. 2007; Fantegrossi et al. 2010; Canal et al. 2013). Taken together, these findings indicate that 5-HT2C activation inhibits the HTR. Conversely, one group has claimed that pretreatment with a 5-HT2C antagonist reduces the magnitude of the HTR produced by DOI by ~50% in C57BL/6J and DBA/2J mice (Canal et al. 2010, 2013). Although the latter findings indicate that 5-HT2C activation augments the HTR, it is not clear why 5-HT2C blockade attenuates the HTR in some studies but has no effect in others (see above). Although it has been proposed that strain differences may underlie these differences (Fantegrossi et al. 2010), unpublished studies in our laboratory have confirmed that SB-242,084 does not significantly reduce the intensity of the HTR induced by 1-methylpsilocin in C57BL/6J mice (Fig. 1). Therefore, 5-HT2C antagonists do not appear to consistently attenuate the HTR in C57BL6J mice. Because 1-methylpsilocin has higher affinity for 5-HT2C versus 5-HT2A sites (Sard et al. 2005), we hypothesized that it was an excellent candidate to test whether crosstalk occurs between 5-HT2 subtypes.
Detailed pharmacological analysis of the DOI dose-response curve has provided additional evidence that the 5-HT2C receptor can block or usurp 5-HT2A-mediated responses. DOI produces biphasic dose-responses in some strains of mice and rats, with a marked response decrement occurring at higher doses. This pattern of response occurs in Sprague-Dawley rats (Pranzatelli 1990; Wettstein et al. 1999), outbred Swiss mouse strains (Fantegrossi et al. 2010), and in DBA/2J mice (Canal et al. 2010). Although SB-242,084 has no effect on the ascending arm of the DOI response function in NIH-Swiss and Swiss Webster mice, it shifts the descending arm of the DOI response to the right in those strains, indicating that the response elicited by high doses of DOI is attenuated by recruitment of 5-HT2C (Fantegrossi et al. 2010).
Like DOI, the HTR induced by 25CN-NBOH shows a similar biphasic dose-response function (Fantegrossi et al. 2014). In contrast to DOI, however, 5-HT2C blockade does not alter the descending arm of the response to 25CN-NBOH. The fact that a selective 5-HT2A agonist produces a biphasic response demonstrates that the inhibition of the HTR at high doses is not always a consequence of competing activity at 5-HT2C. Alternatively, high levels of 5-HT2A activation may provoke competing behaviors that interfere with expression of shaking behaviors. Along these lines, high doses of quipazine, 5-MeO-DMT, and LSD are known to produce stereotypic behaviors that can mask head shakes and wet dog shakes in rats (Bedard and Pycock 1977; Matthews and Smith 1980; Heal et al. 1986).
2.6 Effect of Lisuride
The ergoline derivative lisuride is a structural analog of LSD that shows a similar binding profile at monoamine receptors. Although lisuride acts as a 5-HT2A agonist (Egan et al. 1998; Kurrasch-Orbaugh et al. 2003; Cussac et al. 2008), it does not produce hallucinogenic effects (Herrmann et al. 1977; Verde et al. 1980; Raffaelli et al. 1983; Benes et al. 2006). Nevertheless, lisuride produces false-positive results in some animal models used to study hallucinogens. For example, some drug discrimination studies have shown that rats trained with LSD, DOI, and DOM will generalize to lisuride (White and Appel 1982; Glennon and Hauck 1985; Fiorella et al. 1995; Appel et al. 1999). Likewise, both lisuride and LSD produce limb flicks and abortive grooming in cats (Marini et al. 1981). One of the reasons why the HTR is widely used as a behavioral model for human hallucinogen effects is that it can reliably distinguish hallucinogenic and non-hallucinogenic 5-HT2A agonists (González-Maeso et al. 2007). Even high doses of lisuride (e.g., 3.2 mg/kg) fail to elicit HTR in mice; in comparison, LSD produces head twitches at doses as low as 0.05 mg/kg (Halberstadt and Geyer 2013). The effects of LSD and lisuride on HTR are illustrated in Fig. 2. It is not clear why lisuride is not hallucinogenic but the behavioral differences between LSD and lisuride may be a consequence of 5-HT2A functional selectivity (González-Maeso et al. 2007). Specifically, LSD and other hallucinogens may activate signaling mechanisms that are not recruited by lisuride.
Fig. 2.
Effects of LSD (a) and lisuride (b) on the head twitch response in male C57BL/6J mice. Data are shown as mean ± SEM. **p < 0.01 versus vehicle control (Tukey’s test). The data are redrawn from: Halberstadt and Geyer (2013)
An alternative explanation for the lack of a HTR to lisuride is that the drug does not activate the 5-HT2A receptor with sufficient efficacy to induce the behavior. Lisuride acts as a weak 5-HT2A partial agonist (Rabin et al. 2002; Cussac et al. 2008). Results obtained with lisuride in discrimination studies are consistent with the action of a partial agonist. Specifically, lisuride produces full substitution in rats trained to discriminate DOM (1.0 mg/kg IP), but the response to the training drug is attenuated by 50% when it is administered in combination with 0.01 mg/kg lisuride (Glennon 1991). Lisuride reportedly produces head twitches in the least shrew (Darmani et al. 1994), a non-rodent species that is highly sensitive to 5-HT2A activation. Hence, it cannot be excluded that lisuride fails to induce the HTR in rats and mice because it is a weak partial agonist.
2.7 Effect of Quipazine
The arylpiperazine quipazine is another example of a drug that is commonly classified as a hallucinogen false-positive. Quipazine acts as a nonselective 5-HT receptor agonist. In addition to inducing the HTR in rats and mice (Malick et al. 1977; Schreiber et al. 1995), quipazine also produces cross-generalization with serotonergic hallucinogens in drug discrimination studies (White et al. 1977; Winter 1979; Colpaert et al. 1982). Quipazine is considered to be a false-positive result because it did not produce hallucinogenic effects when tested in humans at a dose of 25 mg p.o. (Winter 1994). However, subjects treated with quipazine experienced nausea and gastrointestinal discomfort—potentially due to 5-HT3 receptor activation—which severely limits the dose that can be administered to humans. According to Shulgin, quipazine does produce a psychedelic response when administered in combination with a 5-HT3 antagonist (Shulgin et al. 2011). In summary, quipazine may not actually be a hallucinogen false-positive.
2.8 Neuroanatomical Locus for the Head Twitch Response Induced by 5-HT2A Receptor Activation
The HTR induced by the 5-HT precursor 5-hydroxytryptophan (5-HTP) is not blocked by xylamidine, an antagonist of peripheral 5-HT2 receptors (Matthews and Smith 1980). Furthermore, DOI induces the HTR when administered by the intracerebroventricular (ICV) route (Hawkins et al. 2002; Nakagawasai et al. 2007). These findings are consistent with a central site of action, but there is some disagreement regarding the neuroanatomical locus for the HTR. Injection of DOI directly into the medial prefrontal cortex (PFC) induces the HTR in rats (Willins and Meltzer 1997; Ciccocioppo et al. 1999). Likewise, the loss of the HTR in knockout mice can be rescued by restoration of 5-HT2A receptors to glutamatergic forebrain neurons (González-Maeso et al. 2007). Other evidence, however, indicates that the PFC is not required for the HTR induced by 5-HT agonists. The ability of quipazine and 5-HTP to induce the HTR in rats is unaffected by ablation of the frontal cortex (Lucki and Minugh-Purvis 1987). The HTR still occurs if the brain is sliced transversely at the level of the anterior commissure, but is abolished by transection at the level of the posterior commissure, indicating that the HTR is mediated by structures in the caudal diencephalon and medial brainstem (Bedard and Pycock 1977). According to studies in rats, WDS can be induced by infusion of DOI into the ventromedial brainstem (Watson and Gorzalka 1992) or by infusion of 5-MeO-DMT into the caudal part of the periaqueductal gray (Webster et al. 1982). In summary, there appear to be multiple populations of 5-HT2A receptors in different brain regions that can elicit the HTR, although the region(s) that are responsible for the HTR induced by systemic administration of 5-HT receptor agonists have not been conclusively identified.
2.9 Signal Transduction Mechanisms Underlying the Head Twitch Response
The 5-HT2A receptor can couple to multiple signaling pathways but the specific effector mechanisms responsible for the HTR have not been identified conclusively. The Gq/11-PLCβ cascade is the canonical signaling pathway recruited by 5-HT2A activation, resulting in phosphoinositide (PI) hydrolysis and mobilization of intracellular Ca2+. Although the Gq-PLC-PI cascade is usually assumed to mediate the behavioral response to hallucinogens, that does not actually appear to be the case. There is no correlation between 5–HT2A-induced PI hydrolysis and LSD-like stimulus effects (Rabin et al. 2002). Despite having profound hallucinogenic effects, LSD stimulates PI hydrolysis with low efficacy (Kurrasch-Orbaugh et al. 2003; Knauer et al. 2009). Conversely, lisuride stimulates PI hydrolysis via 5-HT2A receptors (Burris et al. 1991; Rabin et al. 2002; Kurrasch-Orbaugh et al. 2003; González-Maeso et al. 2007; Cussac et al. 2008), but is not hallucinogenic. Indeed, the ability of DOI to induce the HTR is only blunted 35–40% in mice (Garcia et al. 2007), indicating that other signaling pathways are likely involved in the behavior.
We recently found that for several N-benzyl-substituted tryptamines and phenethylamines there is a significant correlation (r = 0.69) between ED50 values for head twitch and EC50 values for increasing levels of intracellular Ca2+ via 5-HT2A (Nichols et al. 2015). Conversely, HTR potency is not correlated with 5-HT2C activation (r = 0.17). Although Ca2+ mobilization is usually thought to be a direct downstream consequence of Gq-PLC activation, other contributing factors may exist. Lisuride and other non-hallucinogenic 5-HT2A agonists exhibit functional selectivity with regard to Gq-PLC activation and Ca2+ release. Compared with its effects on Ca2+, lisuride activates Gq signaling with >1000-fold higher potency (Cussac et al. 2008). According to another study, lisuride is 300-fold selective for PI hydrolysis versus Ca2+ mobilization (Strachan et al. 2010). By contrast, LSD stimulates Gq signaling and Ca2+ mobilization with only an 18-fold difference in potency, and DOI activates both pathways non-selectively (Strachan et al. 2010). Further studies are necessary to identify whether non-Gq-dependent pathways contribute to 5-HT2A receptor-induced Ca2+ mobilization.
2.10 Role of Glutamatergic Signaling in the Head Twitch Response
The glutamatergic system may represent a common final pathway for hallucinogenesis (Vollenweider and Geyer 2001). Serotonergic hallucinogens increase recurrent glutamatergic network activity in the PFC and other cortical regions (Beique et al. 2007), resulting in increased glutamate release (Scruggs et al. 2003; Muschamp et al. 2004) and subsequent activation of AMPA receptors (Aghajanian and Marek 1997; Zhang and Marek 2008). The enhancement of glutamatergic activity may play an important role in the HTR induced by hallucinogens. Most importantly, as we have reviewed elsewhere (Halberstadt 2015), manipulations that suppress recurrent glutamatergic network activity also block the HTR. The HTR is attenuated by activation of mGlu2/3 receptors (Gewirtz and Marek 2000; Klodzinska et al. 2002), which function as terminal autoreceptors and inhibit glutamate release. Additionally, expression of the HTR is dependent on AMPA receptor activation (Gorzalka et al. 2005; Zhang and Marek 2008; Egashira et al. 2011). Based on these findings, increases of glutamatergic signaling and subsequent AMPA receptor activation appear to play a key role in the HTR induced by 5-HT2A receptor activation.
There is evidence that mGlu2 and 5-HT2A receptors can form heterodimers (González-Maeso et al. 2008), potentially explaining the crosstalk between the receptors. Alternatively, the crosstalk may be purely functional and occur at the circuit level (Delille et al. 2012, 2013). The HTR induced by LSD and DOI is absent in mice (Moreno et al. 2011), which indicates that mGlu2 is required for expression of the behavior. Nevertheless, mGlu2 receptors are known to play an important role in the regulation of glutamatergic transmission (Schoepp 2001) and 5-HT2A signaling (Molinaro et al. 2009), so constitutive adaptations that restrain 5-HT2A responses may occur in mice. Although the level of 5-HT2A expression is not altered in mGlu2 knockout mice (Moreno et al. 2011), 5-HT2A receptor desensitization or alterations of glutamatergic signaling may have occurred, potentially explaining why the HTR is absent in the knockout mice.
2.11 Effect of Serotonin Releasers and Precursors
Amphetamine and methamphetamine, which act primarily by increasing carrier-mediated release of dopamine and norepinephrine, do not provoke head twitches (Corne and Pickering 1967; Silva and Calil 1975; Yamamoto and Ueki 1975; Jacobs et al. 1976; Bedard and Pycock 1977; Halberstadt and Geyer 2013). By contrast, the 5-HT releasing drugs fenfluramine and p-chloroamphetamine (PCA) do produce a robust HTR (Singleton and Marsden 1981; Darmani 1998a). Fenfluramine and PCA are thought to act indirectly, by increasing carrier-mediated release of 5-HT, because the response can be blocked by inhibition of the 5-HT transporter (Balsara et al. 1986; Darmani 1998a) or by depletion of 5-HT (Singleton and Marsden 1981; Balsara et al. 1986).
In addition to fenfluramine and PCA, other manipulations that increase the level or functional activity of 5-HT in the central nervous system (CNS) produce head twitches. Examples include the 5-HT precursors L-tryptophan and L-5-hydroxytryptophan (5-HTP), 5-HT1A antagonists (Darmani 1998b; Fox et al. 2010b), and the cannabinoid CB1 receptor antagonist SR 141716A (Darmani and Pandya 2000; Darmani et al. 2003). Head twitches also occur when 5-HT is infused directly into the brain (Drust and Connor 1983). There is evidence that the HTR induced by high central levels of 5-HT may be mediated—at least in part—by hallucinogenic N-methylated metabolites. 5-HT is a substrate for N-methyltranferases, potentially resulting in the formation of N-methylserotonin and N,N-dimethylserotonin (Axelrod 1962; Kärkkäinen et al. 2005). One example is indolethylamine N-methyltransferase (INMT), which is expressed in the brain and spinal cord (Thompson et al. 1999; Mavlyutov et al. 2012) and can be inhibited by N, N′bis-(3-methyl-2-thiazolidinylidene)succinamide (MTZ). According to Schmid and Bohn (2010) the response to 5-HTP is significantly attenuated in mice pre-treated with MTZ, whereas the response to N-methylserotonin and 5-MeO-DMT is not affected. Although MTZ does not completely block the ability of 5-HTP to produce head twitches—indicating that the response reflects the combined effects of N-methyltryptamines and 5-HT—the shaking induced by 5-HT appears to be mediated by a different signaling cascade than the shaking produced by hallucinogens. Specifically, the response to 5-HT is dependent on activation of a β-arrestin2/Src/Akt signaling cascade whereas the response to DOI, 5-MeO-DMT, and N-methylserotonin is independent of β-arrestin2 (Schmid et al. 2008; Schmid and Bohn 2010).
Because indirect 5-HT agonists such as fenfluramine, PCA, and 5-HTP are not hallucinogenic (Van Praag et al. 1971; Brauer et al. 1996; Turner et al. 2006), their effects on HTR can potentially be classified as false-positive responses. There is some disagreement regarding whether the HTR is a valid model of hallucinogenesis or merely serves as a convenient readout of 5-HT2A activation in rodents (Canal and Morgan 2012). Given the possibility that endogenous N-methyltryptamines contribute to the response to 5-HTP and other indirect agonists, and because 5-HT2A functional selectivity may also play a role, further studies are necessary to determine whether they are really false-positive responses.
3 Sensorimotor Gating
The startle reflex is a transient defensive motor response elicited by sudden intense stimuli such as loud and unexpected sounds (Dodge and Louttit 1926; Fleshler 1965). It is well established that the amplitude of the startle response is attenuated if a weak prestimulus is presented immediately prior to the startle pulse (Hoffman and Searle 1965, 1968; Hoffman and Ison 1980). The ability of prepulses to inhibit the startle response—a phenomenon known as PPI—is often used as a laboratory measure of sensorimotor gating. PPI can be assessed in humans and in laboratory animals using similar procedures. PPI deficits have been observed in patients suffering from a variety of illnesses associated with gating or filtering impairments (see Braff et al. 2001 for review), including schizophrenia (Braff et al. 1978; Grillon et al. 1992; Mackeprang et al. 2002), Tourette’s syndrome (Swerdlow et al. 1994), and obsessive compulsive disorder (Swerdlow et al. 1993). Hallucinogens have been postulated to work by disrupting sensory filtering mechanisms, resulting in sensory overload and cognitive dysfunction (Vollenweider 1994; Vollenweider and Geyer 2001). Studies of hallucinogen effects on PPI have been used to assess their influence on gating mechanisms as well as to model the filtering deficits found in schizophrenia patients.
3.1 Hallucinogen Effects on Prepulse Inhibition in Rats
Classical hallucinogens disrupt PPI in rats, indicating that they impair sensorimotor gating. Examples of hallucinogens that have been shown to reduce PPI include DOI (Sipes and Geyer 1994; Varty and Higgins 1995; Padich et al. 1996), DOB (Johansson et al. 1995), LSD (Ouagazzal et al. 2001; Leng et al. 2003; Halberstadt and Geyer 2010; Palenicek et al. 2010), mescaline (Pálenícek et al. 2008), and 2C-B (Pálenícek et al. 2013). The putative 5-HT2A-selective agonist 25CN-NBOH also disrupts PPI in rats (Fig. 3). The effect of DOI on PPI is blocked by M100907, whereas SB 242,084 and the mixed 5-HT2C/2B antagonist SER-082 are ineffective (Sipes and Geyer 1995b; Padich et al. 1996). Likewise, the effect of LSD appears to be solely attributable to 5-HT2A activation because it can be blocked by M100907 and MDL 11,939 but not by the 5-HT2C antagonist SB 242,084, the 5-HT1A antagonist S-(+)-WAY-100,135, the 5-HT6 antagonists Ro 04-6790 and Ro 65-7199, or the dopamine D2 antagonist haloperidol (Ouagazzal et al. 2001; Leng et al. 2003; Halberstadt and Geyer 2010).
Fig. 3.
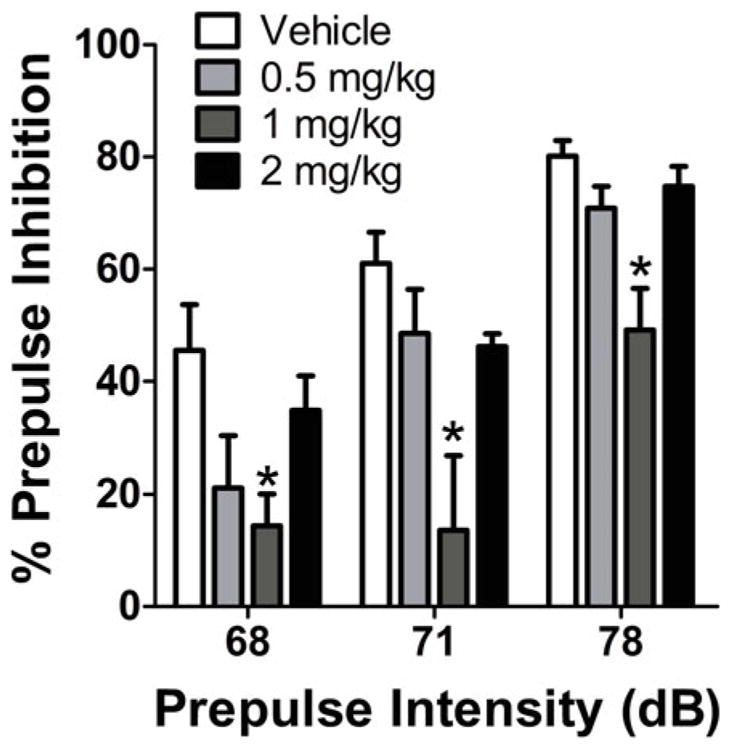
The selective 5-HT2A agonist 25CN-NBOH reduces prepulse inhibition of startle (PPI) in rats. Male Sprague-Dawley rats (n = 8/group, 32 total) were injected SC with vehicle (water containing 5% Tween-80) or 25CN-NBOH hydrochloride 10 min before placement in the startle test chambers. Values represent mean ± SEM for each group. *p < 0.05, significantly different from vehicle control (Tukey’s test)
In contrast to the PPI disruption produced by hallucinogens, 5-HT2C receptor activation tends to increase PPI in rats. For example, the PPI disruption induced by the dopamine D2/3/4 receptor agonist apomorphine is ameliorated by the 5-HT2C agonist CP-809,101 (Siuciak et al. 2007). It has also been shown that the 5-HT2C-selective agonist WAY-163909 antagonizes the PPI disruption produced by DOI and the NMDA receptor antagonist MK-801 (Marquis et al. 2007). The fact that 5-HT2C agonists are capable of reversing PPI deficits produced by a variety of pharmacological manipulations is consistent with evidence that they have an antipsychotic-like profile of effects. Furthermore, given the effects of CP-809,101 and WAY-163909 on PPI, it is unlikely that the 5-HT2C receptor is responsible for mediating the PPI disruption produced by DOI and other hallucinogens.
The ventral pallidum (VP) is a critical neural substrate for the PPI-disruptive effects of DOI. The VP is part of a descending corticostriatal circuit that regulates PPI (Swerdlow et al. 2001). DOI produces a significant disruption of PPI when infused directly into the VP, but has no effect when infused into the nucleus accumbens (Sipes and Geyer 1997). Likewise, infusion of M100907 into the VP blocked the PPI disruption produced by subcutaneous administration of DOI. Although these findings indicate that 5-HT2A receptors in the VP mediate the PPI disruption induced by DOI, the results of the M100907 infusion experiment are confounded by the fact that the drug was infused in a vehicle containing dimethyl sulfoxide (DMSO). Because DMSO is a highly lipophilic solvent that exhibits poor tissue retention, some of the M100907 infused into the VP may have diffused into surrounding regions. Indeed, the effect of DOI was partially blocked by infusing M100907 into the caudate nucleus, indicating the VP may not be the only site where the 5-HT2A receptors mediating the PPI-disruptive effects of DOI are located.
There is some disagreement in the literature regarding whether dopamine receptor antagonists block the PPI disruption produced by DOI. Although it has been reported that haloperidol can ameliorate the effect of DOI on PPI (Sipes and Geyer 1994; Brea et al. 2006), most studies have found that haloperidol and the selective dopamine D2/3 antagonist raclopride are ineffective at blocking the response to DOI (Varty and Higgins 1995; Padich et al. 1996; Marquis et al. 2007). It is unlikely that DOI acts directly through dopamine receptors to disrupt PPI because it has low affinity for D2 receptors (Ki > 10,000 nM; Mos et al. 1992). However, it is now recognized that 5-HT2A and D2 receptors can form heterodimers (Borroto-Escuela et al. 2010; Lukasiewicz et al. 2010), so D2 ligands could potentially modulate the response to DOI by binding to D2-5-HT2A oligomers.
5-MeO-DMT has also been found to disrupt PPI in rats (Rigdon and Weatherspoon 1992; Krebs-Thomson and Geyer 1996; Halberstadt 2016). However, in contrast to other hallucinogens, the effect of 5-MeO-DMT on PPI is blocked by the 5-HT1A antagonist WAY-100635 but not by M100907 (Krebs-Thomson and Geyer 1996). These findings demonstrate that the PPI disruption produced by 5-MeO-DMT is mediated by 5-HT1A but not by 5-HT2A receptors. 5-MeO-DMT binds to 5-HT1A sites with high nanomolar affinity (Spencer et al. 1987; Glennon et al. 1988; McKenna et al. 1990) and acts as a potent agonist at 5-HT1A receptors negatively coupled to adenylyl cyclase (Blair et al. 2000). Like 5-MeO-DMT, other potent 5-HT1A agonists, including 8-OH-DPAT, buspirone, and gepirone, disrupt PPI in rats (Rigdon and Weatherspoon 1992; Sipes and Geyer 1995a; Johansson et al. 1995). Many of the behavioral effects of 5-MeO-DMT in rodents are mediated by 5-HT1A, with the 5-HT2A receptor playing only a minor role (Lucki et al. 1984; Spencer et al. 1987; Berendsen et al. 1989; Sanchez et al. 1996; Winter et al. 2000; van den Buuse et al. 2011).
We recently demonstrated that it is possible to enhance the interaction of 5-MeO-DMT with 5-HT2A receptors by increasing the accumulation of the drug in the central nervous system (Halberstadt 2016). The primary route of metabolism for 5-MeO-DMT is oxidative deamination by monoamine oxidase-A (MAOA) (Agurell et al. 1969; Squires 1975; Suzuki et al. 1981; Shen et al. 2010). Studies in rats have shown that inhibition of MAOA increases the concentration of 5-MeO-DMT in the brain by more than an order of magnitude and reduces the clearance rate (Sitaram et al. 1987; Halberstadt 2016). We found that pretreatment with a behaviorally inactive dose of the MAOA inhibitor clorgyline or the MAOA/B inhibitor pargyline markedly prolongs the effect of 5-MeO-DMT on PPI in rats (Halberstadt 2016). Furthermore, the combined effect of 5-MeO-DMT and pargyline on PPI can be antagonized by pretreatment with either WAY-100635 or MDL 11,939, indicating the effect is mediated by both 5-HT1A and 5-HT2A receptors. These results confirm that 5-HT2A receptors can play a significant role in the behavioral response to 5-MeO-DMT, at least under certain conditions.
Studies have also compared the effects of LSD and lisuride on PPI in rats (Halberstadt and Geyer 2010). Lisuride produces a marked disruption of PPI and is even more potent than LSD. However, in contrast to LSD, the effect of lisuride was not blocked by MDL 11,939 but was antagonized by the dopamine D2/3 antagonist raclopride. Therefore, the effects of LSD and lisuride on PPI are mediated by different receptor mechanisms. The fact that lisuride disrupts PPI by activating D2/3 receptors is consistent with extensive evidence that lisuride is a potent dopaminergic drug in vitro (Piercey et al. 1996; Newman-Tancredi et al. 2002) and in vivo (Holohean et al. 1982; Kimura et al. 1991; Baladi et al. 2010).
3.2 Hallucinogen Effects on Prepulse Inhibition in Mice
In contrast to rats, 5-HT2A receptor activation does not consistently alter PPI in mice. Dulawa and Geyer reported that administration of DOM at doses ranging from 0.5 to 4 mg/kg had no effect on PPI in C57BL/6, 129 Sv, or ICR mice (Dulawa and Geyer 2000). Another study found that 1 mg/kg DOI had no effect on PPI in wild-type (WT) mice on the C57BL/6J × 129S6/SvEvTac hybrid background, although it did disrupt PPI in pituitary adenylate cyclase-activating polypeptide (PACAP) +/− mice on the same background (Hazama et al. 2014). It was reported, however, that 1 and 5 mg/kg DOI significantly disrupted PPI in WT mice on a mixed 129/Sv and C57BL/6 background but had no effect on PPI in caveolin-1−/− mice (Allen et al. 2011).
Another difference between rats and mice is that tryptamine hallucinogens increase PPI in mice. The ability of tryptamine hallucinogens to increase PPI in mice was first observed with DMT (Freedland and Mansbach 1999) and was later found to occur with 5-MeO-DMT and psilocin (Halberstadt and Geyer 2011). The 5-HT1A receptor appears to play a role in the PPI increase produced by tryptamine hallucinogens because the effect of 5-MeO-DMT is partially attenuated by WAY-100635. Likewise, although selective 5-HT1A agonists disrupt PPI in rats, 8-OH-DPAT has been shown to increase PPI in mice (Dulawa et al. 2000; Gogos et al. 2008).
3.3 Hallucinogen Effects on Prepulse Inhibition in Humans
Hallucinogens alter PPI in humans but their effects depend on the interstimulus interval (ISI). Psilocybin reduces PPI at a short ISI (30 ms), but increases PPI at longer ISIs (100–2000 ms)(Gouzoulis-Mayfrank et al. 1998; Vollenweider et al. 2007). The PPI reduction produced by psilocybin at 30 ms ISI is completely blocked by pretreatment with 40 mg p.o. ketanserin (Quednow et al. 2012), indicating that the effect is mediated by 5-HT2A receptors. Administration of LSD at a moderately high dose (200 μg p.o.) reduces PPI at 30–120 ms ISIs (Schmid et al. 2015). In contrast to psilocybin and LSD, which alter PPI in humans, the hallucinogen DMT appears to be an exception. DMT had no effect on PPI when administered in an ayahuasca preparation (Riba et al. 2002) or by continuous i.v. infusion (Heekeren et al. 2007). Interestingly, the 5-HT2A A-1438G and T102C polymorphisms influence PPI levels in schizophrenia patients (Quednow et al. 2008) and in healthy control subjects (Quednow et al. 2009; Bräuer et al. 2009). These findings provide additional confirmation that the 5-HT2A receptor plays a role in the regulation of sensorimotor gating.
4 Exploratory and Investigatory Behavior
Animals are motivated to explore their surroundings in order to reduce perceptual uncertainty about the environment (Berlyne 1950, 1955, 1966; Fowler 1965). Measures of exploratory behavior, such as the amount of locomotor activity exhibited by rats in an open field, are often used to characterize the effects of psychoactive drugs. Studies investigating the effects of hallucinogens on the behavior of rats in an open field have produced inconsistent findings and fail to distinguish hallucinogens from other drug classes (Brimblecombe 1963; Dandiya et al. 1969; Gupta et al. 1971; Silva and Calil 1975). The inconclusive results are not surprising given the complexity of hallucinogen effects; univariate measures of spontaneous activity can detect arousing or sedating drug effects but reveal nothing about the qualitative nature of behavior. Locomotion alone is not necessarily the most reliable measure of exploration because it does not distinguish between nonspecific motor activity and specific exploratory responses to environmental stimuli (Hughes 1972).
The Behavioral Pattern Monitor (BPM) was developed to address some of the weaknesses associated with activity measurements in rodents. The BPM combines the features of activity and holeboard chambers and assesses both the quantity and several aspects of the quality of activity by monitoring response frequencies and spatial and temporal sequences of activity (Geyer et al. 1986). Investigatory holepokes and rearings are used as specific measures of inspective and diversive exploration, respectively (Berlyne 1960, 1966). By comparing measures of locomotor activity and investigatory responding, it is possible to discriminate changes in the responsivity of animals to environmental stimuli from more general stimulant or depressant effects. Statistical assessment of the geometrical and dynamical structure of motor behavior in the BPM has proven very useful in characterizing drug effects in rodents (Geyer et al. 1986; Gold et al. 1988; Paulus and Geyer 1991; Geyer and Paulus 1992). This approach has proven to be especially useful for studies of different classes of psychostimulants, which at certain doses produce comparable increases in locomotion but marked qualitative differences in behavior.
4.1 Effects in Rats
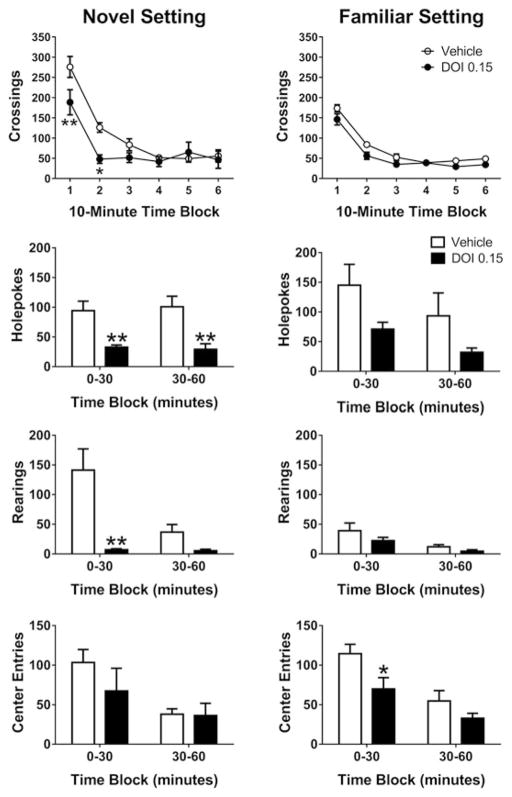
Phenylalkylamine hallucinogens (mescaline, DOI, DOM, and DOET) and indoleamine hallucinogens (LSD, DMT, 5-MeO-DMT, and psilocin) produce a characteristic pattern of effects when rats are tested in novel BPM chambers: (1) locomotor activity is reduced; (2) investigatory behaviors (rearings and holepokes) are suppressed; and (3) the animals spend less time in the center of the chamber (Adams and Geyer 1985a, b; Wing et al. 1990; Krebs-Thomson et al. 1998a, 2006). The effects of hallucinogens in the BPM are most apparent during the period immediately after the animals are placed in the chambers. When hallucinogens are tested in a familiar environment, the effects on locomotor activity and investigatory behavior are markedly attenuated (see Fig. 4), and therefore likely reflect potentiation of the neophobia exhibited by rats in novel settings (Adams and Geyer 1985a, b; Wing et al. 1990). In other words, hallucinogens enhance the threatening nature of the unfamiliar test environment, but hallucinogen-treated animals are more likely to explore the BPM chambers once the stimuli associated with the apparatus become less threatening due to habituation. Other groups have reported that hallucinogens reduce locomotor activity in a novel environment (Tilson et al. 1975; Hillegaart et al. 1996; Reyes-Parada et al. 1996; Scorza et al. 1996; Palenicek et al. 2010) but have no effect or increase activity in a familiar environment (Tilson et al. 1975; Ouagazzal et al. 2001; Filip et al. 2004; Zaniewska et al. 2009; Baisley et al. 2012). Hallucinogens increase center avoidance in novel and familiar settings, suggesting they probably also act by enhancing the fear of open spaces (agoraphobia), which is normally displayed by rodents even after habituation. In humans, it is well-known that the effects of LSD and other hallucinogens are dependent on environmental setting, with reactivity to the environment, and especially to unpleasant or threatening stimuli, being markedly enhanced (Salvatore and Hyde 1956; Cohen 1960). Hence, the increased avoidance of novel and open areas observed in rats after administration of hallucinogens may have construct validity as being analogous to the enhanced reactivity to environmental stimuli observed in humans.
Fig. 4.
Effect of habituation on the response to DOI in the rat Behavioral Pattern Monitor (BPM). Male Sprague-Dawley rats were tested in novel BPM chambers (Left panels) or familiar BPM chambers (Right panels). In the novel environment paradigm, rats (n = 5/group, 10 total) were injected SC with vehicle (saline) or DOI (0.15 mg/kg), 10 min prior to being placed in the BPM for a 60-min test session. In the familiar environment paradigm, rats (n = 8/group, 16 total) were tested in the BPM on three occasions at 48-h intervals, but only injected with the drug prior to their third exposure to the BPM chamber; rats were injected SC with vehicle (saline) or DOI (0.15 mg/kg), 10 min prior to being placed in the BPM for a 60-min test session. Data from the test sessions are presented in 10-min time blocks (crossings, a measure of locomotor activity) or 30-min time blocks (holepokes, rearings, center entries). Data are shown as mean ± SEM. *p < 0.05, **p < 0.01 versus vehicle control (Bonferroni’s multiple comparisons test)
In addition to the characteristic effects described above, certain hallucinogens produce behavioral profiles with greater complexity. Like other hallucinogens, LSD reduces investigatory behavior and center duration (Adams and Geyer 1985b), but it has biphasic effects on locomotor activity, initially suppressing activity but then increasing it as time progresses (Mittman and Geyer 1991). 5-MeO-DMT alone produces a brief reduction in locomotion (Wing et al. 1990; Krebs-Thomson et al. 2006), but administration of 5-MeO-DMT in combination with a behaviorally inactive dose of an MAO inhibitor (MAOI) such as harmaline, clorgyline, or pargyline produces biphasic effects on locomotor activity that are identical to those of LSD (Halberstadt et al. 2008, 2012). As with other hallucinogen effects, the delayed hyperactivity is not observed in rats habituated to the BPM chambers, indicating that the animals may be exhibiting an increased drive to explore the novel environment. Further work demonstrated that α,α,β,β-tetradeutero-5-MeO-DMT, a 5-MeO-DMT isotopologue that is resistant to metabolism by MAO, does not require an MAOI to produce a biphasic locomotor profile (Halberstadt et al. 2012). The latter finding indicates that MAOIs alter the behavioral profile of 5-MeO-DMT by slowing its biotransformation, which allows high levels of the drug to accumulate in the brain. Indeed, we found that the concentration of 5-MeO-DMT in the CNS is increased ~20-fold in animals pretreated with an MAOI (Halberstadt 2016). Reports have also appeared in the literature demonstrating that very high doses of mescaline (100 mg/kg), 2C-B (25 mg/kg), and DOM (≥ 5 mg/kg) can produce biphasic locomotor effects in rats (Yamamoto and Ueki 1975; Pálenícek et al. 2008, 2013).
The effects of hallucinogens on exploratory and investigatory behavior in the BPM are unique and are not reproduced by other pharmacological classes and thus have considerable predictive validity. Hallucinogen effects are distinct from those of 5-HT releasers (e.g., MDA and MDMA), phencyclidine (PCP) and other NMDA receptor antagonists, psychostimulants such as cocaine and (+)-amphetamine, DA receptor agonists, cholinergic agonists and antagonists, and antidepressants (Geyer et al. 1986, 1987; Gold et al. 1988; Callaway et al. 1990; Lehmann-Masten and Geyer 1991; Rempel et al. 1993; Halberstadt et al. 2016b). Selective 5-HT1A agonists such as 8-OH-DPAT, ipsapirone, and gepirone produce hallucinogen-like on effects on exploratory and investigatory behavior, but these effects occur in both novel and familiar settings and therefore are thought to reflect a generalized sedative influence rather than a change in responsiveness to environmental stimuli (Mittman and Geyer 1989). It is also possible to distinguish the effects of lisuride from those of LSD and other hallucinogens. The effects of lisuride are similar to those of apomorphine and other dopamine receptor agonists, with low doses (5–15 μg/kg SC) producing sedative effects and higher doses (60–80 μg/kg SC) producing highly stereotyped preservative patterns of hyperactivity (Adams and Geyer 1985c; Paulus and Geyer 1991).
Most of the effects of hallucinogens in the BPM are mediated by activation of the 5-HT2A receptor. The effects of DOI, DOM, and mescaline on locomotor activity, investigatory behavior, and center activity are blocked by pretreatment with the 5-HT2 antagonists ritanserin and ketanserin (Wing et al. 1990; Mittman and Geyer 1991; Hillegaart et al. 1996; Krebs-Thomson et al. 1998b). Additional studies demonstrated that the effects of DOI are blocked by M100907 but not by SER-082 (Krebs-Thomson et al. 1998a), demonstrating that they are mediated by 5-HT2A but not by 5-HT2C receptors. Compared with phenylalkylamine hallucinogens, the mechanism for the effects of indoleamines is more complex. For example, the mixed 5-HT1/β-adrenergic antagonist propranolol (Mittman and Geyer 1991) and the selective 5-HT1A antagonist WAY-100635 (Krebs-Thomson and Geyer 1996) block the initial suppression of locomotor activity induced by LSD, whereas ritanserin (Mittman and Geyer 1991) and M100907 (Ouagazzal et al. 2001) block the hyperactivity induced by LSD. Additionally, chronic pretreatment with either 8-OH-DPAT or DOI produces cross-tolerance to the effects of LSD on exploratory behavior (Krebs and Geyer 1994). For 5-MeO-DMT, the effects of low doses are blocked by WAY-100635 but not by M100907 (Krebs-Thomson et al. 2006). However, the delayed hyperactivity induced by administration of 5-MeO-DMT in combination with the MAOI clorgyline is blocked by the highly selective 5-HT2A antagonist MDL 11,939 but not by WAY-100635 (Halberstadt et al. 2008). Thus, it appears that the effects of LSD and 5-MeO-DMT in the BPM are mediated by 5-HT1A and 5-HT2A receptors.
4.2 Effects in Mice
We have also tested hallucinogens in a mouse version of the BPM system (Tanaka et al. 2012). Administration of phenylalkylamine hallucinogens to C57BL/6J mice by the IP route produces a reduction in investigatory behavior, as well as effects on locomotor activity that follow a bell-shaped dose-response function, increasing activity at low to moderate doses and reducing activity at high doses. This specific profile of effects occurs with DOI, DOM, DOET, and mescaline, as well as with TCB-2 (Halberstadt et al. 2009, 2013). Consistent with our findings, other groups have reported that low doses of phenylalkylamine hallucinogens increase locomotor activity and high doses reduce activity in mice (Huang and Ho 1973; Yamamoto and Ueki 1975; Darmani et al. 1996; Brookshire and Jones 2009; Carlsson et al. 2011). The increase in locomotor activity produced by a low dose of DOI (1 mg/kg), mescaline (25 mg/kg), or TCB-2 (3 mg/kg) is blocked by pretreatment with M100907, and does not occur in 5-HT2A−/− knockout mice (Halberstadt et al. 2009, 2013; Fig. 5). By contrast, the reduction in locomotor activity produced by a high dose of DOI (10 mg/kg) is potentiated in 5-HT2A−/− knockout mice and attenuated by SER-082. Taken together, these findings indicate that low doses of phenylalkylamine hallucinogens increase locomotor activity by activating the 5-HT2A receptor, and high doses reduce activity by activating the 5-HT2C receptor. This conclusion is supported by the fact that selective 5-HT2C agonists such as WAY-161,503 and CP-801,909 reduce locomotor activity in mice (Halberstadt et al. 2009; Fletcher et al. 2009). It is also interesting to note that the hypoactivity induced by atypical antipsychotics is reportedly mediated by blockade of the 5-HT2A receptor, and that 5-HT2A−/− but not 5-HT2C−/− knockout mice are resistant to the suppression of locomotor activity by clozapine and olanzapine (McOmish et al. 2012). By contrast, 5-HT2C blockade with the selective antagonist SB-242,084 increases locomotor activity in mice. Hence, it appears that 5-HT2A and 5-HT2C receptors have a countervailing influence on locomotor activity.
Fig. 5.
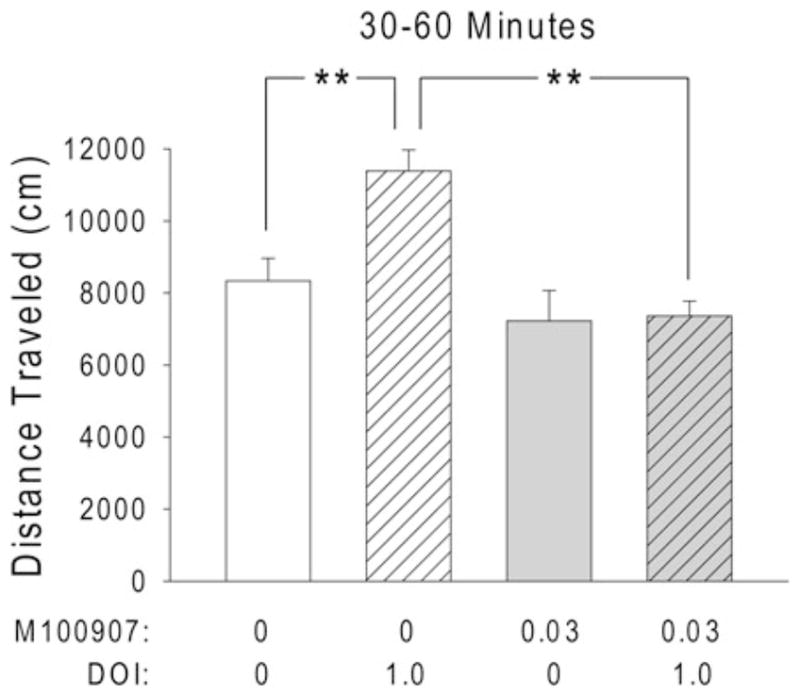
Pretreatment with M100907 blocks the hyperactivity induced by DOI in mice. Male C57BL/6J mice (n = 10/group, 40 total) were treated SC with vehicle (water containing 5% Tween-80) or M100907 (0.03 mg/kg) 15 min prior to IP vehicle (saline) or DOI hydrochloride (1 mg/kg). Animals were placed in the mouse Behavioral Pattern Monitor (BPM) chambers 15 min after treatment with DOI and tested for 60 min. Administration of 1 mg/kg DOI increased locomotor activity (measured as distance traveled, in cm) during the second half of the test session; the effect of DOI was completely blocked by 0.03 mg/kg M100907. Data are shown as mean ± SEM for the second half of the test session (30–60 min). **p < 0.01 (Tukey’s test)
Compared with phenylalkylamines, tryptamine hallucinogens produce a disparate profile of effects in the mouse BPM. Administration of psilocin or 5-MeO-DMT produces a profound suppression of locomotor activity, investigatory holepokes and rearings, and center duration in C57BL/6J mice (Halberstadt et al. 2011). Most of these effects are blocked by pretreatment with the 5-HT1A antagonist WAY-100635, whereas the 5-HT2C antagonist SB242084 is ineffective. Likewise, the reduction of locomotor activity induced by 5-MeO-DMT is largely absent in 5-HT1A−/− knockout mice (van den Buuse et al. 2011). The 5-HT1A agonist 8-OH-DPAT was found to produce a similar profile of effects that were not observed in mice lacking the 5-HT1A receptor. It appears that tryptamines alter exploratory and investigatory activity in mice by activating 5-HT1A receptors, whereas phenylalkylamines act through a mechanism involving both 5-HT2A and 5-HT2C receptors. The fact that phenylalkylamine and tryptamine hallucinogens produce different effects in the mouse BPM indicates this paradigm may have utility in the detection of subtle behavioral differences between these two classes of hallucinogens.
5 Conclusions
The HTR, PPI, and BPM paradigms are three of the most popular behavioral assays used to study the pharmacology of classical hallucinogens in laboratory animals. As is the case in humans, the behavioral responses induced by hallucinogens in animals are primarily mediated by 5-HT2A receptor activation, although 5-HT1A and 5-HT2C receptors also contribute to or modulate the responses induced by certain compounds. The HTR has been widely adopted due to its insensitivity to false-positive responses and because it can be used to probe interactions between the 5-HT2A receptor and other receptors and signaling pathways. In contrast to HTR, which has limited construct validity with regard to the effects of hallucinogens in humans, PPI is a cross-species readout that can be assessed in humans and animals using similar methodologies and represents clearly analogous and potentially homologous behaviors across species. Finally, the BPM paradigm has been used to assess hallucinogen effects in rodents and has clear conceptual relevance to human hallucinogen phenomenology and hence substantial construct validity. In rats, it appears that the BPM model also has predictive validity for identifying the class of hallucinogenic drugs. Importantly, however, a human version of the BPM has been developed (Young et al. 2007; Perry et al. 2009), potentially enabling human hallucinogen studies to be conducted.
This chapter has focused on the acute effects of hallucinogens, as assessed using measures of unconditioned behavior. Operant tasks such as drug discrimination have demonstrated considerable utility in studies of hallucinogen structure-activity relationships and have helped to elucidate some of the neurochemical effects of hallucinogens. However, the drug discrimination paradigm requires extended training and repeated administration of the hallucinogens. By contrast, unconditioned behavioral responses are more suited to the study of the acute effects of hallucinogens, which exhibit rapid tolerance and are more likely to be related to the subjective effects reported by humans. Though behavioral paradigms that necessitate repeated drug treatment have taught us much about the actions of hallucinogens, it is important that our field also employ behavioral tests in which acute effects that do exhibit tolerance are examined. Further, because the category of hallucinogens is defined by subjective reports of distortions of perception and affect, it is useful for animal studies of hallucinogens to incorporate specific measures of the responsivity to exteroceptive stimuli and their sensitivity to novelty (Mittman and Geyer 1989, 1991; Wing et al. 1990; Geyer 1998). Use of such measures increases the likelihood of obtaining results in laboratory animals that have construct validity and are translatable to man.
Acknowledgments
This work was supported by grants from NIDA (R01 DA002925 and R01 DA041336), NIMH (K01 MH100644), and the Veteran’s Affairs VISN 22 MIRECC.
Contributor Information
Adam L. Halberstadt, Department of Psychiatry, University of California San Diego, La Jolla, CA 92093-0804, USA. Research Service, VA San Diego Healthcare System, San Diego, CA, USA
Mark A. Geyer, Department of Psychiatry, University of California San Diego, La Jolla, CA 92093-0804, USA. Research Service, VA San Diego Healthcare System, San Diego, CA, USA
References
- Abramson HA, Sklarofsky B, Baron MO, Fremont-Smith N. Lysergic acid diethylamide (LSD-25) antagonists. II. Development of tolerance in man to LSD-25 by prior administration of MLD-41 (1-methyl-d-lysergic acid diethylamide) Arch Neurol Psychiatry. 1958;79:201–207. [PubMed] [Google Scholar]
- Acuña-Castillo C, Villalobos C, Moya PR, Sáez P, Cassels BK, Huidobro-Toro JP. Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 2002;136:510–519. doi: 10.1038/sj.bjp.0704747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry. 1985a;9:121–132. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. A proposed animal model for hallucinogens based on LSD’s effects on patterns of exploration in rats. Behav Neurosci. 1985b;99:881–900. doi: 10.1037//0735-7044.99.5.881. [DOI] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. Patterns of exploration in rats distinguish lisuride from lysergic acid diethylamide. Pharmacol Biochem Behav. 1985c;23:461–468. doi: 10.1016/0091-3057(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Agurell S, Holmstedt B, Lindgren JE. Metabolism of 5-methoxy-N,-N dimethyltryptamine-14C in the rat. Biochem Pharmacol. 1969;18:2771–2781. doi: 10.1016/0006-2952(69)90185-3. [DOI] [PubMed] [Google Scholar]
- Allen JA, Yadav PN, Setola V, Farrell M, Roth BL. Schizophrenia risk gene CAV1 is both pro-psychotic and required for atypical antipsychotic drug actions in vivo. Transl Psychiatry. 2011;1:e33. doi: 10.1038/tp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel JB, West WB, Rolandi WG, Alici T, Pechersky K. Increasing the selectivity of drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:353–358. doi: 10.1016/s0091-3057(99)00089-1. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Facilitation of 8-OHDPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur J Pharmacol. 1989;161:45–51. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- Axelrod J. The enzymatic N-methylation of serotonin and other amines. J Pharmacol Exp Ther. 1962;138:28–33. [PubMed] [Google Scholar]
- Baisley SK, Fallace KL, Rajbhandari AK, Bakshi VP. Mutual independence of 5-HT2 and α1 noradrenergic receptors in mediating deficits in sensorimotor gating. Psychopharmacology. 2012;220:465–479. doi: 10.1007/s00213-011-2490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Dopamine D3 receptors mediate the discriminative stimulus effects of quinpirole in free-feeding rats. J Pharmacol Exp Ther. 2010;332:308–315. doi: 10.1124/jpet.109.158394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsara JJ, Bapat TR, Nandal NV, Gada VP, Chandorkar AG. Head-twitch response induced by ergometrine in mice: behavioural evidence for direct stimulation of central 5-hydroxytryptamine receptors by ergometrine. Psychopharmacology. 1986;88:275–278. doi: 10.1007/BF00180824. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard P, Pycock CJ. “Wet-dog” shake behaviour in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology. 1977;16:663–670. doi: 10.1016/0028-3908(77)90117-4. [DOI] [PubMed] [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes H, Deissler A, Rodenbeck A, Engfer A, Kohnen R. Lisuride treatment of Restless Legs Syndrome: first studies with monotherapy in de novo patients and in combination with levodopa in advanced disease. J Neural Transm. 2006;113:87–92. doi: 10.1007/s00702-005-0386-1. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Broekkamp CL. Behavioural evidence for functional interactions between 5-HT-receptor subtypes in rats and mice. Br J Pharmacol. 1990;101:667–673. doi: 10.1111/j.1476-5381.1990.tb14138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen HH, Jenck F, Broekkamp CL. Selective activation of 5HT1A receptors induces lower lip retraction in the rat. Pharmacol Biochem Behav. 1989;33:821–827. doi: 10.1016/0091-3057(89)90477-2. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Novelty and curiosity as determinants of exploratory behavior. Br J Psychol. 1950;41:68–80. [Google Scholar]
- Berlyne DE. The arousal and satiation of perceptual curiosity in the rat. J Comp Physiol Psychol. 1955;48:238–246. doi: 10.1037/h0042968. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Conflict, arousal and curiosity. Macgraw-Hill; New-York: 1960. [Google Scholar]
- Berlyne DE. Curiosity and exploration. Science. 1966;153:25–33. doi: 10.1126/science.153.3731.25. [DOI] [PubMed] [Google Scholar]
- Bigwood J, Ott J, Thompson C, Neely P. Entheogenic effects of ergonovine. J Psychedelic Drugs. 1979;11:147–149. doi: 10.1080/02791072.1979.10472099. [DOI] [PubMed] [Google Scholar]
- Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, Nichols DE. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem. 2000;43:4701–4710. doi: 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Marcellino D, Ciruela F, Agnati LF, Fuxe K. Dopamine D2 and 5-hydroxytryptamine 5-HT2A receptors assemble into functionally interacting heteromers. Biochem Biophys Res Commun. 2010;401:605–610. doi: 10.1016/j.bbrc.2010.09.110. [DOI] [PubMed] [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- Braff DL, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Colestock T, Burrow TE, Chapman SJ, Stratford A, Nichols DE, Halberstadt AL. Return of the lysergamides. Part II: analytical and behavioural characterization of N6-allyl-6-norlysergic acid diethylamide (AL-LAD) and (2′S,4′S)-lysergic acid 2,4-dimethylazetidide (LSZ) Drug Test Anal. 2017;9:38–50. doi: 10.1002/dta.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Hoang K, Wallach J, Halberstadt AL. Return of the lysergamides. Part I: Analytical and behavioral characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD) Drug Test Anal. 2016;8:891–902. doi: 10.1002/dta.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer LH, Johanson CE, Schuster CR, Rothman RB, de Wit H. Evaluation of phentermine and fenfluramine, alone and in combination, in normal, healthy volunteers. Neuropsychopharmacology. 1996;14:233–241. doi: 10.1016/0893-133X(95)00113-R. [DOI] [PubMed] [Google Scholar]
- Bräuer D, Strobel A, Hensch T, Diers K, Lesch KP, Brocke B. Genetic variation of serotonin receptor function affects prepulse inhibition of the startle. J Neural Transm. 2009;116:607–613. doi: 10.1007/s00702-009-0222-0. [DOI] [PubMed] [Google Scholar]
- Brea J, Castro M, Loza MI, Masaguer CF, Raviña E, Dezi C, Pastor M, Sanz F, Cabrero-Castel A, Galán-Rodríguez B, Fernández-Espejo E, Maldonado R, Robledo P. QF2004B, a potential antipsychotic butyrophenone derivative with similar pharmacological properties to clozapine. Neuropharmacology. 2006;51:251–262. doi: 10.1016/j.neuropharm.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Brimblecombe RE. Effects of psychotropic drugs on open-field behaviour in rats. Psychopharmacologia. 1963;4:139–147. doi: 10.1007/BF00413331. [DOI] [PubMed] [Google Scholar]
- Brookshire BR, Jones SR. Direct and indirect 5-HT receptor agonists produce gender-specific effects on locomotor and vertical activities in C57 BL/6J mice. Pharmacol Biochem Behav. 2009;94:194–203. doi: 10.1016/j.pbb.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris KD, Breeding M, Sanders-Bush E. (+)Lysergic acid diethylamide, but not its nonhallucinogenic congeners, is a potent serotonin 5HT1C receptor agonist. J Pharmacol Exp Ther. 1991;258:891–896. [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–464. [PubMed] [Google Scholar]
- Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012;4:556–576. doi: 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology. 2010;209:163–174. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D. Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology. 2013;70:112–121. doi: 10.1016/j.neuropharm.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB. The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N, N-dimethyltryptamine and N, N-diisopropyltryptamine in rats and mice. Psychopharmacology. 2015;232:275–284. doi: 10.1007/s00213-014-3658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutt DJ. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA. 2012;109:2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson ML, Burstein ES, Kloberg A, Hansson S, Schedwin A, Nilsson M, Rung JP, Carlsson A. In vivo evidence for partial agonist effects of (−)-OSU6162 and (+)-OSU6162 on 5-HT2A serotonin receptors. J Neural Transm. 2011;118:1511–1522. doi: 10.1007/s00702-011-0704-8. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Colombo G, Gessa G, Massi M. Autoradiographic analysis of 5-HT2A binding sites in the brain of Sardinian alcohol-preferring and nonpreferring rats. Eur J Pharmacol. 1999;373:13–19. doi: 10.1016/s0014-2999(99)00239-3. [DOI] [PubMed] [Google Scholar]
- Cohen M. LSD: Side effects and complications. J Nerv Ment Dis. 1960;130:39–45. doi: 10.1097/00005053-196001000-00005. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJ, Janssen PA. A drug discrimination analysis of lysergic acid diethylamide (LSD): in vivo agonist and antagonist effects of purported 5-hydroxytryptamine antagonists and of pirenperone, a LSD-antagonist. J Pharmacol Exp Ther. 1982;221:206–214. [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warner BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly-Lestienne I. Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol. 2008;594:32–38. doi: 10.1016/j.ejphar.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Dandiya PC, Gupta BD, Gupta ML, Patni SK. Effects of LSD on open field performance in rats. Psychopharmacologia. 1969;15:333–340. doi: 10.1007/BF00401687. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Cocaine and selective monoamine uptake blockers (sertraline, nisoxetine, and GBR 12935) prevent the d-fenfluramine-induced head-twitch response in mice. Pharmacol Biochem Behav. 1998a;60:83–90. doi: 10.1016/s0091-3057(97)00548-0. [DOI] [PubMed] [Google Scholar]
- Darmani NA. The silent and selective 5-HT1A antagonist, WAY 100635, produces via an indirect mechanism, a 5-HT2A receptor-mediated behaviour in mice during the day but not at night. Short communication. J Neural Transm. 1998b;105:635–643. doi: 10.1007/s007020050085. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Pandya DK. Involvement of other neurotransmitters in behaviors induced by the cannabinoid CB1 receptor antagonist SR 141716A in naive mice. J Neural Transm. 2000;107:931–945. doi: 10.1007/s007020070043. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Mock OB, Towns LC, Gerdes CF. The head-twitch response in the least shrew (Cryptotis parva) is a 5-HT2- and not a 5-HT1C-mediated phenomenon. Pharmacol Biochem Behav. 1994;48:383–396. doi: 10.1016/0091-3057(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Shaddy J, Gerdes CF. Differential ontogenesis of three DOI-induced behaviors in mice. Physiol Behav. 1996;60:1495–1500. doi: 10.1016/s0031-9384(96)00323-x. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Janoyan JJ, Kumar N, Crim JL. Behaviorally active doses of the CB1 receptor antagonist SR 141716A increase brain serotonin and dopamine levels and turnover. Pharmacol Biochem Behav. 2003;75:777–787. doi: 10.1016/s0091-3057(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology. 2012;62:2184–2191. doi: 10.1016/j.neuropharm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Delille HK, Mezler M, Marek GJ. The two faces of the pharmacological interaction of mGlu2 and 5-HT2A—relevance of receptor heterocomplexes and interaction through functional brain pathways. Neuropharmacology. 2013;70:296–305. doi: 10.1016/j.neuropharm.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Dodge R, Louttit CM. Modification of the pattern of the guinea pig’s reflex response to noise. J Com Psychol. 1926;60:267–285. [Google Scholar]
- Drust EG, Connor JD. Pharmacological analysis of shaking behavior induced by enkephalins, thyrotropin-releasing hormone or serotonin in rats: evidence for different mechanisms. J Pharmacol Exp Ther. 1983;224:148–154. [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology. 2000;39:2170–2179. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Gross C, Stark KL, Hen R, Geyer MA. Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharmacology. 2000;22:650–659. doi: 10.1016/S0893-133X(99)00164-5. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Handley SL. Similarities in the pharmacology of spontaneous and DOI-induced head-shakes suggest 5HT2A receptors are active under physiological conditions. Psychopharmacology. 1996;128:198–205. doi: 10.1007/s002130050125. [DOI] [PubMed] [Google Scholar]
- Egan CT, Herrick-Davis K, Miller K, Glennon RA, Teitler M. Agonist activity of LSD and lisuride at cloned 5HT2Aand 5HT2Creceptors. Psychopharmacology. 1998;136:409–414. doi: 10.1007/s002130050585. [DOI] [PubMed] [Google Scholar]
- Egashira N, Shirakawa A, Okuno R, Mishima K, Iwasaki K, Oishi R, Fujiwara M. Role of endocannabinoid and glutamatergic systems in DOI-induced head-twitch response in mice. Pharmacol Biochem Behav. 2011;99:52–58. doi: 10.1016/j.pbb.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Ettrup A, Holm S, Hansen M, Wasim M, Santini MA, Palner M, Madsen J, Svarer C, Kristensen JL, Knudsen GM. Preclinical safety assessment of the 5-HT2A receptor agonist PET radioligand [11C]Cimbi-36. Mol Imaging Biol. 2013;15:376–383. doi: 10.1007/s11307-012-0609-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, Leach PT, Van Martin C, Karabenick RL, Chen X, Ohizumi Y, Ullrich T, Rice KC, Woods JH. Nantenine: an antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology. 2004;173:270–277. doi: 10.1007/s00213-003-1741-2. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology. 2005a;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, De la Garza R, Woods JH. Serotonin synthesis inhibition reveals distinct mechanisms of action for MDMA and its enantiomers in the mouse. Psychopharmacology. 2005b;181:529–536. doi: 10.1007/s00213-005-0005-8. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N, N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–129. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. Hallucinogen-like effects of N, N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav. 2008;88:358–365. doi: 10.1016/j.pbb.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther. 2010;335:728–734. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gray BW, Bailey JM, Smith DA, Hansen M, Kristensen JL. Hallucinogen-like effects of 2-([2-(4-cyano-2,5-dimethoxyphenyl) ethylamino]methyl)phenol (25CN-NBOH), a novel N-benzylphenethylamine with 100-fold selectivity for 5-HT2A receptors, in mice. Psychopharmacology. 2014;232:1039–1047. doi: 10.1007/s00213-014-3739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J Pharmacol Exp Ther. 2004;310:1246–1254. doi: 10.1124/jpet.104.068841. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: reassessment of LSD false positives. Psychopharmacology. 1995;121:357–363. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- Fleshler M. Adequate acoustic stimulus for the startle reflex in the rat. J Com Physiol Psychol. 1965;60:200–207. doi: 10.1037/h0022318. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. Characterizing the effects of 5-HT2C receptor ligands on motor activity and feeding behaviour in 5-HT2C receptor knockout mice. Neuropharmacology. 2009;57:259–267. doi: 10.1016/j.neuropharm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Fowler H. Curiosity and exploratory behavior. Macmillan; New York: 1965. [Google Scholar]
- Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. The serotonin 5-HT2A receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology. 2010a;212:13–23. doi: 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- Fox MA, Stein AR, French HT, Murphy DL. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT) Br J Pharmacol. 2010b;159:879–887. doi: 10.1111/j.1476-5381.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland CS, Mansbach RS. Behavioral profile of constituents in ayahuasca, an Amazonian psychoactive plant mixture. Drug Alcohol Depend. 1999;54:183–194. doi: 10.1016/s0376-8716(98)00154-9. [DOI] [PubMed] [Google Scholar]
- Garcia EE, Smith RL, Sanders-Bush E. Role of Gq protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology. 2007;52:1671–1677. doi: 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Behavioral studies of hallucinogenic drugs in animals: implications for schizophrenia research. Pharmacopsychiatry. 1998;31:73–79. doi: 10.1055/s-2007-979350. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs KM. Serotonin receptor involvement in an animal model of the acute effects of hallucinogens. NIDA Res Monograph Ser. 1994;146:124–156. [PubMed] [Google Scholar]
- Geyer MA, Paulus MP. Multivariate and nonlinear approaches to characterizing drug effects on the locomotor and investigatory behavior of rats. NIDA Res Monogr. 1992;124:203–235. [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Segal DS, Kuczenski R. Effects of apomorphine and amphetamine on patterns of locomotor and investigatory behavior in rats. Pharmacol Biochem Behav. 1987;28:393–399. doi: 10.1016/0091-3057(87)90460-6. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Discriminative stimulus properties of hallucinogens and related designer drugs. NIDA Res Monogr. 1991;116:25–44. [PubMed] [Google Scholar]
- Glennon RA, Hauck AE. Mechanistic studies on DOM as a discriminative stimulus. Pharmacol Biochem Behav. 1985;23:937–941. doi: 10.1016/0091-3057(85)90096-6. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA. Discriminative stimulus properties of DOM and several molecular modifications. Pharmacol Biochem Behav. 1982a;16:553–556. doi: 10.1016/0091-3057(82)90413-0. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA. A comparison of the behavioral effects of DOM homologs. Pharmacol Biochem Behav. 1982b;16:557–559. doi: 10.1016/0091-3057(82)90414-2. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA. Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT2 antagonists. Eur J Pharmacol. 1983;91:189–196. doi: 10.1016/0014-2999(83)90464-8. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, McKenney JD, Lyon RA, Titeler M. 5-HT1 and 5-HT2 binding characteristics of 1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane analogues. J Med Chem. 1986;29:194–199. doi: 10.1021/jm00152a005. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Seggel MR, Lyon RA. N-Methyl derivatives of the 5-HT2 agonist 1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane. J Med Chem. 1987;30:930–932. doi: 10.1021/jm00388a032. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Lyon RA, Slusher RM. N, N-di-n-propylserotonin: binding at serotonin binding sites and a comparison with 8-hydroxy-2-(di-n-propylamino)tetralin. J Med Chem. 1988;31:867–870. doi: 10.1021/jm00399a031. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Dukat M, el-Bermawy M, Law H, De los Angeles J, Teitler M, King A, Herrick-Davis K. Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines. J Med Chem. 1994;37:1929–1935. doi: 10.1021/jm00039a004. [DOI] [PubMed] [Google Scholar]
- Gogos A, Bogeski M, van den Buuse M. Role of serotonin-1A receptors in the action of antipsychotic drugs: comparison of prepulse inhibition studies in mice and rats and relevance for human pharmacology. Behav Pharmacol. 2008;19:548–561. doi: 10.1097/FBP.0b013e32830cd822. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Sun JC. Functional role of the endocannabinoid system and AMPA/kainate receptors in 5-HT2A receptor-mediated wet dog shakes. Eur J Pharmacol. 2005;516:28–33. doi: 10.1016/j.ejphar.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Thelen B, Lindenblatt H, Kovar KA, Sass H, Geyer MA. Effects of the hallucinogen psilocybin on habituation and prepulse inhibition of the startle reflex in humans. Behav Pharmacol. 1998;9:561–566. doi: 10.1097/00008877-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187:268–283. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Charney DS, Krystal J, Braff DL. Startle gating deficits occur across prepulse intensities in schizophrenic patients. Biol Psychiatry. 1992;32:939–943. doi: 10.1016/0006-3223(92)90183-z. [DOI] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68:71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Chu H, Sanders-Bush E. m-Chlorophenylpiperazine and m-trifluoromethylphenylpiperazine are partial agonists at cloned 5-HT2A receptors expressed in fibroblasts. J Pharmacol Exp Ther. 1994;271:1122–1126. [PubMed] [Google Scholar]
- Gupta BD, Dandiya PC, Gupta ML, Gabba AK. An examination of the effect of central nervous system stimulant and anti-depressant drugs on open field performance in rats. Eur J Pharmacol. 1971;13:341–346. doi: 10.1016/0014-2999(71)90224-x. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. Recent advances in the neuropsychopharmacology of hallucinogens. Behav Brain Res. 2015;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL. Behavioral and pharmacokinetic interactions between monoamine oxidase inhibitors and the hallucinogen 5-methoxy-N, N-dimethyltryptamine. Pharmacol Biochem Behav. 2016;143:1–10. doi: 10.1016/j.pbb.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. LSD but not lisuride disrupts prepulse inhibition in rats by activating the 5-HT2A receptor. Psychopharmacology. 2010;208:179–189. doi: 10.1007/s00213-009-1718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology. 2013;227:727–739. doi: 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Effect of the hallucinogen 2C–I and two superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology. 2014;77:200–207. doi: 10.1016/j.neuropharm.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten V, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology. 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman M, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. Opposing effects of 5-HT2A and 5-HT2C receptors on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol. 2011;25:1548–1561. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Nichols DE, Geyer MA. Behavioral effects of α, α, β, β-tetradeutereo-5-MeO-DMT in rats: comparison with 5-MeO-DMT administered in combination with a monoamine oxidase inhibitor. Psychopharmacology. 2012;221:709–718. doi: 10.1007/s00213-011-2616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Powell SB, Geyer MA. Role of the 5-HT2A receptor in the locomotor hyperactivity produced by phenylalkylamine hallucinogens in mice. Neuropharmacology. 2013;70:218–227. doi: 10.1016/j.neuropharm.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Sindhunata I, Scheffers K, Flynn AD, Sharp RF, Geyer MA, Young JW. Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice. Neuropharmacology. 2016a;107:364–375. doi: 10.1016/j.neuropharm.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Slepak N, Hyun J, Buell MR, Powell SB. The novel ketamine analog methoxetamine produces dissociative-like behavioral effects in rodents. Psychopharmacology. 2016b;233:1215–1225. doi: 10.1007/s00213-016-4203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL. Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci. 2014;5:243–249. doi: 10.1021/cn400216u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins MF, Uzelac SM, Baumeister AA, Hearn JK, Broussard JI, Guillot TS. Behavioral responses to stress following central and peripheral injection of the 5-HT2 agonist DOI. Pharmacol Biochem Behav. 2002;73:537–544. doi: 10.1016/s0091-3057(02)00822-5. [DOI] [PubMed] [Google Scholar]
- Hazama K, Hayata-Takano A, Uetsuki K, Kasai A, Encho N, Shintani N, Nagayasu K, Hashimoto R, Reglodi D, Miyakawa T, Nakazawa T, Baba A, Hashimoto H. Increased behavioral and neuronal responses to a hallucinogenic drug in PACAP heterozygous mutant mice. PLoS ONE. 2014;9:e89153. doi: 10.1371/journal.pone.0089153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Philpot J, O’Shaughnessy KM, Davies CL. The influence of central noradrenergic function on 5-HT2-mediated head-twitch responses in mice: possible implications for the actions of antidepressant drugs. Psychopharmacology. 1986;89:414–420. doi: 10.1007/BF02412113. [DOI] [PubMed] [Google Scholar]
- Heekeren K, Neukirch A, Daumann J, Stoll M, Obradovic M, Kovar KA, Geyer MA, Gouzoulis-Mayfrank E. Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and N, N-dimethyltryptamine (DMT) models of psychosis. J Psychopharmacol. 2007;21:312–320. doi: 10.1177/0269881107077734. [DOI] [PubMed] [Google Scholar]
- Herrmann WM, Horowski R, Dannehl K, Kramer U, Lurati K. Clinical effectiveness of lisuride hydrogen maleate: a double-blind trial versus methysergide. Headache. 1977;17:54–60. doi: 10.1111/j.1526-4610.1977.hed1702054.x. [DOI] [PubMed] [Google Scholar]
- Hillegaart V, Estival A, Ahlenius S. Evidence for specific involvement of 5-HT1A and 5-HT2A/C receptors in the expression of patterns of spontaneous motor activity of the rat. Eur J Pharmacol. 1996;295:155–161. doi: 10.1016/0014-2999(95)00666-4. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Nabeshima T, Kameyama T, Maeda Y, Cho AK. The effect of optical isomers of 3,4-methylenedioxymethamphetamine (MDMA) on stereotyped behavior in rats. Pharmacol Biochem Behav. 1989;33:343–347. doi: 10.1016/0091-3057(89)90511-x. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in the modification of startle reaction in the rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic and temporal factors in the evocation of startle. J Acoustic Soc Am. 1968;43:269–282. doi: 10.1121/1.1910776. [DOI] [PubMed] [Google Scholar]
- Holohean AM, White FJ, Appel JB. Dopaminergic and serotonergic mediation of the discriminable effects of ergot alkaloids. Eur J Pharmacol. 1982;81:595–602. doi: 10.1016/0014-2999(82)90349-1. [DOI] [PubMed] [Google Scholar]
- Huang JT, Ho BT. Differentiation of pressor and behavioral effects of d-amphetamine and 2,5-dimethoxy-4-methylamphetamine (STP) by cinanserin. Can J Physiol Pharmacol. 1973;51:976–980. doi: 10.1139/y73-146. [DOI] [PubMed] [Google Scholar]
- Hughes RN. Chlordiazepoxide modified exploration in rats. Psychopharmacologia. 1972;24:462–469. doi: 10.1007/BF00423436. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Trulson ME, Stern WC. An animal behavior model for studying the actions of LSD and related hallucinogens. Science. 1976;194:741–743. doi: 10.1126/science.982043. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Trulson ME, Stern WC. Behavioral effects of LSD in the cat: proposal of an animal behavior model for studying the actions of hallucinogenic drugs. Brain Res. 1977;132:301–314. doi: 10.1016/0006-8993(77)90423-1. [DOI] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav. 1995;52:649–654. doi: 10.1016/0091-3057(95)00160-x. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Hoffman AJ, Nichols DE. Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices. Eur J Pharmacol. 1986;132:269–276. doi: 10.1016/0014-2999(86)90615-1. [DOI] [PubMed] [Google Scholar]
- Kantor RE, Dudlettes SD, Shulgin AT. 5-Methoxy-α-methyltryptamine (α, O-dimethylserotonin) a hallucinogenic homolog of serotonin. Biol Psychiatry. 1980;15:349–352. [PubMed] [Google Scholar]
- Kärkkäinen J, Forsström T, Tornaeus J, Wähälä K, Kiuru P, Honkanen A, Stenman UH, Turpeinen U, Hesso A. Potentially hallucinogenic 5-hydroxytryptamine receptor ligands bufotenine and dimethyltryptamine in blood and tissues. Scand J Clin Lab Invest. 2005;65:189–199. doi: 10.1080/00365510510013604. [DOI] [PubMed] [Google Scholar]
- Keller DL, Umbreit WW. Permanent alteration of behavior in mice by chemical and psychological means. Science. 1956;124:723–724. doi: 10.1126/science.124.3225.723. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Glen A, Grewal S, Forbes I, Gadre A, Blackburn TP. In vivo properties of SB 200646A, a 5-HT2C/2B receptor antagonist. Br J Pharmacol. 1994;111:797–802. doi: 10.1111/j.1476-5381.1994.tb14808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura KK, Akai TT, Nakamura KK, Yamaguchi MM, Nakagawa H, Oshino NN. Dual activation by lisuride of central serotonin 5-HT1A and dopamine D2 receptor sites: drug discrimination and receptor binding studies. Behav Pharmacol. 1991;2:105–112. [PubMed] [Google Scholar]
- Klodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of in mice. Pharmacol Biochem Behav. 2002;73:327–332. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Knauer CS, Campbell JE, Chio CL, Fitzgerald LW. Pharmacological characterization of mitogen-activated protein kinase activation by recombinant human 5-HT2C, 5-HT2A, and 5-HT2B receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:461–471. doi: 10.1007/s00210-008-0378-4. [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Jäncke L, Vollenweider FX. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci. 2013;33:10544–10551. doi: 10.1523/JNEUROSCI.3007-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs KM, Geyer MA. Cross-tolerance studies of serotonin receptors involved in behavioral effects of LSD in rats. Psychopharmacology. 1994;113:429–437. doi: 10.1007/BF02245219. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. The role of 5-HT1A receptors in the locomotor-suppressant effects of LSD: WAY-100635 studies of 8-OH-DPAT, DOI and LSD in rats. Behav Pharmacol. 1996;7:551–559. [PubMed] [Google Scholar]
- Krebs-Thomson K, Lehmann-Masten V, Naiem S, Paulus MP, Geyer MA. Modulation of phencyclidine-induced changes in locomotor activity and patterns in rats by serotonin. Eur J Pharmacol. 1998a;343:135–143. doi: 10.1016/s0014-2999(97)01557-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998b;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology. 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther. 2003;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- Lehmann-Masten VD, Geyer MA. Spatial and temporal patterning distinguishes the locomotor activating effects of dizocilpine and phencyclidine in rats. Neuropharmacology. 1991;30:629–636. doi: 10.1016/0028-3908(91)90083-n. [DOI] [PubMed] [Google Scholar]
- Leng A, Ouagazzal A, Feldon J, Higgins GA. Effect of the 5-HT6 receptor antagonists Ro04-6790 and Ro65-7199 on latent inhibition and prepulse inhibition in the rat: comparison to clozapine. Pharmacol Biochem Behav. 2003;75:281–288. doi: 10.1016/s0091-3057(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Niemegeers CJ, Van Nueten JM, Laduron PM. [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982;21:301–314. [PubMed] [Google Scholar]
- Lucki I, Minugh-Purvis N. Serotonin-induced head shaking behavior in rats does not involve receptors located in the frontal cortex. Brain Res. 1987;420:403–406. doi: 10.1016/0006-8993(87)91265-0. [DOI] [PubMed] [Google Scholar]
- Lucki I, Nobler MS, Frazer A. Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J Pharmacol Exp Ther. 1984;228:133–139. [PubMed] [Google Scholar]
- Lukasiewicz S, Polit A, Kędracka-Krok S, Wędzony K, Maćkowiak M, Dziedzicka-Wasylewska M. Hetero-dimerization of serotonin 5-HT2A and dopamine D2 receptors. Biochim Biophys Acta. 2010;1803:1347–1358. doi: 10.1016/j.bbamcr.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Lyon RA, Glennon RA, Titeler M. 3,4-Methylenedioxymethamphetamine (MDMA): stereoselective interactions at brain 5-HT1 and 5-HT2 receptors. Psychopharmacology. 1986;88:525–526. doi: 10.1007/BF00178519. [DOI] [PubMed] [Google Scholar]
- Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naïve schizophrenic patients. Biol Psychiatry. 2002;52:863–873. doi: 10.1016/s0006-3223(02)01409-9. [DOI] [PubMed] [Google Scholar]
- Malick JB, Doren E, Barnett A. Quipazine-induced head-twitch in mice. Pharmacol Biochem Behav. 1977;6:325–329. doi: 10.1016/0091-3057(77)90032-6. [DOI] [PubMed] [Google Scholar]
- Marini JL, Jacobs BL, Sheard MH, Trulson ME. Activity of a non-hallucinogenic ergoline derivative, lisuride, in an animal behavior model for hallucinogens. Psychopharmacology. 1981;73:328–331. doi: 10.1007/BF00426460. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Sabb AL, Logue SF, Brennan JA, Piesla MJ, Comery TA, Grauer SM, Ashby CR, Jr, Nguyen HQ, Dawson LA, Barrett JE, Stack G, Meltzer HY, Harrison BL, Rosenzweig-Lipson S. WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole]: a novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther. 2007;320:486–496. doi: 10.1124/jpet.106.106989. [DOI] [PubMed] [Google Scholar]
- Matthews WD, Smith CD. Pharmacological profile of a model for central serotonin receptor activation. Life Sci. 1980;26:1397–1403. doi: 10.1016/0024-3205(80)90042-9. [DOI] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Liu P, Verbny YI, Ziskind-Conhaim L, Ruoho AE. Development of the sigma-1 receptor in C-terminals of motoneurons and colocalization with the N, N′-dimethyltryptamine forming enzyme, indole-N-methyl transferase. Neuroscience. 2012;206:60–68. doi: 10.1016/j.neuroscience.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JA, Dantanarayana AP, Zinke PW, McLaughlin MA, Sharif NA. 1-((S)-2-aminopropyl)-1H-indazol-6-ol: a potent peripherally acting 5-HT2 receptor agonist with ocular hypotensive activity. J Med Chem. 2006;49:318–328. doi: 10.1021/jm050663x. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Repke DB, Lo L, Peroutka SJ. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology. 1990;29(3):193–198. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- McOmish CE, Lira A, Hanks JB, Gingrich JA. Clozapine-induced locomotor suppression is mediated by 5-HT2A receptors in the forebrain. Neuropsychopharmacology. 2012;37:2747–2755. doi: 10.1038/npp.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Effects of 5HT-1A agonists on locomotor and investigatory behaviors in rats differ from those of hallucinogens. Psychopharmacology. 1989;98:321–329. doi: 10.1007/BF00451682. [DOI] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Disassociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology. 1991;105:69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- Molinaro G, Traficante A, Riozzi B, Di Menna L, Curto M, Pallottino S, Nicoletti F, Bruno V, Battaglia G. Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice. Mol Pharmacol. 2009;76:379–387. doi: 10.1124/mol.109.056580. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493:76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mos J, Olivier B, Tulp MThM. Ethopharmacological studies differentiate the effects of various serotonergic compounds on agression in rats. Drug Dev Res. 1992;26:343–360. [Google Scholar]
- Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–1061. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL. Endocrine and neurochemical effects of 3,4-methylenedioxymethamphetamine and its stereoisomers in rhesus monkeys. J Pharmacol Exp Ther. 2010;334:642–650. doi: 10.1124/jpet.110.166595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA. Lysergic acid diethylamide and [−]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 2004;1023:134–140. doi: 10.1016/j.brainres.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Nakagawasai O, Murata A, Arai Y, Ohba A, Wakui K, Mitazaki S, Niijima F, Tan-No K, Tadano T. Enhanced head-twitch response to 5-HT-related agonists in thiamine-deficient mice. J Neural Transm (Vienna) 2007;114:1003–1010. doi: 10.1007/s00702-007-0655-2. [DOI] [PubMed] [Google Scholar]
- Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA. Effect of the R(−) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neurosci Lett. 1994;177:111–115. doi: 10.1016/0304-3940(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Audinot V, Nicolas JP, De Ceuninck F, Boutin JA, Millan MJ. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D2-like receptor and alpha1/alpha2-adrenoceptor. J Pharmacol Exp Ther. 2002;303:805–814. doi: 10.1124/jpet.102.039875. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Structure-activity relationships of serotonin 5-HT2A agonists. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:559–579. [Google Scholar]
- Nichols DE, Pfister WR, Yim GK. LSD and phenethylamine hallucinogens: new structural analogy and implications for receptor geometry. Life Sci. 1978;22:2165–2170. doi: 10.1016/0024-3205(78)90567-2. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ. N-benzyl-5-methoxytryptamines as potent serotonin 5-HT2 receptor family agonists and comparison with a series of phenethylamine analogues. ACS Chem Neurosci. 2015;6:165–1175. doi: 10.1021/cn500292d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouagazzal A, Grottick AJ, Moreau J, Higgins GA. Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology. 2001;25:565–575. doi: 10.1016/S0893-133X(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Padich RA, McCloskey TC, Kehne JH. 5-HT modulation of auditory and visual sensorimotor gating: II. Effects of the 5-HT2A antagonist MDL 100,907 on disruption of sound and light prepulse inhibition produced by 5-HT agonists in Wistar rats. Psychopharmacology. 1996;124:107–116. doi: 10.1007/BF02245610. [DOI] [PubMed] [Google Scholar]
- Palenicek T, Hlinak Z, Bubenikova-Valesova V, Novak T, Horacek J. Sex differences in the effects of N, N-diethyllysergamide (LSD) on behavioural activity and prepulse inhibition. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:588–596. doi: 10.1016/j.pnpbp.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Pálenícek T, Balíková M, Bubeníková-Valesová V, Horácek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology. 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- Pálenícek T, Fujáková M, Brunovskỳ M, Horácek J, Gorman I, Balíková M, et al. Behavioral, neurochemical and pharmaco-EEG profiles of the psychedelic drug 4-bromo-2,5-dimethoxyphenethylamine (2C-B) in rats. Psychopharmacology (Berl) 2013;225:75–93. doi: 10.1007/s00213-012-2797-7. [DOI] [PubMed] [Google Scholar]
- Parrish JC, Braden MR, Gundy E, Nichols DE. Differential phospholipase C activation by phenylalkylamine serotonin 5-HT2A receptor agonists. J Neurochem. 2005;95:1575–1584. doi: 10.1111/j.1471-4159.2005.03477.x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology. 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercey MF, Hoffmann WE, Smith MW, Hyslop DK. Inhibition of dopamine neuron firing by pramipexole, a dopamine D3 receptor-preferring agonist: comparison to other dopamine receptor agonists. Eur J Pharmacol. 1996;312:35–44. doi: 10.1016/0014-2999(96)00454-2. [DOI] [PubMed] [Google Scholar]
- Pranzatelli MR. Evidence for involvement of 5-HT2 and 5-HT1C receptors in the behavioral effects of the 5-HT agonist 1-(2,5-dimethoxy-4-iodophenyl aminopropane)-2 (DOI) Neurosci Lett. 1990;115:74–80. doi: 10.1016/0304-3940(90)90520-j. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kühn K-U, Mössner R, Schwab SG, Schuhmacher A, Maier W, Wagner M. Sensorimotor gating of schizophrenia patients is influenced by 5-HT2A receptor polymorphisms. Biol Psychiatry. 2008;64:434–437. doi: 10.1016/j.biopsych.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Schmechtig A, Ettinger U, Petrovsky N, Collier DA, Vollenweider FX, Wagner M, Kumari V. Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study. Biol Psychiatry. 2009;66:614–620. doi: 10.1016/j.biopsych.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology. 2012;37:630–640. doi: 10.1038/npp.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin RA, Regina M, Doat M, Winter JC. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol Biochem Behav. 2002;72:29–37. doi: 10.1016/s0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Raffaelli E, Jr, Martins OJ, dos Santos P, Dãgua Filho A. Lisuride in cluster headache. Headache. 1983;23:117–121. doi: 10.1111/j.1526-4610.1983.hed2303117.x. [DOI] [PubMed] [Google Scholar]
- Rempel NL, Callaway CW, Geyer MA. Serotonin1B receptor activation mimics behavioral effects of presynaptic serotonin release. Neuropsychopharmacology. 1993;8:201–211. doi: 10.1038/npp.1993.22. [DOI] [PubMed] [Google Scholar]
- Reyes-Parada M, Scorza C, Romero V, Silveira R, Medina JH, Andrus D, Nichols DE, Cassels BK. (±)-1-(2,5-Dimethoxy-4-ethylthiophenyl)-2-aminopropane (ALEPH-2), a novel putative anxiolytic agent lacking affinity for benzodiazepine sites and serotonin-1A receptors. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:579–585. doi: 10.1007/BF00170831. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Barbanoj MJ. Effects of ayahuasca on sensory and sensorimotor gating in humans as measured by P50 suppression and prepulse inhibition of the startle reflex, respectively. Psychopharmacology. 2002;165:18–28. doi: 10.1007/s00213-002-1237-5. [DOI] [PubMed] [Google Scholar]
- Rigdon GC, Weatherspoon JK. 5-Hydroxytryptamine1a receptor agonists block prepulse inhibition of acoustic startle reflex. J Pharmacol Exp Ther. 1992;263:486–493. [PubMed] [Google Scholar]
- Rusterholz DB, Spratt JL, Long JP, Kelly TF. Serotonergic and dopaminergic involvement in the mechanism of action of R-(−)-2,5-dimethoxy-4-bromoamphetamine (DOB) in cats. Life Sci. 1978;23:1499–1506. doi: 10.1016/0024-3205(78)90132-7. [DOI] [PubMed] [Google Scholar]
- Salvatore S, Hyde RW. Progression of effects of lysergic acid diethylamide (LSD) Arch Neurol Psychiat. 1956;2:50–59. doi: 10.1001/archneurpsyc.1956.02330250052007. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Arnt J, Moltzen E. Assessment of relative efficacies of 5-HT1A receptor ligands by means of in vivo animal models. Eur J Pharmacol. 1996;315:245–254. doi: 10.1016/s0014-2999(96)00621-8. [DOI] [PubMed] [Google Scholar]
- Sard H, Kumaran G, Morency C, Roth BL, Toth BA, He P, Shuster L. SAR of psilocybin analogs: discovery of a selective 5-HT2C agonist. Bioorg Med Chem Lett. 2005;15:4555–4559. doi: 10.1016/j.bmcl.2005.06.104. [DOI] [PubMed] [Google Scholar]
- Schlemmer RF, Jr, Davis JM. A primate model for the study of hallucinogens. Pharmacol Biochem Behav. 1986;24:381–392. doi: 10.1016/0091-3057(86)90368-0. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Müller F, Borgwardt S, Liechti ME. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78:544–553. doi: 10.1016/j.biopsych.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-Dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT)2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Scorza MC, Reyes-Parada M, Silveira R, Viola H, Medina JH, Viana MB, Zangrossi H, Jr, Graeff FG. Behavioral effects of the putative anxiolytic (±)-1-(2,5-dimethoxy-4-ethylthiophenyl)-2-aminopropane (ALEPH-2) in rats and mice. Pharmacol Biochem Behav. 1996;54:355–361. doi: 10.1016/0091-3057(95)02149-3. [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett. 2003;346:137–140. doi: 10.1016/s0304-3940(03)00547-0. [DOI] [PubMed] [Google Scholar]
- Segal DS, Geyer MA. Animal models of psychopathology. In: Judd LL, Groves PM, editors. Psychobiolgical foundations of clinical psychiatry. J.B. Lippincott Co; Philadelphia: 1985. pp. 1–14. [Google Scholar]
- Shen HW, Jiang XL, Winter JC, Yu AY. Psychedelic 5-methoxy-N, N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr Drug Metabol. 2010;11:659–666. doi: 10.2174/138920010794233495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin AT. Stereospecific requirements for hallucinogenesis. J Pharm Pharmacol. 1973;25:271–272. doi: 10.1111/j.2042-7158.1973.tb10642.x. [DOI] [PubMed] [Google Scholar]
- Shulgin AT, Nichols DE. Characterization of three new psychotomimetics. In: Stillman RC, Willette RE, editors. The psychopharmacology of hallucinogens. Pergamon Press; New York: 1978. p. 74. [Google Scholar]
- Shulgin AT, Shulgin A. PIHKAL: a chemical love story. Transform Press; Berkeley, CA: 1991. [Google Scholar]
- Shulgin AT, Sargent T, Naranjo C. 4-Bromo-2,5-dimethoxyphenylisopropylamine, a new centrally active amphetamine analog. Pharmacology. 1971;5:103–107. doi: 10.1159/000136181. [DOI] [PubMed] [Google Scholar]
- Shulgin AT, Manning T, Daley PF. Psychedelic phenethylamines and related compounds. Vol. 1. Transform Press; Berkeley, CA: 2011. The shulgin index. [Google Scholar]
- Silva MT, Calil HM. Screening hallucinogenic drugs: systematic study of three behavioral tests. Psychopharmacologia. 1975;42:163–171. doi: 10.1007/BF00429548. [DOI] [PubMed] [Google Scholar]
- Singleton C, Marsden CA. Circadian variation in the head twitch response produced by 5-methoxy-N1, N1-dimethyltryptamine and p-chloroamphetamine in the mouse. Psychopharmacology. 1981;74:173–176. doi: 10.1007/BF00432688. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. 8-OH-DPAT disruption of prepulse inhibition in rats: reversal with (+)WAY 100,135 and localization of site of action. Psychopharmacology. 1995a;117:41–48. doi: 10.1007/BF02245096. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT2A and not by 5-HT2C receptors. Behav Pharmacol. 1995b;6:839–842. [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res. 1997;761:97–104. doi: 10.1016/s0006-8993(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Sitaram BR, Lockett L, Talomsin R, Blackman GL, McLeod WR. In vivo metabolism of 5-methoxy-N, N-dimethyltryptamine and N, N-dimethyltryptamine in the rat. Biochem Pharmacol. 1987;36:1509–1512. doi: 10.1016/0006-2952(87)90118-3. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, Iredale PA. CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology. 2007;52:279–290. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Smith DA, Bailey JM, Williams D, Fantegrossi WE. Tolerance and cross-tolerance to head twitch behavior elicited by phenethylamine- and tryptamine-derived hallucinogens in mice. J Pharmacol Exp Ther. 2014;351:485–491. doi: 10.1124/jpet.114.219337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH, Unger S, Blatchley R, Barfknecht CF. Stereospecific actions of DOET (2,5-dimethoxy-4-ethylamphetamine) in man. Arch Gen Psychiatry. 1974;31:103–106. doi: 10.1001/archpsyc.1974.01760130079013. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Jr, Glaser T, Traber J. Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N, N-dimethyltryptamine. Psychopharmacology. 1987;93:158–166. doi: 10.1007/BF00179927. [DOI] [PubMed] [Google Scholar]
- Squires RF. Evidence that 5-methoxy-N, N-dimethyltryptamine is a specific substrate for MAO-A in the rat: implications for the indoleamine dependent behavioral syndrome. J Neurochem. 1975;24:47–50. doi: 10.1111/j.1471-4159.1975.tb07626.x. [DOI] [PubMed] [Google Scholar]
- Strachan RT, Sciaky N, Cronan MR, Kroeze WK, Roth BL. Genetic deletion of p90 ribosomal S6 kinase 2 alters patterns of 5-hydroxytryptamine2A serotonin receptor functional selectivity. Mol Pharmacol. 2010;77:327–338. doi: 10.1124/mol.109.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki O, Katsumata Y, Oya M. Characterization of eight biogenic indoleamines as substrates for type A and type B monoamine oxidase. Biochem Pharmacol. 1981;30:1353–1358. [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder (OCD) Biol Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Zinner S, Hartston H, Filion D, Magulac M. Central inhibitory deficits in obsessive compulsive disorder and Tourette syndrome. Biol Psychiatry. 1994;35(664):1994. [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Tadano T, Neda M, Hozumi M, Yonezawa A, Arai Y, Fujita T, Kinemuchi H, Kisara K. alpha-Methylated tryptamine derivatives induce a 5-HT receptor-mediated head-twitch response in mice. Neuropharmacology. 1995;34:229–234. doi: 10.1016/0028-3908(94)00119-d. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behav Brain Res. 2012;233:55–61. doi: 10.1016/j.bbr.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Moon E, Kim UJ, Xu J, Siciliano MJ, Weinshilboum RM. Human indolethylamine N-methyltransferase: cDNA cloning and expression, gene cloning, and chromosomal localization. Genomics. 1999;61:285–297. doi: 10.1006/geno.1999.5960. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Baker TG, Chamberlain JH. Behavioral and neuropharmacological analysis of amphetamine and 2,5-dimethoxy-4-methylamphetamine in rats. Psychopharmacologia. 1975;44:229–239. doi: 10.1007/BF00428899. [DOI] [PubMed] [Google Scholar]
- Turner EH, Loftis JM, Blackwell AD. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther. 2006;109:325–338. doi: 10.1016/j.pharmthera.2005.06.004. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Ruimschotel E, Martin S, Perrin K, Risbrough VB, Halberstadt AL. Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT1A receptor knockout mice: implications for schizophrenia. Neuropharmacology. 2011;61:209–216. doi: 10.1016/j.neuropharm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag HM, Schut T, Bosma E, van den Bergh R. A comparative study of the therapeutic effects of some 4-chlorinated amphetamine derivatives in depressive patients. Psychopharmacologia. 1971;20:66–76. doi: 10.1007/BF00404060. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology. 1995;122:15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- Verde G, Chiodini PG, Liuzzi A, Cozzi R, Favales F, Botalla L, et al. Effectiveness of the dopamine agonist lisuride in the treatment of acromegaly andpathological hyperprolactinemic states. J Endocrinol Invest. 1980;3:405–414. doi: 10.1007/BF03349379. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT2A receptor-mediated head-twitch behaviour in the rat by 5-HT2C receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX. Evidence for a cortical–subcortical imbalance of sensory information processing during altered states of consciousness using positron emission tomography and [18F]fluorodeoxyglucose. In: Pletscher A, Ladewig D, editors. 50 years of LSD: current status and perspectives of hallucinogens. Parthenon; London: 1994. pp. 67–86. [Google Scholar]
- Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- Wasson RG, Hofmann A, Ruck CAP. The road to Eleusis: unveiling the secret of the mysteries. Harcourt, Brace, Jovanovich; New York: 1978. [Google Scholar]
- Watson NV, Gorzalka BB. Concurrent wet dog shaking and inhibition of male rat copulation after ventromedial brainstem injection of the 5-HT2 agonist DOI. Neurosci Lett. 1992;141:25–29. doi: 10.1016/0304-3940(92)90326-3. [DOI] [PubMed] [Google Scholar]
- Webster VA, Griffiths EC, Slater P. Induction of wet-dog shaking in rats by analogues and metabolites of thyrotrophin-releasing hormone (TRH) Regul Pept. 1982;5:43–51. doi: 10.1016/0167-0115(82)90074-x. [DOI] [PubMed] [Google Scholar]
- Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioral effects of1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:533–544. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]
- White FJ, Appel JB. Lysergic acid diethylamide (LSD) and lisuride: differentiation of their neuropharmacological actions. Science. 1982;216:535–537. doi: 10.1126/science.7071600. [DOI] [PubMed] [Google Scholar]
- White FJ, Kuhn DM, Appel JB. Discriminative stimulus properties of quipazine. Neuropharmacology. 1977;16:827–832. doi: 10.1016/0028-3908(79)90054-6. [DOI] [PubMed] [Google Scholar]
- Wieland S, Kreider MS, McGonigle P, Lucki I. Destruction of the nucleus raphe obscurus and potentiation of serotonin-mediated behaviors following administration of the neurotoxin 3-acetylpyridine. Brain Res. 1990;520:291–302. doi: 10.1016/0006-8993(90)91718-v. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology. 1990;100:417–425. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- Winter JC. Quipazine-induced stimulus control in the rat. Psychopharmacology. 1979;60:265–269. doi: 10.1007/BF00426666. [DOI] [PubMed] [Google Scholar]
- Winter JC. The stimulus effects of serotonergic hallucinogens in animals. NIDA Res Monograph Ser. 1994;146:157–182. [PubMed] [Google Scholar]
- Winter CA, Flataker L. Effects of lysergic acid diethylamide upon performance of trained rats. Proc Soc Exp Biol Med. 1956;92:285–289. doi: 10.3181/00379727-92-22453. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N, N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Behavioral effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rats and mice. Eur J Pharmacol. 1975;32:156–162. doi: 10.1016/0014-2999(75)90278-2. [DOI] [PubMed] [Google Scholar]
- Yarosh HL, Katz EB, Coop A, Fantegrossi WE. MDMA-like behavioral effects of N-substituted piperazines in the mouse. Pharmacol Biochem Behav. 2007;88:18–27. doi: 10.1016/j.pbb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neurosci Biobehav Rev. 2007;31:882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Filip M. Interactions of serotonin (5-HT)2 receptor-targeting ligands and nicotine: locomotor activity studies in rats. Synapse. 2009;63:653–661. doi: 10.1002/syn.20645. [DOI] [PubMed] [Google Scholar]
- Zhang C, Marek GJ. AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:62–71. doi: 10.1016/j.pnpbp.2007.07.009. [DOI] [PubMed] [Google Scholar]