Abstract
The potent estrogen 17β-estradiol (E2) has long been known to regulate the hippocampus and hippocampal-dependent memories in females, and research from the past decade has begun to shed light on the molecular mechanisms through which E2 mediates memory formation in females. Although E2 can also regulate hippocampal function in males, relatively little is known about how E2 influences memory formation in males, or whether sex differences in underlying mechanisms exist. This review, based on a talk given in April 2017 at the American University symposium entitled, “Sex Differences: From Neuroscience to the Clinic and Beyond”, first provides an overview of the molecular mechanisms in the dorsal hippocampus through which E2 enhances memory consolidation in ovariectomized female mice. Next, newer research is described demonstrating key roles for the prefrontal cortex and de novo hippocampal E2 synthesis to the memory-enhancing effects of E2 in females. The review then discusses the effects of de novo and exogenous E2 on hippocampal memory consolidation in both sexes, and putative sex differences in the underlying molecular mechanisms through which E2 enhances memory formation. The review concludes by discussing the importance and implications of sex differences in the molecular mechanisms underlying E2-induced memory consolidation for human health.
Keywords: Estradiol, cell signaling, dendritic spine density, prefrontal cortex, sex differences, ERK
1. Introduction
Sex differences are currently a hot topic in biomedical research, thanks to recent policies enacted by funding agencies, including the National Institutes of Health, that require consideration of sex as a biological variable in all proposals [1, 2]. The purpose of these policies is clear: they seek to reverse the perennial lack of females in both basic and clinical research to better understand how potential sex differences in brain and behavior may influence human health and response to therapeutic drugs. The relative merits of such policies have been debated of late on both practical and conceptual grounds. On a practical level, examining sex as a biological variable poses certain challenges [3]. Additional time and money are required to include both sexes in research studies, which strains already slim grant budgets in a time of unprecedented funding competition. Forcing researchers without backgrounds in endocrinology and genetics to address sex differences in their studies also raises potential problems for study design and interpretation. Conceptually, it has been argued that considering sex as a biological variable does not make sense for all lines of investigation, in part because this ignores social, cultural, and psychological (i.e., gender) influences on human health [3]. It has further been countered that sex is not a simple binary variable, but rather a complex phenotype involving genetic and hormonal components that are influenced by factors such as age and environment [3]. Despite these arguments, however, ignoring possible sex differences in form and function is simply no longer acceptable, given the potential adverse consequences of doing so. For example, women metabolize the drug zolpidem, the active ingredient in the sleeping pill Ambien, more slowly than men, leading to impairments in tasks such as driving the morning after women take this medication [4, 5]. As such, the Food and Drug Administration reduced the recommended Ambien dosage for women by half in 2013 [5], spurring calls for increased attention to sex-specific responses to therapeutic drugs. Compelling arguments in favor of both the inclusion of females and direct examination of sex differences in biomedical research have been provided by numerous investigators [6–9], which have served to increase awareness among researchers. In addition, workshops such as that held at American University in April 2017 (“Sex Differences: From Neuroscience to the Clinic and Beyond”), and meetings sponsored by the Organization for the Study of Sex Differences, the Society for Women’s Health Research, and the Society for Behavioral Neuroendocrinology, have been important venues for bringing researchers together from a variety of perspectives to discuss sex differences in multiple functional systems. Nevertheless, sex differences have yet to truly penetrate the consciousness of most researchers, precipitating the need for special issues such as this and others (e.g., [10, 11]).
Sex differences in all aspects of human health are interesting and important. However, the sex difference that most piques our laboratory’s interest pertains to the relative risk of Alzheimer’s disease in men and women. Although age is the single greatest risk factor for Alzheimer’s, women are at substantially greater risk of developing Alzheimer’s than men, even when accounting for women’s longer lifespans [12, 13]. According to recent reports from the Alzheimer’s Association, women’s estimated lifetime risk of developing Alzheimer’s at ages 65, 75, and 85 is approximately twice that of men [14, 15]. One notable aspect of the sex difference in Alzheimer’s disease risk is that it appears after menopause. Menopause marks reproductive senescence in women, and is characterized by a loss of menstrual cycling and significant hormonal alterations, including dramatic increases in gonadotropin secretion and decreases in circulating estrogen and progestin levels, that result from ovarian and hypothalamic aging. In particular, the ovarian estrogens produced by reproductively mature women are important trophic factors for neurons in regions of the brain, such as the hippocampus and prefrontal cortex [16, 17], that mediate cognitive functions like learning and memory. As such, the loss of estrogens during menopause is thought to render these neurons more vulnerable to age-related decline and neurodegenerative diseases such as Alzheimer’s. Indeed, elderly women with low endogenous estrogen levels experience greater risks of cognitive decline than those with higher estrogen levels [18–21].
If estrogen loss in post-menopausal women contributes to memory deficits, then estrogen replacement could potentially mitigate this loss. However, the promise of estrogen therapy for reducing and/or reversing memory loss in older women has not borne fruit. For example, treatment with conjugated equine estrogens, with or without an accompanying synthetic progestin, does not maintain or improve cognitive function in post-menopausal women over age 65, and in fact, can be detrimental to cognitive function in this population [22, 23]. Moreover, hormone replacement carries small, but statistically significant, risks of breast cancer, heart disease, and stroke [24]. Despite benefits to colorectal and bone health [24], estrogen therapy is no longer generally recommended for women over age 65, including for purposes of maintaining cognition. Estrogen therapy, particularly that involving the potent estrogen 17β-estradiol (E2), appears to have no adverse effects on cognitive function in perimenopausal women in their 50’s [25–27], suggesting altered responsiveness to estrogen therapy from middle- to old-age. Somewhat similar effects have been reported in rat models of aging, in which long-term ovariectomy lasting throughout middle age diminishes the beneficial effects of E2 on hippocampal synaptic plasticity and hippocampal-dependent memory [28–30]. As such, determining how estrogens affect brain function and why the brain’s responsiveness to estrogens decreases with advanced age are important to understanding why women are at greater risk of developing Alzheimer’s than men.
To address these questions as they relate to learning and memory, many researchers, including ourselves, have focused on females. This approach makes sense from the perspective of understanding how estrogens work to regulate memory function in the sex most affected by Alzheimer’s. Historically, our own rationale has been to first understand how estrogens influence memory in female rodents before examining this issue in males. Other labs have taken the opposite approach by examining hippocampal function in male rodents, and the resulting studies often report similar effects to those in females [31, 32]. In addition, high levels of E2 can be found endogenously in the hippocampus of both male and female rats [33, 34]. Thus, numerous pieces of evidence suggest that E2 not only affects the functioning of cognitive brain regions in males, but also that its effects are generally similar in both sexes. However, recent reports suggest that similar functional effects of E2 in both sexes (e.g., on memory and synaptic plasticity) may be driven by different molecular mechanisms in males and females [35], which could have critical implications for the design of therapeutic interventions for men and women. As discussed below, future work must examine potential sex differences at the cellular and molecular level to determine if distinct sex-specific mechanisms underlie phenotypic differences.
In this vein, our laboratory has spent the past decade identifying molecular mechanisms in the hippocampus through which E2 enhances hippocampal memory consolidation in female mice (for recent reviews, see [36, 37]). We have primarily examined these issues in young adult females to better understand how E2 influences memory formation in an optimally functioning system. We believe that these data from young subjects can then provide the foundation for determining how E2, and its loss at reproductive senescence, may influence age-related memory decline and dementia in aging subjects. Therefore, most of this review discusses data collected in young females, but data from aging females is discussed at appropriate points where available. More recently, we have begun to examine these the molecular mechanisms through which E2 may regulate memory consolidation in young males as well, and have found potentially interesting sex differences that support the notion that E2 may exploit different molecular means in males and females to achieve similar behavioral ends. As such, the bulk of this review will focus on our data from females, with particular emphasis on new directions that illustrate the importance of hippocampally-synthesized E2 and interactions between the hippocampus and prefrontal cortex. The remainder of the review will discuss work from our lab and others describing effects of E2 on hippocampal function in males, and putative roles for sex differences in underlying mechanism. We then conclude with recommendations for future research.
2. Molecular mechanisms through which E2 regulates memory consolidation in female mice
2.1. Background
Our laboratory’s work on this subject has focused on the hippocampus because this brain region regulates the formation of numerous types of memory (e.g., spatial, contextual, object recognition) that are affected by aging and Alzheimer’s disease [38–42]. The hippocampus is also exquisitely sensitive to levels of E2. For example, acute E2 treatment in young female rodents increases dendritic spine density in the CA1 region, neurogenesis in the dentate gyrus, and various forms of synaptic plasticity including long-term potentiation (LTP) (e.g., [43–53]). These effects can occur quite rapidly, as increases in CA1 dendritic spine density have been observed in vitro or in vivo as early as 20–30 minutes after bath application, systemic injection, or dorsal hippocampal infusion [54–58]. E2 also swiftly triggers hippocampal cell signaling within minutes of application (e.g., [59–62]), suggesting rapid effects through non-classical estrogen receptor (ER) mechanisms in addition to potentially longer-lasting classical ER mechanisms that regulate gene transcription via estrogen response elements on DNA. Indeed, the canonical ERs, ERα and ERβ, can act both classically as nuclear transcription factors and non-classically by interacting at the membrane with neurotransmitter receptors to stimulate cell signaling [63–65]. Although both classical and rapid mechanisms influence gene transcription, the genes influenced by both processes are unlikely to be identical. Of the identified ERs, intracellular ERα and ERβ, as well as the membrane ER termed G protein-coupled estrogen receptor (GPER), are localized throughout the hippocampus in dendrites, dendritic spines, axons, and terminals [66–68], where they are poised to mediate rapid non-classical effects of estrogens. Given that E2-induced memory consolidation is a relatively fast process lasting between 1–3 hours after treatment [69, 70], these findings render the hippocampus an ideal brain region in which to study the rapid effects of E2 on memory consolidation.
Memory consolidation can be examined using treatments administered prior to training (pre-training) or immediately after training (post-training). Numerous studies have shown that pre-training administration of E2 or ER agonists given systemically or directly into the hippocampus can rapidly enhance various forms of hippocampus-mediated memories including spatial, object, and social memories [57, 70–73]. However, the timing of such treatments make it difficult to tease apart effects on acquisition vs. consolidation and performance vs. memory. Thus, to pinpoint effects of E2 specifically on consolidation, several laboratories, including our own, have used immediate post-training treatments.
We have primarily used two object-based one-trial learning tasks: object recognition and object placement (aka., object location) (Fig. 1). Both tasks take advantage of rodent’s natural proclivity to explore novelty. In both tasks, subjects are habituated within the testing apparatus, which is an empty white square arena (e.g., for 5 minutes/day for 1 or 2 days), after which they are then given the opportunity to explore two identical objects. In some protocols, subjects are given a total of 5 minutes to explore the objects, but our laboratory prefers for subjects to remain in the box until they have accumulated 30 seconds exploring the objects to ensure that all animals accrue the same amount of object exploration [74, 75]. Immediately after training, pharmacological treatments (e.g., intracranial infusions, systemic injections, oral gavage) are administered, after which subjects are returned to their home cages. After a delay (24 or 48 hours for object recognition and 4 or 24 hours for object placement in our laboratory), subjects are returned to the box and again allowed to explore two objects. In object recognition, one object is identical to training and the other is a novel object. If subjects remember the identity of the familiar object, then they should spend more time than chance (15 seconds) exploring the novel object. In object placement, one of the training objects is moved to a different corner of the box. If subjects remember the locations of the training objects, then they should spend more time than chance with the moved object. Thus, the key difference between these two tasks involves the nature of the memory expressed during testing: “what” the object is in object recognition versus “where” the object is in object placement. As described in more detail elsewhere [69, 74, 76], these tasks are advantageous to study the molecular mechanisms underlying memory consolidation because they involve one-trial learning and rapid consolidation (within 3 hours). They also use the same general procedure and apparatus to test multiple types of hippocampal memory and do not require potentially confounding motivational stimuli (e.g., aversive or appetitive) to encourage exploratory behavior.
Fig. 1. Schematic of the object recognition and object placement tasks.
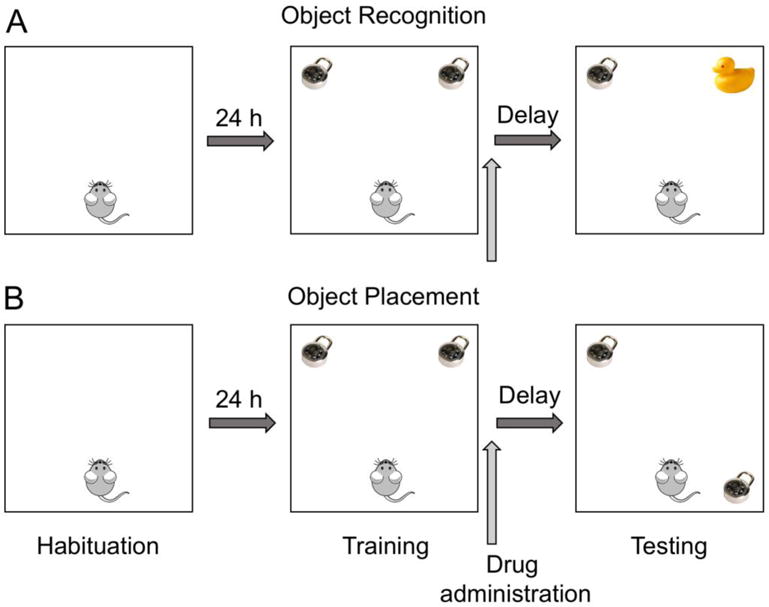
Both tasks begin with a habituation phase in which subjects explore an empty box, typically once or twice for 5 minutes each. During the training phase, subjects explore two identical objects placed near the corners of the box. Training ends after a fixed amount of time (e.g., 5 minutes) or after subjects have accumulated 30 seconds of object exploration. Drugs are administered immediately post-training to assess effects on memory consolidation. After a delay (e.g., 24–48 hours), the testing phase occurs, during which one training object is replaced with a new object (A) or moved to a new location (B). Testing ends after a fixed duration or after 30 seconds of object exploration.
We and others have shown consistently that E2 significantly enhances hippocampal-dependent spatial and object recognition memory consolidation in young male and female mice and rats. A comprehensive discussion of these effects is beyond the scope of this review, but they have been detailed recently in numerous reviews to which we refer the reader [36, 70, 74, 77, 78]. These studies employ acute systemic injections or infusions delivered into the dorsal hippocampus or dorsal third ventricle immediately or within 1–3 hours after behavioral training to pinpoint effects of E2 on the consolidation phase of memory formation (see [69, 70] for further discussion of this post-training rationale). This work has shown that E2 administered immediately, but not 1–3 hours, after training enhances memory as measured in the Morris water maze, object recognition, and object placement tasks. Most of these studies used ovariectomized mice and rats as subjects, although similar effects have been reported in gonadally-intact males (e.g., [31]). The consistency of E2’s ability to enhance memory consolidation across various labs and species, in both sexes, and in behavioral tasks tapping into different types of memory, makes the post-training paradigm an excellent tool for probing the molecular mechanisms through which E2 regulates hippocampal memory formation. Thus, the sections below describe the current state of knowledge about the molecules and molecular processes necessary for post-training E2 treatment to enhance memory consolidation.
2.2. Cell-signaling and receptor mechanisms mediating E2’s effects on memory in females
Nearly ten years ago, our laboratory discovered that phosphorylation of the mitogen activated protein (MAP) kinase called extracellular signal-regulated kinase (ERK) was necessary for E2 to enhance object recognition memory in ovariectomized female mice [59, 62]. We have since extended this finding to object placement (spatial memory) as well [79–82]. Systemic injection (0.2 mg/kg) or dorsal hippocampal infusion (5 μg bilaterally) of E2 increased phosphorylation of the p42 isoform of ERK within 60 or 5 minutes, respectively, and dorsal hippocampal inhibition of ERK phosphorylation prevented E2 from enhancing object recognition memory consolidation [59, 83]. These findings demonstrated for the first time that the memory-enhancing effects of E2 depended on phosphorylation (i.e., activation) of a cell-signaling kinase. Dorsal hippocampal ERK phosphorylation is also necessary for dorsal hippocampal infusion of 5 μg E2 to enhance object recognition memory consolidation in middle-aged ovariectomized mice [62]. However, 5 μg E2 has no effect on object recognition or ERK phosphorylation in aged ovariectomized mice [62], suggesting a loss of responsiveness to E2 in the hippocampus after middle age in female mice. In subsequent work with young and middle-aged ovariectomized mice, we have shown that the beneficial effects of E2 on memory consolidation are mediated in the dorsal hippocampus by complex interactions among cell-signaling pathways and receptors for estrogens and glutamate neurotransmission. For example, upstream from ERK, we found that the ability of E2 to activate p42-ERK and enhance memory consolidation in young ovariectomized mice depended on activation of protein kinase A (PKA), phosphatidylinositol 3-kinase (PI3K), N-methyl-D-aspartate (NMDA) receptors [83, 84], and interactions between metabotropic glutamate receptor 1a (mGluR1a) and ERα or ERβ [79] (Fig. 2, left). Similarly in middle-aged ovariectomized mice, the ability of E2 to enhance object recognition memory consolidation depended on PI3K-induced activation of ERK [62]. Unpublished work from our laboratory suggests that activation of canonical Wnt/β-catenin signaling in the dorsal hippocampus of young ovariectomized mice is also necessary for E2 to enhance object recognition and object placement memory consolidation (Taxier and Frick, unpublished observations; Fig. 2, center; [85]), but it is currently unclear if estrogenic regulation of this signaling pathway is associated with ERK or upstream signaling.
Fig. 2. Schematic illustration of the putative molecular mechanisms underlying estrogenic regulation of memory consolidation in female mice.
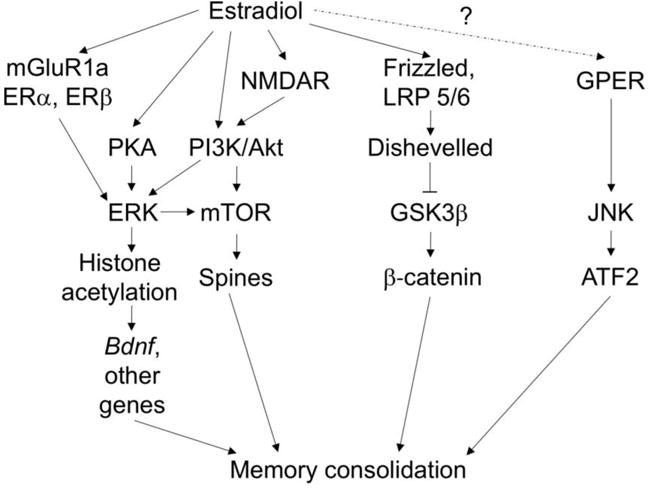
Published work indicates that E2enhances memory consolidation in ovariectomized female mice by rapidly activating ERK via ERα/β-mGluR1a interactions, NMDA receptors, and activation of PI3K/Akt and PKA [79, 83, 84]. ERK phosphorylation triggers activation of mTOR signaling and CA1 dendritic spinogenesis [56, 83], as well as histone H3 acetylation of Bdnf and transcription of multiple other genes [81]. These alterations presumably lead to enhanced memory consolidation. Unpublished data suggest that E2 also enhances memory consolidation by activating canonical Wnt/β-catenin signaling, presumably via activation of the Frizzled receptor-LRP5/6 complex and recruitment of Dishevelled, which dephosphorylates glycogen synthase kinase 3β (GSK3β) and allows the transcription factor β-catenin to translocate into the nucleus and promote gene transcription [85]. The mechanisms through which E2 may interact with NMDA and Frizzled receptors are unknown. Other published findings indicate that activation of GPER enhances object recognition and spatial memory consolidation by activating JNK and the transcription factor ATF2, although the data suggest that E2 does not play a role in the effects of GPER on memory consolidation [86].
Bilateral infusions of agonists for ERα, ERβ, or GPER into the dorsal hippocampus of young ovariectomized mice mimic the beneficial effects of E2 on object recognition and object placement [79, 86], suggesting that activation of any of the ERs can enhance memory consolidation. However, the signaling kinases used by these receptors to influence memory differ. Whereas ERα and ERβ regulate memory via ERK [79], GPER enhances memory in young ovariectomized mice by activating a different MAP kinase, c-Jun N-terminal kinase (JNK) [86] (Fig. 2, left). Indeed, our work showed that E2 does not increase JNK phosphorylation, nor did infusion of a JNK inhibitor or GPER antagonist prevent E2 from enhancing object recognition or object placement memory [86]. Thus, these data suggest the interesting possibility that GPER does not interact with E2 to regulate hippocampal memory. Instead, E2 appears to act via ERα and ERβ to activate ERK and related kinases to influence memory formation.
Downstream from ERK, we have demonstrated multiple ways in which E2 may rapidly regulate gene transcription and protein translation. In one line of research, we showed that epigenetic processes, such as histone acetylation and DNA methylation, were necessary for E2 to enhance object recognition memory in young ovariectomized mice (see [87, 88] for recent reviews). Within 30 minutes of a dorsal hippocampal infusion, E2 significantly increased acetylation of histone H3 in the hippocampus in an ERK-dependent manner, and this acetylation was necessary for E2 to enhance object recognition memory [89, 90]. Subsequent work in young and middle-aged ovariectomized mice showed that E2 rapidly (within 30 minutes) increased H3 acetylation of specific promoters of the gene for brain derived neurotrophic factor (Bdnf) [81], a neurotrophin that is both essential for hippocampal memory formation and is regulated by E2 [91–93]. Not only did E2 increase H3 acetylation of Bdnf promoters II and IV in middle-aged females, but treatment also significantly increased levels of BDNF and Pro-BDNF proteins in the dorsal hippocampus [81]. Collectively, these data suggest that E2 treatment triggers the activation of numerous cell-signaling cascades that converge on ERK to rapidly promote gene transcription and protein translation via epigenetic mechanisms including histone acetylation.
In addition to altering protein translation via gene transcription, E2 can rapidly influence local protein synthesis by activating the mammalian target of rapamycin (mTOR) signaling pathway. mTOR mediates local protein synthesis within hippocampal dendrites and is necessary for hippocampal memory formation [94]. Because mTOR signaling is activated by both ERK and PI3K [94–96], we surmised that it may play a role in estrogenic regulation of memory formation. In young ovariectomized mice, we found that E2 activated dorsal hippocampal mTOR signaling within 5 minutes of a bilateral dorsal hippocampal infusion, and that this activation was necessary for E2 to enhance object recognition memory consolidation [83]. This finding was particularly intriguing because of previous reports from Drs. Victoria Luine, Maya Frankfurt, and colleagues that systemically-injected E2 could increase dendritic spine density in the CA1 and medial prefrontal cortex of ovariectomized rats within just 30 minutes [54, 55]. In young ovariectomized mice, several studies show a similarly rapid increase in CA1 dendritic spine density by systemic or dorsal hippocampal administration of E2 or agonists of ERα and GPER [57, 71, 72, 97]. The rapid timeframe in which E2 and ER agonists increases spines in rats and mice suggested to us that local protein synthesis, such as that mediated by mTOR, could play a major role in E2-induced spinogenesis. Therefore, in collaboration with Drs. Luine and Frankfurt, we examined in young ovariectomized mice whether rapid activation of ERK and/or mTOR contributed to E2’s effects on dendritic spines. We first found that a bilateral dorsal hippocampal infusion of E2 significantly increased basal and apical spine density on CA1 dendrites within 30 minutes, and this effect lasted for two hours [56]. The effect was specific to the CA1, as infusions did not affect spine density in the dentate gyrus. Next, to examine whether ERK or mTOR activation was necessary for E2 to increase dendritic spine density, we infused inhibitors of ERK or mTOR phosphorylation (U0126 and rapamycin, respectively) bilaterally into the dorsal hippocampus in conjunction with an infusion of E2 into the dorsal third ventricle (this protocol allows us to deliver E2 adjacent to the dorsal hippocampus without risking tissue damage from two successive infusions into the hippocampus). As we previously observed with memory consolidation [83], inhibition of ERK or mTOR phosphorylation prevented E2 from increasing CA1 dendritic spine density 2 hours after infusion [56] (Fig. 3A–C). These data demonstrate that rapid activation of ERK and mTOR signaling regulates E2-induced spinogenesis in CA1. Indeed, these data provided the first in vivo evidence that E2 influences dendritic morphology in females via activation of cell signaling. The findings are also consistent with in vitro data from adult male rat hippocampus and embryonic cortical rat cultures showing that E2-induced spinogenesis depends on activation of ERK and other signaling cascades [32, 58, 98, 99]. Current studies in our laboratory are investigating which ERs may mediate these effects, although previous work suggests the involvement of ERα and GPER [57]. Importantly, how might this rapid E2-induced spinogenesis relate to E2-induced memory consolidation? Numerous studies link increased spine density with enhanced memory and synaptic plasticity (e.g., [100–102]). Although evidence of a direct relationship between the two remains circumstantial, the fact that both E2-induced memory consolidation and CA1 spinogenesis depend on ERK and mTOR phosphorylation provides evidence supporting the notion that E2-induced spinogenesis underlies the enhanced memory consolidation. This relationship is also bolstered by timing, in that the increased spine density observed 30 min and 2 hours after E2 infusion occurs well within the 3-hour time window in which E2 enhances memory consolidation (e.g., [59, 103, 104]). In other work, a single injection of E2 increased CA1 dendritic spine density in ovariectomized rats 24, 48, and 72 hours later [105], suggesting that E2-induced spine density increases may last through object placement and object recognition testing 24 and 48 hours later, respectively. As such, the E2 data lend support to the idea that rapid effects of E2 on cell signaling trigger CA1 spinogenesis, which then provides a morphological substrate for memory consolidation.
Fig. 3. Effects of E2 on apical and basal dendritic spine density in hippocampal CA1 and the mPFC are dependent on activation of ERK or mTOR in the dorsal hippocampus. (A).
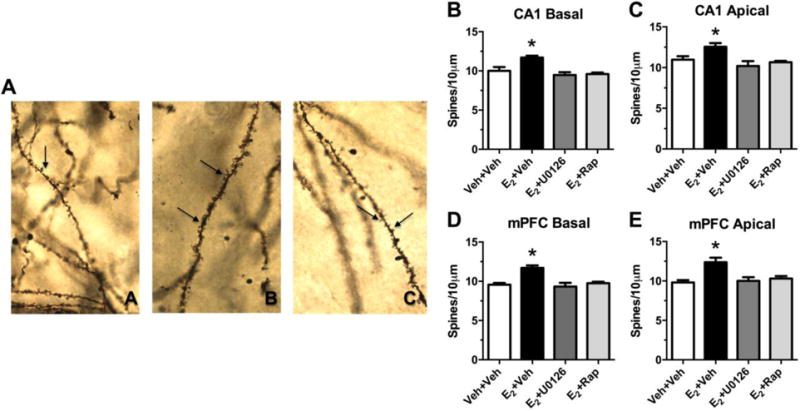
Photomicrograph of Golgi-impregnated secondary basal dendrites of CA1 pyramidal cells (image A = vehicle+vehicle, image B = E2+vehicle, image C = E2+U0126). Arrows denote spines. Under oil 63×. (B–E) Two hours after an intracerebroventricular infusion of E2, basal and apical spine density was significantly increased on pyramidal neurons in CA1 (A, B, C) and mPFC (D, E) relative to vehicle. These effects were blocked by dorsal hippocampal infusion of inhibitors of ERK (U0126) or mTOR (rapamycin) phosphorylation. Bars represent the mean ± SEM, *p < 0.05 relative to all other groups. Adapted from [56] with permission.
3. Interactions between the hippocampus and medial prefrontal cortex
Research on estrogens and cognition has been dominated by a primary focus on the hippocampus. However, accumulating evidence suggests that E2 can influence various forms of learning and memory in other brain regions, such as the prefrontal cortex, striatum, amygdala, and perirhinal cortex (e.g., [106–108]). As mentioned above, systemic injections of E2 increase dendritic spine density not only in the dorsal hippocampus, but also in the medial prefrontal cortex [54, 55]. Both brain regions are essential for similar types of learning and memory, and accumulating evidence suggests a functional connection between the two [109–113]. Therefore, in our aforementioned spine density study, we examined the effects of dorsal hippocampal E2 infusion on spine density in the medial prefrontal cortex. As a control for non-specific effects on brain regions not directly involved in learning and memory, we also examined the ventromedial hypothalamic nucleus as an estrogen-sensitive brain region involved in a different type of behavior (lordosis) [114]. Although dorsal hippocampal infusion of 5 μg E2 had no effect on spine density in the hypothalamus, it increased basal dendritic spine density in the medial prefrontal cortex two hours later [56], suggesting that estrogenic regulation of dorsal hippocampal function influences dendritic morphology in the prefrontal cortex. To determine if the effects on cortical spinogenesis depended on rapid activation of ERK or mTOR signaling, as in the CA1, we examined spine density in the prefrontal cortex of mice infused with 10 μg E2 into the dorsal third ventricle and inhibitors of ERK or mTOR phosphorylation into the dorsal hippocampus. Ventricular infusion of E2 increased both basal and apical dendritic spine density in the medial prefrontal cortex [56]. As in the CA1, inhibitors of ERK or mTOR blocked this effect [56] (Fig. 3D–E), demonstrating that E2-induced spinogenesis in the medial prefrontal cortex depends on ERK and mTOR activation in the dorsal hippocampus. These data suggest the intriguing possibility that the dorsal hippocampus and medial prefrontal cortex work in concert to mediate the memory-enhancing effects of E2 in the dorsal hippocampus. Moreover, the results raise numerous questions about the role of E2 in the medial prefrontal cortex in mediating memory consolidation. To address these issues, our laboratory has conducted preliminary work showing that bilateral infusion of E2 into the medial prefrontal cortex enhances object recognition and object placement memory consolidation in ovariectomized mice (Tuscher and Frick, unpublished observations; [115]). Interestingly, our preliminary data also suggest that temporary post-training inactivation of the medial prefrontal cortex blocks the memory-enhancing effects of dorsal hippocampal E2 infusion (Tuscher, Taxier, and Frick, unpublished observations; [116]). If confirmed, these data would support the notion that the dorsal hippocampus and medial prefrontal cortex interact to mediate the effects of E2 on memory consolidation in females. Indeed, the data suggest a more circuit-level effect of E2 on memory that may involve not only the medial prefrontal cortex but also other brain regions to which the hippocampus is connected, such as the basal forebrain, amygdala, entorhinal cortex, and perirhinal cortex. Recent work employing a contextual fear conditioning paradigm indicates that the development and maturation of engram cells in the prefrontal cortex of mice depends on input from several brain regions including the hippocampus, medial entorhinal cortex, and basolateral amygdala [113]. Thus, identifying the regions involved in the putative circuit involved in E2’s effects on memory is an area ripe for future investigation.
4. Role of hippocampally-synthesized estradiol
Estrogens are synthesized in multiple tissues through the body. The primary sources of estrogens in females are the ovaries, however, the brain also makes estrogens. The hippocampus contains all of the enzymes necessary to synthesize estrogens [117], and indeed, the concentration of E2 in the hippocampus of male and female rats is higher than in plasma [33, 34]. Although ovariectomy significantly decreases hippocampal E2 levels, measureable levels remain present, and indeed, levels in ovariectomized females are comparable to intact females in diestrus [34]. That sufficient levels of E2 remain after ovariectomy to compare to endogenous estrous cycle stages suggests that de novo hippocampal E2 synthesis may contribute to memory formation. This idea was first tested in male song birds using hippocampal implants containing an inhibitor of aromatase, the enzyme that converts testosterone into E2. In gonadally-intact male zebra finches, such pre-training aromatase inhibition blocks spatial memory formation [118, 119]. This effect appears to depend at least in part on activation of GPER [119]. In addition, aromatase inhibition during fear extinction training impairs extinction recall in gonadally-intact male rats [120]. Interestingly, the hippocampus of female rats appears to be more sensitive to aromatase inhibition than that of males, as illustrated by data showing that systemic treatment with the aromatase inhibitor letrozole reduces CA1 spine density and LTP significantly more in females than in males [121–123]. Based on these collective data, we reasoned that aromatase inhibition might prevent memory consolidation in females. Ovariectomized mice received bilateral dorsal hippocampal infusion of letrozole immediately or three hours after training in the object recognition and object placement tasks. Infusion of letrozole immediately, but not two or three hours, after training dose-dependently blocked memory consolidation in both tasks [80] (Fig. 4A,B), suggesting that de novo hippocampal E2 synthesis is necessary for females to form object recognition and spatial memories. A role for rapid E2 synthesis was supported by data showing that E2 levels were transiently elevated 30 minutes after object training, an effect that was blocked by letrozole [80] (Fig. 4C). Together, these data suggest that object training triggers local E2 synthesis, which then binds to ERs and facilitates memory consolidation.
Fig. 4. Aromatase inhibition impairs memory consolidation and reduces hippocampal E2 levels. (A, B).
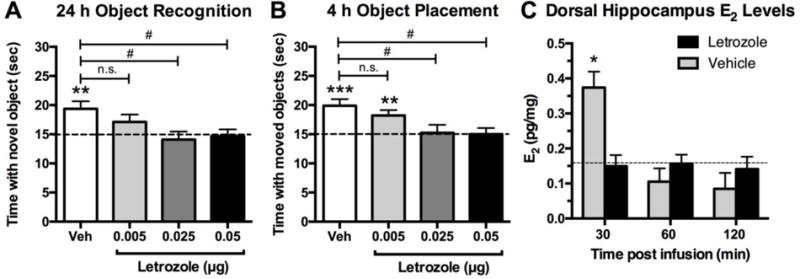
Mice receiving bilateral dorsal hippocampal infusion of 0.025 or 0.05 μg letrozole immediately after training were significantly impaired in both object recognition (A) and object placement (B) relative to chance (dashed line at 15 seconds, **p < 0.01, ***p < 0.001) and to vehicle (#p < 0.05), suggesting that these doses blocked memory consolidation. A 0.005 μg dose of letrozole had no effect on object placement and a minimal effect on object recognition. (C) Mice receiving bilateral dorsal hippocampal infusion of 0.025 μg letrozole had significantly lower dorsal hippocampal E2 levels than vehicle-treated mice 30 minutes after infusion (*p < 0.05 as measured by enzyme-linked immunosorbent assay (EIA)). E2 levels in vehicle-treated mice dropped to the level of those in letrozole-treated mice by 60 minutes after training. Dashed horizontal line indicates the average background E2 concentration as reported by EIA for control wells. Adapted from [80] with permission.
To study the role of ERs in mediating the effects of de novo E2, we more recently infused ERα or ERβ antagonists into the dorsal hippocampus of ovariectomized mice and measured effects on memory. Inhibition of a single ER by an ER antagonist in ovariectomized subjects can provide indirect information about the role of individual ERs in the memory-enhancing effects of de novo hippocampal E2 synthesis because any hippocampal E2 in ovariectomized females would presumably be derived from de novo synthesis rather than the gonads. Preliminary data suggest that ERα antagonism blocked memory consolidation in the object placement, but not object recognition, task, whereas ERβ antagonism blocked consolidation of both types of memory (Kim and Frick, unpublished observations; [124]). These data suggest that the newly synthesized E2 induced by object training mediates spatial memory via either ER, but regulates object recognition via ERβ. We should note that E2 can be made in other non-gonadal tissues (e.g., adrenals, fat), however, the fact that aromatase inhibition in the hippocampus blocks spatial and object memory consolidation [80, 118] strongly suggests that the learning-induced E2 that influences memory consolidation is hippocampally derived. Nevertheless, although these findings support a primary role for de novo hippocampal E2 synthesis in memory consolidation, considerably more work must be done to fully understand the extent to which hippocampal E2 influences memory processes.
5. Sex differences in the molecular mechanisms regulating estradiol’s effects on memory consolidation
Thus far, this review has focused exclusively on molecular mechanisms underlying estrogenic regulation of memory formation in females because the vast majority of work on this subject has been conducted in this sex. However, E2 also regulates hippocampal function in males, and emerging data suggest interesting sex differences in the molecular mechanisms through which E2 mediates memory consolidation in males and females. In both young males and females, gonadectomy has been reported to impair hippocampal memory (e.g., spatial reference memory, object recognition) and reduce CA1 dendritic spine density, and these effects can be reversed by E2 or dihydrotestosterone [78, 125–130]. Relatively few studies have examined the effects of E2 on memory in males, but the balance suggests a beneficial effect on memory. For example, several studies of gonadectomized male rats report that chronic pre-training E2 treatment reverses gonadectomy-induced deficits in spatial reference and working memory, as well as conditioned taste aversion and operant learning [127, 131, 132]. Relevant to the present discussion of consolidation, a single systemic post-training injection of E2 given immediately after Morris water maze training enhanced spatial reference memory consolidation in gonadally-intact male rats [31]. Because more thorough reviews describing the effects of exogenous E2 on hippocampal function in both sexes have been published previously [78, 133, 134], the section below will focus solely on information relevant to putative sex differences in the molecular mechanisms underlying estrogenic regulation of hippocampal memory consolidation.
As discussed above, the ability of E2 to enhance memory consolidation in ovariectomized female mice depends on estrogen- and glutamate receptor-driven activation of numerous cell signaling pathways, including ERK, PI3K, PKA, and mTOR [59, 62, 83]. Moreover, the ability of E2 to regulate dendritic spine density in the dorsal hippocampus and prefrontal cortex of ovariectomized females depends on ERK and mTOR activation in the dorsal hippocampus [115]. Similarly, Suguru Kawato’s group has shown that bath application of E2 to hippocampal slices from gondally-intact adult males increases CA1 dendritic spine density within 2 hours in a manner dependent on activation of ERK, PI3K, PKA, protein kinase C (PKC), and calcium calmodulin kinase II (CaMKII) [32, 99, 135–138]. Activation of these cell-signaling cascades is also necessary for E2 to potentiate theta-burst-stimulated LTP in males [32]. Also in males, bath application of testosterone and the non-aromatizable androgen dihydrotestosterone produce similar effects on spine density and LTP as E2, and these effects are blocked by inhibitors of ERK, PKA, PKC, LIM kinase (LIMK), and calcineurin [135, 139]. Thus, these data indicate that both androgens and estrogens can regulate spine density and LTP in males. Interestingly, LIMK signaling also plays a role in E2’s ability to increase CA1 dendritic spine density and LTP in ovariectomized female rats [46], suggesting similar cell-signaling mechanisms underlying E2’s effects on spinogenesis and synaptic plasticity in males and females.
The overlap between cell-signaling mechanisms involved in spinogenesis and synaptic plasticity in females and males suggested to us that E2 might employ similar cellular mechanisms to regulate memory in males and females. As such, we recently began to investigate the effects of E2 on hippocampal cell signaling and memory consolidation in young male mice. We first needed to establish that E2 could enhance memory consolidation in our behavioral paradigms. Our first experiments have used bilateral dorsal hippocampal infusions of 5 μg E2 per hemisphere because this dose enhances spatial and object memory consolidation in female mice [59, 62, 79–82]. Ovariectomized female, castrated male, and sham castrated male mice received bilateral dorsal hippocampal infusions of 5 μg E2 immediately after object recognition and object placement training. Preliminary data indicate that E2 enhanced memory consolidation in both tasks in all groups (Koss and Frick, unpublished observations; [140, 141]). These findings suggest two interesting points. First, that dorsal hippocampal infusion of E2 enhances spatial and object memory consolidation in male mice, which is consistent with the beneficial effects of dorsal hippocampal E2 infusion on spatial and object memory consolidation in ovariectomized female mice [59, 79, 80, 86, 142]. This effect in males is also consistent with a previous report that post-training dorsal hippocampal E2 infusion enhances spatial memory consolidation in gonadally-intact male rats [31]. Effects of dorsal hippocampal E2 infusion on middle-aged and aged males have yet to be examined as in females [62], so the ability of this treatment to reverse age-related memory decline in males is an open question for future investigation. The second point raised by these data is that E2 appears to enhance memory consolidation in males regardless of gonadal status, suggesting that exogenous E2 can regulate memory in the absence of circulating estrogens and/or androgens. Supporting a role for de novo hippocampal E2 in males, our preliminary data also suggest that dorsal hippocampal infusion of letrozole blocks memory consolidation in castrated male mice, as it does in ovariectomized female mice [80], but not in sham castrated mice [141]. Other studies have shown that aromatase inhibition produces a much more robust reduction of LTP and CA1 dendritic spine density in ovariectomized and/or gonadally-intact female mice than in gonadally-intact male mice [121–123]. Although methodological differences (e.g., age, gonadal status, duration of letrozole treatment) make it somewhat difficult to directly compare these studies, the balance of data indicates that the testes may contribute to sex differences in the role of hippocampal E2 in hippocampal function. Nevertheless, in vivo data in adult mice suggest that both hippocampally-synthesized E2 and exogenous E2 can positively regulate memory in males and females.
Interestingly, the biochemical mechanisms underlying the memory-enhancing effects of E2 may differ between the sexes. Recall that the ability of E2 to enhance object recognition and object placement memory consolidation in females depends on phosphorylation of ERK in the dorsal hippocampus [59, 79]. Infusion of 5 μg E2 in females results in a robust and reliable increase in p42 ERK phosphorylation within 5 minutes [59, 79, 86]. However, our pilot work shows no effect of 5 μg E2 on p42 or p44 ERK in the dorsal hippocampus of males (Koss and Frick, unpublished observations; [140]). Moreover, blocking ERK phosphorylation in males does not prevent E2 from enhancing memory in the object tasks as observed in females (Koss and Frick, unpublished observations; [141]). These preliminary findings indicate that E2 regulates memory consolidation in males via a signaling mechanism different from ERK. This finding is contrary to in vitro reports showing that blocking ERK phosphorylation in gonadally-intact adult male mice and rats prevents E2-induced LTP induction and CA1 dendritic spinogenesis [32, 99, 136], suggesting potentially important differences between the in vivo and in vitro preparations. We are currently trying to determine which signaling pathways are involved in E2-induced memory consolidation in males. If supported by additional studies, this putative sex difference in underlying mechanism suggests potentially important sex differences in the way in which E2 regulates memory.
There is some precedence for sex differences in the mechanisms through which E2 regulates hippocampal function. For example, in hippocampal cultures from neonatal rats, E2 interacts with mGluRs to increase ERK-dependent phosphorylation of cAMP response element binding (CREB) protein in females, but not in males [63]. Because mGluR1a activation is necessary for E2 to increase ERK phosphorylation and enhance memory consolidation in adult females [79], the inability of E2 to stimulate ERK-dependent CREB phosphorylation in neonatal males could provide insight into our observed sex difference in E2-induced ERK activation. Sex differences in E2-stimulated cell signaling may result from distinct effects of ERs on cell signaling in males and females. Alternatively, sex differences may result from differences in the specific ERs used in males and females to influence hippocampal function. This possibility is supported by a recent study showing similar potentiating effects of E2 on glutamatergic synaptic transmission in male and females rats that were mediated by different ERs in each sex. In females, the probability of glutamate release depended on presynaptic activation of ERβ, whereas glutamate sensitivity was regulated post postsynaptically by GPER [35]. In males, glutamate release was mediated presynaptically by ERα, and glutamate sensitivity was regulated postsynaptically by ERβ [35]. These data suggest that different ERs act at different parts of the synapse in male and female rats to produce the same potentiating effects of E2 on glutamatergic transmission. This phenomenon is reminiscent of our observation that E2 produces similar memory-enhancing effects in male and female mice by apparently activating different cell-signaling pathways in each sex. Although we clearly must do more work to better understand how E2 regulates memory consolidation in males and females, these preliminary observations suggest the presence of interesting, and potentially important, sex differences in the neural mechanisms underlying estrogenic mediation of memory.
6. Conclusions
This review has highlighted the molecular mechanisms thus far known to be essential for E2 to enhance memory consolidation in females, and presented the intriguing possibility that these mechanisms may be different in males. Much of the literature on sex differences to date has focused on whether a sex difference is present in measureable outcomes, such as memory function, synaptic plasticity, or neuronal morphology. The advent of the new “sex as a biological variable” policy in the United States promises many more such reports in the future. The presence of observable sex differences leads to obvious next steps in trying to figure out the cause of these sex differences. However, we would caution against concluding that a variable is not affected by sex if no observable sex difference is present. As seen from our work and that from the Woolley laboratory [35, 133], E2 can produce similar effects on memory consolidation and synaptic transmission in both males and females, leading to the potential conclusion of no sex differences in response to E2. However, these data belie the fact that the molecular mechanisms underlying these effects of E2 (i.e., cell signaling and ER involvement) differ between the sexes. In both cases, the causes of these sex differences are currently unknown, but future work will undoubtedly address this question. Considerable possibilities abound, potentially involving genetic and epigenetic regulation of signaling kinases and ERs.
Why might it matter if males and females differ in their molecular responses to E2 if the ultimate result of treatment (e.g., enhanced memory) is similar? We would argue that sex differences in molecular means to a phenotypic end could be vitally important to the development of new therapeutic drugs for neuropsychiatric and neurodegenerative diseases. If E2 enhances memory consolidation via different mechanisms in males and females, then disease processes may differentially act upon those processes to alter the effects of E2 on memory (Fig. 5). Even if disease processes have similar effects on the brain, using a one-size-fits-all strategy for the treatment of any condition makes no sense if the molecular mechanisms underlying the condition differ between men and women. If, as an exceedingly simplistic example, ERK phosphorylation is necessary for E2 to enhance memory in women with Alzheimer’s disease but not men with Alzheimer’s disease, then drugs that potentiate ERK phosphorylation could improve memory in female, but not male, patients. Thus, the more we learn about putative sex differences in the molecular mechanisms underlying cognitive dysfunction in neuropsychiatric and neurodegenerative diseases, the more likely it seems that sex-specific approaches to new drug development will be needed. Such approaches could provide unique opportunities for the development of therapeutics that more effectively reduce cognitive dysfunction in both sexes than those in current use. This exciting possibility should be embraced with open arms by the research community, rather than with dread at having to consider another sex, as it may lead to improvements in human health that are not possible when considering only a single sex.
Fig. 5. Potential impact of sex differences in molecular mechanisms underlying estrogenic regulation of memory consolidation.
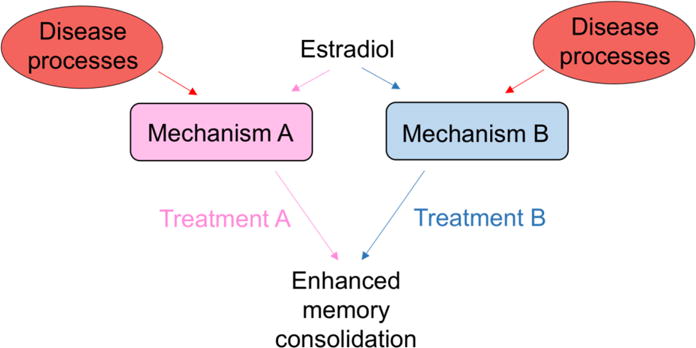
If E2 mediates memory consolidation in each sex via different molecular mechanisms (e.g., Mechanism A for females and Mechanism B for males), then different treatments (Treatment A for females and Treatment B for males) may be warranted during aging or in conditions such as Alzheimer’s or depression for E2 or related drugs to enhance memory formation. Different treatments may also be necessary if disease processes differentially affect Mechanisms A and B, resulting in sex differences in response to E2.
Highlights.
Estradiol (E2) rapidly enhances hippocampal memory consolidation in both sexes
Many underlying molecular mechanisms are known in females, but not males
Putative sex differences in these molecular mechanisms are described
Role for the prefrontal cortex in effects of hippocampal E2 infusion is discussed
Involvement of de novo hippocampal E2 synthesis in both sexes is considered
Acknowledgments
Karyn Frick would like to thank Drs. Colin Saldanha and Terry Davidson, Ms. Bernadette Storey-Laubach, and the Center for Behavioral Neuroscience at American University for organizing the sex differences workshop upon which this review is based and for the invitation to speak at this workshop. During the writing of this manuscript, the authors were supported by National Institutes of Health (R01MH107886), Alzheimer’s Association (SAGA-17-419092), and University of Wisconsin-Milwaukee Research Growth Initiative (101×334) awards to K.M.F., the University of Wisconsin-Milwaukee College of Letters & Science, and the University of Wisconsin-Milwaukee Graduate School. Empirical work from our laboratory described herein was supported by the National Institutes of Health (R01MH107886, R01AG022525, R03MH065460), two University of Wisconsin-Milwaukee Research Growth Initiative Awards (101×334, 101×240), the American Federation for Aging Research, the University of Wisconsin-Milwaukee, and Yale University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J. 2016;30:519–24. doi: 10.1096/fj.15-279554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tannenbaum C, Schwarz JM, Clayton JA, De Vries GJ, Sullivan C. Evaluating sex as a biological variable in preclinical research: the devil in the details. Biol Sex Differ. 2016;7:13. doi: 10.1186/s13293-016-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eliot L, Richardson SS. Sex in context: Limitations of animal studies for addressing human sex/gender neurobehavioral health disparities. J Neurosci. 2016;36:11823–30. doi: 10.1523/JNEUROSCI.1391-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verster JC, Roth T. Gender differences in highway driving performance after administration of sleep medication: a review of the literature. Traffic Inj Prev. 2012;13:286–92. doi: 10.1080/15389588.2011.652751. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration FDA Drug Safety Communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist) 2013 http://www.fda.gov.proxy.lib.mcw.edu/drugs/drugsafety/ucm334033.htm. Accessed May 22, 2017.
- 6.Shansky RM, Woolley CS. Considering sex as a biological variable will be valuable for neuroscience research. J Neurosci. 2016;36:11817–22. doi: 10.1523/JNEUROSCI.1390-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32:2241–7. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks CE, Clayton JA. Sex/gender influences on the nervous system: Basic steps toward clinical progress. J Neurosci Res. 2017;95:14–6. doi: 10.1002/jnr.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy MM. Multifaceted origins of sex differences in the brain. Philosophical Transactions B of the Royal Society. 2016;371:20150106. doi: 10.1098/rstb.2015.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahill L. An issue whose time has come. J Neurosci Res. 2017;95:12–3. doi: 10.1002/jnr.23972. [DOI] [PubMed] [Google Scholar]
- 12.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA. 2002;288:2123–9. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 13.Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, et al. Rates and risk factors for dementia and Alzheimer’s disease: Results from EURODEM pooled analyses. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 14.Alzheimer’s Association 2012 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2012;8:131–68. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Alzheimer’s Association 2015 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2015;11:332–84. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Brann D, Raz L, Wang R, Vadlamudi R, Zhang Q. Oestrogen signalling and neuroprotection in cerebral ischaemia. J Neuroendocrinol. 2012;24:34–47. doi: 10.1111/j.1365-2826.2011.02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohrabji F, Williams M. Stroke neuroprotection: oestrogen and insulin-like growth factor-1 interactions and the role of microglia. J Neuroendocrinol. 2012;25:1173–81. doi: 10.1111/jne.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaffe K, Barnes D, Lindquist K, Cauley J, Simonsick EM, Penninx B, et al. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28:171–8. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. The Lancet. 2000;356:708–12. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- 20.Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm Behav. 2002;41:259–66. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs EG, Weiss BK, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, et al. Impact of sex and menopausal status on episodic memory circuitry in early midlife. J Neurosci. 2016;36:10161–73. doi: 10.1523/JNEUROSCI.0951-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 23.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 24.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperbert C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 25.Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affect Study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699–708. doi: 10.1212/WNL.0000000000002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, LeBlanc ES, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Int Med. 2013;173:1429–36. doi: 10.1001/jamainternmed.2013.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in milddle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2003;147:607–14. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- 29.Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen’s ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci USA. 2010;107:19543–8. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vedder LC, Bredemann TM, McMahon LL. Estradiol replacement extends the window of opportunity for hippocampal function. Neurobiol Aging. 2014;35:2183–92. doi: 10.1016/j.neurobiolaging.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: Interaction with cholinergic systems. Behav Neurosci. 1996;110:626–32. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, et al. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res. 2015;1621:147–61. doi: 10.1016/j.brainres.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 33.Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, et al. Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology. 2009;150:5106–12. doi: 10.1210/en.2009-0305. [DOI] [PubMed] [Google Scholar]
- 34.Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, et al. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oberlander JG, Woolley CS. 17β-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci. 2016;36:2677–90. doi: 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frick KM. Building a better hormone therapy?: How understanding the rapid effects of sex steroid hormones could lead to novel therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.deToledo-Morrell L, Stoub TR, Wang C. Hippocampal atrophy and disconnection in incipient and mild Alzheimer’s disease. Prog Brain Res. 2007;163:741–53. doi: 10.1016/S0079-6123(07)63040-4. [DOI] [PubMed] [Google Scholar]
- 39.Driscoll I, Sutherland RJ. The aging hippocampus: Navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- 40.Cohen SJ, Stackman RW., Jr Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res. 2015;285:105–17. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 42.Eichenbaum H. The Cognitive Neuroscience of Memory. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 43.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–54. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 45.Gu Q, Moss RL. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–9. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, et al. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–93. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–8. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–22. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17ß-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–9. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 50.Sharrow KM, Kumar A, Foster TC. Calcineurin as a potential contributor in estradiol regulation of hippocampal synaptic function. Neuroscience. 2002;113:89–97. doi: 10.1016/s0306-4522(02)00151-3. [DOI] [PubMed] [Google Scholar]
- 51.Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–48. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rat. J Neurobiol. 2003;55:247–60. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- 53.Barker JM, Galea LA. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 54.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–93. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 55.Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: Role of dendritic spines. Endocrinology. 2012;1534:3357–67. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuscher JJ, Luine VN, Frankfurt M, Frick KM. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J Neurosci. 2016;36:1483–9. doi: 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phan A, Suschkov S, Molinaro L, Reynolds K, Lymer JM, Bailey CD, et al. Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc Natl Acad Sci USA. 2015;112:16018–23. doi: 10.1073/pnas.1522150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, et al. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA. 2008;105:14650–5. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, et al. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–7. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci USA. 2000;97:3602–7. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–8. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 62.Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–78. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai M-J, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 65.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, et al. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 67.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–71. [PubMed] [Google Scholar]
- 68.Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, et al. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci. 2015;35:2384–97. doi: 10.1523/JNEUROSCI.1298-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frick KM, Fernandez SM, Harburger LL. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta Gen Subj. 2010;1800:1045–55. doi: 10.1016/j.bbagen.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luine VN. Estradiol and cognitive function: Past, present and future. Horm Behav. 2014;66:602–18. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, et al. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology. 2012;37:2299–309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- 73.Daniel JM. Effects of oestrogen on cognition: What have we learned from basic research? J Neuroendocrinol. 2006;18:787–95. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- 74.Tuscher JJ, Fortress AM, Kim J, Frick KM. Regulation of object recognition and object placement by ovarian sex steroid hormones. Behav Brain Res. 2015;285:140–57. doi: 10.1016/j.bbr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–91. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 76.Fortress AM, Frick KM. Pharmacologically manipulating learning and memory. In: Bimonte-Nelson HA, editor. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. New York: Springer Science+Business Media; 2015. pp. 165–210. [Google Scholar]
- 77.Luine V. Recognition memory tasks in neuroendocrine research. Behav Brain Res. 2015;285:158–64. doi: 10.1016/j.bbr.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frick KM. Sex steroid hormones matter for learning and memory: Estrogenic regulation of hippocampal function in male and female rodents. Learn Mem. 2015;22:472–93. doi: 10.1101/lm.037267.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–94. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, et al. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav. 2016;83:60–7. doi: 10.1016/j.yhbeh.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM. 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn Mem. 2014;21:457–67. doi: 10.1101/lm.034033.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J, Boulware MI, Frick KM. Role of G-protein-coupled estrogen receptor (GPER/GPR30) in hippocampal memory and cell signaling in female mice. Soc Neurosci Abstr. 2013 Poster 376.05. [Google Scholar]
- 83.Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation on mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–55. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–21. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taxier LR, Keifer MM, Philippi SM, Fortress AM, Frick KM. Dorsal hippocampal Wnt/β-catenin signaling is required for 17β-estradiol to enhance object memory consolidation in female mice. Soc Neurosci Abstr. in press. [Google Scholar]
- 86.Kim J, Szinte JS, Boulware MI, Frick KM. 17β-estradiol and agonism of G-protein Coupled Estrogen Receptor (GPER) enhance hippocampal memory via different cell-signaling mechanisms. J Neurosci. 2016;36:3309–21. doi: 10.1523/JNEUROSCI.0257-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frick KM. Epigenetics, oestradiol and hippocampal memory consolidation. J Neuroendocrinol. 2013;25:1151–62. doi: 10.1111/jne.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Front Neuroendocrinol. 2014;35:530–49. doi: 10.1016/j.yfrne.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32:2344–51. doi: 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate the estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA. 2010;107:5605–10. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–70. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: A potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–52. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–68. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- 94.Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84:275–91. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signaling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 97.Gabor C, Lymer J, Phan A, Choleris E. Rapid effects of the G-protein coupled oestrogen receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice. Physiol Behav. 2015;149:53–60. doi: 10.1016/j.physbeh.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 98.Sellers KJ, Erli F, Raval P, Watson IA, Chen D, Srivastava DP. Rapid modulation of synaptogenesis and spinogenesis by 17β-estradiol in primary cortical neurons. Front Cell Neurosci. 2015;9:137. doi: 10.3389/fncel.2015.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murakami G, Hojo Y, Ogiue-Ikeda M, Mukai H, Chambon P, Nakajima K, et al. Estrogen receptor KO mice study on rapid modulation of spines and long-term depression in the hippocampus. Brain Res. 2014;1621:133–46. doi: 10.1016/j.brainres.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Jedlicka P, Vlachos A, Schwarzacher SW, Deller T. A role for the spine apparatus in LTP and spatial learning. Brain Res. 2008;192:12–9. doi: 10.1016/j.bbr.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 101.Segal M. Dendritic spines: Morphological building blocks of memory. Neurobiol Learn Mem. 2017;138:3–9. doi: 10.1016/j.nlm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 102.Moser B, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA. 1994;91:12673–5. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;26:8517–22. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gervais NJ, Jacob S, Brake WG, Mumby DG. Systemic and intra-rhinal-cortical 17-β estradiol administration modulate object-recognition memory in ovariectomized female rats. Horm Behav. 2013;64:642–52. doi: 10.1016/j.yhbeh.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 107.Maeng LY, Cover KK, Taha MB, Landau AJ, Milad MR, Lebrón-Milad K. Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J Neurosci Res. 2017;95:163–75. doi: 10.1002/jnr.23826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 110.Warburton EC, Brown MW. Neural circuitry for rat recognition memory. Behav Brain Res. 2015;285:131–9. doi: 10.1016/j.bbr.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatoryaminoacid pathway from the hippocampus to the prefrontal cortex: contribution of AMPA receptors in hippocampo-prefrontal cortex transmission. Eur J Neurosci. 1992;4:1285–95. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 112.Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behav Brain Res. 2011;225:389–95. doi: 10.1016/j.bbr.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, et al. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017;356:73–8. doi: 10.1126/science.aam6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frankfurt M. Gonadal steroids and neuronal plasticity. Studies in the adult rat hypothalamus. Ann N Y Acad Sci. 1994;743:45–59. doi: 10.1111/j.1749-6632.1994.tb55786.x. [DOI] [PubMed] [Google Scholar]
- 115.Tuscher JJ, Fortress AM, Frick KM. The role of the dorsal hippocampus and medial prefrontal cortex in estradiol-mediated enhancement of object memory consolidation in female mice. Soc Neurosci Abstr. 2016 Poster 179.08. [Google Scholar]
- 116.Tuscher JJ, Taxier LR, Frick KM. Chemogenetic investigation of dorsal hippocampal-medial prefrontal interactions in estradiol-mediated enhancement of object memory consolidation in female mice. Soc Neurosci Abstr. in press. [Google Scholar]
- 117.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology. 2013;154:4707–14. doi: 10.1210/en.2013-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bailey DJ, Makeyeva YV, Paitel ER, Pedersen AL, Hon AT, Gunderson JA, et al. Hippocampal aromatization modulates spatial memory and characteristics of the synaptic membrane in the male zebra finch. Endocrinology. 2017;158:852–589. doi: 10.1210/en.2016-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn Mem. 2014;21:347–50. doi: 10.1101/lm.034926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, et al. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology. 2010;151:1153–60. doi: 10.1210/en.2009-0254. [DOI] [PubMed] [Google Scholar]
- 122.Fester L, Prange-Kiel J, Zhao L, Blittersdorf BV, Böhm J, Jarry H, et al. Estrogen-regulated synaptogenesis in the hippocampus: Sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol. 2012;131:24–9. doi: 10.1016/j.jsbmb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 123.Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, et al. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–26. doi: 10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim J, Szinte JS, Frick KM. Distinct effects of estrogen receptor inhibition on novel object recognition and spatial memory consolidation in ovariectomized mice. Soc Neurosci Abstr Poster. 2014;451(11) [Google Scholar]
- 125.Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–12. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- 126.Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–82. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 127.Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–83. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- 129.Mendell AL, Atwi S, Bailey CDC, McCloskey D, Scharfman HE, MacLusky NJ. Expansion of mossy fibers and CA3 apical dendritic length accompanies the fall in dendritic spine density after gonadectomy in male, but not female, rats. Brain Struct Funct. 2017;222:587–601. doi: 10.1007/s00429-016-1237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–92. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–6. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- 132.Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–77. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koss WA, Frick KM. Sex differences in hippocampal function. J Neurosci Res. 2017;95:539–62. doi: 10.1002/jnr.23864. [DOI] [PubMed] [Google Scholar]
- 134.McCarthy MM, Konkle ATM. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 135.Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, et al. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol. 2012;131:37–51. doi: 10.1016/j.jsbmb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 136.Murakami G, Tsurugizawa T, Hatanaka Y, Komatsuzaki Y, Tanabe N, Mukai H, et al. Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun. 2006;351:553–8. doi: 10.1016/j.bbrc.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 137.Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, et al. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–67. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 138.Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, et al. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res Rev. 2008;57:363–75. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 139.Hatanaka Y, Hojo Y, Mukai H, Murakami G, Komatsuzaki Y, Kim J, et al. Rapid increase of spines by dihydrotestosterone and testosterone in hippocampal neurons: Dependence on synaptic androgen receptor and kinase networks. Brain Res. 2014;1621:121–32. doi: 10.1016/j.brainres.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 140.Koss WA, Frick KM. Memory-enhancing effects of 17β-estradiol in male and female mice. Soc Neurosci Abstr. 2016 Poster 179.06. [Google Scholar]
- 141.Koss WA, Gremminger RL, Philippi SM, Frick KM. Effects of dorsal hippocampal estradiol treatment and aromatase inhibition on memory consolidation in male mice. Soc Neurosci Abstr. in press. [Google Scholar]
- 142.Pereira LM, Bastos CP, de Souza JM, Ribeiro FM, Pereira GS. Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor α. Neurobiol Learn Mem. 2014;114:1–9. doi: 10.1016/j.nlm.2014.04.001. [DOI] [PubMed] [Google Scholar]


