Abstract
The activities of antioxidative enzymes, i.e. superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX), in the leaves and roots of Zea mays L. plants exposed to abiotic (methyl jasmonate, MJ, or/and copper, Cu) and biotic (Trigonotylus caelestialium) factors were examined. The contribution of MJ as a signal molecule in the defense mechanism against abiotic and biotic stresses was studied. All plants were cultivated hydroponically and divided into three groups: not treated by abiotic factors (control), treated by MJ only (MJ) and by MJ and Cu (MJ + Cu) and in each group half of the plants were exposed to T. caelestialium attack. The enzymatic activities of SOD, CAT, APX, and GPX in the leaves were higher in the insect-treated than non-insect-treated control plants, but lower in both MJ + Cu- or MJ- and insect-treated plants. In the roots, the enzyme activities were elevated in all insect-treated plants with the highest rise in MJ + Cu, in comparison with the MJ-treated plants. The results showed that MJ and MJ + Cu were efficient in reducing the activity of the antioxidative enzymes in the leaves under the insect influence by elevating enzyme activity in the roots.
Keywords: Catalase, Insect, Peroxidase, Maize, Methyl jasmonate, Superoxide dismutase
Both abiotic (e.g. Cu excess) and biotic (e.g. insect attack) stresses trigger cross-talk among hormones, including jasmonic acid signaling pathway (Paudel et al. 2013) and generate reactive oxygen species (ROS), which can be directly toxic towards insects (Wasternack and House 2013). Therefore, the activities of enzymes, used as markers of oxidative stress, are crucial, i.e. superoxide dismutase (SOD) degrading O·−2 to H2O2 and catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidases (GPX) converting H2O2 to H2O.
Plants attacked by herbivorous insects induce volatile organic compounds, such as methyl jasmonate (MJ) to regulate the attack by attracting other organisms that destroy damaging insects (Turlings et al. 2012), or to inform other plants about a coming danger to prepare for fighting (Kim and Felton 2013). The defense signaling against chewing insects is mediated by the biosynthesis, transport, and perception of molecules of jasmonates pathway and the following interaction of these molecules with other plant hormones and messengers (Boughton et al. 2006; Tian et al. 2014).
Maize (Z. mays L.) is a crucial agricultural crop in the world after wheat and rice. Moreover, it is used not only as a food, but also as a pharmaceutical and industrial product. Also it is worth studying insect infestations by the Holarctic plant bug T. caelestialium (Kirkaldy) (Heteroptera: Miridiae, Stenodemini) as one of the possible biotic stresses to maize limiting its productivity. Moreover, there is a limited number of papers studying T. caelestialium on plants used in agriculture (Ito 2004) and exposed to exogenous jasmonates (Koramutla et al. 2014).
The information about the exact regulation of ROS by MJ in the insect attack is still patchy. The aim of this study was to determine the activities of the antioxidative enzymes, SOD, CAT, APX, and GPX, in the leaves and roots of Z. mays L. plants exposed to abiotic factors, Cu and MJ (in concentrations chosen on the grounds of the previously published data; Hanaka et al. 2015, 2016) and a biotic factor, T. caelestialium.
Trigonotylus caelestialium insects were collected from grasslands near Lublin, Poland. The insects were reared in a plastic cage in an insect-rearing room at 25 ± 1 °C under a 16 h photoperiod and maintained on grass and maize leaves, which were exchanged with new ones every 3 days. The sucking insects at the 4th larval stage were taken to the experiment with plants.
Zea mays L. cv. Reduta plants were grown hydroponically on the Hoagland nutrient solution (3 plants per pot). The plants were cultivated in a growth chamber at 25 °C (day)/18 °C (night) under a 16 h photoperiod and photosynthetic photon flux density of 130 µmol m−2 s−1. 10-day-old plants were divided into three groups: cultivated without abiotic stress (control), treated by MJ for 24 h (MJ) and treated by MJ for 24 h and then transferred to a fresh Hoagland nutrient solution with addition of Cu2+ for 5 h (MJ + Cu). MJ at 10 µM and Cu at 50 µM (in the form of CuSO4 × 5 H2O) were used and applied straight to the Hoagland solution. In each group, half of the 13-day plants were additionally exposed to insect attack, then covered with a plastic cage enabling gas exchange for the following 7 days. After that period, all insects were adult. 20-day-old leaves and roots were cut and frozen in liquid nitrogen for analyses of SOD, CAT, APX, and GPX.
Proteins concentration were determined according to Bradford (1976). Activities of SOD, CAT, APX, and GPX were measured following Verma and Dubey (2003), Aebi (1984), Nakano and Asada (1987), and Milosevic and Slusarenko (1996), respectively.
All data were analyzed using one-way ANOVA and Fisher’s post hoc test with significance determined at p < 0.05 on Statistica ver. 6 software (SoftStat, Inc., USA). The mean values (three to five separate experiments with at least three replicates each) ± SE were presented.
The total protein concentration in the leaves was from 1.99 to 3.29 times higher than in the roots, with the highest difference detected for the MJ + Cu- and MJ-treated plants in the presence of insects (Table 1). In the leaves, all agents applied to the plants, compared with the control, significantly elevated the protein concentration, and the most pronounced rise was detected for both the MJ- and insect-treated plants in the following order (and statistical significance): MJ + insect (a) > MJ − insect (b) = MJ + Cu + insect (b) > MJ + Cu − insect (c) = control + insect (c) > control − insect (d) (Table 1). In the roots, the MJ + Cu- or MJ-treated plants without the presence of insects achieved the highest protein concentration and the exact order was as follows (with statistical significance): MJ + insect (a) = MJ − insect (a) = MJ + Cu − insect (a) > control − insect (b) > control + insect (c) = MJ + Cu + insect (c) (Table 1).
Table 1.
Protein concentration in Zea mays leaves and roots exposed to abiotic, i.e. MJ (10 µM applied for 24 h) or both MJ (10 µM applied for 24 h) and Cu (50 µM applied for 5 h), and biotic (T. caelestialium) factors
| Treatment | Protein concentration [% of control without insect] | |
|---|---|---|
| Leaves | Roots | |
| MJ + Cu without insect | 114 c | 128 a |
| MJ without insect | 128 b | 119 a |
| Control with insect | 113 c | 86 c |
| MJ + Cu with insect | 124 b | 84 c |
| MJ with insect | 145 a | 102 a |
Different letters indicate significant differences between the treatments. Values are the means of three–five separate experiments ± SE (three replicates each)
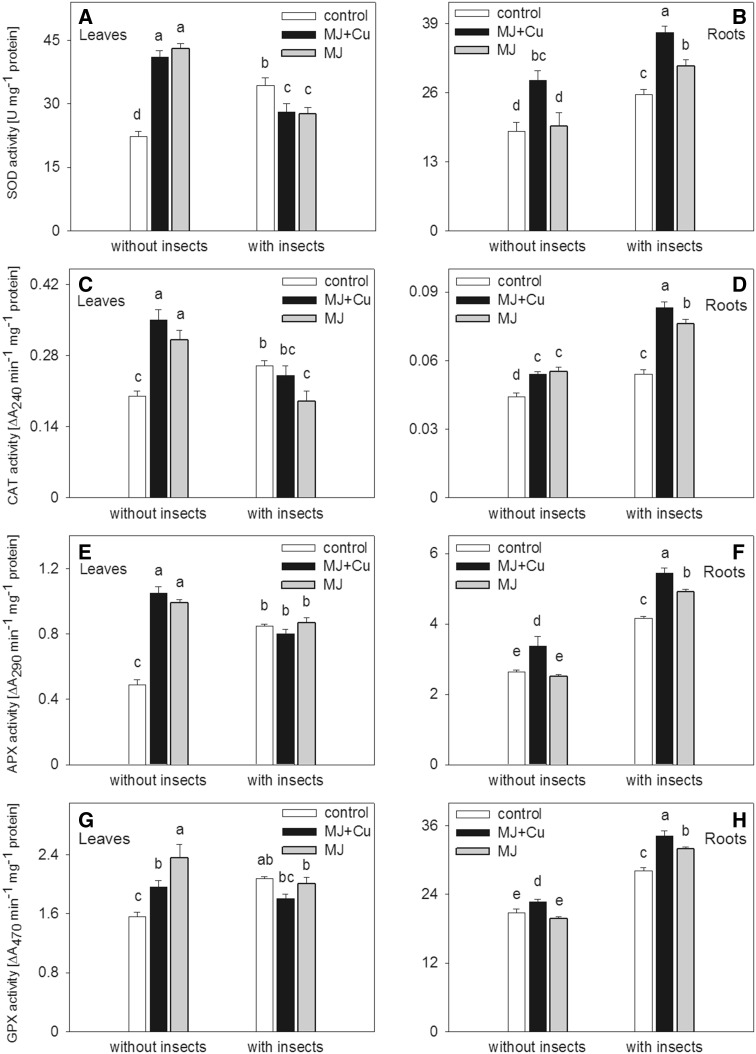
The higher activities of SOD and CAT in the leaves than in the roots were accompanied by higher activities of APX and GPX in the roots than in the leaves (Fig. 1a–h). Both in the leaves and roots, higher activity of the enzymes was detected in the control plants with T. caelestialium, in comparison with the control plants not sucked by the insect (Fig. 1a–h).
Fig. 1.
Activities of SOD (a), CAT (b), APX (c), and GPX (d) in Zea mays leaves and roots exposed to abiotic, i.e. MJ (10 µM applied for 24 h) or both MJ (10 µM applied for 24 h) and Cu (50 µM applied for 5 h), and biotic (T. caelestialium) factors. Different letters indicate significant differences between the treatments. Values are the means of three–five separate experiments ± SE (three replicates each)
In the leaves, the most pronounced enzyme activity was detected without insect sucking after MJ + Cu or MJ supplementation, but it declined after the insect treatments (Fig. 1a, c, e, g). In the leaves of the insect-treated plants, after MJ + Cu or MJ addition, the enzyme activity was the same or lower than that in the insect-treated control (Fig. 1 a, c, e, g).
In the roots of the T. caelestialium-stimulated maize plants, the enzyme activities were significantly higher than in all corresponding treatments without the insect infestation (Fig. 1b, d, f, h). The most pronounced activities were detected in the MJ + Cu- and MJ-treated plants after the insect attack with the highest activity observed in the first of the cases mentioned (Fig. 1b, d, f, h).
The insects themselves caused enhanced stress measured as elevated activity of antioxidant enzymes in all root treatments and in the leaves of control plants (Fig. 1a–h). Contrary to this statement, the insects reduced stress interpreted as reduced activity of enzymes in the leaves of the MJ + Cu- or MJ-treated plants in comparison with the non-insect-treated plants (Fig. 1a–h).
The concentration of total proteins, which take part in protection against stress conditions, was significantly higher in the leaves of Z. mays than in the roots. Such a result suggests that leaves can be better protected than the roots. Moreover, in our research, exogenous MJ under insect attack caused production and significant accumulation of proteins in the leaves, but to a lesser extent also in the roots. Maize plants tested in the experiment represent diversified activity of antioxidative enzymes between the leaves and the roots, which is in accordance with our previous results (Strubińska and Hanaka 2011; Hanaka et al. 2016). Zea mays “enzymatic kit” seems to be well-balanced because the activity of two enzymes, SOD and CAT, is higher in the leaves, but activity of both peroxidases, APX and GPX, is higher in the roots. In the plant reaction to adverse conditions, a broad range of proteins is involved, thus besides detoxifying enzymes, e.g. SOD, CAT, APX, and GPX, also genes encoding pathogenesis-related proteins are induced by MJ (Faurie et al. 2009). At the early stage of insect attack, it seems that MJ treatment causes rapid increase of O·−2 and H2O2 and subsequently elevated activities of SOD, CAT, and peroxidases detoxify ROS molecules (Boughton et al. 2006; Koramutla et al. 2014; Farooq et al. 2016). In our experiment as well as in the papers of Botelho-Júnior et al. (2008), Ye et al. (2013), and Rigsby et al. (2016), MJ application elevates activity of peroxidases. It is known that peroxidases participate in lignification, as a response to the pathogen infection and wounding (Rigsby et al. 2016). Moreover, in the response to herbivore attack, concentrations of detoxifying enzymes in plants could be elevated in a MJ dose-dependent manner (Boughton et al. 2006). We proved that the roots react statistically more spectacularly than the leaves because in all measurements with the insects, the activity of enzymes increased. It means that the information about a stimulus acting in one part of plant body (an insect sucking the leaf) is quickly moved to the distant parts (the roots), e.g. by MJ. Thus, the defense system works systemically and helps the plant to survive in a changing environment.
Cellular redox changes appear in response to the mechanical damage after insect feeding (Paudel et al. 2013). In our experiment, the insect attack elicited response in the plant organism by elevating antioxidant enzyme activities in all root treatments and in the leaves of control plants, which was connected with increased O·−2 and H2O2 accumulation in plant tissues (Wasternack and Hause 2013). In the roots, independent of the presence of insects, MJ addition reduced enzyme activities in comparison with MJ + Cu supplementation (except for CAT activity). Furthermore, during metal treatment, the level of jasmonates is reduced, which suggests a defensive role in mitigation of excess ROS (Dar et al. 2015). The present results have shown the effect of Cu on defense against insects by reduction of the stress level in the leaves and simultaneously enhancement thereof in the roots.
Exogenous application of MJ reduced feeding damage caused by insects through minimizing the number and size of feeding scars (Fedderwitz et al. 2016), or reducing the growth rate of insect population (Koramutla et al. 2014), but not through deterring the insects from attacking the plant (Fedderwitz et al. 2016). Furthermore, MJ stimulated defense genes expression and increased density of glandular trichomes on leaves, which negatively influenced the herbivore population (Boughton et al. 2006; Horgan et al. 2009; Tian et al. 2014). Moreover, MJ applied exogenously induced reactions similar to the natural wounding, which proves that MJ can be a substantial molecule in stimulating plant resistance in the natural environment (Thaler et al. 2012). On the other hand, endogenous MJ production is stimulated by the damage signals for which the molecular pattern seems to be similar to the insect attack (Wasternack and Hause 2013).
It should be also mentioned that MJ pathway do not play its role in the isolation from other defense systems. For instance, MJ treatment may result in the enhancement of trypsin protease inhibitor levels (Ye et al. 2013) or production of enzymes involved in the SA and JA biosynthesis (i.e. lipoxygenase, allene oxide synthase) (Botelho-Júnior et al. 2008). Moreover, most scientists confirm the JA–SA antagonistic interaction (Zhang et al. 2017). But in Z. mays plants attacked by caterpillars, no antagonistic crosstalk between the JA and SA pathways was stated (Rostás et al. 2013). On the other hand, it was proved that the elevated levels of SA in Cu treated maize plants were directly responsible for JA induction (Engelberth et al. 2011). It means that in Zea plants relationship between the JA and SA pathways in a specific stress can be confusing and should not be generalized.
In conclusion, our results have shown that, compared with the control, the MJ treatment did not change the antioxidative enzyme activities in the plant roots not subjected to the insect attack (except for the elevation of CAT activity), whereas in the same conditions, MJ + Cu elevated the enzyme activities. Moreover, both MJ and Cu were potent molecules in modulating enzyme activity in the presence of the sucking insect because they prepared the roots for reception of the stress, in consequence limiting the stress in the leaves. This implies involvement of the MJ-dependent signaling pathway in the plant defense against the insect attack under the experimental conditions used. Thus, MJ has a potential in agricultural research and application.
Acknowledgements
Authors express their gratitude to Prof. Waldemar Maksymiec from Maria Curie-Skłodowska University in Poland for the encouragement and helpful advises.
Abbreviations
- APX
Ascorbate peroxidase
- CAT
Catalase
- MJ
Methyl jasmonate
- GPX
Guaiacol peroxidase
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
Compliance with ethical standards
Conflict of interest
The authors declare there is no conflict of interest.
References
- Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Botelho-Júnior S, Siqueira-Júnior CL, Jardim BC, Machado OLT, Neves-Ferreira AGC, Perales J, Jacinto T. Trypsin inhibitors in passion fruit (Passiflora f. edulis flavicarpa) leaves: accumulation in response to methyl jasmonate, mechanical wounding, and herbivory. J Agric Food Chem. 2008;56(20):9404–9409. doi: 10.1021/jf8013266. [DOI] [PubMed] [Google Scholar]
- Boughton AJ, Hoover K, Felton GW. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol Exp Appl. 2006;120:175–188. doi: 10.1111/j.1570-7458.2006.00443.x. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dar TA, Uddin M, Khan MMA, Hakeem KR, Jaleel H. Jasmonates counter plant stress: a review. Environ Exp Bot. 2015;115:49–57. doi: 10.1016/j.envexpbot.2015.02.010. [DOI] [Google Scholar]
- Engelberth J, Viswanathan S, Engelberth MJ. Low concentrations of salicylic acid stimulate insect elicitor responses in Zea mays seedlings. J Chem Ecol. 2011;37:263–266. doi: 10.1007/s10886-011-9926-3. [DOI] [PubMed] [Google Scholar]
- Farooq M, Gill RA, Islam F, Ali B, Liu H, Xu J, He S, Zhou W. Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front Plant Sci. 2016;7:468. doi: 10.3389/fpls.2016.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurie B, Cluzet S, Merillion J-M. Implication of signaling pathways involving calcium, phosphorylation and active oxygen species in methyl jasmonate-induced defense responses in grapevine cell cultures. J Plant Physiol. 2009;166:1863–1877. doi: 10.1016/j.jplph.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Fedderwitz F, Nordlander G, Ninkovic V, Björklund N. Effects of jasmonate-induced resistance in conifer plants on the feeding behaviour of a bark-chewing insect, Hylobius abietis. J Pest Sci. 2016;89:97–105. doi: 10.1007/s10340-015-0684-9. [DOI] [Google Scholar]
- Hanaka A, Maksymiec W, Bednarek W. The effect of methyl jasmonate on selected physiological parameters of copper-treated Phaseolus coccineus plants. Plant Growth Regul. 2015;77:167–177. doi: 10.1007/s10725-015-0048-8. [DOI] [Google Scholar]
- Hanaka A, Wójcik M, Dresler S, Mroczek-Zdyrska M, Maksymiec W. Does methyl jasmonate modify the stress response in Phaseolus coccineus treated with Cu? Ecotoxicol Environ Safe. 2016;124:480–488. doi: 10.1016/j.ecoenv.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Horgan FG, Quiring DT, Lagnaoui A, Pelletier Y. Effects of altitude of origin on trichome-mediated anti-herbivore resistance in wild Andean potatoes. Flora. 2009;204:49–62. doi: 10.1016/j.flora.2008.01.008. [DOI] [Google Scholar]
- Ito K. The role of the feeding habits of Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae) on the production of pecky rice grains with special reference to the occurrence of split-hull paddy. Jpn J Appl Entomol Zool. 2004;48:23–32. doi: 10.1303/jjaez.2004.23. [DOI] [Google Scholar]
- Kim J, Felton GW. Priming of antiherbivore defensive responses in plants. Insect Sci. 2013;20:273–285. doi: 10.1111/j.1744-7917.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- Koramutla MK, Kaur A, Negi M, Venkatachalam P, Bhattacharya R. Elicitation of jasmonate-mediated host defense in Brassica juncea (L.) attenuates population growth of mustard aphid Lipaphis erysimi (Kalt.) Planta. 2014;240:177–194. doi: 10.1007/s00425-014-2073-7. [DOI] [PubMed] [Google Scholar]
- Milosevic N, Slusarenko AJ. Active oxygen metabolism and lignification in the hypersensitive response in bean. Physiol Mol Plant Pathol. 1996;49:143–158. doi: 10.1006/pmpp.1996.0045. [DOI] [Google Scholar]
- Nakano Y, Asada K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28:131–140. [Google Scholar]
- Paudel J, Copley T, Amirizian A, Prado A, Bede JC. Arabidopsis redox status in response to caterpillar herbivory. Front Plant Sci. 2013;4:1–11. doi: 10.3389/fpls.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigsby CM, Herms DA, Bonello P, Cipollini D. Higher activities of defense-associated enzymes may contribute to greater resistance of manchurian ash to emerald ash borer than a closely related and susceptible congener. J Chem Ecol. 2016;42(8):782–792. doi: 10.1007/s10886-016-0736-5. [DOI] [PubMed] [Google Scholar]
- Rostás M, Winter TR, Borkowski L, Zeier J. Copper and herbivory lead to priming and synergism in phytohormones and plant volatiles in the absence of salicylate-jasmonate antagonism. Plant Signal Behav. 2013;8(6):e24264-1–e24264-3. doi: 10.4161/psb.24264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubińska J, Hanaka A. Adventitious root system reduces lead uptake and oxidative stress in sunflower seedlings. Biol Plant. 2011;55(4):771–774. doi: 10.1007/s10535-011-0185-5. [DOI] [Google Scholar]
- Thaler JS, Humphrey PT, Whiteman NK. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012;17:260–270. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Tian D, Peiffer M, De Moraes CM, Felton GW. Roles of ethylene and jasmonic acid in systemic induced defense in tomato (Solanum lycopersicum) against Helicoverpa zea. Planta. 2014;239:577–589. doi: 10.1007/s00425-013-1997-7. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Hiltpold I, Rasmann S. The importance of root-produced volatiles as foraging cues for entomopathogenic nematodes. Plant Soil. 2012;358:51–60. doi: 10.1007/s11104-012-1295-3. [DOI] [Google Scholar]
- Verma S, Dubey RS. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003;164:645–655. doi: 10.1016/S0168-9452(03)00022-0. [DOI] [Google Scholar]
- Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Song Y, Long J, Wang R, Baerson SR, Pan Z, Zhu-Salzman K, Xie J, Cai K, Luo S, Zeng R. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. PNAS. 2013;110(38):E3631–E3639. doi: 10.1073/pnas.1305848110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang F, Melotto M, Yao J, He SY. Jasmonate signaling and manipulation by pathogens and insects. J Exp Bot. 2017;68(6):1371–1385. doi: 10.1093/jxb/erw478. [DOI] [PMC free article] [PubMed] [Google Scholar]