Abstract
Aegilops cylindrica, a salt-tolerant gene pool of wheat, is a useful plant model for understanding mechanism of salt tolerance. A salt-tolerant USL26 and a salt-sensitive K44 genotypes of A. cylindrica, originating from Uremia Salt Lake shores in Northwest Iran and a non-saline Kurdestan province in West Iran, respectively, were identified based on screening evaluation and used for this work. The objective of the current study was to investigate the expression patterns of four genes related to ion homeostasis in this species. Under treatment of 400 mM NaCl, USL26 showed significantly higher root and shoot dry matter levels and K+ concentrations, together with lower Na+ concentrations than K44 genotype. A. cylindrica HKT1;5 (AecHKT1;5), SOS1 (AecSOS1), NHX1 (AecNHX1) and VP1 (AecVP1) were partially sequenced to design each gene specific primer. Quantitative real-time PCR showed a differential expression pattern of these genes between the two genotypes and between the root and shoot tissues. Expressions of AecHKT1;5 and AecSOS1 was greater in the roots than in the shoots of USL26 while AecNHX1 and AecVP1 were equally expressed in both tissues of USL26 and K44. The higher transcripts of AecHKT1;5 in the roots versus the shoots could explain both the lower Na+ in the shoots and the much lower Na+ and higher K+ concentrations in the roots/shoots of USL26 compared to K44. Therefore, the involvement of AecHKT1;5 in shoot-to-root handover of Na+ in possible combination with the exclusion of excessive Na+ from the root in the salt-tolerant genotype are suggested.
Keywords: HKT1;5, NHX1, Salinity, Sodium exclusion, SOS1
Introduction
Increasing soil salinity due to human activities, climate changes as well as natural mineral weathering and secondary salinization is one of the major abiotic constraints affecting agricultural productivity worldwide. Increased salinization of arable land is estimated to result in land loss (by up to 30%) with devastating global consequences by 2050, when world population is projected to be 9.1 billion (FAO 2011). The conventional method of introgressing genes from related wild germplasm to a cultivated species encounters difficulty due to incompatibility barriers. Therefore, an alternative way to transfer alien genes to a crop species is transgenic technology (Arzani and Ashraf 2016). However, the molecular marker-aided traditional breeding and transformation techniques of gene transfer from salt-tolerant wild species require candidate genes to be identified whose expression is associated with salt tolerance.
The genetic basis of sodium and potassium selectivity has been investigated in relation to salt tolerance at the molecular, cellular and whole plant levels (Arzani and Ashraf 2016; Schachtman and Munns 1992; Shabala et al. 2013). One key mechanism of salt tolerance is the ability of a plant to regulate Na+ transport at both the cellular and tissue levels, either by minimizing the amount of Na+ entering the plant through its roots, or by secreting Na+ into organelles, cells and tissues where it can do far less harm (Apse and Blumwald 2007; Munns and Tester 2008). Sodium exclusion does not allow the accumulation of Na+ to toxic levels within leaves and that leads to a high K+/Na+ discrimination, has frequently been described as an important mechanism involved in salt tolerance in cereal crops such as bread wheat (Gorham 1990; Schachtman and Munns 1992), durum wheat (Houshmand et al. 2005), barley (Garthwaite et al. 2005; Wei et al. 2003) and rice (Zhu et al. 2001). The plasma membrane Na+/H+ SOS1 (salt overly sensitive) antiporters, the high-affinity potassium transporters (HKTs) and the intracellular Na+/H+ antiporters (NHXs) are known to be involved in the Na+ homeostasis (Arzani and Ashraf 2016; Hamamoto et al. 2015; Shi et al. 2002). For instance, the key role that HKT1;5 (previously HKT8) plays in salt tolerance through sequestering Na+ to the non-photosynthetic reservoir, leaf sheaths and roots has been reported in wheat (Byrt et al. 2007) and rice (Platten et al. 2013). In addition, the role of the vacuolar NHX1 gene has been well documented as contributing to the cytoplasmic Na+ extrusion into the vacuole (Bassil and Blumwald, 2014). Vacuolar H+-pyrophosphatase (VPs) genes are also required to power the Na+/H+ antiporter through the electrochemical gradient for H+ across the tonoplast (Zhao et al. 2007). In this regard, earlier reports showed that transgenic plants co-overexpressing the H+-PPase VP1 and vacuolar Na+/H+ antiporter NHX1 have a far greater tolerance to high concentrations of NaCl (Brini et al. 2007; Zhao et al. 2007). Nevertheless, the plant ability to discriminate between Na+ and K+ in soil solution and to preferentially exclude Na+ and accumulate K+ has also been described as a crucial salt tolerance mechanism in the Triticeae tribe (Arabbeigi et al. 2014; Arzani and Ashraf 2016; Dvorak et al. 1994; Shabala et al. 2013).
The wild relatives of cultivated plants, which include their ancestors and more or less distantly related species, perform superior under adverse environments, and hence constitute a valued gene pool for tolerance to abiotic stress (Arzani and Ashraf 2016; Saha et al. 2016). Aegilops cylindrica Host., commonly known as Jointed goat grass, is an amphidiploid (2n = 4x = 28; CCDD) originated from the ancestors of A. markgrafii (Greuter) Hammer (2n = 2x= 14; CC) and A. tauschii Coss. (2n = 2x = 14; DD) species and represents a valuable source of salt tolerance genes (Arabbeigi et al. 2014; Arzani and Ashraf 2016; Colmer et al. 2006; Kiani et al. 2015). Our previous research identified a hyper-salt tolerant genotype originated from the Uremia Salt Lake shores (North-West Iran) with the ability to exclude high Na+ ions (Arabbeigi et al. 2014; Kiani et al. 2015). The observations for the germplasm exploration conducted in the West Iran showed that A. cylindrica is the sole member of Triticeae tribe that grows in the presence of high concentrations of salt in salinized shore area of Uremia Salt Lake (Arzani and Ashraf 2016). Knowledge about the sequences and expression profiles of the genes responsible for Na+ exclusion would contribute towards better understanding of the mechanisms leading to hyper-salt tolerance of A. cylindrica. The aim of this work was, therefore, to investigate the expression profiles of some of the most important Na+ transporter related genes (namely, AecHKT1;5, AecSOS1, AecNHX1 and AecVP1) in the root and shoot tissues of both salt tolerant and salt sensitive genotypes of A. cylindrica.
Materials and methods
Plant material, growth conditions and treatments
A hyper-salt tolerant genotype (USL26, originating from Uremia Salt Lake shores Northwest Iran) and a salt sensitive genotype (K44, originating from Kurdestan province, West Iran) were selected based on our previous studies (Arabbeigi et al. 2014; Kiani et al. 2015) and used in this study. Data on growth parameters and physiological measurements of the roots and shoots of 44 genotypes under control and 400 mM NaCl treatments (4 weeks) were presented in this study. All the details related to salt tolerance evaluation experiment are given in our previous publication (Arabbeigi et al. 2014). The whole set of A. cylindrica genotypes (88) were also assessed for salt tolerance at a lower stress level (350 mM NaCl) due to the poor growth and survival rates of the genotypes originated from non-saline habitat (Kiani et al. 2015; for the list of the genotypes see its Supplementary Table online at: doi:10.1007/s11738-015-1881-0).
For gene expression analysis, seeds were germinated for 4 days at 4 °C on moist filter paper in Petri dishes. Germinated seeds were planted into 60 × 40 cm plastic trays containing gravel, under greenhouse conditions. The excess solution passing through the drainage holes at the bottom of each tray was collected by an underneath plate. Seedlings were watered initially with tap water, and then irrigated with half-strength Hoagland nutrient solution 3 days after emergence. At second leaf emergence, the nutrient solution was replaced with full strength Hoagland nutrient solution to which was added NaCl in three gradual steps of irrigation until final concentrations reached 400 mM (EC ≈ 40 ds m−1). Greenhouse air temperature ranged from 25 °C (day) to 17 °C (night). Treatments were replicated four times with ten plants per replication. The experiment was carried out based on a factorial design with three factors; NaCl treatments (0 and 400 mM NaCl at 24 h and 400 mM at 7 days), two genotypes and two tissues as well as two biological and two technical replications. Shoot and root tissues of each genotype were collected during sampling, snap frozen in the liquid nitrogen, and immediately stored at − 80 °C until use.
Na+ and K+ concentrations
The root and shoot samples were dried at 80 °C for 48 h to determine the dry matter (DM). One gram of the ground shoot and root dried sample was further dried to ash at 550 °C for 4 h; mixed with 2 M hot HCl, filtered, and brought to a final volume of 50 ml with distilled water. The Na+ and K+ concentrations of the solutions were analyzed using flame photometry (Jenway PFP7, UK).
RNA extraction and cDNA synthesis
In total, 100 mg of leaf and root tissues were ground into a fine powder using liquid nitrogen. Total RNA was isolated from 50 mg of the ground tissue using the Spectrum Plant RNA Kit (Sigma-Aldrich). Tissues were mixed thoroughly in 1 ml of G1 buffer in an RNase-free microfuge tubes and incubated at room temperature for 5 min. The tubes were then centrifuged at 12,000×g for 10 min at 4 °C. After transferring into a new tube, the supernatant from each sample was mixed with 200 µl of chloroform and the tube was incubated at room temperature for 3 min. After centrifugation at 12,000×g for 15 min at 4 °C, the supernatant was precipitated into a new tube with half the volume of isopropanol and the same volume from G2 buffer for 10 min at room temperature. The RNA was pelleted by centrifugation at 10,000×g for 10 min at 4 °C and washed using 70% ethanol. After brief drying, RNA was re-suspended in 30 µl of RNase-free water. The purity of the total RNA was quantified by Eppendorf AG22331 BioPhotometer Spectrophotometer (Hamburg, Germany). DNase treatment of total RNA was carried out using RQ1 (RNA Qualified) RNase-Free DNase Kit (Promega, USA) according to the manufacturer’s instructions. Synthesis of cDNA from 1 µg RNA was carried out using ImProm-II™ Reverse Transcription System Kit (Promega, USA) as per the manufacturer’s instructions in a 20 µl final volume.
Sequencing genes for salt tolerance proteins and primer design
In order to develop HKT1;5, SOS1, NHX1, and VP1 gene-sequence specific primers for A. cylindrica, nucleotide sequences of these genes from wheat, rice, maize and Arabidopsis were aligned by ClustalW tool. A pair of primers was designed from the conserved aligned sequences for each gene and the details of the primers used for screening the genes of interest are provided in Table 1. Fragments of the AecHKT1;5, AecSOS1, AecNHX1 and AecVP1 genes were amplified following the PCR conditions reported earlier (Saha and Blumwald 2014) and sequenced from Macrogen (South Korea). Sequences were subjected to the basic local alignment search tool using a nucleotide query (BLASTn) of the National Center for Biotechnology Information (NCBI) for similarity search. The partial sequences of the genes were deposited in NCBI (AecHKT1;5 and AecSOS1) and EMBL (AecNHX1 and AecVP1) databases.
Table 1.
Sequences of primers used for genomic DNA amplification and qRT-PCR in this study
| Primera | Sequence | Amplicon length (bp) | Tm (°C) |
|---|---|---|---|
| HKT1;5-G | F: CATCTCCTTCTTCGGTTTGG R: GATCATCCCTTCGTTGTTCG |
610 | 55 |
| HKT1;5-R | F: GCAAACCAGCACCATCATCT R: CGCTCAGGTAGACCAGCATC |
198 | 60 |
| SOS1-G | F: CAAGGATTTTGCTCCCTCAG R: CTTCTGGTTCGCTTCCACAT |
804 | 58 |
| SOS1-R | F: CCATTGAGCTTGGGCATAAT R:TGCTCTAGCCTCCACCTGAT |
197 | 60 |
| NHX1 | F: ATTCTTCACCAGCACCGTTC R: TCAGATCCAGCAGCATTGAC |
158 | 60 |
| VP1 | F: GGAGCCATCACATCTCTGGT R: TGACAACAAGAAGCCCATGA |
150 | 60 |
| 18S | F:GAATTGACGGAAGGGCACCACCAG R:ACTAAGAACGGCCATGCACCACCA |
149 | 60 |
aG denotes amplification of genomic DNA and R denotes qRT-PCR
Quantitative real-time PCR
A pair of primers from each gene was developed using Oligo 7 software (Molecular Biology Insights, Inc., USA) for quantitative real-time PCR (qRT-PCR). The qRT-PCR was carried out in a 10-µl volume containing 5 μl of 2x Fast SYBR Green PCR Master Mix (Applied Biosystems), 2 μl of 1 µM each of reverse and forward primers, 2 μl of diluted cDNA and 1 μl of RNAse free water following the procedure described by Saha and Blumwald (2014). The qRT-PCR was performed in an optical 96 well plate (Applied Biosystems, Foster City, CA) using a StepOnePlus™ real time PCR system (Applied Biosystems). The PCR reactions were conducted with following the cycling conditions: 50 °C for 2 min, 95 °C for 20 s, followed by 40 cycles of 95 °C for 3 s and 60 °C for 30 s. After 40 cycles, a melting curve analysis was performed (60–95 °C) to verify the specificity of amplicons. All amplification reactions were carried out in duplicate and reverse transcriptase negative controls were included to test for genomic DNA contamination. The 18S rRNA and Gapdh reference genes were used as an endogenous control to normalize gene expression and the fold expression changes of target mRNAs were determined using the 2−ΔΔCT method (Livak and Schmittgen 2001). The data are presented as the fold change of mRNA expression in plant tissues (roots and shoots) relative to normal tissues after normalization to an endogenous control.
Phylogenetic analysis of salt tolerance genes
Related nucleotide sequences of the AecHKT1;5, AecSOS1, AecNHX1 and AecVP1 genes were obtained from NCBI GenBank using BLASTn search. Multiple sequence alignments of all homologous sequences for each gene from closely related grass species were performed in ClustalW (Thompson et al. 1994) and phylogenetic analysis was conducted using the maximum likelihood (ML) method with 1000 bootstrap replication in the MEGA v. 5.2.1 software (Tamura et al. 2011).
Statistical analysis
Analysis of variance (ANOVA) was carried out to examine the effects of NaCl treatments (0, 400 mM at 24 h and 400 mM at 7 days) on the gene expressions of plant tissues (shoot and root) in salt tolerant and salt sensitive genotypes using factorial ANOVA in SAS version 9.3 (SAS Institute 2011). Assumptions of the normality of distribution and the homogeneity of variances were assessed with the Kolmogorov–Smirnov test and Bartlett’s test, respectively, before being subjected to ANOVA. Data were analyzed as a factorial design, where salt stress treatments were used as a fixed factor while genotype, tissue and their interactions as random effects using the PROC GLM procedure of SAS. Mean comparisons were conducted using the Fisher’s least significant differences test (LSD0.05).
The data obtained from the physiological response of the 44 A. cylindrica genotypes to salt stress (400 mM NaCl) were subjected to principal component analysis using PROC FACTOR (method = prin) using the Kaiser’s criterion (i.e., Eigenvalue ≥ 1.0 in SAS 9.3) (SAS Institute 2011). A genotype-by-trait biplot was then constructed using the first two principal components (PC1 and PC2).
A backward stepwise regression analysis was also performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, United States). The backward procedure first calculated the statistics for a model that included DM as the dependent variable and Na+, K+, K+/Na+, AecHKT1;5, AecSOS1, AecNHX1 and AecVP1 as the independent ones.
Results
Root and shoot growth and physiological traits
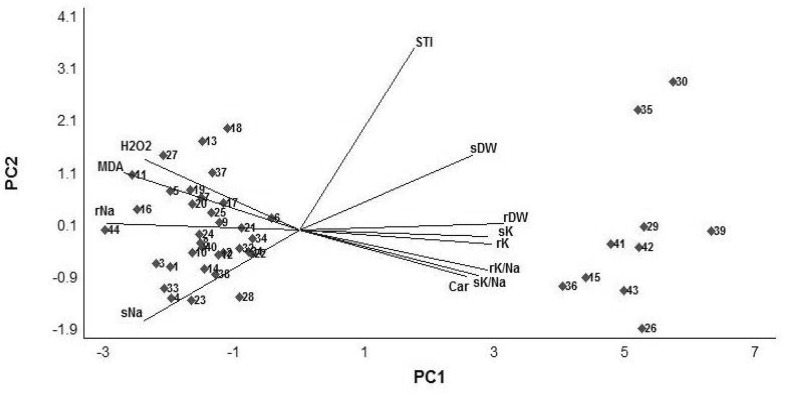
Salt tolerance of 44 A. cylindrica genotypes collected from the Uremia Salt Lake shores and Kurdestan was evaluated using such physiological traits as shoot H2O2, MDA, carbohydrate content, Na+ and K+ concentrations, K+/Na+ ratio and DM as well as root Na+ and K+ concentrations, K+/Na+ ratio and DM under 0 and 400 mM NaCl in greenhouse conditions. Genotype-by-trait biplot was constructed as a tool to visualize discrimination among the 44 A. cylindrica genotypes on the basis of multiple traits (Fig. 1). The experimental results, except root data, accompanied by molecular markers associated with salt tolerance were described in our earlier article (Arabbeigi et al. 2014). The overall shoot and root data of our trial presented in Fig. 1 revealed that USL26 is the most salt-tolerant genotype collected from saline Uremia Salt Lake shore area, while K44 is the most salt-sensitive genotype originated from non-saline Kurdestan province. The hyper-salt tolerant genotype USL26 not only survived completely the whole experimental period but also showed superior root and shoot DM as well as physiological traits compared to other genotypes (Fig. 1).
Fig. 1.
Biplot of 44 genotypes of A. cylindrica (1–44) and physiological traits (vectors) affected by 400 mM NaCl for 4 weeks. The PC1 and PC2 explain 53.4 and 31.5% of the total variations, respectively. Traits abbreviations: STi: salt tolerance index, rNa: root Na+, rK: root K+, rK/Na: root K+/Na+, sNa: shoot Na+, sK: shoot K+, sK/Na: shoot K+/Na+, rDW: root dry matter, sDW: shoot dry matter; MDA: shoot malondialdehyde, H2O2: shoot hydrogen peroxide and Car: shoot carbohydrate
In the current study, these two contrasting genotypes were further characterized for root and shoot Na+ and K+ concentrations as well as expressions of genes related to ion homeostasis under 0 mM (control) 400 mM for 24 h and 7 days of NaCl treatments. The results revealed that shoot and root DM of the genotypes decreased under salt stress (400 mM NaCl) compared to control, while the salt tolerant genotype USL26 showed threefold higher DM compared to salt-sensitive genotype K44 (Table 2).
Table 2.
Mean comparisons of root and shoot ionic concentrations affected by salt treatments in two contrasting A. cylindrica genotypes
| Treatment/tissue (mM NaCl) | Tolerant genotype (USL26) | Sensitive genotype (K44) | ||||||
|---|---|---|---|---|---|---|---|---|
| Na+ (mM gDM−1) | K+ (mM gDM−1) | K+/Na+ | DM (g/plant) | Na+ (mM gDM−1) | K+ (mM gDM−1) | K+/Na+ | DM (g/plant) | |
| Control (0)/root | 1.46 ha | 1.04 e | 0.62 c | 0.11 c | 1.31 h | 0.99 f | 0.54 c | 0.08 c |
| 24 h (400)/root | 3.45 de | 3.58 c | 1.03 b | 0.07 cd | 6.36 b | 1.48 e | 0.23 e | 0.03 d |
| 7 d (400)/root | 5.65 c | 5.25 b | 0.92 b | 0.03 d | 8.53 a | 2.93 cd | 0.34 d | 0.01 d |
| Control (0)/shoot | 1.22 h | 0.99 f | 0.81 bc | 0.45 a | 1.26 h | 1.12 e | 0.63 c | 0.40 a |
| 24 h (400)/shoot | 1.99 g | 3.88 c | 1.94 a | 0.4 a | 4.23 cd | 1.32 e | 0.31 d | 0.24 b |
| 7 d (400)/shoot | 2.89 f | 6.95 a | 2.05 a | 0.18 bc | 6.63 b | 2.23 d | 0.33 d | 0.06 cd |
aMeans followed by the same letter in each column are not significantly different at P < 0.05
The Na+ and K+ content as well as K+/Na+ ratio in the shoot and root tissues showed that not only salt stress significantly altered the ionic concentrations of both the genotypes, but also that USL26 and K44 responded significantly differently to NaCl treatments by differential accumulation of the ions in their tissues (Table 2). Although a significant increase was observed in Na+ content of both the root and shoot tissues in the salt treated plants compared to those of the control, the USL26 possessed lower Na+ content and higher K+ content and K+/Na+ ratio in the roots and shoots (Table 2). The comparison of Na+ content in the roots and shoots between the treatment periods showed that Na+ concentration was much lower at 24 h than 7 days of salt treatment. In addition, a higher Na+ accumulation was occurred in the roots than in the shoots at both the treatment periods.
Sequence homology and diversity of salt tolerance genes
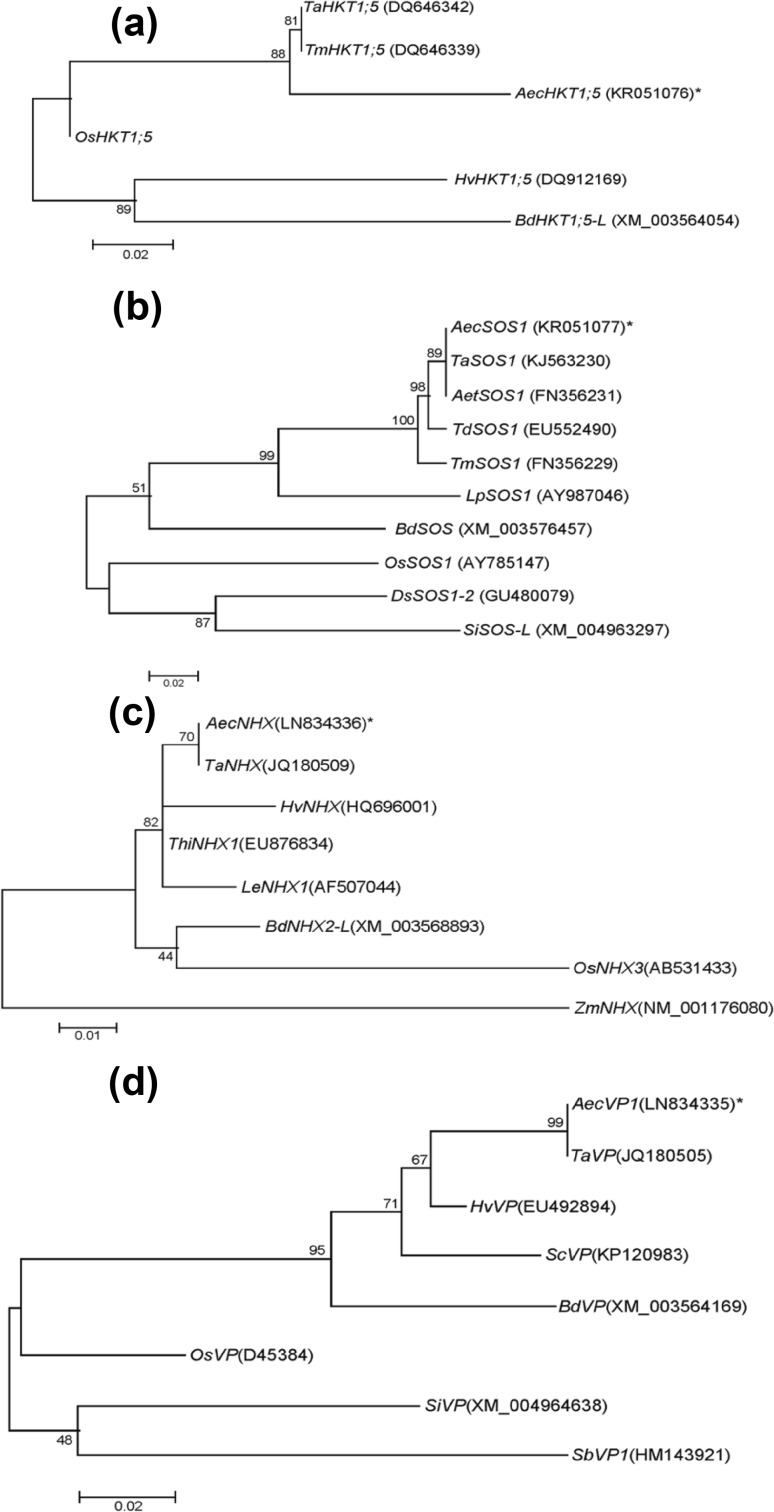
The PCR amplified the 580, 782, 158 and 150 bp amplicons of AecHKT1;5, AecSOS1, AecNHX1 and AecVP1 genes respectively, and were gel eluted and sequenced. The sequences were deposited to the NCBI GenBank database (accession numbers KR051076-KR051077 for AecHKT1;5 and AecSOS1) and EMBL/GenBank database (accession numbers LN834336 for AecNHX1 and LN834335 for AecVP1). BLAST analysis of nucleotide sequences revealed that AecHKT1;5 gene had a homology of 99% with TaHKT1;5-D, 97% with TmHKT1;5, 94% with TdHKT1;5-B2 and TaHKT1;5-B2, 94% with TdHKT1;5-B3, 87% with HvHKT1;5 and 86% with TaHKT1;5-B1 (for details see Table 3). A relatively lower identity (80–81%) was observed between AecHKT1;5 and OsHKT1;5. The comparison of nucleotide sequences for the consensus sequences of the AecHKT1;5 gene from USL26 and K44 revealed their identity. A motif search using Pfam and NCBI CDD (http://www.genome.jp/tools-bin/search_motif_lib/) was performed. The predicted polypeptide (150 aa) from the AecHKT1;5 partial sequence indicated the presence of three domains including a conserved domain TrKH (cation transport protein), an ISK_channel (slow voltage-gated potassium channel) and the QueT (QueT transporter).
Table 3.
Results of BLAST homology search of AecHKT1;5 gene using NCBI BLASTn tool
| Species | Gene | Accession number | Identity (%) |
|---|---|---|---|
| Triticum aestivum | TaHKT1;5-D |
DQ646342.2 DQ646338.1 |
99 |
| T. aestivum | TaHKT1;5-B2 | DQ646341.1 DQ646337.1 | 94 |
| T. aestivum | TaHKT1;5-B1 | DQ646340.1 DQ646336.1 DQ646333.1 | 86 |
| T. monococcum | TmHKT1;5 |
DQ646339.2 DQ646332.1 |
97 |
| T. turgidum subsp. durum | TdHKT1;5-B2 | DQ646334.1 | 94 |
| T. turgidum subsp. durum | TdHKT1;5-B3 | DQ646335.1 | 94 |
| Hordeum vulgare subsp. vulgare | HvHKT1;5 | DQ912169.1 | 87 |
| Oryza sativa | OsHKT1;5 | JQ695816.1 | 80 |
Phylogenetic analysis of nucleotide sequences of the AecHKT1;5, AecSOS1, AecNHX1 and AecVP1 genes with homologous sequences from closely related species are presented in Fig. 2. The phylogenetic tree for HKT1;5 gene revealed three distinct clusters, where AecHKT1;5 remained in a group with highly similar two HKT1;5 genes (TaHKT1;5 and TmHKT1;5) from wheat species. In another cluster OsHKT1;5 (HKT1;5 from Oryza sativa) stayed at the center close to the root, while the third cluster included distinctly related HvHKT1;5 and BdHKT1;5L from barley and Brachypodium distachyon, respectively (Fig. 2a). Phylogenetic analysis of SOS1 gene demonstrated that AecSOS1 was mostly related to TaSOS1 and AetSOS1, while poorly related to LpSOS1 (from Lolium perenne), BdSOS1, OsSOS1, DsSOS1 (from Distichlis spicata) and SiSOS1 (from Setaria italica) (Fig. 2b). The NHX1 tree revealed AecNHX1 and TaNHX1 were closely related, whereas the phylogenetic analysis of VP1 clustered AecVP1, TaVP and HvVP together indicating their close relationship in the evolutionary process (Fig. 3d).
Fig. 2.
Phylogenetic tree based on nucleotide sequences, showing the relation of a AecHKT1;5, b AecSOS1, c AceNHX1 and d AecVP1to the correspondences of members of the genus Triticum spp. and the species Aegilops tauschii, Hordeum vulgare, Oryza sativa, Zea mays, Brachypodium distachyon, Setaria italica and other
Fig. 3.
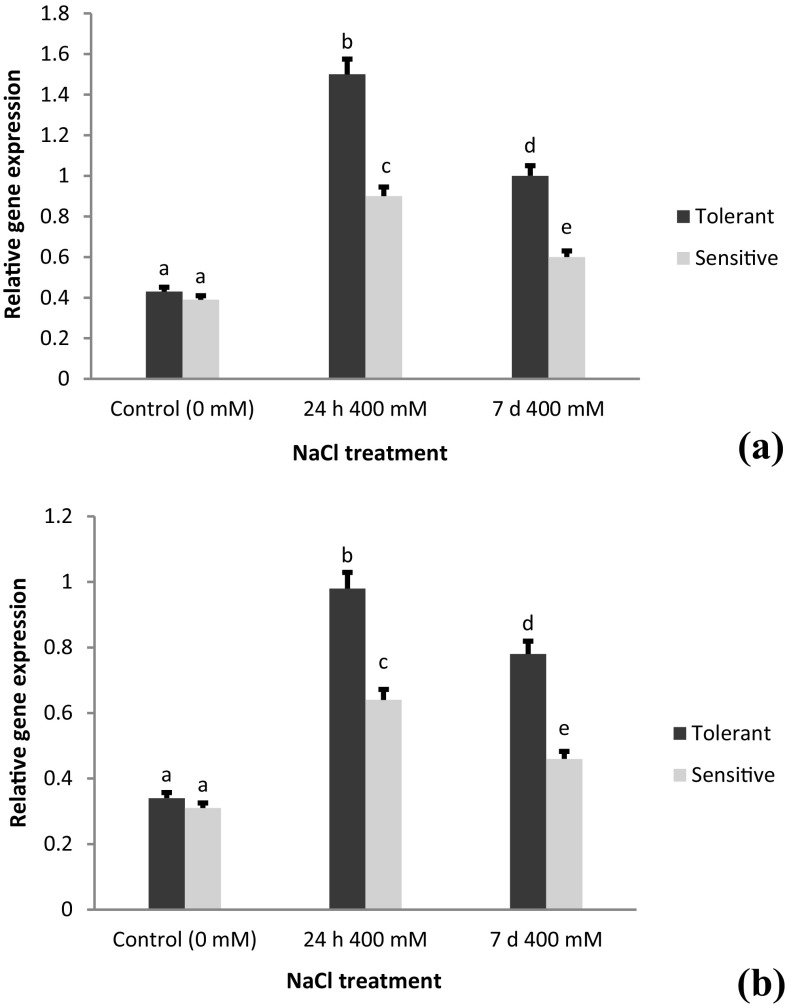
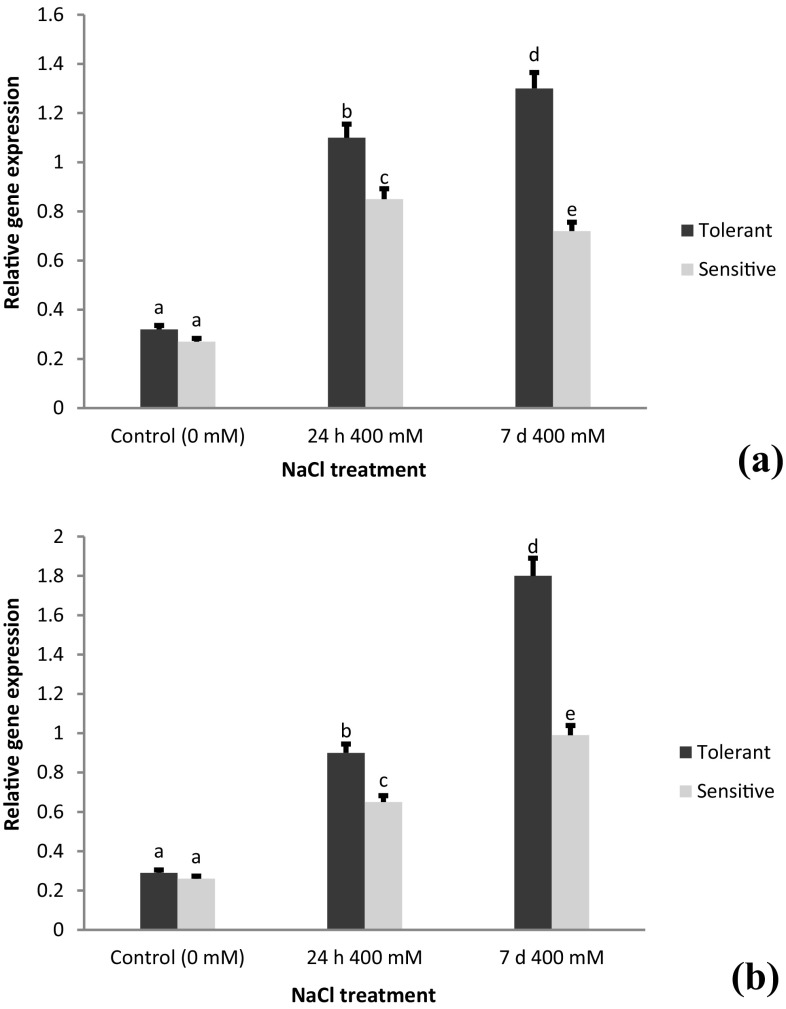
Expression analysis of the AecHKT1;5 gene in a root and b shoot tissues of A. cylindrica tolerant (USL26) and sensitive (K44) genotypes. Bars represent means ± SE and bars headed by the same letter represent not significantly different at P < 0.05
Expression analysis of salt tolerance genes
In this study, the results of ANOVA for the gene expression data revealed a significant effect of salt stress on the expression profiles of four genes in the root and shoot tissues (Table 4). The results of real-time PCR showed that the transcript levels of the all genes were significantly different between the root and shoot tissues. The salt treatment up-regulated (3–5 fold) the expression of AecHKT1;5 compared to control in both the genotypes. In the tissue level, the expression of this gene was 4-fold higher in the roots than in the shoots (Fig. 3). In addition, the accumulation of AecHKT1;5 transcript was 2-fold higher at 24 h than 7 days of 400 mM NaCl treatment (Fig. 3a). The expression of AecHKT1;5 in the root tissues was much higher in USL26 than K44, while their transcript level in the shoots indifferently upregulated under NaCl stress conditions (Fig. 3a, b). The expression analysis of AecSOS1 gene indicated that overall expression level was relatively low compared to AecHKT1;5. The expression level of AecSOS1 was significant upregulated in the roots and shoots of salt treated plants with 1.5-fold higher transcript levels in the roots than shoots (Fig. 4). The expression levels of AecSOS1 were significantly surpassed by the salt-tolerant USL26 genotype in both the root and shoot tissues.
Table 4.
Results of analysis of variance for the effects of NaCl treatments (0, 400 mM at 24 h and 400 mM at 7 days) on relative gene expressions of the plant tissues (shoot and root) in the salt-tolerant and salt-sensitive A. cylindrica genotypes
| Source of variations | Degree of freedom | Mean squares | |||
|---|---|---|---|---|---|
| Salt stress (S) | 2 | 4.89** | 3.54** | 2.89* | 3.11* |
| Genotype (G) | 1 | 3.18* | 1.76* | 3.09* | 3.15* |
| Tissue (T) | 1 | 3.19* | 3.65** | 1.01 | 0.09 |
| S × G | 2 | 3.35* | 3.34** | 4.65** | 5.2** |
| S × T | 2 | 2.36* | 3.99** | 3.01* | 2.23* |
| G × T | 1 | 3.27* | 1.82* | 2.92* | 4.83** |
| S × G × T | 2 | 2.32* | 0.65 | 3.97** | 1.94* |
| Residual | 36 | 0.08 | 0.09 | 0.10 | 0.14 |
*, ** Significant at 0.05 and 0.01 probability levels, respectively
Fig. 4.
Expression analysis of the AecSOS1 gene in a root and b shoot tissues of A. cylindrica tolerant (USL26) and sensitive (K44) genotypes. Bars represent means ± SE and bars headed by the same letter represent not significantly different at P < 0.05
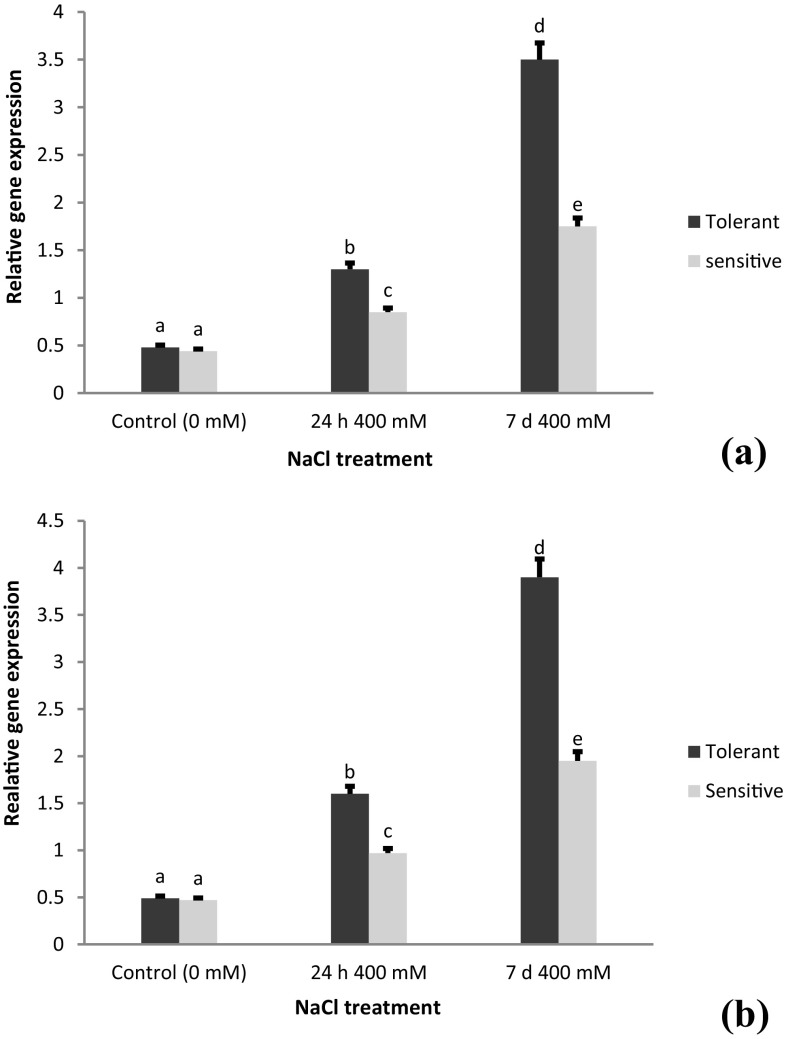
The expression patterns of gene expression analyses for AecNHX1 and AecVP1 showed similar results with 2-fold higher transcript levels of AecNHX1 than AecVP1 under the 400 mM NaCl stress (Figs. 5, 6). In comparison to control, a 8-fold and a 5-fold increase of AecNHX1 transcript levels in roots and in shoots of USL26 was observed. In the control condition, no significant difference was observed between the salt-tolerant and salt-sensitive genotypes in the expression level of these genes either in root or the shoot tissues.
Fig. 5.
Expression analysis of the AecNHX1 gene in a root and b shoot tissues of A. cylindrica tolerant (USL26) and sensitive (K44) genotypes. Bars represent means ± SE and bars headed by the same letter represent not significantly different at P < 0.05
Fig. 6.
Expression analysis of AecVP1 gene in a root and b shoot tissues of A. cylindrica tolerant (USL26) and sensitive (K44) genotypes. Bars represent means ± SE and bars headed by the same letter represent not significantly different at P < 0.05
Association of ion content and ion-related transcripts to DM
Regression analysis was employed to define the combination of the explanatory variables that best predicted DM under salt stress conditions. Results showed that Na+ and AecHKT1;5 were the strongest predictors of DM under salt stress (Table 5). Clearly, the regression model including these two variables explained 51% of the variability in DM. Among these two explanatory variables which significantly affected salt tolerance, plant Na+ concentration (R2 = 0.82; r = − 0.90**) contributed the most towards the variation in DM.
Table 5.
Results of multiple regression analysis with dry matter (DM) as the dependent variable under salt stress conditions
| Independent variablea | Unstandardized coefficient (B) | Standard error | Standardized coefficient (β) | Adjusted R squareb (R2) | P |
|---|---|---|---|---|---|
| Intercept | 0.215 | 0.030 | 0.0001 | ||
| AecHKT1;5 | 0.039 | 0.021 | 0.375 | 0.347 | 0.0001 |
| Plant Na+ | − 0.022 | 0.007 | 0.826 | 0.819 | 0.001 |
| R2 = 0.51 and Cp = 2.1 | |||||
aSum of root and shoot data for the dependent (DM) and independent variables used
bCalculated by linear regression analysis
Discussion
Over the past decades, molecular candidates for voltage-gated channels of K+, Na+ and Ca2+ ions, as well as ion transporters have been identified and biochemically characterized using model plants and crop species (Arzani and Ashraf 2016; Hasegawa 2013). However, the molecular and physiological identities of transporters involved in high salt tolerance in A. cylindrica are still elusive. Byrt et al. (2007) reported that TaHKT1;5-D and TdHKT1;5 are likely candidates for the major QTLs responsible for Na+ exclusion of in durum wheat (Nax2) and in bread wheat (Kna1), respectively. However, compelling evidence have been presented recently (Zhu et al. 2016) suggesting that Nax loci contributes in the reduction of Na+ content in xylem by two mechanisms, one that retrieves Na+ back into the root stele via HKT1;4 or HKT1;5, while the other leading to reduction in the rate of Na+ loading into the xylem via SOS1. HKT transporters can be classified into at least two subfamilies, class I and class II (Horie et al. 2009). In contrast to the class II subfamily that in general shows Na+–K+ co-transport activity, class I HKT transporters exhibit Na+-selective transport with poor K+ permeability (Horie et al. 2009). AtHKT1;1, a class I transporter in Arabidopsis thaliana, was demonstrated to function in Na+ transport (Uozumi et al. 2000); HKT1 transporter also operated as highly selective Na+ channel (with no permeation for K+) in Dionea glands (Böhm et al. 2015). Very recently, a novel function of HKT1;5 in mediating Na+ exclusion in the phloem was shown in rice (Kobayashi et al. 2017). The results of the current study revealed a high identity (over 90%) of AecHKT1;5 with wheat HKT1;5 genes, which suggest that is an orthologue of TaHKT1;5-D.
This study examined whether or not an interrelationship exists between the explanatory variables and DM in a multivariate analysis. Based on our multiple regression analysis, AecHKT1;5 is the only gene that could significantly contribute to salt tolerance, though its association was lower than that of plant Na+ concentration (Table 5). Interestingly, it was found that incorporation of TmHKT1;5-A into bread wheat that already contains endogenous TaHKT1;5-D, can further improve the plant’s ability to exclude leaf Na+ (James et al. 2011). This may also show that salt tolerance in wheat could be further improved by incorporating HKT1;5 genes from wild relatives, such as AecHKT1;5. An argument can be made for the evolution of HKT1;5 in Triticum spp. and Aegilops spp. from a single ancestor by the results obtained from phylogenetic tree analysis (Fig. 2a). Likewise, a non-allelic variation for the HKT1;5-A gene has been described in wheat genotypes with different levels of salt tolerance by Byrt et al. (2007). Adding to this line of evidence, in a phylogeny analysis of 115 rice accessions conducted by Platten et al. (2013), seven major and three minor alleles of OsHKT1;5 have been categorized, one of which represented the highest Na+ exclusion. The high homology of AecHKT1;5 and TaHKT1;5-D coupled with the location of this gene on wheat 4D chromosome could suggest the assumption that TaHKT1;5 is originated from the D genome of the common ancestor of wheat species such as A. tauschii and A. cylindrica.
The high expression of AecHKT1;5 in roots than shoots observed in the current study was consistent with those of Byrt et al. (2007) and Byrt et al. (2014) who reported that the TaHKT1;5-D gene was expressed in the root rather than in the shoot. Zamani Babgohari et al. (2013) also reported that the HKT1;5 expression followed a genotype- and tissue-specific pattern in wheat and its wild relatives. The AecHKT1;5 may counteract Na+ root to shoot transport in a different or complementary way to SOS1 because, expression level of AecHKT1;5 in root were higher than AceSOS1 expression at 24 h NaCl treatment.
The high homology of AecSOS1 with TaSOS1 and AetSOS1, and that of AecNHX1 with TaNHX1 as well as AecVP1 with TaVP1 may suggest their close evolutionary relationships in the tribe Triticeae (Fig. 2). In spite of the hypothesis of similar ancestral lineage among the studied genes and those of bread wheat (T. aestivum L.), transcriptional and post-transcriptional regulations can be unique for each of the A. cylindrica and T. aestivum genes. The recent work by Nawaz et al. (2014) provided strong evidence in support to the specific role played by the operation of SOS1 and NHX1 genes in the halophytic and glychophytic Brassicaceae species.
Maintaining a low Na+ but a high K+ in the cell cytosol as well as the coordinated long-distance transport of Na+ in the xylem and phloem in plants are the key aspects in plant salt tolerance (Arzani and Ashraf 2016). The role of SOS1 in xylem loading/unloading, Na+ export by roots, retention in stems, and the differential accumulation/distribution in old leaves may be even overtly manifested in plants with high levels of salt tolerance (Ji et al. 2013; Shi et al. 2000, 2002). However, SOS1 transcripts are also highly abundant at the xylem parenchyma (Shi et al. 2000), and it was shown both theoretically (Shabala 2013) and experimentally (Zhu et al. 2017) that SOS1 mediates xylem Na+ loading under saline conditions. As we did not separated epidermal and stellar tissues for their PCR analysis, higher SOS1 transcript levels in tolerant genotype may potentially indicate their better ability to deliver Na+ to the shoot for the purpose of osmotic adjustment (if expressed in the stele). Real time PCR analysis of AecSOS1 expression revealed the differential expressions not only in the salt-sensitive and salt-tolerant genotypes but also in root and shoot tissues. The higher AecSOS1 transcripts obtained for roots than for shoots in the two periods of salt stress treatments are consistent with those reported for the glycophytic AtSOS1 (Shi et al. 2002) and halophytic SbSOS1 genes (Yadav et al. 2012). In rice, Martinez et al. (2007) also reported salt treatment induced OsSOS1 mRNA accumulation exclusively in the roots. The results of the current study revealed that AecSOS1 transcripts were higher in the initial stage (24 h) than at a later stage (7 days) after salt treatment. Likewise, Ramezani et al. (2013) reported that TaSOS1 transcript levels in wheat were found to be down regulated at a very early stage and up-regulated 24 h after salt treatment. However, Niu et al. (1995) noted that plants increase expression of transporters when they are adapting to new salt conditions, but that once acceptable ion concentrations have been attained under saline conditions they are retained by passive mechanisms.
Na+ vacuolar sequestration is reportedly essential to prevent the toxic effects of Na+ in the cytosol, and enhanced function of NHX tonoplast Na+/H+ exchangers has been accordingly exhibited to improve salt tolerance in plants (Bassil and Blumwald 2014). In the current study, we observed the remarkable induced expression of AecNHX1 of the salt tolerant genotype (Fig. 5). These results are in agreement with our ion data of 2-fold Na+ content in the roots than in the shoots in the 400 mM NaCl treatment of the salt-tolerant genotype. This in turn, indicates the essential role of AecNHX1 in the sequestration of Na+ into the root vacuoles to alleviate Na+ toxicity in stems and leaves. The expression response in the current study was comparable with that reported in wheat (Yousfi et al. 2016), but somewhat more stringent than that of Adem et al. (2014), who found no change in the transcript level of the NHX1 in barley. The expression levels of the AecNHX1 and AecVP1 genes followed an identical pattern in both root and shoot tissues. These findings are consistent with the observations reported elsewhere that the expression of SsNHX1 in Suaeda salsa was increased by NaCl treatment in the whole plant including roots and shoots (Ma et al. 2004). It is also believed that the compartmentalization of sodium ions into the vacuole occurs simultaneously in all tissues (Bartels and Dinakar 2013). On the other hand, NHX1 has been found to be more strongly expressed in the shoots of the halophytic and glycophytic Brassicaceae species than in their roots (Nawaz et al. 2014). Our results may extend the findings from others suggesting that co-overexpression of VP1/NHX1 results in higher salt tolerance levels in plants than either VP1 or NHX1 single-gene-overexpression (Shen et al. 2015). The highest expression increments upon 7 d exposure to 400 mM NaCl indicated that the synchronous function of these genes to sequester the excess Na+ into the vacuole might occur with Na+ accumulation in the cytosol.
The consistency between the results observed in the sequences and expression of the AecHKT1;5, AecSOS1, AecNHX1 and AecVP1 genes, on one hand, and Na+ concentrations in the roots and shoots of the contrasting A. cylindrica genotypes, on the other, support the critical roles of these genes in salt tolerance. The lower Na+ concentration in shoot tissues compared to that in root tissues in the salt-tolerant genotype and its significant lower level than in the salt-sensitive genotype are in coordination with the functions of the AecSOS1 and AecHKT1;5 genes. These findings are in support of our prior observations that Na+ exclusion is a vital salt tolerance mechanism in the A. cylindrica species (Arabbeigi et al. 2014; Kiani et al. 2015). Colmer et al. (2006) suggested that Na+ concentration in leaves could be used as an indicator of the relative ability to ‘exclude’ Na+ within a species, or even within a genus. A functional link and possible interplay between AtHKT1;1 and AtSOS1 to achieve Na+ (and K+) homeostasis has been previously suggested (Rus et al. 2004). Our results follow a model in which HKT1 loads Na+ into the shoot phloem where handover to roots occurs through the downward stream of phloem, whereas SOS1 mediate the transfer of Na+ from the xylem parenchyma to xylem vessels, preferably in the roots. The coordinated functions of these transporters would eventually regulate the amount of Na+ that is transferred to the shoots. It seems that despite the crucial role of the AecSOS1 gene in ion homeostasis, AecHKT1;5 gene plays a more important role in Na+ exclusion from shoots to roots than does SOS1 in the studied species. Similarly, Munns and Tester (2008) reported that both HKT1;5 and SOS1 genes had functions in Na+ recirculation from shoots to roots, by loading Na+ from the shoots into the phloem and then unloading it into the roots for efflux. Recently, in parallel to our findings, Zhang et al. (2017) tested the expression patterns of PtHKT1;5, PtSOS1 and PtNHX1 under low salt stress (25 and 150 mM NaCl) in a halophytic grass Puccinellia tenuiflora and suggested that these genes may play a synergistically crucial role in regulating Na+ homeostasis. Flowers (2004) argues for the hypothesis that manipulation of a single salt tolerance gene cannot be expected to be effective in improving a trait as complex as salt tolerance. To support his argument, he analyzed 13 species that had been transformed with nearly 40 genes between 1993 and 2003 to show that the majority of the experiments had used rice, tobacco and Arabidopsis for transformation. To mitigate this unpromising perspective it is clear that the majority of the over-expressed genes used so far originated from salt-sensitive species such as those listed by Flowers (2004). There is an example of successful introgression of the T. monococcum TmHKT1;5 gene into a susceptible commercial durum wheat variety which led to significant increase in grain yield under saline field conditions, far exceeding the expected yield increase of up to 25% (Munns et al. 2012).
In the current study, a significant negative association was found between Na+ content and DM under the 400 mM NaCl stress treatment, indicating the vital role of Na+ ion exclusion mechanisms on salt tolerance in A. cylindrica. Of great importance, however, is the finding that the root and shoot K+ contents were profoundly higher in the salt-tolerant than in the salt-sensitive genotype under the 400 mM NaCl conditions. To date, there is no experimental evidence—to the authors’ knowledge—for a significant role of HKT transporters in K+ transport in plants. It is clear, however, that not only are transport and sequestration of Na+ essential for avoiding the toxic effects of salt, but cytosolic K+ maintenance is also important for enhancing salt tolerance in plants, as also highlighted by Pottosin and Shabala (2014). Ion homeostasis under salt stress conditions is crucial in salt tolerance and implicates a network of transport processes controlling uptake, extrusion through the plasma membrane, recirculation of ions through the plant organs and compartmentalization of salts into cell vacuoles, thus regulating the osmotic adjustment and retention of high K+/Na+ ratios in plant cytosol (Apse and Blumwald 2007; Arzani and Ashraf 2016). Our results of higher potassium contents in the salt tolerant genotype coupled with the genotypically differential upregulation of the AecHKT1;5 gene in the roots and the presence of conserved domain TrKH (cation transport protein) in this gene might also highlight yet another possible role in K+ uptake and transport in plant (Hamamoto et al. 2015). Low concentration of leaf Na+ and high K+/Na+ ratio lead to improved discrimination and preference of K+ over Na+ in their transport from the root to the shoot, while no effects have been suggested on root Na+ concentration as the likely roles of Nax2 and Kna1 (Byrt et al. 2007; James et al. 2006). Our findings, therefore, lend support to the hypothesis that the capacity to discriminate between Na+ and K+ uptakes and to preferentially exclude Na+ and accumulate K+ is one of the key salt tolerance mechanisms in the tribe Triticeae (Arzani and Ashraf 2016; Dvorak et al. 1994; Kiani et al. 2015; Shabala et al. 2013). It is clear, hence, that not only are Na+ transport and sequestration needed, but cytosolic K+ maintenance is also essential for mediating salt tolerance in plants (Anschutz et al. 2014; Pottosin and Shabala 2014; Shabala and Pottosin 2014).
All of these evidences along with the inference of A. cylindrica species is the only member of Triticeae tribe coping hyper-saline conditions of shore land of Uremia Salt Lake could be explained on the grounds that polyploidy has the positive roles in the adaptive response to saline conditions (see the review by Arzani and Ashraf (2016) for further details on the effects of polyploidy on salt tolerance), C genome has impacted on salt tolerance (Kiani et al. 2015) and that the saline-habitat genetic resources perform better than normal-habitat genetic resources screened for salt tolerance (Arzani and Ashraf 2016). While this study illustrates neatly the importance of the D-genome originated salt related genes on Na+ alleviation in A. cylindrica plants, future molecular studies are required to determine the ion homeostasis genes associated with the C genome of this species. Thus, the analysis requires a sequenced genome as a prelude to enhanced analysis and understanding of salt tolerance genes in this grass species.
Conclusions
The results of our experiments suggest a functional link and a likely interplay between AecHKT1;5 and AecSOS1 to achieve Na+ (and K+) homeostasis in A. cylindrica. We observed lower Na+ and higher K+ concentrations in the roots/shoots in addition to higher levels of expression of these genes in the salt-tolerant genotype compared to its salt-sensitive counterpart. The higher Na+ concentration in the roots than in the shoots was in agreement with the transcript profiles of AecHKT1;5 in these tissues. AecHKT1;5 expression and Na+ concentration were identified as the strongest predictors of DM. Therefore, it appears that the involvement of AecHKT1;5 in Na+ sequestration in the root tissue possibly in combination with the activity of Na+ exclusion from roots, resulted in salt-tolerant plants ability to retain Na+ in their roots and thereby avert it from reaching the photosynthetic tissues. Since about half the variation observed in the DM of the salt-tolerant genotypes can be explained with reference to the root and shoot Na+ concentrations and the AecHKT1;5 gene that is likely to be located in the D genome, future work should focus on genes conferring the salt tolerance in the panel of the C genome of A. cylindrica. In this particular species, it appears likely that tolerance to high salt concentration must be dealt with the ability of plant to maintain low Na+ concentration in the tissues, particularly in the shoots.
Acknowledgements
The authors are grateful to Dr. Andre Kim, from the University of California Davis, for his fruitful comments on the manuscript and Dr. M. Talebi, from Isfahan University of Technology, for his technical assistance. This research was partially supported by Grant No. 91-03-18/9275 from Isfahan University of Technology.
References
- Adem GD, Roy SJ, Zhou M, Bowman JP, Shabala S. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol. 2014 doi: 10.1186/1471-2229-14-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anschutz U, Becker D, Shabala S. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol. 2014;171:670–687. doi: 10.1016/j.jplph.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Apse MP, Blumwald E. Na+ transport in plants. FEBS Lett. 2007;581:2247–2254. doi: 10.1016/j.febslet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Arabbeigi M, Arzani A, Majidi MM, Kiani R, Sayed Tabatabaei BE, Habibi F. Salinity tolerance of Aegilops cylindrica genotypes collected from hyper-saline shores of Uremia Salt Lake using physiological traits and SSR markers. Acta Physiol Plant. 2014;36:2243–2251. doi: 10.1007/s11738-014-1602-0. [DOI] [Google Scholar]
- Arzani A, Ashraf M. Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. Crit Rev Plant Sci. 2016;35:146–189. doi: 10.1080/07352689.2016.1245056. [DOI] [Google Scholar]
- Bartels D, Dinakar C. Balancing salinity stress responses in halophytes and non-halophytes: a comparison between Thellungiella and Arabidopsis thaliana. Func Plant Biol. 2013;40:819–831. doi: 10.1071/FP12299. [DOI] [PubMed] [Google Scholar]
- Bassil E, Blumwald E. The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr Opin Plant Biol. 2014;22:1–6. doi: 10.1016/j.pbi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Böhm J, Scherzer S, Shabala S, Krol E, Neher E, Mueller TD, Hedrich R. Venus flytrap HKT1-type channel provides for prey sodium uptake into carnivorous plant without conflicting with electrical excitability. Mol Plant. 2015;9:428–436. doi: 10.1016/j.molp.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K. Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J Exp Bot. 2007;58:301–308. doi: 10.1093/jxb/erl251. [DOI] [PubMed] [Google Scholar]
- Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R. HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol. 2007;143:1918–1928. doi: 10.1104/pp.106.093476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrt CS, Xu B, Krishnan M, Lightfoot DJ, Athman A, Jacobs AK, Watson-Haigh NS, Plett D, Munns R, Tester M, Gilliham M. The Na+ transporter, TaHKT1;5-D, limits shoot Na+ accumulation in bread wheat. Plant J. 2014;80:516–526. doi: 10.1111/tpj.12651. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Flowers TJ, Munns R. Use of wild relatives to improve salt tolerance in wheat. J Exp Bot. 2006;57:1059–1078. doi: 10.1093/jxb/erj124. [DOI] [PubMed] [Google Scholar]
- Dvorak J, Noaman MM, Goyal S, Gorham J. Enhancement of the salt tolerance of Triticum turgidum by the kna1 locus transferred from the Triticum aestivum chromosome 44 by homoeologous recombination. Theor Appl Genet. 1994;87:872–877. doi: 10.1007/BF00221141. [DOI] [PubMed] [Google Scholar]
- FAO . The state of the world’s land and water resources for food and agriculture (SOLAW)—managing systems at risk. London: Food and Agriculture Organization of the United Nations, Rome and Earthscan; 2011. [Google Scholar]
- Flowers TJ. Improving crop salt tolerance. J Exp Bot. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- Garthwaite AJ, von Bothmer R, Colmer TD. Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl− into the shoots. J Exp Bot. 2005;56:2365–2378. doi: 10.1093/jxb/eri229. [DOI] [PubMed] [Google Scholar]
- Gorham J. Salt tolerance in the Triticeae: K/Na discrimination in synthetic hexaploid wheats. J Exp Bot. 1990;41:623–627. doi: 10.1093/jxb/41.5.623. [DOI] [Google Scholar]
- Hamamoto S, Horie T, Hauser F, Deinlein U, Schroeder JI, Uozumi N. HKT transporters mediate salt stress resistance in plants: from structure and function to the field. Curr Opin Biotechnol. 2015;32:113–120. doi: 10.1016/j.copbio.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Hasegawa PM. Sodium (Na+) homeostasis and salt tolerance of plants. Environ Exp Bot. 2013;92:19–31. doi: 10.1016/j.envexpbot.2013.03.001. [DOI] [Google Scholar]
- Horie T, Hauser F, Schroeder JI. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009;14:660–668. doi: 10.1016/j.tplants.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshmand S, Arzani A, Maibody SAM, Feizi M. Evaluation of salt-tolerant genotypes of durum wheat derived from in vitro and field experiments. Field Crops Res. 2005;91:345–354. doi: 10.1016/j.fcr.2004.08.004. [DOI] [Google Scholar]
- James RA, Davenport RJ, Munns R. Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol. 2006;142:1537–1547. doi: 10.1104/pp.106.086538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RA, Blake C, Byrt CS, Munns R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J Exp Bot. 2011;62:2939–2947. doi: 10.1093/jxb/err003. [DOI] [PubMed] [Google Scholar]
- Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X. The salt overly sensitive (SOS) pathway: established and emerging roles. Mol Plant. 2013;6:275–286. doi: 10.1093/mp/sst017. [DOI] [PubMed] [Google Scholar]
- Kiani A, Arzani A, Habibi F. Physiology of salinity tolerance in Aegilops cylindrica. Acta Physiol Plant. 2015;37:135. doi: 10.1007/s11738-015-1881-0. [DOI] [Google Scholar]
- Kobayashi NI, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Fujii-Kashino M, Horiuchi T, Al Nayef M, Shabala S, An G, Ma JF, Horie T. OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J. 2017;91:657–670. doi: 10.1111/tpj.13595. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma XL, Zhang Q, Shi HZ, Zhu JK, Zhao YX, Ma CL, Zhang H. Molecular cloning and different expression of a vacuolar Na+/H+ antiporter gene in Suaeda salsa under salt stress. Biol Plant. 2004;48:219–225. doi: 10.1023/B:BIOP.0000033448.96998.44. [DOI] [Google Scholar]
- Martinez J, Jiang XY, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, Quintero FJ. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007;143:1001–1012. doi: 10.1104/pp.106.092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Munns R, James RA, Xu B, Athman A, Conn SJ, Jordans C, Byrt CS, Hare RA, Tyerman SD, Tester M, Plett D, Gilliham M. Wheat grain yield on saline soils is improved by an ancestral transporter gene. Nat Biotechnol. 2012;30:360–364. doi: 10.1038/nbt.2120. [DOI] [PubMed] [Google Scholar]
- Nawaz I, Iqbal M, Hakvoort HWJ, Bliek M, Boerc B, Schat H. Expression levels and promoter activities of candidate salt tolerance genes in halophytic and glycophytic Brassicaceae. Environ Exp Bot. 2014;99:59–66. doi: 10.1016/j.envexpbot.2013.10.006. [DOI] [Google Scholar]
- Niu X, Bressan RA, Hasegawa PM, Pardo JM. Ion homeostasis in NaCl stress environments. Plant Physiol. 1995;109:735–742. doi: 10.1104/pp.109.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten JD, Egdane JA, Ismail AM. Salinity tolerance, Na+ exclusion and allele mining of HKT1;5 in Oryza sativa and O. glaberrima: many sources, many genes, one mechanism. BMC Plant Biol. 2013 doi: 10.1186/1471-2229-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin I, Shabala S. Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Plant Sci. 2014;5:1–16. doi: 10.3389/fpls.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani A, Niazi A, Abolimoghadam AA, Zamani Babgohari M, Deihimi T, Ebrahimi M, Akhtardanesh H, Ebrahimie E. Quantitative expression analysis of TaSOS1 and TaSOS4 genes in cultivated and wild wheat plants under salt stress. Mol Biotechnol. 2013;53:189–197. doi: 10.1007/s12033-012-9513-z. [DOI] [PubMed] [Google Scholar]
- Rus A, Lee BH, Munoz-Mayor A, Sharkhuu A, Miura K, Zhu JK. AtHKT1 facilitates Na+ homeostasis and K+ nutrition in plants. Plant Physiol. 2004;136:2500–2511. doi: 10.1104/pp.104.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P, Blumwald E. Assessing reference genes for accurate transcript normalization using quantitative real-time PCR in pearl millet [Pennisetum glaucum (L.) R. Br.] PLoS ONE. 2014;9(8):e106308. doi: 10.1371/journal.pone.0106308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P, Sade N, Arzani A, Wilhelmi MMR, Coe KM, Li B, Blumwald E. Effects of abiotic stress on physiological plasticity and water use of Setaria viridis (L.) Plant Sci. 2016;251:128–138. doi: 10.1016/j.plantsci.2016.06.011. [DOI] [PubMed] [Google Scholar]
- SAS Institute . Base SAS 9.3 procedures guide. Cary: SAS Institute Inc.; 2011. [Google Scholar]
- Schachtman DP, Munns R. Sodium accumulation in leaves of Triticum species that differ in salt tolerance. Funct Plant Biol. 1992;19:331–340. [Google Scholar]
- Shabala S. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot. 2013;112:1209–1221. doi: 10.1093/aob/mct205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Pottosin I. Regulation of potassium transport in plant under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol Plant. 2014;151:257–279. doi: 10.1111/ppl.12165. [DOI] [PubMed] [Google Scholar]
- Shabala S, Hariadi Y, Jacobson SE. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J Plant Physiol. 2013;170:906–914. doi: 10.1016/j.jplph.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Shen G, Wei J, Qiu X, Hu R, Kuppu S, Auld D, Blumwald E, Gaxiola R, Payton P, Zhang H. Co-overexpression of AVP1 and AtNHX1 in cotton further improves drought and salt tolerance in transgenic cotton plants. Plant Mol Biol Rep. 2015;33:167–177. doi: 10.1007/s11105-014-0739-8. [DOI] [Google Scholar]
- Shi H, Ishitani M, Kim C, Zhu J. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins D, Gibson T. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice CLUSTAL W. Nucl Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JI. The Arabidopsis HKT1 gene homolog mediates inward currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol. 2000;122:1249–1259. doi: 10.1104/pp.122.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Bilsborrow PE, Hooley P, Fincham DA, Lombi Forster BP. Salinity induced differences in growth, ion distribution and partitioning in barley between the cultivar Maythorpe and its derived mutant Golden Promise. Plant Soil. 2003;250:183–191. doi: 10.1023/A:1022832107999. [DOI] [Google Scholar]
- Yadav NS, Shukla PS, Jha A, Agarwal PK, Jha B. The SbSOS1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol. 2012;12:188. doi: 10.1186/1471-2229-12-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousfi S, Marquez AJ, Betti M, Araus JL, Serret MD. Gene expression and physiological responses to salinity and water stress of contrasting durum wheat genotypes. J Integr Plant Biol. 2016;58:48–66. doi: 10.1111/jipb.12359. [DOI] [PubMed] [Google Scholar]
- Zamani Babgohari M, Niazi A, Moghadam AA, Deihimi T, Ebrahimie E. Genome-wide analysis of key salinity-tolerance transporter (HKT1;5) in wheat and wild wheat relatives (A and D genomes) In Vitro Cell Dev Biol Plant. 2013;49:97–106. doi: 10.1007/s11627-012-9478-4. [DOI] [Google Scholar]
- Zhang WD, Wang P, Bao Z, Ma Q, Duan LJ, Bao AK, Zhang JL, Wang SM. SOS1, HKT1; 5 and NHX1 synergistically modulate Na+ homeostasis in the halophytic grass Puccinellia tenuiflora. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhi D, Xue Z, Liu H, Xia G. Enhanced salt tolerance of transgenic progeny of tall fescue (Festuca arundinacea) expressing a vacuolar Na+/H+ antiporter gene from Arabidopsis. J Plant Physiol. 2007;164:1377–1383. doi: 10.1016/j.jplph.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Zhu GY, Kinet JM, Lutts S. Characterization of rice (Oryza sativa L.) F3 populations selected for salt resistance. I. Physiological behaviour during vegetative growth. Euphytica. 2001;121:251–263. doi: 10.1023/A:1012016431577. [DOI] [Google Scholar]
- Zhu M, Shabala L, Cuin TA, Huang X, Zhou M, Munns R, Shabala S. Nax loci affect SOS1-like Na+/H+ exchanger expression and activity in wheat. J Exp Bot. 2016;67:835–844. doi: 10.1093/jxb/erv493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Zhou M, Shabala L, Shabala S. Physiological and molecular mechanisms mediating xylem Na+ loading in barley in the context of salinity stress tolerance. Plant Cell Environ. 2017;40:1009–1020. doi: 10.1111/pce.12727. [DOI] [PubMed] [Google Scholar]