Abstract
The present work makes an effort to assess and standardize some redox metabolic and molecular parameters for screening drought tolerant indigenous aromatic rice cultivars of West Bengal, India. PEG-induced dehydration stress during early germination caused disruption of redox-homeostasis and oxidative damage in four IARVs (Jamainadu, Tulaipanji, Sitabhog and Badshabhog) by enhancing the accumulation of pro-oxidants [assessed in terms of oxidation of 2′,7′-dichlorofluorescindiacetate (DCFDA), accumulation of and H2O2 and in situ staining of reactive oxygen species (ROS) in germinating tissue], significant reduction of antioxidative defence (total antioxidant and radical scavenging capacity, total thiol content and activities of antioxidative defence enzymes) and aggravating protein oxidation and lipid peroxidation (assessed in terms of free carbonyl content and accumulation of thiobarbituric acid reactive substances). When compared between the indigenous aromatic rice cultivars, a clear trend in differential redox regulatory properties in which ROS-antioxidant interaction acts at metabolic interface for redox homeostasis was observed in the order Badshabhog > Tulaipanji > Sitabhog > Jamainadu. Moreover, when the efficacy of ascorbate–glutathione cycle for scavenging H2O2 generated during dehydration stress was assessed and compared between the landraces exposed to PEG-induced dehydration stress in germinating tissue, it also exhibited almost the same trend with the landrace Tulaipanji and Badsabhog exhibiting maximum and Jamainadu the minimum efficiencies of the redox cycle. The indigenous aromatic rice cultivars Tulaipanji and Badsabhog resist dehydration stress better than the other two landraces due to its early preparedness to combat oxidative stress by up-regulating expression of genes of some enzymes of ascorbate–glutathione cycle along with some other antioxidative enzymes. A model of redox homeostasis in which ROS-antioxidant (ascorbate–glutathione system) acts at metabolic interface for up-regulation of antioxidative gene expression necessary for differential drought stress tolerance among the indigenous aromatic rice varieties is suggested.
Electronic supplementary material
The online version of this article (10.1007/s12298-017-0484-1) contains supplementary material, which is available to authorized users.
Keywords: Ascorbate–glutathione cycle, Drought stress, Reactive oxygen species, Redox regulation, Indigenous aromatic rice cultivars
Introduction
Aromatic rice assumes immense significance for their aroma, taste, kernel elongation after cooking and better market demand and price. Although the most popular aromatic rice cultivars across the globe are mostly long grained but the Indigenous Aromatic Rice Cultivars (IARCs) in India are small and medium grained (Yadav et al. 2014). The state West Bengal, India is still a good source of IARC with excellent grain quality parameters in spite of biodiversity erosion due to green revolution. More than seventy IARCs are cultivated in different agro-climatic zones of the state (Deb and Bhattacharya 2005). Though, most of the IARCs are adapted to marginal lands requiring fewer inputs but reports related to their stress-responsiveness trait, particularly drought, is very scanty. In fact, rice exhibits variation in their sensitivity to drought and salinity stress (Basu et al. 2010a, b). Although, there are some reports on indica varieties of rice as drought and salinity tolerant but not much is known about drought tolerance of some aromatic rice cultivars traditionally grown in Gangetic West Bengal of India.
Water is a significant factor in agriculture and food production and its deficit pose severe threat to rice production (Wang et al. 2012; Serraj et al. 2011). Drought stress is primarily characterized by continuous loss of water through transpiration while the uptake of water by plant is significantly reduced due to depleted water level in soil. This eventually causes excessive loss of agricultural productivity, thus recognized as serious threat to sustainable agriculture (Khush 2005; Serraj et al. 2011). The adverse effect of drought stress to large extent depends on its duration, severity and the developmental stage of the plant. In all cases, depending on severity and duration, dehydration stress causes serious metabolic impairment in plants (Li and Liu 2016; RoyChoudhury et al. 2007). Generation of excess reactive species (ROS) and loss of redox homeostasis of the cell is one of the most important output of such metabolic impairment during dehydration stress (Li and Liu 2016; Li et al. 2012). Generally, ROS like , H2O2, ·OH, RCO·, RO·, 1O2, are produced by successive reduction of molecular O2 and associated oxidative damage ensued during dehydration stress (Faize et al. 2011). Both photosynthetic and respiratory redox cascades along with Photosynthetic carbon oxidation cycle (PCOC) contribute significantly in the excessive accumulation of ROS in plant cell under drought.
Since any change in redox status of the cell towards pro-oxidant always triggers oxidative deterioration and metabolic impairment, drought tolerance of crops largely depends on the efficiency of antioxidant defense systems and their interaction with ROS at metabolic interface to trigger and regulate the signaling role of ROS. Generally plants are equipped with an array of enzymatic and non-enzymatic defense system not only to reduce oxidative damage but also to regulate the titer of ROS under dehydration stress (Foyer and Noctor 2005; Zhang and Kirkham 1996). Out of various antioxidative pathways, the ascorbate–glutathione (ASC–GSH) cycle along with other H2O2 processing system has been regarded as the most important one (Noctor and Foyer 1998; Seckin and Aksoy 2013). In this antioxidative defense cycle, H2O2, the major long lived ROS, is reduced to H2O by ascorbate peroxidase (APOX), using ascorbate as electron donor (Smirnoff, 2000). Dehydroascorbate (DHA) produced as by-product then subsequently regenerates ascorbate by dehydroascorbate reductase (DHAR) at the expense of reduced glutathione (GSH). Finally GSSG is converted to GSSH by receiving electron from NADPH by NADPH dependent glutathione reductase (GR).
With increasing intensity of drought stress in rice, the level of ascorbic acid and glutathione increased with corresponding enhancement of some ascorbate–glutathione (ASC–GSH) cycle enzymes (Selote and Khanna-Chopra 2004; Sharma and Dubey 2005). The enhanced activities of these antioxidative defense systems in rice represent one of the most significant protective activity to counteract oxidative injury promoted by drought conditions in rice (Pandey and Shukla 2015; Yang et al. 2014). Therefore, up-regulation of antioxidative defense comprising of H2O2 processing system, particularly ascorbate–glutathione (ASC–GSH) cycle may be one of the important strategy not only for reducing oxidative damage but also to control ROS titer necessary for maintenance of redox homeostasis and signaling (Hasanuzzaman et al. 2014; Zagorchev et al. 2016; Yang et al. 2014).
At present, our poor understanding about inherent complexity of drought stress tolerance mechanism including several signaling processes with ROS-antioxidant interaction as the central mechanism lead to our inability to improve drought stress tolerance. Most significantly, scant attention is being paid to prove the significance of ascorbate–glutathione (ASC–GSH) cycle and its key components involved in redox-regulation in aromatic indica rice cultivars under dehydration stress. Here an effort have been made, to assess the efficacy of ascorbate–glutathione (ASC–GSH) cycle for scavenging H2O2 and maintenance of redox homeostasis in four IARCs commonly cultivated in west Bengal, India. The study was also undertaken to determine more clearly the basis of differential redox regulation and oxidative stress tolerance under PEG-induced drought stress during early imbibitional phase of germination of four important IARCs. Further, the work will also help us to ascertain whether redox-regulated traits should be targeted by plant breeders for selection or genetic manipulation for improving the ability of aromatic rice cultivars to grow under drought stress.
Materials and methods
Plant growth and treatment of PEG-6000 to induce imbibitional dehydration stress
Seeds of the four Indigenous Aromatic Rice Cultivars (IARCs (Oryza sativa L, Cultivars Jamainadu, Tulaipanji, Sitabhog and Badshabhog), selected as experimental material, have been collected from Chinsurah Rice Research Institute, West Bengal, India. Seeds of the experimental cultivar were washed with distilled water and were treated with 0.2% HgCl2 for 5 min and then washed thrice with sterile distilled water. The surface sterilized seeds were imbibed in distilled water for 48 h in darkness at 25° ± 2 °C and thereafter, were sown on moist filter paper in petri plates and were placed in standardized conditions of thermostat-controlled seed germinator cum stability chamber (Remi 82 BL, India) maintained at 25° ± 2 °C temperature. For imposing imbibitional dehydration stress of different magnitude, three different water-imbibed seed lots were treated with − 0.344, − 0.851 and − 1.619 MPa PEG-6000 for 7 days, with intermittent change of treating solutions in petriplates (24 h interval). For untreated control set, water imbibed seeds were sown directly in petriplates and exposed at 25° ± 2 °C. All the seed lots were allowed to grow at 25° ± 2 °C with 14 h photo period (light intensity 270 µmol m−2 s−1) and 78 ± 2% relative humidity. For all biochemical analysis, 168 h old seedlings raised from aforesaid conditions were used.
Determination of reactive oxygen species (ROS)
Estimation of “total ROS” generation
Total ROS generation was estimated by performing an in vitro assay. Seedling tissue (30 mg) was placed in 8 mL 40 mM TRIS–HCl buffer (pH-7.0) in the presence of 100 µM 2′,7′-dichlorofluorescindiacetate (DCFDA, Sigma) at 30 °C. Supernatant was removed after 60 min and fluorescence was monitored in a spectrofluorometer (Hitachi, Model F-4500 FL Spectrophotometer) with excitation at 504 nm and emission at 525 nm (Simontacchi et al. 1993). To differentiate ROS from other long-lived substances able to react with DCFDA, additional controls were performed. For additional controls, seedling tissues were incubated without DCFDA for 60 min and then tissues were removed followed by addition of DCFDA which is 60 min before fluorescence was determined. This florescence values was subtracted from all readings to assess the fluorescence that depend on ROS.
Estimation of “and H2O2” generation
Hydrogen peroxide was extracted and estimated by the procedure of MacNevin and Uron (1953) using titanic sulfate. For this, 1 g of tissue was extracted with 5 mL of cold acetone and filtered through Whatman No. 1 filter paper and volume made up to 10 mL with distilled water. Now 1 mL of 5% titanic sulfate (in 20% H2SO4) was added to this, which was followed by addition of 2 mL of concentrated NH4OH and finally centrifuged at 6000 rpm for 10 min. Pellet obtained was washed with 5 mL of acetone (thrice) and then centrifuged at 5000 rpm for 10 min. Then, the pellet was dissolved in 3 mL of 2(N) H2SO4 and absorbance was taken at 420 nm against a blank.
In case of determination of superoxide, the process of Chaitanya and Naithani (1994) was followed with some necessary modifications. 500 mg of tissues was homogenized in cold with 5 mL of 0.2 M sodium phosphate buffer (pH 7.2), with addition of diethyldithiolcarbomate (10−3 M) to inhibit SOD activity. The homogenates was immediately centrifuged at 2000g at 4 °C for 1 min. In the supernatant, superoxide anion was measured by its capacity to reduce NBT (nitrobluetetrazolium, 2.5 × 10−4 M). The absorbance of the end product was measured at 540 nm. Formation of superoxide was expressed as ΔA540 g−1 (dm) min−1.
In situ staining for visualization of superoxide and hydrogen peroxide
For the detection of superoxide and hydrogen peroxide, the process of He et al. (2009) was followed. In case of superoxide, the seeds of different stressed conditions as well as control set were incubated separately in 6 mM Nitroblue tetrazolium in 10 mM TRIS–HCl buffer (pH-7.4) at room temperature for 15 min. The accumulation of superoxide anion was detected by observing the dark blue colour as compared to untreated control set. Hydrogen peroxide was detected by soaking the stressed and untreated control seedlings in 0.42 mM TMB (3, 5, 3′5′-tetramethylbenzidine) solution in TRIS–acetate buffer (pH-5) for 2 h. Blue–green colour can be monitored to indicate the accumulation of H2O2.
Determination of indices of oxidative membrane damage (free carbonyl content and thiobarbituric acid reactive substances)
Free carbonyl content
Oxidative damage to proteins was estimated as the content of carbonyl groups following the procedure of Jiang and Zhang (2001). 500 mg of tissues (root and shoot) were homogenized with 3 mL of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM PMSF (phenyl methyl sulfonyl fluoride), 10 mM DTT (dithithreitol) and 5 μg mL−1 leupeptin, 5 μg mL−1 aprotinin and 5 μg mL−1 antipain (protease inhibitors). The homogenate was centrifuged at 15000×g for 25 min and the supernatant was made free from contaminating nucleic acids by treatment with streptomycin sulfate. An equal volume of 10 mM DNPH in 2 M HCl was added to supernatant containing the oxidized protein. These were allowed to stand in the dark at room temperature for 1 h, with vortex every 10 min. Samples were precipitated with trichloroacetic acid (TCA; 20% final concentration) and centrifuged in a table-top micro centrifuge for 5 min. The supernatants were discarded and the protein pellets were washed twice more with TCA, and then washed three times with 1 mL portions of ethanol/ethylacetate (1:1) to remove any free DNPH. The protein samples were re-suspended in 1 mL of 6 M guanidine hydrochloride (dissolved in 20 mM phosphate buffer, pH 2.3) at 37 °C for 15 min with vortex mixing. Carbonyl contents were determined from the absorbance at 370 nm using a molar absorption coefficient of 22 mM cm−1.
Thiobarbituric acid reactive substances
To estimate membrane lipid peroxidation, test for thiobarbituric acid reactive substances (TBARS) was performed using the procedure of Heath and Packer (1968). 200 mg of sample was homogenized in 5 mL 0.1% trichloroacetic acid (TCA) and then centrifuged at 10,000 rpm for 15 min and finally supernatant was taken. To 1 mL of supernatant, 3 mL of 5% TCA containing 1% thiobarbituric acid (TBA) was added and heated in a hot water bath for 30 min and cooled quickly in cold water bath. It was finally centrifuged at 10,000 rpm for 10 min. The absorbance of the supernatant was measured at 530 nm. The concentration of TBARS was measured from its extinction coefficient of 155 μM cm−1. The non-specific turbidity was corrected by subtracting A600 from A530 value. The TBARS content is finally expressed in n mol g−1 dry mass of tissue.
Determination of antioxidative defense
ABTS decolorization assay
ABTS (2,2′ azinobis (3-ethylbenzthiazoline)-6-sulfonic acid) free radical decolourization assay was done according to Re et al. (1999) with some necessary modifications. For the preparation of tissue extract required for ABTS assay, 1.5 g of dry sample (seedling tissue kept at 45 °C for 2 days) was extracted with 30 mL 80% methanol at 28 °C for 24 h in shaking incubator. Extracts were centrifuged at 3500 rpm for 20 min at 4 °C. Supernatant was collected and filtered and filtrate was used for ABTS assay. The preformed radical monocation of ABTS was generated by making ABTS solution (7 mM) react with 2.42 mM potassium persulfate at room temperature for 18 h in dark. The solution was then diluted to obtain absorbance of 0.7 ± 0.02 at 734 nm. The aliquot of 300 µL of sample was the mixed with 3000 µL of ABTS free radical cation solution. The absorbance monitored for 5 min was measured spectrophotometrically. Appropriate solvent blanks were run in each assay. The percentage of inhibition was calculated against control and compared against standard curve with trolox as antioxidant.
DPPH (2,2′-diphenyl-1-pycryl hydrazyl) free radical scavenging activity
For determination of DPPH free radical scavenging activity, the process of Mensor et al. (2001) was followed with slight modification. 1.5 g of dry sample (seedling tissue kept at 45 °C for 2 days) was extracted with 30 mL of 80% methanol at 28 °C for 24 h in shaking incubator. Extracts were centrifuged at 3500 rpm for 20 min at 4 °C. Supernatant was collected and filtered and filtrate was used for DPPH radical scavenging activity. For estimating the radical scavenging activity, 1 mL sample was mixed with 3 mL DPPH (0.04 mg mL−1 ethanol) and incubated for 30 min in darkness and then absorbance was taken at 517 nm. Total antioxidant capacity (TAC) was calculated as:
where Ai = 1 mL sample + 3 mL DPPH, Aj = 1 mL sample + 3 mL ethanol, Ac = 1 mL ethanol + 3 mL DPPH.
FRAP assay
For FRAP (Ferric reducing antioxidant power) assay the procedure was carried out according to Benzie and Strain (1996) with slight modification. The working FRAP reagent was produced by mixing 300 mM acetate buffer (pH 3.6), 10 mM 2, 4, 6 tripyridyl-s-trizine (TPTZ) solution and 20 mM FeCl3·6H2O in 10:1:1 ratio prior to use and heated at 37 °C in water bath. For the preparation of tissue extract, 1.5 g of dry sample (seedling tissue kept at 45 °C for 2 days) was extracted with 30 mL 80% methanol at 28 °C for 24 h in shaking incubator. Extracts were centrifuged at 3500 rpm for 20 min at 4 °C. Supernatant was collected and filtered and filtrate was used for FRAP assay. A total of 150 µL of tissue extract with 2850 µL of freshly prepared FRAP reagent was mixed in a cuvette and kept for 30 min in dark. Finally the absorbance was taken at 593 nm. The standard curve using grades of Trolox was prepared for estimating the unknown. The final result was expressed as antioxidant capacity in µM trolox equivalent g−1 dm.
Extraction and estimation of enzymatic antioxidants
Ascorbate peroxidase (APOX) activity was determined according to Nakano and Asada (1981) using homogenates previously supplemented with 0.5 mM ascorbic acid and 0.1 mM EDTA. Parallel experiments in presence of p-chloromercuribenzoate (50μM) were performed to rule out any interference from guaiacol peroxidases. Glutathione reductase (GR) activity was measured according to Schaedle and Bassham (1977). The reaction mixture contained 50 mM Tris–HCl (pH 7.6), 0.15 mM NADPH, 0.5 mM oxidized glutathione (GSSG), 3 mM MgCl2 and 100 μL homogenate (7 mg protein mL−1). NADPH oxidation was followed at 340 nm. The enzyme activity in all cases was expressed as enzyme unit min−1 g−1 d.m. according to Fick and Qualset (1975).
The extraction and estimation of superoxide dismutase (SOD) activity was determined by measuring the photochemical reduction ability of nitroblue tetrazolium (NBT) according to Giannopolities and Ries (1977) with some necessary modifications. The reaction mixture contained 0.05 M Na2CO3, 0.1 mM EDTA, 63 mM NBT and 13 mM riboflavin. The assay mixture was placed under 40 W florescent lamp at a distance of 30 cm at 25 °C for 30 min and absorbance was read at 560 nm. The non-irradiated sample served as control.
For the extraction and estimation of catalase (CAT), the process of Snell and Snell (1971) was followed with some necessary modifications. 100 mg of tissue was homogenized with 0.1 M sodium phosphate buffer (pH 7.0) containing 1% polyvinylpolypyrillidone (PVPP) and the homogenate was centrifuged at 5000 rpm for 10 min at 4 °C. For enzyme assay, 1 mL of enzyme extract was added to 1 mL of 0.5 mM H2O2 and incubated for 15 min at 37 °C. The reaction mixture was stopped by adding 2 mL 1% TiSO4 (in 25% H2SO4). The assay mixture was further centrifuged at 5000 rpm and the absorbance of the supernatant was read at 420 nm.
Determination of components of ascorbate–glutathione pathway
One gram seedling tissue was homogenised in 10 mL cold 5% metaphosphoric acid. After centrifugation at 15,000g for 30 min at 4 °C, the supernatant was collected for analyses of ascorbate and glutathione. This extraction procedure was slightly modified from the method given by Gossett et al. (1994). The measurement of total ascorbate and reduced ascorbate (AsA) contents was modified from the method of Law et al. (1983). Total ascorbate contents were determined in a 3 mL mixture. Enzyme extract was mixed with 10 mM DTT and 150 mM phosphate buffer (pH 7.4) containing 5 mM EDTA and was incubated at 25 °C for 10 min, followed by addition of 0.5% of N-ethylmaleimide. Then 10% TCA, 44% orthophosphoric acid, and 4% of α,α′-bipyridyl were added. Finally, 3% of FeCl3 was added and the mixture was incubated in 40 °C for 40 min and the absorbance was detected at A525. AsA contents were determined by adding distilled water instead of DTT and N-ethylmaleimide and then followed the same method as above. Total and reduced contents were estimated from the standard curve of 0–100 µg mL−1 L-AsA determined by the above method. DHA contents were calculated by the subtraction of AsA from total AsA. Total glutathione contents were determined by the absorbance at 412 nm according to the method, reported by Zhang and Kirkham, 1996. The contents of glutathione (reduced form) were estimated from the standard curve of 0–30 µmol mL−1 glutathione. After the removal of glutathione (GSH) by 2-vinylpyridine derivative, glutathione disulfide (GSSG) contents were determined, and the glutathione (GSH) contents were calculated by the subtraction of glutathione disulfide (GSSH) contents from total glutathione contents.
The estimation of dehydroascorbate reductase (DHAR) activity was done by following the process of Nakano and Asada (1981) with some modification. The enzyme extract was mixed with 50 mM phosphate buffer (pH-7.0), 2.5 mM GSH, 0.2 mM DHA, 0.1 mM EDTA and reaction rates were measured by the increase in absorbance at 265 nm in 10 s and in 30 s after adding the enzyme.
Extraction and estimation of total thiol content
For the estimation of total thiol content (TTC), the process of Tietze (1969) was followed. Total-SH content was assayed in acid soluble extract (500 mg of tissue in 3% w/v TCA solution) followed by a brief centrifugation. The supernatant was then diluted tenfold in 100 mM phosphate buffer (pH 7.5). Thiol contents was determined measuring absorbance at 412 nm in presence of 0.5 mM 5,5′-dithiobis 2-nitrobenzoic acid (DTNB), 0.5 U mL−1 glutathione reductase and 0.2 mM NADPH.
RNA isolation, preparation of cDNA and analysis of transcript profile by semi quantitative RT-PCR
RNA isolation
RNA was extracted from untreated control, imbibitional heat and chilling stress-raised seedlings of both the experimental cultivars of rice using Guanidium isothiocyanate-phenol based reagent (RNA-XPress™ reagent, HiMedia) according to manufacturer’s instructions. To ensure the comparability of the resulting band intensity, quantification of RNA was done using Nano drop Spectrophotometer (ND1000, Nanodrop technologies, USA) and confirmed by applying equal amounts of total RNA to an agarose gel using ethidium bromide staining.
Preparation of cDNA
First strand cDNA synthesis was done by using Revert Aid First Strand cDNA synthesis kit (Fermentas, Thermo Scientific) according to the manufacturer’s protocol and quality of cDNA was checked by running 2 µl of total cDNA in 1.1% agarose gel using tris–acetate-EDTA (TAE, pH-8) buffer. Quantification of cDNA was performed by Nano Drop spectrophotometer (ND1000, Nanodrop technologies, USA) at 260 nm and stored at − 80 °C for further use.
Differential accumulation of transcript using reverse-transcriptase RT-PCR
Semiquantitative RT-PCR was performed as described by Burch-Smith et al. (2006). After synthesis, cDNA was diluted 1:10 and 4 µL of cDNA was used as template for PCR amplification in a 25 µL reaction mixture. Reaction contained selected couples of the following gene-specific primers: SodCc2 (accession no. L19434) F, 5′-ATTGGCCGAGCTGTTGTTGTCC-3′, SodCc2 R, 5′-TAACCCTGGAGTCCGATGATTCCG-3′, CatA (accession no EF371902) F, 5′-AAGCTGTTCGTCCAGGTGATCG-3′, CatA, R, 5′-TGTCGACGTTGCGGTTGAGAAC-3′, OsAPx2 F (accession no. AK068430) 5′-TCTTCCTGATGCCACACAAGGTTC-3′, OsAPx2 R,5′-TCCTTGTCACTCAAACCCATCTGC-3′, GRase F, (accession no. NM001055020) 5′-CGACCTTTGACAGCACTGTTGG-3′, GRase R, 5′-TGGTCAAGGTCCGCATTGTCAC-3′, 18S rRNA (Hsk) F, (accession no. AB047313) 5′-CTACGTCCCTGCCCTTTGTACA-3′ 18S R, 5′-ACACTTCACCGGACCATTCAA-3′. PCR conditions were as follows: initial denaturation of 2 min at 95 °C, followed by 30 cycles of denaturation for 30 s at 95 °C, annealing at 54 °C for 30 s, extension at 72 °C for 30 s, and a final extension of 8 min at 72 °C. The conditions of semi-quantitative RT-PCR were chosen so that none of the mRNAs analyzed reached a plateau at the end of amplification cycles, i.e. they were in exponential phase of amplification, and that the two sets of primers (one set of gene specific primer and other set of 18S rRNA primers) used in the reaction did not compete with each other. Appropriate number of cycles was determined by testing different cycles of 15, 20 and 25 for both antioxidant gene transcripts and 18S rRNA (internal control) amplification. It was important to select the appropriate number of cycles so that the amplified product was clearly visible on agarose gel and can be quantified, while the amplification was in exponential range and did not reach plateau yet. Moreover, the optimal number of cycles was strictly maintained to be in the same range for specific mRNA of interest and the internal control, so that both can be measured on the same gel. Equal amounts of PCR products were the run on the same 1.2% agarose gel for comparison and quantification. Images of the RT-PCR products in ethidium bromide stained agarose gels were acquired in BIO-Rad Molecular Imager Gel Doc XR systemwith high resolution CCD camera and quantification of bands was performed by Bio Rad image Densitometer GS-700 using quantity one software (version-4.6.9).
Determination of early growth performances
For studying early growth performances, relative growth index (RGI), T50 value, relative germination performance (RGP) and vigor index (VI) were calculated according to Rubio-Casal et al. (2003) and Bhattacharjee (2008).
Relative growth index was calculated as:
Vigor index (VI) was measured as:
Relative germination performance (RGP) was measured as:
Statistical analysis
Each treatment consisted of three replicates and each experiment was carried out twice at different times. Results are mean of three replicates ± standard error. For statistical analysis of the data for significance, the paired two sample t test was done with the help of Microsoft Excel 2010, which shows the significant variations between untreated control and different imbibitional dehydration stress-raised seedlings.
Results
Modulation of endogenous redox cues by different magnitude of PEG-induced imbibitional dehydration stress (IDS) in four indigenous aromatic rice cultivars
When imbibitional dehydration stress (IDS) of different magnitude (− 0.344 MPa, IDS − 0.851 MPa, IDS − 1.619 MPa) was imposed separately to all four experimental aromatic rice cultivars (Jamainadu, Tulaipanji, Sitabhog, Badshabhog) by treatment with PEG 6000, corresponding changes in internal redox cues of growing seedlings were observed in terms of accumulation of pro-oxidants (DCFDA oxidation, , H2O2 content), antioxidative defense (ABTS, FRAP and DPPH radical scavenging activities, total thiol content, activities of some antioxidative defense enzymes). Figures 1a, b and 2d shows the level of endogenous total ROS (assessed in terms of and H2O2 accumulation and oxidation of DCFDA) in IDS-raised seedlings of four experimental IARCs. The result also showed a dose-dependent accumulation of ROS, with maximum accumulation being noticed in IDS − 1.619 MPa treatment raised seedlings of Jamainadu and Sitabhog and minimum in Badsabhog and Tulaipanji under the same magnitude of IDS. When total ROS concentration was measured (DCFDA oxidation) and compared between the IDS-raised seedlings of all the four experimental rice cultivars, Tulaipanji exhibited minimum accumulation followed by Badsabhog, Jamainadu and Sitabhog, indicating loss of redox homeostasis in the order Jamainadu > Sitabhog > badsabhog > Tulaipanji.
Fig. 1.
Effect of different magnitude of PEG induced imbibitional dehydration stress (− 0.344, − 0.851 and − 1.619 MPa) on the accumulation of superoxide (a), and hydrogen peroxide (b); protein oxidation [assessed in terms of free carbonyl content (c)] and membrane lipid peroxidation [assessed in terms of accumulation of thiobarbituric acid reactive substances (d)] of four indigenous aromatic rice varieties of Oryza sativa L. (Tulaipanji, Sitabhog, Badshabhog and Jamainadu). Results are mean of three replicates ± standard error. *Significant from control at 0.05 level (t test). **Significant from control at 0.01 level (t test)
Fig. 2.
Effect of different magnitude of PEG induced imbibitional dehydration stress (− 0.344, − 0.851 and − 1.619 MPa) on total antioxidant capacity [assessed in terms of ABTS assay (a)]; radical scavenging activity [assessed in terms of DPPH radical scavenging assay (b) and assessed in terms of FRAP assay (c)] and accumulation of total reactive oxygen species [assessed in terms of DCFDA oxidation (d)] of four indigenous aromatic rice varieties of Oryza sativa L.(Tulaipanji, Sitabhog, Badshabhog and Jamainadu). Results are mean of three replicates ± standard error. *Significant from control at 0.05 level (t test). **Significant from control at 0.01 level (t test)
Assessment of antioxidant capacity (ABTS, DPPH and FRAP assay) to IDS-raised seedlings of all the four experimental rice cultivars clearly exhibited enhanced radical scavenging activities in the seedlings of cultivars Tulaipanji and Badshabhog under all treatment, whereas the cultivars Sitabhog and Jamainadu exhibited significant reduction under − 0.851 and − 1.619 MPa IDS treatment (Fig. 2). The reductions of radical scavenging activities measured by ABTS, DPPH and FRAP assays, in Jamainadu, Sitabhog cultivars under − 0.851 and − 1.619 MPa IDS were found to be statistically significant (P ≤ 0.05). Similarly, the enhancement of radical scavenging activities for the cultivar Tulaipanji and Badshabhog were found to be significant. The same pattern of restoration of total thiol content in experimental rice cultivars raised from different magnitude of IDS (− 0.344, − 0.851 and − 1.619 MPa) have been compared (Table 3), corroborating well with the data total radical scavenging properties measured by ABTS, DPPH and FRAP assay. Histochemical staining for in situ localization of , H2O2 in IDS raised seedlings also corroborate the same finding with maximum intensification of staining being noticed in the cultivar Jamainadu and minimum in Tulaipanji (Fig 5A & B in ESM). Result clearly exhibited maximum accumulation of both the ROS (superoxide and hydrogen peroxide) in the cultivar Jamainadu and minimum in Tulaipanji and Badshabhog under the same magnitude of IDS.
Table 3.
Effect of different magnitude of PEG induced imbibitional dehydration stress (− 0.344, − 0.851 and − 1.619 MPa) on glutathione (total, oxidised and reduced) and total thiol content of four indigenous aromatic rice varieties of Oryzasativa L. (Tulaipanji, Sitabhog, Badshabhog and Jamainadu)
| Landraces of aromatic rice | Treatment | Total glutathione (µg g−1 dm) | Oxidized glutathione (µg g−1 dm) | Reduced glutathione (µg g−1 dm) | Total thiol content (m mol g−1 dm) |
|---|---|---|---|---|---|
| Jamainadu | Untreated control | 750 ± 2.42 | 193.18 ± 1.96 | 556.82 ± 0.46 | 2.342 ± 0.032 |
| IDS (− 0.344 MPa) | 416.67 ± 2.54** | 82.07 ± 2.42** | 334.60 ± 0.13** | 1.865 ± 0.03** | |
| IDS (− 0.851 MPa) | 325.31 ± 2.69** | 140.88 ± 2.69** | 184.43 ± 0.00** | 1.386 ± 0.022** | |
| IDS (− 1.619 MPa) | 537.26 ± 2.31** | 125.65 ± 2.59** | 411.61 ± 0.27** | 1.199 ± 0.027** | |
| Tulaipanji | Untreated control | 385.9 ± 1.39 | 184.56 ± 1.55 | 201.34 ± 0.16 | 4.157 ± 0.008 |
| IDS (− 0.344 MPa) | 241.6 ± 1.58** | 28.01 ± 1.33** | 213.59 ± 0.25 | 3.591 ± 0.016** | |
| IDS (− 0.851 MPa) | 199.11 ± 1.91** | 25.58 ± 1.39** | 73.53 ± 0.52** | 3.435 ± 0.025** | |
| IDS (− 1.619 MPa) | 180.26 ± 1.96** | 29.55 ± 1.50** | 150.71 ± 0.46** | 3.364 ± 0.014** | |
| Sitabhog | Untreated control | 412.31 ± 1.52 | 199.61 ± 1.69 | 212.70 ± 0.16 | 1.844 ± 0.015 |
| IDS (− 0.344 MPa) | 485.03 ± 1.69 | 97.66 ± 1.80** | 387.37 ± 0.11 | 1.421 ± 0.02** | |
| IDS (− 0.851 MPa) | 378.79 ± 2.04** | 244.95 ± 2.31 | 133.84 ± 0.27** | 1.449 ± 0.016** | |
| IDS (− 1.619 MPa) | 496.96 ± 2.26 | 192.70 ± 1.99** | 304.26 ± 0.27 | 1.663 ± 0.027* | |
| Badshabhog | Untreated control | 327.75 ± 1.66 | 64.79 ± 1.39 | 262.96 ± 0.27 | 2.339 ± 0.024 |
| IDS (− 0.344 MPa) | 318.28 ± 1.61** | 69.44 ± 2.10 | 248.84 ± 0.49* | 1.499 ± 0.018** | |
| IDS (− 0.851 MPa) | 229.13 ± 2.01** | 20.83 ± 1.42** | 208.30 ± 0.60** | 1.492 ± 0.024** | |
| IDS (− 1.619 MPa) | 159.53 ± 2.40** | 114.29 ± 2.56 | 45.24 ± 0.16** | 1.431 ± 0.03** |
Results are mean of three replicates ± standard error
* Significant from control at 0.05 level (t test); ** significant from control at 0.01 level (t test)
Efficacy of ascorbate–glutathione cycle in seedlings of four indigenous aromatic rice cultivars raised from PEG-induced imbibitional dehydration stress
Imbibitional dehydration stress of different magnitude to all four cultivars of experimental rice revealed significant variation in germination (Table 1) and activities of all the enzymes of ascorbate–glutathione (ASC–GSH) cycle (APOX, DHAR and GR) in both the cultivar Tulaipanji (Fig. 3 and Table 2). Both the cultivasr Tulaipanji and Badsabhog exhibited maximum significant up-regulation in the activities of APOX under different magnitude of IDS, as compared to the cultivars Jamainadu and Sitabhog which exhibited significant reduction in higher doses of IDS (− 0.851 and − 1.619 MPa). When GR activity was measured and compared, Tulaipanji though exhibited significant enhancement in activities in higher doses of IDS (− 0.851 and − 1.619 MPa), but the cultivars Badsabhog, Sitabhog showed a declining trend in the activities of GR under the same higher doses of IDS. In fact, the activities of all these ascorbate–glutathione (ASC–GSH) cycle enzymes in seedlings of the cultivars Sitabhog and Jamainadu, particularly at higher magnitude of IDS (− 0.851 and − 1.619 MPa) exhibited significant decline over untreated control, corroborating well with the data of elevated accumulation of ROS and reduced total antioxidant capacity. Thus, when compared between the four experimental landraces, the efficacy of enzyme activities associated with ascorbate–glutathione (ASC–GSH) cycle showed significant variation with maximum efficiency in terms of the activities of APOX, DHAR and GR was exhibited by the cultivars Tulaipanji and Badshabhog followed by Sitabhog and Jamainadu under the same magnitude of IDS.
Table 1.
Effect of different magnitude of PEG-induced imbibitional dehydration stress (− 0.344, − 0.851 and − 1.619 MPa) on germination and early growth performances [assessed in terms of T50, Vigour Index (VI), Relative Germination Performance (RGP), Relative Growth Index (RGI) and Relative Water Content (RWC)] of four indigenous aromatic rice varieties of Oryza sativa L. (Cultivars Tulaipanji, Sitabhog, Badshabhog and Jamainadu)
| Early growth performances | Landraces of aromatic rice | Imbibitional dehydration stress [IDS] (MPa) | |||
|---|---|---|---|---|---|
| Untreated control | − 0.344 | − 0.851 | − 1.619 | ||
| T50 (h) | Jamainadu | 26.09 ± 0.05 | 22.22 ± 0.14** | 31.58 ± 0.19 | 150 ± 0.54 |
| Tulaipanji | 26.09 ± 0.08 | 19.35 ± 0.05** | 24 ± 0.03** | 25 ± 0.04** | |
| Sitabhog | 37.5 ± 0.23 | 40 ± 0.25 | 60 ± 0.19 | 300 ± 1.4 | |
| Badshabhog | 75 ± 0.23 | 120 ± 0.41 | 100 ± 0.82 | 100 ± 0.27 | |
| Vigor index (VI) | Jamainadu | 0.148 ± 0.001 | 0.083 ± .0003** | 0.106 ± 0.007 | 0.067 ± 0.001** |
| Tulaipanji | 0.082 ± 0.001 | 0.052 ± 0.0003** | 0.060 ± 0.001** | 0.079 ± 0.0003** | |
| Sitabhog | 0.084 ± 0.001 | 0.074 ± 0.001** | 0.063 ± 0.001** | 0.099 ± 0.001 | |
| Badshabhog | 0.122 ± 0.016 | 0.069 ± 0.003 | 0.060 ± 0.002 | 0.138 ± 0.005 | |
| Relative germination performance (RGP) | Jamainadu | 100.00 ± 0 | 104.44 ± 0.27 | 86.66 ± 0.19** | 51.11 ± 0.25** |
| Tulaipanji | 100.00 ± 0 | 100.00 ± 0 | 100.00 ± 0 | 100.00 ± 0 | |
| Sitabhog | 100.00 ± 0 | 88.88 ± 0.26** | 77.77 ± 0.19** | 80.55 ± 0.15** | |
| Badshabhog | 100.00 ± 0 | 120.00 ± 0.68 | 100.00 ± 0.27 | 92.00 ± 0.22** | |
| Relative growth index (RGI) | Jamainadu | 100.00 ± 0 | 99.03 ± 0.23 | 101.44 ± 0.54 | 95.65 ± 0.24* |
| Tulaipanji | 100.00 ± 0 | 106.03 ± 0.82 | 108.62 ± 0.54 | 104.31 ± 0.27 | |
| Sitabhog | 100.00 ± 0 | 102.02 ± 0.41 | 91.91 ± 0.27** | 102.02 ± 0.54 | |
| Badshabhog | 100.00 ± 0 | 104.12 ± 0.68 | 101.03 ± 0.41 | 109.28 ± 0.98 | |
| Relative water content (RWC) | Jamainadu | 91.7 ± 0.22 | 89.20 ± 0.19** | 88.78 ± 0.15** | 83.50 ± 0.17** |
| Tulaipanji | 90.23 ± 0.18 | 90.96 ± 0.16 | 89.27 ± 0.15** | 86.25 ± 0.14** | |
| Sitabhog | 92.00 ± 0.21 | 89.20 ± 0.19** | 83.62 ± 0.17** | 81.20 ± 0.16** | |
| Badshabhog | 87.05 ± 0.20 | 84.32 ± 0.17** | 82.62 ± 0.13** | 82.44 ± 0.14** | |
Results are mean of three replicates ± standard error
* Significant from control at 0.05 level (t test); ** significant from control at 0.01 level (t test)
Fig. 3.
Effect of different magnitude of PEG induced imbibitional dehydration stress (− 0.344, − 0.851 and − 1.619 MPa) on the activities of ascorbate peroxidase (a), glutathione reductase (b), superoxide dismutase (c) and catalase (d) of four indigenous aromatic rice varieties of Oryza sativa L. (Tulaipanji, Sitabhog, Badshabhog and Jamainadu). Results are mean of three replicates ± standard error. *Significant from control at 0.05 level (t test). **Significant from control at 0.01 level (t test)
Table 2.
Effect of different magnitude of PEG-induced imbibitional dehydration stress (− 0.344, − 0.851 and − 1.619 MPa) on ascorbate (total ascorbate, ascorbate and dehydroascorbate) and dehydroascorbate reductase of four indigenous aromatic rice varieties of Oryzasativa L. (Tulaipanji, Sitabhog, Badshabhog and Jamainadu)
| Landraces of aromatic rice | Treatment | Total ascorbate (mg g−1 dm) | Ascorbate (mg g−1 dm) | Dehydroascorbate (mg g−1 dm) | Dehydroascorbate reductase (A265 min−1 g−1 dm) |
|---|---|---|---|---|---|
| Jamainadu | Untreated control | 2.682 ± 0.03 | 2.424 ± 0.02 | 0.258 ± 0.016 | 0.447 ± 0.020 |
| IDS (− 0.344 MPa) | 2.121 ± 0.02** | 1.995 ± 0.02** | 0.126 ± 0.009 | 0.297 ± 0.019** | |
| IDS (− 0.851 MPa) | 1.721 ± 0.03** | 1.619 ± 0.03** | 0.103 ± 0.002 | 0.218 ± 0.022** | |
| IDS (− 1.619 MPa) | 1.568 ± 0.02** | 1.430 ± 0.02** | 0.139 ± 0.005* | 0.141 ± 0.016** | |
| Tulaipanji | Untreated control | 2.466 ± 0.02 | 2.282 ± 0.01 | 0.185 ± 0.003 | 0.356 ± 0.010 |
| IDS (− 0.344 MPa) | 2.101 ± 0.02** | 1.933 ± 0.02** | 0.168 ± 0.004* | 0.347 ± 0.011 | |
| IDS (− 0.851 MPa) | 2.046 ± 0.02** | 1.841 ± 0.02** | 0.205 ± 0.002 | 0.300 ± 0.009** | |
| IDS (− 1.619 MPa) | 2.139 ± 0.01** | 1.773 ± 0.02** | 0.366 ± 0.007 | 0.179 ± 0.014** | |
| Sitabhog | Untreated control | 2.068 ± 0.01 | 2.029 ± 0.02 | 0.039 ± 0.001 | 0.076 ± 0.008 |
| IDS (− 0.344 MPa) | 2.266 ± 0.02 | 2.148 ± 0.02 | 0.117 ± 0.006 | 0.062 ± 0.006 | |
| IDS (− 0.851 MPa) | 1.576 ± 0.03** | 1.556 ± 0.02** | 0.020 ± 0.005 | 0.047 ± 0.008** | |
| IDS (− 1.619 MPa) | 1.379 ± 0.03** | 1.349 ± 0.02** | 0.030 ± 0.007 | 0.035 ± 0.005 | |
| Badshabhog | Untreated control | 1.829 ± 0.02 | 1.784 ± 0.01 | 0.046 ± 0.004 | 0.144 ± 0.026 |
| IDS (− 0.344 MPa) | 1.435 ± 0.02** | 1.389 ± 0.01** | 0.046 ± 0.01 | 0.165 ± 0.027 | |
| IDS (− 0.851 MPa) | 1.479 ± 0.03** | 1.313 ± 0.02* | 0.167 ± 0.04 | 0.185 ± 0.027 | |
| IDS (− 1.619 MPa) | 1.295 ± 0.03** | 1.200 ± 0.03** | 0.095 ± 0.001 | 0.044 ± 0.006 |
Results are mean of three replicates ± standard error
* Significant from control at 0.05 level (t test); ** significant from control at 0.01 level (t test)
IDS of different magnitude further modify the endogenous level of both the low molecular weight antioxidants ascorbate (ASC) and glutathione (GSH) in all IARCs (Tables 2 and 3). The cultivars Jamainadu and Sitabhog suffered maximum loss of ASC and GSH in comparison to Tulaipanji and Badsabhog, under the same magnitude of IDS. In fact, the loss of low molecular weight antioxidant ascorbate was found to be minimum in Tulaipanji. The result, therefore, corroborate strongly the fact that the efficacy of ASC–GSH cycle found to be maximum in Tulaipanji and Badshabhog followed by Sitabhog and Jamainadu.
When the activities of other antioxidative defense enzymes (SOD and CAT) were assessed and compared between all four experimental aromatic rice cultivars, raised from different magnitude of IDS, a clear difference were observed (Fig. 3). IDS of different magnitude significantly reduce the activities of SOD and CAT for the cultivar Jamainadu, in a dose-dependent manner (Fig. 3). When compared, the seedlings of cultivar Badshabhog exhibited significant rise in the activities of SOD in a concentration-dependent manner. The cultivar Tulaipanji, on the other hand does not show much change in the activities of SOD over untreated control whereas the cultivar Sitabhog exhibited significant rise in activities for more severe dehydration stress (− 0.851 and − 1.619 MPa). When the activities of catalase (CAT) from IDS-raised seedlings of all four experimental cultivars of rice were assessed and compared, it showed more or less the same trend, with maximum reduction in activities for the cultivars Jamainadu and Sitabhog, whereas the cultivars Badshabhog and Tulaipanji though exhibited decline over untreated control, but not to the extent of Jamainadu and Sitabhog.
Oxidation of protein and lipid of newly assembled membrane system and early growth performance of seedlings of four aromatic rice cultivars raised from varying magnitude of imbibitional dehydration stress
The oxidation of membrane protein and lipid that aggravate under oxidative stress is a reliable indicator of loss of redox homeostasis of the cell that largely conveys the changes in internal redox cues of the stressed tissue. In order to ascertain the impact of different magnitude of IDS (− 0.344, − 0.851 and − 1.619 MPa) on oxidative protein and lipid damages of newly assembled membrane system of the four germinating experimental rice cultivars, free carbonyl content (RC=O) and thiobarbituric acid reactive substances (TBARS) were assessed and compared (Fig. 1c, d). But when compared, the cultivar Tulaipanji exhibited little change in protein oxidation over untreated control, indicating lesser extent of oxidative damage under different magnitude of IDS. On the other hand, the extent of protein oxidation enhances maximally in the cultivar Jamainadu. The cultivar Badshabhog exhibited significant reduction in protein oxidation, particularly under higher magnitude of IDS. The same pattern of result was seen in case of TBARS accumulation, which reflect membrane lipid peroxidation, where it was found to be induced maximally in the cultivar Jamainadu followed by Sitabhog, Tulaipanji and Badshabhog (Fig. 1c, d).
For adjudging whether the differential loss of redox homeostasis and associated oxidative damages to newly assembled membrane system of all four experimental indigenous aromatic rice cultivars have an impact on germination and subsequent early growth performances, relative germination performance (RGP), relative growth index (RGI), vigor index (VI) and T50 value of germination was assessed and compared (Table 1). Imbibational dehydration stress (IDS) of higher magnitude (− 0.851 and − 1.619 MPa) significantly enhance the T50 value of germination and reduce the relative germination performance (RGP) of the cultivars Jamainadu and Sitabhog as compared to the cultivars Tulaipanji and Badshabhog, indicating serious germination impairment for the cultivars Jamainadu and Sitabhog. When post-germinative growth were assessed and compared in terms of Vigor index (VI) and Relative growth index (RGI), it also exhibited the same trend corroborating the significant role of internal redox cues regulating germination under imbibitional dehydration stress. Further, the assessment of relative water content (RWC) of all four IARCs treated with different IDS also showed significant differences with the cultivars Jamainadu and Sitabhog exhibiting maximum impact on internal water content as compared to the cultivars Badsabhog and Tulaipanji (Table 1). So, taken all the parameters of germination, early growth performances and internal water content together, the cultivars Badsabhog and Tulaipanji exhibited significantly better early growth performances and maintenance of internal water content under different magnitude of IDS as compared to the cultivars Sitabhog and Jamainadu (Table 1).
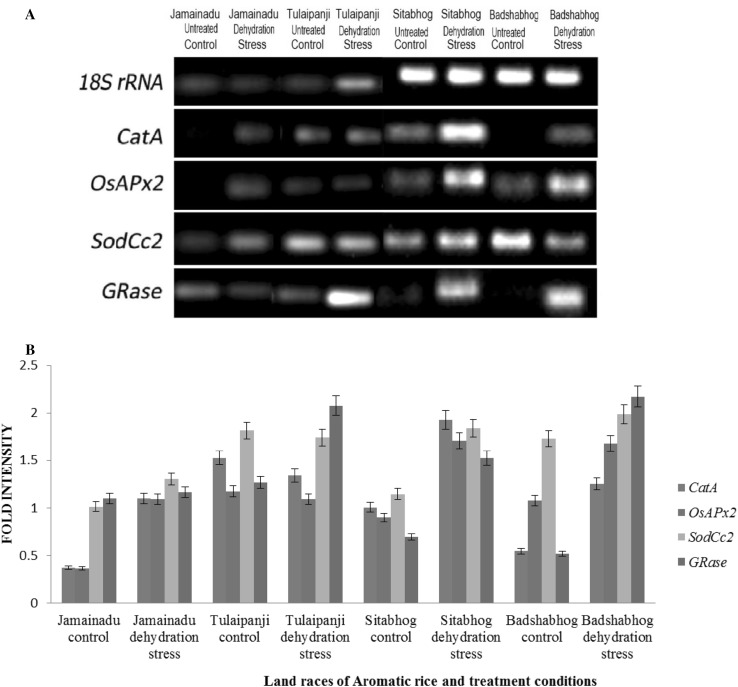
Effect of imbibitional dehydration stress on transcript abundance of genes of antioxidant encoding enzymes of four aromatic rice cultivars using semi-quantitative RT-PCR
Gene transcripts encoding the major antioxidative defense enzymes that eliminate and H2O2 were determined relative to 18S rRNA using semi-quantitative RT-PCR, specific primers and cDNA from untreated control and IDS stressed (− 1.619 MPs) seedlings of four experimental aromatic rice cultivars. Rice 18S rRNA remained constant in both control and IDS-raised seedlings and hence suitably used as gel loading control for antioxidant related transcript analysis (Fig. 4). Transcript abundance of genes of SodCc2, CatA, OsAPx2 and GRase, encoding cytosolic Cu/Zn SOD, CAT, APOX and GR respectively exhibited differential relative expression for seedlings raised from IDS of all four aromatic rice cultivars. Seedlings of aromatic rice cultivar Badshabhog exhibited significant enhancement of transcript abundance for all genes of antioxidative enzymes tested (CatA, OsAPx2, SodCc2 and GRase) over untreated control, corroborating well with the data of corresponding enzymatic activities. The cultivar Tulaipanji, on the other hand, exhibited significant enhancement of transcript abundance of GRase, whereas the transcript abundance of other genes (CatA, OsAPx2 and SodCc2) showed no significant changes. The cultivar Sitabhog on the other hand exhibited a moderate enhancement in transcript abundance of genes of all antioxidative enzymes over untreated control. Although the abundance of transcript for the genes CatA and OsAPx2 enhanced marginally, but for other two genes i.e. SodCc2 and GRase there were no significant enhancement in transcript abundance.
Fig. 4.
Transcript abundance of SodCc2, OsAPx2, GRase and CatA genes of imbibitional dehydration stress raised seedlings of four indigenous aromatic rice varieties [Oryza sativa L., Cultivars Jamainadu, Tulaipanji, Sitabhog and Badshabhog (a)]. Semi-quantitative RT-PCR was performed as described in section “Materials and Methods”. The rice 18S rRNA control reaction above each set of corresponding antioxidant gene reactions was conducted on equivalent cDNA batches to verify equivalent loading reaction volumes on the gel. Antioxidant genes were amplified for 25 cycles. Bar diagram represents mean intensity of relative expression of genes (SodCc2, OsAPx2, GRase and CatA) found in semi-quantitative RT-PCR in four indigenous aromatic rice varieties (Oryza sativa L., Cultivars Jamainadu, Tulaipanji, Sitabhog and Badshabhog) under imbibitional dehydration stress (b)
Therefore, the ability of maintenance of higher antioxidative enzyme activities of ascorbate–glutathione (ASC–GSH) cycle was accompanied by enhanced expression of two important genes of ascorbate–glutathione cycle tested (OsAPx2 and GRase) in two aromatic rice cultivars Badshabhog and Tulaipanji, which offer better capacity to maintain redox homeostasis and mitigation of oxidative deterioration during early germination as compared to cultivars Sitabhog and Jamainadu.
Discussion
The present work makes an effort to assess and standardize some redox metabolic and molecular parameters for screening IARCs commonly cultivated in west Bengal, India. In fact, the integration of metabolic and physiological data along with the genomic data always ensues a better understanding of complex responses of plant to abiotic stress including drought. The present work, in this perspective, which exhibit variations of redox parameters (efficacy and expression of genes of ascorbate–glutathione cycle, oxidative membrane damage, and redox status) among the four indigenous aromatic rice cultivars under drought stress may be used as stress tolerance biomarkers for screening of the germplasms of aromatic rice.
In the present investigation, differential responses of four IARCs raised from different level of osmotic stress by using PEG-6000 in the growing media during early imbibitional phase of germination were studied. PEG being an inert, water binding polymer with non-ionic and impermeable long chain compound, exactly mimic drought stress associated with dry soil (Couper and Eley 1984). In fact, the application of osmoticum like PEG-6000 is considered as a common alternative of assessing performance of the plant under dehydration stress in laboratory conditions (Sinhababu and Kar 2003, Basu et al. 2010a).
Drought stress-induced loss of redox homeostasis due to over accumulation of ROS is well recognized and is related largely with the susceptibility of plant to drought (Cruz de Carvalho 2008; Reddy et al. 2004; Mittler 2002; Basu et al. 2010a; Yang et al. 2014). The aromatic rice cultivars Tulaipanji and Badshabhog though suffered imbibitional dehydration stress-induced loss of redox homeostasis and associated oxidative damage to newly assembled membrane, but the extent of oxidative damage was found to be significantly lesser as compared to the cultivars Jamainadu and Sitabhog. This result exhibiting differential oxidative damage to rice cultivars concurs with the findings of Basu et al. (2010b), Roychoudhury et al. (2008), Faize et al. (2011). The extent of oxidative damage assessed in terms of accumulation of free carbonyl content and thiobarbituric acid reactive substances (RC=O and TBARS), caused by different magnitude of IDS, generally correlated with early growth performances and internal osmotic status of germinating seedlings (assessed in terms of vigor index, relative growth index and relative germination performance) of all four landraces of rice. Protein carbonylation is significantly more sensitive of oxidative stress than lipid peroxidation as the later gets catabolized rapidly than oxidized protein (Palma et al. 2002). Further, the differential susceptibility of newly assembled membrane system of four landraces of rice towards IDS-induced oxidative stress not only suggests the importance of intactness of membrane system in post-germinative growth but also determine the differential tolerance of the germplasm towards dehydration stress. In fact, both cell elongation and division got hampered under the influence of IDS induced oxidative stress and associated oxidative damages to membrane components, leading to delayed germination and stunted growth (Gong et al. 1988; Chakraborty and Bhattacharjee 2015). Although oxidative membrane injury (assessed in terms of TBARS and RC=O) and early growth performances are not completely linked, but for all the landraces of rice, the early growth performances declined with higher accumulation of RC=O and TBARS. This finding strongly suggests that early growth performances under IDS require the ability to tolerate and mitigate oxidative damages to newly assembled membrane system of all four germinating experimental rice cultivars (Gong et al. 1988; Larkindale and Huang 2004; He et al. 2009; Bhattacharjee 2008). It is also quite evident that cultivar Tulaipanji and Badshabhog were more tolerant in relation to oxidative injury and maintenance of internal water status of juvenile germinating tissues, which might be further corroborated by the data of redox status of the tissue under IDS. In fact, the oxidative stress resistance feature of Tulaipanji and Badshabhog largely confer dehydration stress resistance attribute of the cultivars.
In several earlier studies, lower efficiency of antioxidative defense along with aggravation of oxidative damages has been held responsible for poor germination and delayed post germination growth (Wahid et al. 2007; Devi et al. 2008; Chakraborty and Bhattacharjee 2015). The altered internal redox cues due to varying magnitude of IDS in all four experimental aromatic rice cultivars are reflected in terms of endogenous level of pro-oxidants and antioxidants. These ratios of pro-oxidant-antioxidant level (assessed in terms of total ROS, , H2O2, total thiol content and radical scavenging activities) and in situ staining properties of ROS in germinating seedling, which got explicitly enhanced for the cultivars Jamainadu and Sitabhog, made them extremely susceptible towards IDS in a dose-dependent manner (Reddy et al. 2004).
The enzymatic components of antioxidative defense system work in a well-coordinated manner at the advent of any abiotic or biotic stresses, largely to regulate and control redox homeostasis (Foyer and Noctor 2013; Turan and Tripathy 2013). Here, we have investigated the changes in activities of key enzymes of Halliwell–Asada pathway i.e., ascorbate–glutathione cycle and correlated with the expression of corresponding genes. Since ascorbate–glutathione cycle involves efficient removal of H2O2 in plant cell (Anjum et al. 2011; Hossain et al. 2013), particularly under environmental stress, we studied this metabolism as an important metabolic marker for screening four experimental aromatic landraces. Comparative assessment of activities of all three enzymes of the pathway, namely APOX, DHAR and GR exhibited significant differences among the cultivars, with cultivars Jamainadu and Sitabhog exhibiting significant decline, whereas the cultivars Tulaipanji and Badshabhog showed significant up-regulation under the same magnitude of IDS. This enhancement of activities in Tulaipanji and Badshahbhog under IDS increases regeneration of ascorbate, which can subsequently serve as electron donor for detoxification of H2O2. The enhanced activity of DHAR coupled with APOX in the cultivars Tulaipanji and Badshabhog as compared to Sitabhog and Jamainadu, under all three magnitudes of IDS was accompanied by significant restoration of ASC and GSH under imbibitional dehydration stress (IDS). The down regulation in DHAR and APOX in the cultivars Jamainadu and Sitabhog, accompanied by significant reduction in accumulation of ASC and GSH, caused less processing of H2O2, greater oxidative damage and early growth impairment (Hossain et al. 2013; Noctor et al. 1998). The result is in concurrence with the findings of Sreenji et al. (2010), Hossain et al. (2013), Anjum et al. (2011), who also noticed the enhancement of DHAR activities in drought, salinity and heavy metal stressed plants for the maintenance of ascorbate. The increased DHAR activity in turn reflects enhanced utilization of GSH and greater accumulation of GSSG. The enhanced accumulation of GSSG may be regarded as oxidative stress and hence need to be reduced back to GSH by glutathione reductase (GR). Our study also exhibited differential glutathione reductase (GR) activity for dehydration stress susceptible cultivars Sitabhog and Jamainadu as compared to comparatively resistant cultivars Tulaipanji. The cultivar Tulaipanji did not allow the accumulation of GSSG by up-regulating the activities of glutathione reductase (GR) under IDS. So, the maintenance of GSH pool indirectly plays pivotal role under IDS to combat oxidative damage in Tulaipanji and Badshabhog as compared to Sitabhog and Jamainadu, confirming once again the significance of ascorbate–glutathione (ASC–GSH) cycle to combat dehydration stress during early germination in aromatic rice cultivars (Zagorchev et al. 2016; Hossain et al. 2013; Moradi and Ismail 2007). In a similar recent study by Cao et al. (2015) on PEG induced dehydration stress raised tomato plants showed simultaneous and gradual increase in ROS production and metabolic dysfunction. PEG induced dehydration stress raised plants showed an increase in activities of APOX, SOD and MDHAR accompanied by decrease in DHAR and GR up to third day of stress imposition (Cao et al. 2015). Whereas, in prolonged dehydration stress imposed by PEG up to 12 days, all five enzymes of ascorbate–glutathione cycle reduced significantly. Thus, it may be inferred that, in drought sensitive plants the chloroplast-antioxidant system depends mainly on ascorbic acid for H2O2 scavenging through APOX with subsequent reduction of MDA and no GSH-dependent reduction by DHAR. This mechanism, however, is not found to be efficient method of ROS detoxification for longer spell of dehydration stress. The cultivars with better GR and DHAR activities impart complete detoxification even under longer spell of dehydration stress (Cao et al. 2015).
We have also investigated the transcript abundance of some of the important genes of ascorbate–glutathione (ASC–GSH) cycle and H2O2 metabolizing enzymes. In our study, IDS-raised seedlings of Badshabhog exhibited greater transcript level of CatA, OsAPx2, SodCc2 and GRase genes as compared to cultivars Jamainadu and Sitabhog, corroborating well with the data of corresponding enzyme activities. The cultivar Tulaipanji, on the other hand, exhibited significant enhancement of transcript abundance of GRase, whereas the transcript abundance of other genes (CatA, OsAPx2 and SodCc2) showed no significant changes. Reduced expression of genes of all tested antioxidative enzymes and of other isoforms of these enzymes may be responsible for lesser efficiency of ascorbate–glutathione (ASC–GSH) cycle and radical scavenging properties in the cultivars Jamainadu and Sitabhog as compared to Badshabhog and Tulaipanji (Zagorchev et al. 2016; Chakraborty and Bhattacharjee 2015). Therefore, IDS induced transcriptional activation of OsAPx2 and GRase along with Cat A and SodCc2 corresponds to better maintenance of Halliwell–Asada pathway and H2O2 processing system to protect the cellular compartments from oxidative damage against adverse effect of ROS produced as a consequence of redox imbalance (Chakraborty and Bhattacharjee 2015; Seckin and Aksoy 2013). The ascorbate–glutathione (ASC–GSH) cycle corresponds to one of the central antioxidative defense mechanism in plants, which is primarily responsible for detoxification of H2O2 and thereby restore homeostasis in major cellular compartments (Zagorchev et al. 2016; Cao et al. 2015). The regulation and maintenance of redox cues of major water soluble redox couples like GSH/GSSG and ASA/DHA as mentioned by the activities of GR, DHAR, APOX is of crucial importance as the survival strategy under drought stress during early germination of the cultivars Badshabhog and Tulaipanji. Therefore, the higher or induced expression of the genes of important enzymes of the pathway along with their activities is associated with better detox scavenging and tolerance to water deficit condition and may be used as standard redox parameters for screening indigenous aromatic rice cultivars (Abbas et al. 2014; Devi et al. 2012; Zhang et al. 2014).
Conclusion
The present work showed the differential impact of imbibitional dehydration stress on the changes in internal redox cues and corresponding oxidative damages in four aromatic landraces (Jamainadu, Tulaipanji, Sitabhog and Badshabhog) of West Bengal, India. When compared between the landraces, a clear trend in differential redox-regulatory properties was observed in the order of the landraces Badshabhog > Tulaipanji > Sitabhog > Jamainadu. The role of redox homeostasis in which ROS-antioxidant (ascorbate–glutathione system) acts at metabolic interface for up-regulation of antioxidative gene expression necessary for differential drought stress tolerance among the landraces of aromatic rice is suggested. The application of redox metabolic and molecular parameters for screening IARCs for drought stress tolerance is extremely important, though not always decisive.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Effect of different magnitude of PEG induced imbibitional dehydration stress (− 0.344, − 0.851 and − 1.619 MPa) on visualization of superoxide (A) and hydrogen peroxide (B) of four indigenous aromatic rice varieties of Oryza sativa L. (Tulaipanji, Sitabhog, Badshabhog and Jamainadu) (DOC 601 kb)
Acknowledgements
ND acknowledges The University of Burdwan, West Bengal, for State Funded Research Fellowship (136/35, 31.07.31.07.2014, Government of West Bengal, India). Assistance from Anirban Bhar, Faculty Ramakrishna Mission Vivekananda Centenary College, Kolkata, for Semi-quantitative RT-PCR is thankfully acknowledged. Instrumentation facility of CAS (UGC), Govt. of India to the Department of Botany, University of Burdwan is also gratefully acknowledged.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12298-017-0484-1) contains supplementary material, which is available to authorized users.
References
- Abbas S, Ahmed S, Sabir S, Shah A. Detection of drought tolerant sugarcane genotypes (Saccharum officinarum) using lipid peroxidation, antioxidant activity, glycine betaine and proline contents. J Soil Sci Plant Nutr. 2014;14(1):233–243. [Google Scholar]
- Anjum NA, Umar S, Iqbal M, Khan NA. Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbate–glutathione cycle metabolism. Russ J Plant Physiol. 2011;58:92–99. doi: 10.1134/S1021443710061019. [DOI] [Google Scholar]
- Basu S, Roychoudhury A, Saha PP, Sengupta DN. Comparative analysis of some biochemical responses of three indica rice varieties during polyethylene glycol-mediated water stress exhibits distinct varietal differences. Acta Physiol Plant. 2010;32(3):551–563. doi: 10.1007/s11738-009-0432-y. [DOI] [Google Scholar]
- Basu S, Roychoudhury A, Saha PP, Sengupta DN. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. 2010;60:51–59. doi: 10.1007/s10725-009-9418-4. [DOI] [Google Scholar]
- Benzie IFE, Strain JJ. The ferric reducing ability of plasma (FRAP) as measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S. Calcium-dependent signaling pathway in heat-induced oxidative injury in Amaranthus lividus. Biol Plant. 2008;52:1137–1140. doi: 10.1007/s10535-008-0028-1. [DOI] [Google Scholar]
- Burch-Smith TM, Schiff M, Liu Y, Dinesh-Kumar SP. Efficient virus induced gene silencing in Arabidopsis. Plant Physiol. 2006;42:21–27. doi: 10.1104/pp.106.084624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao BL, Ma Q, Zhao Q, Wang L, Xu K. Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Sci Hortic. 2015;194:53–62. doi: 10.1016/j.scienta.2015.07.037. [DOI] [Google Scholar]
- Chaitanya KSK, Naithani SC. Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability of Shorea robusta Gaertn F. New Phytol. 1994;126:623–627. doi: 10.1111/j.1469-8137.1994.tb02957.x. [DOI] [Google Scholar]
- Chakraborty A, Bhattacharjee S. Differential competence of redox-regulatory mechanism under extremes of temperature determines growth performances and cross tolerance in two indica rice cultivars. J Plant Physiol. 2015;176:65–77. doi: 10.1016/j.jplph.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Couper A, Eley D. Surface tension of polyethylene glycolsolutions. J Polym Sci. 1984;3:345–349. doi: 10.1002/pol.1948.120030306. [DOI] [Google Scholar]
- Cruz de Carvalho MH. Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav. 2008;3(3):156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb D, Bhattacharya D. Seeds of traditional, seeds of future: folk rice varieties of Eastern India. New Delhi: Research Foundation for Science, Technology, and Ecology (RFSTE)/(vrihi); 2005. [Google Scholar]
- Devi S, Angrish R, Datta KS, Kumar B. Antioxidant defense system in wheat seedlingsunder sodium chloride stress: an inductive role of sodium chloride stress. Ind J Plant Physiol. 2008;13(2):118–124. [Google Scholar]
- Devi R, Kaur N, Gupta AK. Potential antioxidant enzymes in depicting drought tolerance of wheat (Triticum aestivum L.) Indian J Biochem Biophys. 2012;49(4):257–265. [PubMed] [Google Scholar]
- Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba-Espin G, Clemente-Moreno MJ, Alcobendas R, Artlip T, Hernandez JA. Involvement of cytosolic ascorbate peroxidase and Cu/Zn superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62(8):2599–2613. doi: 10.1093/jxb/erq432. [DOI] [PubMed] [Google Scholar]
- Fick NG, Qualset CD. Genetic control of plant amylase activity. Proc Natl Acad Sci USA. 1975;72:852–862. doi: 10.1073/pnas.72.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005;28:1056–1107. doi: 10.1111/j.1365-3040.2005.01327.x. [DOI] [Google Scholar]
- Foyer CH, Noctor G. Redox signaling in plants. Antioxid Redox Signal. 2013;18(16):2087–2096. doi: 10.1089/ars.2013.5278. [DOI] [PubMed] [Google Scholar]
- Giannopolities CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59:309–319. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M, Li YJ, Chan SZ. ABA induced thermotolerance in maize seedlings is mediated by calcium and associated antioxidant systems. J Plant Physiol. 1988;153:488–497. doi: 10.1016/S0176-1617(98)80179-X. [DOI] [Google Scholar]
- Gossett DR, Millhollon ER, Lucas MC. Antioxidant response to NaCl stress in salt tolerant and salt-sensitive cultivars of cotton. Crop Sci. 1994;34:706–714. doi: 10.2135/cropsci1994.0011183X003400030020x. [DOI] [Google Scholar]
- Hasanuzzaman M, Nahar K, Gill SS, Gill R, Fujita M. Drought stress responses in plants, oxidative stress and antioxidant defense. In: Gill SS, Tuteja N, editors. Climate change and plant abiotic stress tolerance. Germany: Blackwell, Wiley; 2014. pp. 209–249. [Google Scholar]
- He L, Gao Z, Li R. Pretreatment of seed with H2O2 enhances drought tolerance of wheat (Triticum aestivum L.) seedlings. Afr J Biotechnol. 2009;08(22):6151–6157. doi: 10.5897/AJB09.490. [DOI] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Ismail MR, Uddin MdK, Islam MZ, Ashrafuzzaman M. Efficacy of ascorbate–glutathione cycle for scavenging H2O2 in two contrasting rice genotypes during salinity stress. Aust J Crop Sci. 2013;7(12):1801–1808. [Google Scholar]
- Jiang M, Zhang J. Effect of abscisic acid on active oxygen species, antioxidative defense system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001;42:1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- Khush GS. What it will take to feed 5.0 billion rice consumers in 2030? Plant Mol Biol. 2005;59(1):1–6. doi: 10.1007/s11103-005-2159-5. [DOI] [PubMed] [Google Scholar]
- Larkindale J, Huang B. Thermotolerance and antioxidant system in Agrostisstolonifera: involvement of SA, ABA, Ca, H2O2 and ethylene. J Plant Physiol. 2004;161:405–413. doi: 10.1078/0176-1617-01239. [DOI] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. Biochem J. 1983;210(3):899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu F. Drought stress memory and drought stress tolerance in plants: biochemical and molecular basis. In: Hossain MA, editor. Drought stress tolerance in plants. Switzerland: Springer; 2016. [Google Scholar]
- Li XM, Zhang LH, Li YY. Preconditioning alters antioxidative enzyme responses in rice seedlings to water stress. Proc Environ Sci. 2012;11:1346–1351. doi: 10.1016/j.proenv.2011.12.202. [DOI] [Google Scholar]
- MacNevin WM, Uron PF. Spectrum of hydrogen peroxide from organic hydroperox-ides. Anal Chem. 1953;25:1760–1761. doi: 10.1021/ac60083a052. [DOI] [Google Scholar]
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–441. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Moradi F, Ismail AAM. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann Bot. 2007;99:1161–1173. doi: 10.1093/aob/mcm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–729. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Palma JM, Sandalio LM, Corpas FJ, Romero-Puertas MC, Mccarthy I, Delrio LA. Plant proteases, protein degradation and oxidative stress: role of peroxisomes. Plant Physiol Biochem. 2002;40:521–530. doi: 10.1016/S0981-9428(02)01404-3. [DOI] [Google Scholar]
- Pandey V, Shukla A. Acclimation and tolerance strategies of rice under drought stress. Rice Sci. 2015;22(4):147–161. doi: 10.1016/j.rsci.2015.04.001. [DOI] [Google Scholar]
- Re R, Pellegrinni N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activities applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- RoyChoudhury A, Roy C, Sengupta DN. Transgenic tobacco plants over expressing the heterologous lea gene Rab16A from rice during high salt and water deficit display enhanced tolerance to salinity stress. Plant Cell Rep. 2007;26:1839–1859. doi: 10.1007/s00299-007-0371-2. [DOI] [PubMed] [Google Scholar]
- Roychoudhury A, Basu S, Sarkar SN, Sengupta DN. Comparative physiological and molecular responses of a common aromatic indica rice cultivar to high salinity with non-aromatic indica rice cultivars. Plant Cell Rep. 2008;27:1395–1410. doi: 10.1007/s00299-008-0556-3. [DOI] [PubMed] [Google Scholar]
- Rubio-Casal AE, Castillo JM, Lucue C, Fig Ureo ME. Influence of salinity on germination and seed viability of two primary colonizers of Mediterranean salt plants. J Arid Environ. 2003;53:145–152. doi: 10.1006/jare.2002.1042. [DOI] [Google Scholar]
- Schaedle M, Bassham JA. Chloroplast glutathione reductase. Plant Physiol. 1977;59(5):1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckin B, Aksoy M. The responses of ascorbate–glutathione cycle enzymes in seedlings of Pancratium maritimum L. under Drought Treatments. J Stress Physiol Biochem. 2013;9(2):148–158. [Google Scholar]
- Selote DS, Khanna-Chopra R. Drought-induced spikelet sterility is associated with an inefficient antioxidant defence in rice panicles. Physiol Plant. 2004;121(3):462–471. doi: 10.1111/j.1399-3054.2004.00341.x. [DOI] [Google Scholar]
- Serraj R, McNally KL, Slamet-Loedin I, Kohli A, Haefele SM, Atlin G, Kumar A. Drought resistance improvement in rice: an integrated genetic and resource management strategy. Plant Prod Sci. 2011;14(1):1–14. doi: 10.1626/pps.14.1. [DOI] [Google Scholar]
- Sharma P, Dubey RS. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005;46:209–221. doi: 10.1007/s10725-005-0002-2. [DOI] [Google Scholar]
- Simontacchi M, Caro A, Fraga CG, Puntarulo S. Oxidative stress affects α-tocopherol content in soyabean embryonic axes upon imbibitions. Plant Physiol. 1993;103:949–953. doi: 10.1104/pp.103.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinhababu A, Kar RK. Comparative responses of three fuel wood yielding plants to PEG-induced water stress at seedling stage. Acta Physiol Plant. 2003;25:403–409. doi: 10.1007/s11738-003-0022-3. [DOI] [Google Scholar]
- Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol. 2000;3:229–235. doi: 10.1016/S1369-5266(00)00069-8. [DOI] [PubMed] [Google Scholar]
- Snell FD, Snell CT. Colorimetric methods of analysis. New York: Van Nostard Reinford Co; 1971. [Google Scholar]
- Sreenji M, Hideg E, Bebes A, Gyorgyey J. Transcriptional differences in gene families of the ascorbate–glutathione cycle in wheat during mild water deficit. Plant Cell Rep. 2010;29:37–50. doi: 10.1007/s00299-009-0796-x. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidised glutathione: application to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Turan S, Tripathy BC. Salt and genotype impact on antioxidative enzymes and lipid peroxidation in two rice cultivars during de-etiolation. Protoplasma. 2013;250(1):209–222. doi: 10.1007/s00709-012-0395-5. [DOI] [PubMed] [Google Scholar]
- Wahid A, Parveen M, Gelani S, Basra SMA. Pretreatment of seeds with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol. 2007;164:283–294. doi: 10.1016/j.jplph.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Wang JH, Geng LH, Zhang CM. Research on the weak signal detecting technique for crop water stress based on wavelet denoising. Adv Mat Res. 2012;424(425):966–970. [Google Scholar]
- Yadav M, Pal A, Bhowmick K, Adhikary B, Bhowmick MK, Santra CK. Indigenous aromatic rice: quality seed production and area expansion in West Bengal. Annu Tech Issue Seed Hope Harvest. 2014;18:72–93. [Google Scholar]
- Yang PM, Huang QC, Qin GY, Zhao SP, Zhou JG. Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica. 2014;52(2):193–202. doi: 10.1007/s11099-014-0020-2. [DOI] [Google Scholar]
- Zagorchev L, Teofanova D, Odjakova M (2016) Ascorbate–glutathione cycle: controlling the redox environment for drought tolerance. In: Hossain M, Wani S, Bhattacharjee S, Burritt D, Tran LS (eds) Drought stress tolerance in plants, vol 1. Springer, Cham, pp 187–226
- Zhang J, Kirkham MB. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996;132:361–373. doi: 10.1111/j.1469-8137.1996.tb01856.x. [DOI] [PubMed] [Google Scholar]
- Zhang LS, Lai JH, Liang ZS, Ashraf M. Interactive effects of sudden and gradual drought stress and foliar applied glycinebetaine on growth, water relations, osmolyte accumulation and antioxidant defense system in two maize cultivars differing in drought tolerance. J Agron Crop Sci. 2014;200(06):425–433. doi: 10.1111/jac.12081. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of different magnitude of PEG induced imbibitional dehydration stress (− 0.344, − 0.851 and − 1.619 MPa) on visualization of superoxide (A) and hydrogen peroxide (B) of four indigenous aromatic rice varieties of Oryza sativa L. (Tulaipanji, Sitabhog, Badshabhog and Jamainadu) (DOC 601 kb)