Abstract
Illumina-based paired-end sequencing technology was used for the high-throughput transcriptome sequencing of combined Zingiber striolatum Diels tissues (i.e., root, stem, leaf, flower, and fruit tissues). More than 130 million sequencing reads were generated, and a de novo assembly yielded 287,959 contigs and 112,107 unigenes with an average length of 1029 and 28,891 bp, respectively. Similarity searches with known sequences led to the identification of 51,804 (46.21%) genes. Of the annotated unigenes, 6867 and 51,987 were assigned to Gene Ontology and Clusters of Orthologous Groups categories, respectively. Additionally, 8384 simple sequence repeats (SSRs) were identified as potential molecular markers in the unigenes. Thirty pairs of polymerase chain reaction primers were designed and used to validate the unigenes and assess the associated genomic polymorphism. The PCR amplification products for 25 primer pairs were of the expected size. These primers may represent usable molecular markers. The thousands of SSR markers identified in the present study may be useful for analyses of genetic diversity, genetic linkage mapping, and the identification and improvement of varieties during the breeding of Z. striolatum Diels. The unigene sequences and SSR markers described herein may serve as valuable resources for future investigations of Z. striolatum Diels.
Keywords: Zingiber striolatum Diels, Transcriptome, SSR, Molecular marker
Introduction
Zingiber striolatum Diels (i.e., white myoga ginger), Arum sagittifolium, and Alpinia galanga (L.) Willd. are among the perennial vegetables (family: Zingiberaceae) grown in various Chinese provinces (e.g., Guizhou, Sichuan, Guangxi, Hubei, Hunan, Jiangxi, and Guangdong), often at an altitude of about 800 m above sea level (An editorial committee of flora of China 1981). These plants serve as a source of dietary fiber and can be used as medicine as well as food. Zingiber striolatum Diels contains protein, many amino acids, polysaccharides, several trace elements, and abundant cellulose. Because of its medicinal properties, this plant species can be used to promote blood circulation for regulating menstrual flow. It may also function as an antitussive expectorant, detumescence agent, and detoxifier (Qu et al. 2015). The food products (e.g., pickles) and beverages prepared from Z. striolatum Diels are popular among consumers in major cities around the world. However, there is an insufficient supply of Z. striolatum Diels products to satisfy the global demand.
Zingiber striolatum Diels is a unique vegetable grown in China. Although it is sometimes grown under trees in residential areas (e.g., in gardens), Z. striolatum Diels is currently primarily harvested from the wild (Qu et al. 2015), with minimal cultivation in artificial plots. However, the diversity in wild varieties as well as the limited growth areas, relatively low root yields, and paucity of related research, have resulted in the inability to grow enough Z. striolatum Diels plants to satisfy long-term consumer demands. To date, there have been only a few studies on the cultivation of Z. striolatum Diels or analyses of plant contents (e.g., polysaccharides, trace elements, water-soluble dietary fiber, and medicinal chemical constituents) (Qu et al. 2015). Additionally, there are no reports describing a molecular-level investigation of this plant species in China or elsewhere.
Simple sequence repeats (SSRs) are among the most effective molecular markers in plant genetics (Powell et al. 1996; Zalapa et al. 2012) and breeding because of several factors, including their simplicity of use, specificity, wide genomic distribution, co-dominance, and their ability to screen for multiple alleles (Cheng et al. 2016). Various SSR markers have been used to identify animal and plant varieties (Rongwen et al. 1995), examine hybrids (Provan et al. 1996), assess genetic diversity (Goldstein et al. 1996), map genes (Chen et al. 2014), assign genes (Yu et al. 1993), investigate gene flow (Moe and Weiblen 2011), and characterize molecular evolution (Kelkar et al. 2008; Wang et al. 2014).
In this study, we examined Z. striolatum germplasm resource ZSP11 from Guizhou province, China. An Illumina-based paired-end sequencing technique was used to sequence the transcriptome from different Z. striolatum tissues. Additionally, SSR markers were identified and developed at the whole genome level. These markers may be useful for identifying agriculturally important genes and for the genetic improvement and molecular breeding of Z. striolatum.
Materials and methods
Plant materials and RNA extraction
Zingiber striolatum Diels germplasm ZSP11 plants were grown in an experimental field at the Zunyi Academy of Agricultural Sciences in Xinzhou (town), Xinpu (district), Zunyi (city), China. The root, stem, and leaf tissues were collected at the 4-leaf stage, while the flower and fruit tissues were harvested during the flowering and seed-setting periods, respectively. The collected samples were immediately frozen in liquid nitrogen and stored at − 80 °C.
Total RNA was extracted from each sample using a modified CTAB method (Zong et al. 2012), and then purified with the RNeasy Plant Mini Kit (Qiagen, Valencia, CA). The quality of the purified RNA was assessed using the 2100 Bioanalyzer RNA Nanochip (Agilent, Santa Clara, CA, USA). The RNA Integrity Number was above 8.5 for the five tissues. The RNA concentration was determined with the ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA). A 20-µg combined RNA sample (i.e., 4 μg RNA from each collected tissue) was used as the template for preparing the cDNA library.
Construction and sequencing of the cDNA library
The mRNA in the extracted total RNA sample was enriched using oligo-dT magnetic beads. The mRNA was fragmented in fragmentation buffer, and then used as the template for first-strand cDNA synthesis with random hexamers. The second strand was synthesized after the addition of buffer, dNTPs, RNase H, and DNA polymerase I. The prepared cDNA was purified with the QIAQuick PCR kit and eluted in EB buffer. An A-tail along with a sequencing adapter were added to the end-repaired purified cDNA. The fragments with the expected sizes were purified following agarose gel electrophoresis for use in polymerase chain reaction (PCR) analyses. The Illumina HiSeq™ 2000 PE100 sequencing system was used for the transcriptome sequencing of the completed library.
Data processing and de novo assembly
Some of the original sequencing data included low quality sequences with adapters. Thus, the data had to be filtered to obtain clean reads that were subsequently analyzed. The quality requirements for de novo transcriptome sequencing are much higher than those for re-sequencing because errors can introduce several problems for the algorithms used to assemble short fragments. Therefore, we implemented the following rigorous filtration process: (1) reads in which more than 10% of the sequence was N were removed; (2) reads with more than 50% of the base mass values less than 5 were eliminated; and (3) contaminating adapter sequences were removed.
Sequences were assembled to construct the de novo transcriptome using the Trinity program (version 3.0; http://trinotate.github.io/), which was developed by the Broad Institute and the Hebrew University of Jerusalem. This program assembles a full-length transcript according to the de Bruijn graph theory involving the variable transcript splicing characteristics.
Unigenes were annotated using the NCBI non-redundant (NR) protein database (http://www.ncbi.nlm.nih.gov), Swiss-Prot protein database (http://expasy.ch/sprot), Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg), and the Clusters of Orthologous Groups database (http://www.ncbi.nlm.nih.gov/COG). Annotations were also based on BLASTx comparisons (E value < 10−5). The closest matches were used to determine the unigene sequence direction. If there were any inconsistencies among the results for different databases, the priority was given to the results based on the NR database, followed by the Swiss-Prot, KEGG, and COG databases.
Gene annotation and analysis
The coding regions of the assembled transcripts were identified using the TransDecoder program (https://transdecoder.github.io/) according to the following criteria: (1) the open reading frame (ORF) needed to be greater than a certain length; (2) the log value for the likelihood function for the sequences needed to be greater than 0; (3) the highest of six ORF scores was used; and (4) if an ORF contained another one, the longer one was used. The ORFs and unigenes were annotated using the Trinotate program (http://trinotate.github.io/). Additionally, the predicted sequences were annotated based on the Uniprot, RNAmmer, eggNOG, Gene Ontology (GO), and KEGG databases.
Identification and development of simple sequence repeat markers
The MIcroSAtellite program (MISA; http://pgrc.ipk-gatersleben.de/misa/) was used to detect SSRs in the unigenes. Simple sequence repeats consist of a repeating unit of 1–6 bp, and are divided into three types according to their composition and distribution (i.e., pure, compound, and interrupted). The SSRs in this study comprised at least four consecutive repeats of 2–6 bp. The SSRs occurred at a frequency of one per kb of cDNA. Primer Premier 6.0 (PREMIER Biosoft International, Palo Alto, CA, USA) was used to design PCR primers. The primers were at least 18 bp long with a melting temperature of 46–55 °C, and were designed to produce an amplification product consisting of 100–350 bp.
Results
Paired-end sequencing and de novo assembly
An Illumina-based paired-end sequencing technique was used, and 2 × 100-bp fragments were obtained for the ends of the sequenced DNA fragments. A total of 146,199,712 sequenced 100-bp fragments were obtained for the 200-bp insert library. The Trinity program was used to assemble the sequences for the de novo transcriptome. Following a rigorous quality control and data-filtering step, a total of 13,008,709 high-quality fragments were obtained, and the proportion of the bases with a mass value greater than 30 (error rate less than 0.1%) was 95.16%. An analysis of the assembled sequences revealed 287,959 contigs (Table 1), with an average length of 1029 bp, an N50 of 1684 bp, and a GC content of 43.11%. The contigs were 201–17,062 bp long. Additionally, approximately 38.5% of the contigs were longer than 1000 bp. A total of 112,107 unigenes were de novo assembled, with an average length of 28,891 bp, an N50 of 3356 bp, and a length of 328–25,766 bp.
Table 1.
Length distribution of the assembled contigs and unigenes
| Nucleotides length (bp) | Number of contigs | Number of unigenes |
|---|---|---|
| 200–399a | 97,600 | 2690 |
| 401–599 | 35,815 | 3400 |
| 601–799 | 24,209 | 4980 |
| 800–999 | 19,349 | 10,911 |
| 1000–1199 | 17,116 | 9078 |
| 1200–1399 | 16,015 | 9832 |
| 1400–1599 | 14,140 | 8903 |
| 1600–1799 | 12,266 | 9884 |
| 1800–1999 | 10,294 | 5671 |
| 2000–2199 | 8881 | 5624 |
| 2200–2399 | 6993 | 4905 |
| 2400–2599 | 5571 | 4992 |
| 2600–2799 | 4471 | 5663 |
| 2800–2999 | 3409 | 5902 |
| 3000–3199 | 2628 | 4633 |
| 3200–3399 | 1874 | 2462 |
| 3400–599 | 1553 | 2569 |
| 3600–3799 | 1337 | 1544 |
| 3800–3999 | 895 | 1642 |
| > 4000b | 3543 | 6822 |
| Total | 287,959 | 112,107 |
| Minimum length (bp) | 201 | 328 |
| Maximum length (bp) | 17,062 | 25,766 |
| Average length (bp) | 1029 | 28,891 |
| N50 (bp)c | 1684 | 3356 |
| Total nucleotides length (bp) | 296,520,408 | 221,876,352 |
aNumber of contigs and unigenes longer than 200 bp and shorter than 400 bp
bNumber of contigs and unigenes longer than 4000 bp
cN50 length represents the assembly quality. The N50 length is defined as the shortest contig or unigene length representing 50% of the total assembled length
The sequences for assembling the transcriptome were aligned with the unigene sequences (Table 2) using the Burrows–Wheeler Alignment Tool (http://bio-bwa.sourceforge.net/bwa.shtml). The alignment ratios revealed that 95.29% of the unigene sequences were aligned to both reads 1 and 2. Additionally, 4.71% of the unigene sequences were aligned to only read 1 or 2. These results indicated that the assembly quality was relatively high.
Table 2.
Assessment of assembly quality
| Read type | Count | Percentage (%) |
|---|---|---|
| Pair mapping | 113,767,228 | 95.29 |
| Right only | 2,813,394 | 2.36 |
| Left only | 2,808,662 | 2.35 |
| Total aligned reads | 119,389,284 | 100 |
Functional annotations based on searches of public databases
To validate and annotate the assembled unigenes, a similarity search was used to align the sequences with those in the NCBI Nr and Swiss-Prot databases (see the Materials and Methods for details). A total of 54,865 (48.94%) of the 112,107 unigenes were significantly similar to known sequences in the Nr database. This similarity corresponded to 36,692 unique protein accessions. Additionally, the 51,804 (46.21%) unigenes that matched sequences in the Swiss-Prot database represented 33,561 unique protein accessions. A total of 40,876 unique protein accessions were identified in the sequence alignments, suggesting that the Illumina-based paired-end sequencing likely identified some important Z. striolatum Diels genes.
Gene ontology and clusters of orthologous groups classifications
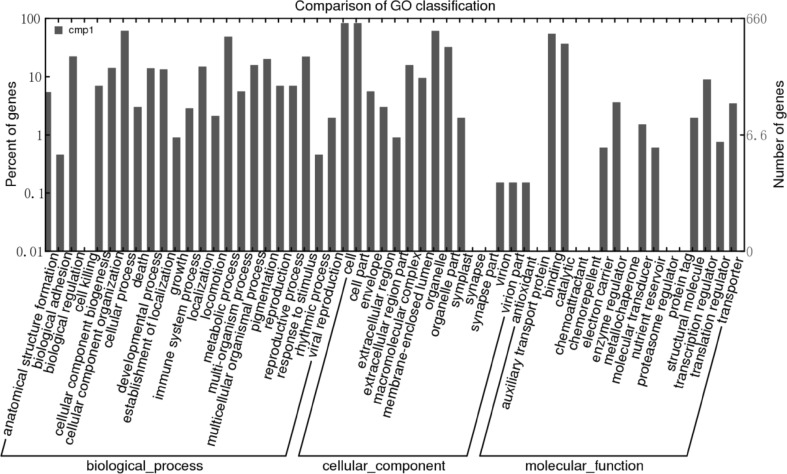
Gene Ontology involves the use of a set of terms to functionally classify eukaryotic genes in cells. These terms are continually accumulated and changed to reflect advances in life science research. Gene Ontology classifications are mainly based on three categories (i.e., biological process, molecular function, and cellular component). Following the annotations based on the Nr database, the Blast2GO program was used to obtain GO annotation details for the unigenes. The WEGO program was then used to functionally classify the unigenes with GO terms. Finally, 6867 unigenes corresponding to known proteins were assigned 18,295 GO terms. Most of the unigenes were associated with the biological process category (7292; 39.86%), followed by the cellular component (6432; 35.16%) and molecular function (4571; 24.98%) categories (Fig. 1).
Fig. 1.
Gene ontology classifications of the assembled unigenes
The unigenes covered a broad range of GO functional categories. Under the biological process category, cellular process (589 unigenes) and metabolic process (526 unigenes) were the most abundant sub-categories, suggesting the importance of some metabolic activities for Z. striolatum Diels development. Interestingly, 135 genes belonged to the pigmentation sub-category. Additionally, 116 unigenes were involved in different stress responses. Under the molecular function category, binding (648 unigenes) and catalytic (632 unigenes) were the most important sub-categories. The most important type of binding was to proteins, followed by ions, ATP, DNA, and then RNA. Under the cellular component category, most genes were related to the cell and cell part sub-categories, while others were associated with the organelle and organelle part sub-categories.
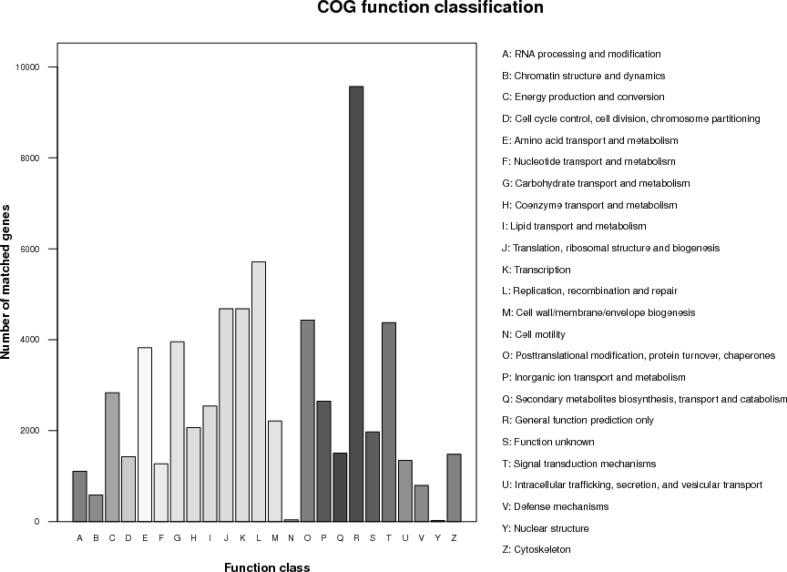
The classifications based on the COG database were determined by individually comparing complete protein-encoding genomic sequences (orthologous genes). The proteins corresponding to each COG were all assumed to come from the same ancestral protein. When considering proteins encoded by a given genome, the comparisons identified the most similar proteins encoded by other genomes. Each of the protein-encoding genes were assessed in turn. The best matches between proteins formed a COG class. All of the unigenes identified in this study were aligned to the COG database sequences to predict and classify their possible functions. A total of 54,865 sequences were attributed to 25 COG classes (Fig. 2).
Fig. 2.
Clusters of orthologous groups (COG) classifications. All unigenes were aligned to COG database sequences to predict and classify possible functions. Of the 102,456 matches to the Nr database sequences, 54,865 sequences were assigned to 25 COG classes
Of the 25 COG classes, general function prediction only consisted of the most unigenes (9825; 18.90%), followed by replication, recombination, and repair (5966; 11.46%), transcription (4750; 9.14%), translation, ribosomal structure, and biosynthesis (4746; 9.13%), post-translational modification, protein turnover, and chaperones (4280; 8.23%), signal transduction mechanisms (4198; 8.08%), carbohydrate transport and metabolism (3980; 7.66%), and amino acid transport and metabolism (3878; 7.46%). A few genes were related to the nuclear and extracellular structures.
Development and characterization of simple sequence repeat markers
The predicted unigenes were used to further assess assembly quality and develop new molecular markers. The MISA program was used to search for SSR loci in the assembled Z. striolatum Diels sequences. A total of 8384 potential SSRs were detected at a frequency of one per 35.4 kb. These SSRs were distributed on 2392 unigenes, with 965 SSRs (11.51%) detected at more than one locus. Additionally, there were 762 compound SSRs (9.09%). The Z. striolatum Diels SSRs mainly comprised six repeats (3766; 44.91%), followed by seven, nine, and 10–20 repeats (3850; 45.92%). Only six SSRs consisted of more than 20 repeats (0.07%, Table 3).
Table 3.
Distribution of simple sequence repeats with different motif types and number of repeats in the Z. striolatum Diels genome
| Motif length/bp | Repeat number | > 20 | Total | Ratio (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 10–20 | ||||
| 2 | 0 | 0 | 0 | 0 | 889 | 1285 | 5 | 2179 | 25.99 |
| 3 | 0 | 3462 | 1668 | 135 | 0 | 5 | 1 | 5271 | 62.87 |
| 4 | 616 | 304 | 1 | 11 | 1 | 1 | 0 | 934 | 11.14 |
| Total | 616 | 3766 | 1669 | 146 | 889 | 1292 | 6 | 8384 | – |
| Ratio /% | 7.35 | 44.92 | 19.91 | 1.74 | 10.6 | 15.41 | 0.07 | – | – |
The repeating units of the detected SSRs were mainly dinucleotides (25.99%) and trinucleotides (62.87%, Tables 3 and 4). These sequences were primarily repeated six, seven, or nine times. The dinucleotide repeat sequences were mainly 18 bp long, corresponding to about 40.80% of the dinucleotide repeats. The most common dinucleotide repeats were AG/CT and GA/TC, accounting for about 35.74 and 28.41% of the dinucleotide repeats, respectively. The GC/GC sequence was the least common dinucleotide repeat (0.21%). The trinucleotide repeat sequences were mainly 18–21 bp long, corresponding to about 97.32% of the trinucleotide repeats, with AGG/CCT being the most common (13.82%), followed by GGA/TCC (8.00%). The TAC/GTA sequence was the least common trinucleotide repeat (0.02%). The tetranucleotide repeats accounted for 11.14% of the repeat sequences.
Table 4.
Distribution of simple sequence repeat types in the Z. striolatum Diels genome
| SSRs motif | Repeat number | SSRs motif | Repeat number | SSRs motif | Repeat number |
|---|---|---|---|---|---|
| AC/GT | 578 | AGA/TCT | 2025 | CGC/GCG | 875 |
| AG/CT | 7685 | AGC/GCT | 1705 | CTA/TAG | 70 |
| AT/AT | 2308 | AGG/CCT | 4647 | CTC/GAG | 2444 |
| GC/GC | 45 | AGT/ACT | 122 | GAA/TTC | 2903 |
| CA/TG | 1930 | ATA/TAT | 553 | GAC/GTC | 302 |
| GA/TC | 6109 | ATC/GAT | 701 | GCA/TGC | 1706 |
| TA/TA | 2846 | ATG/CAT | 431 | GCC/GGC | 2268 |
| AAC/GTT | 298 | CAA/TTG | 339 | GGA/TCC | 2685 |
| AAG/CTT | 2088 | CAC/GTG | 340 | TAA/TTA | 742 |
| AAT/ATT | 1569 | CAG/CTG | 1293 | TAC/GTA | 7 |
| ACA/TGT | 202 | CCA/TGG | 846 | TCA/TGA | 279 |
| ACC/GGT | 468 | CCG/CGG | 1112 | ||
| ACG/CGT | 277 | CGA/TCG | 324 |
Primers were designed for 2392 unigenes containing SSR loci. A total of 5623 pairs of SSR-specific primers were designed, accounting for 55.14% of the SSR loci. Thirty primer pairs specific for different repeating units (i.e., dinucleotides, trinucleotides, and tetranucleotides) were randomly selected for PCR amplifications of Z. striolatum Diels ZSP11 DNA. Twenty-eight primer pairs (93.33%) amplified clear bands (Fig. 3), including 25 amplification products that were consistent with the expected size. Two amplification products were longer than expected, while one product was smaller than expected. The 25 validated primer pairs may be used to analyze the genetic diversity of Z. striolatum germplasms (Fig. 3, Table 5).
Fig. 3.
PCR product of polymorphism of SR primers in different Z. striolatum Diels varieties. a cSSR16; b cSSR21. ZSP1 – ZSP21 represent 1–21 varieties, respectively
Table 5.
Details of 25 primer pairs used for polymerase chain reaction analyses
| Primer no. | Source | Primer sequence (5′→3′) | SSR motif | Length of product |
|---|---|---|---|---|
| cSSR01 | comp101033_c0_seq 3 | CGATCGAGGCGTACACAG | (AG)11 | 224 |
| GAGGAGCGGCTTCTTAGGAT | ||||
| cSSR02 | comp101573_c0_seq 1 | AATGGCTCGGGAGTCAAGAT | (AGG)6 | 233 |
| GGCCAGTTTGAGCGTGTC | ||||
| cSSR04 | comp102711_c0_seq 1 | ATGAAGCCGTGAACGAGAAG | (AGA)7 | 178 |
| TCGATCGTGCTCAGTCTCTG | ||||
| cSSR06 | comp103178_c0_seq 2 | GCATTGCTGAAGAAGGGAAG | (GAT)6 | 200 |
| TTGTTCTCCATTTGGCTTGG | ||||
| cSSR07 | comp104297_c0_seq 2 | ACCCCTCTCGCCCTCTTAT | (GCC)6 | 209 |
| ATGCGGCAGCAGATCATAG | ||||
| cSSR09 | comp105337_c0_seq 4 | GTCAGTTCCGGGGAGGTAAT | (CAA)6 | 225 |
| GACCGAAGACGAAGTCGATG | ||||
| cSSR10 | comp107044_c0_seq 8 | ATTTGTGGACCCGATCTACG | (CGC)6 | 233 |
| GCTTGATGACCCTGTGGAG | ||||
| cSSR11 | comp107202_c0_seq 1 | CCCACCATACCCTACGTTGT | (CCT)6 | 208 |
| GCTCTACCTTTAAGTGCCTTGG | ||||
| cSSR13 | comp108504_c4_seq 9 | TCCCATTCTCCTGCTGAGTT | (GG)9 | 245 |
| GGACGGAAGTCGTAATCTGG | ||||
| cSSR14 | comp109879_c1_seq 1 | CTCCTCTCTTCGCTCCAAAA | (GA)10 | 205 |
| GCCTCCTCTCCCATGTCTCT | ||||
| cSSR15 | comp111587_c0_seq 6 | CGGATCAGAACTTCCCTGAC | (AC)9 | 204 |
| GGACAATTACGCCGACAAAG | ||||
| cSSR16 | comp112622_c1_seq 3 | ACGAAGCTCCCTAGCTGACA | (GAA)6 | 241 |
| TTTTCTTTTGGGTTGCAAGG | ||||
| cSSR17 | comp113200_c2_seq 1 | CTCTTCATTGGTGGAAAGCA | (CAT)6 | 220 |
| GGCATCCTCAAAGACTGCTG | ||||
| cSSR18 | comp27703_c0_seq 1 | ATGGACGGCCATGACTATGT | (AAC)6 | 214 |
| TTTGGGTTGGAGAGAGTTGG | ||||
| cSSR20 | comp50668_c0_seq 1 | CTTACCCACCCTCTCCTTCC | (TTG)6 | 204 |
| ATGAAAGCCCGAGGTCAAG | ||||
| cSSR21 | comp55565_c0_seq 1 | CGACAATTAAAGATAACATCCCAAC | (GAT)6 | 223 |
| CCACGTTATGATCGAAATGG | ||||
| cSSR22 | comp90577_c0_seq 1 | GCGTGTACTCGCTGAAATTG | (GGA)6 | 273 |
| GGCTCACTTATGCCTTCGTC | ||||
| cSSR23 | comp92597_c0_seq 1 | TTGAGAAGGCGTCAGGTACA | (CATC)5 | 205 |
| AAGTCCTGCCATCAAAATGC | ||||
| cSSR25 | comp95230_c0_seq 1 | AGGGAAAGCAAGGAAAGGAA | (TCC)6 | 190 |
| TCGATCCTCTGTTCTGCAAC | ||||
| cSSR26 | comp96728_c0_seq 2 | AGGAGATTGCCATTGACGAC | (TTC)6 | 216 |
| CGGTTCGGTAAGTTCACCTG | ||||
| cSSR27 | comp97410_c3_seq 1 | CGACACGTCTTCATGGATCT | (TGC)7 | 187 |
| TCTATGACGACCCTCGGAAT | ||||
| cSSR28 | comp98468_c0_seq 1 | GACAGACATTATTGGGGGAAAA | (TGAT)5 | 218 |
| GCAGAAAGGCTGCTGGAAT | ||||
| cSSR29 | comp99040_c0_seq 2 | CATGCTCCTCTGCTGGTACA | (GCT)6 | 205 |
| TCATCAATTCCTGGGGAAAA | ||||
| cSSR30 | comp101873_c0_seq 1 | GGGATTGGATTGGTATCTTTGA | (ATTT)5 | 280 |
| TGAAGGGTGTTTTAGTCTTTTCC |
Discussion
The rapid development of second-generation sequencing technology continues to expand the available data regarding whole genomes and transcriptomes, which has increased the abundance of genome-wide SSR markers. In recent years, many researchers have studied SSRs using high-throughput sequencing techniques that have been widely applied for genetic diversity analyses, map building, gene mining, and the identification and improvement of plant varieties (Cheng et al. 2016; Chen et al. 2014; Yi et al. 2006; Portis et al. 2007; Tan et al. 2015).
In this study, 287,959 contigs were obtained for Z. striolatum Diels following high-throughput sequencing, assembly, and alignments. Additionally, 112,107 unigenes were de novo assembled. All of the unigenes were annotated according to alignments with sequences in publically available databases and bioinformatics analyses. A total of 40,876 unique protein accessions were identified. Furthermore, GO and COG functional annotations revealed some important activities related to Z. striolatum Diels development.
The assembled Z. striolatum Diels sequences were used to analyze the distribution of SSRs, suggesting SSR markers may be relevant to future attempts in identifying and classifying Z. striolatum Diels varieties. A search of all unigenes detected 8384 SSR loci (7.48%), with a frequency of one per 35.4 kb. This SSR frequency is higher than that of bananas (5.3%) (Wang et al. 2008), sugarcane (2.9%) (Cordeiro et al. 2001), cotton (4.64%) (Li et al. 2005), rice (4.7%) (Kantety et al. 2002), wheat (3.2%) (Liu et al. 2012), and sorghum (3.6%) (Cordeiro et al. 2001; Kantety et al. 2002). However, it is slightly lower than that of pepper (7.83%) (Liu et al. 2012), and much lower than that of Chinese cabbage (10.4%) (Ge et al. 2005), tea (21.56%) (Jin et al. 2006), coffee (17.3%) (Aggarwal et al. 2007), and Lonicera caerulea (32.51%) (Zhang et al. 2016). These differences may be related to variabilities in the SSR search criteria, database availability, and species.
A previous study concluded that the expressed sequence tag (EST)-SSRs of most plants consist primarily of trinucleotide repeats (Liang et al. 2009). In contrast, the EST-SSRs for a few dicotyledonous plants mainly comprise dinucleotide repeating units (Kumpatla and Mukhopadhyay 2005). Most of the SSRs identified in the current study contained a trinucleotide repeat, followed by a dinucleotide repeating unit. These observations are consistent with the previously reported EST-SSR results for rice, maize, soybean, tomato, cotton, poplar, and Arabidopsis thaliana (Cardle et al. 2000). Similar findings were described for EST analyses of major cereal crops (Varshney et al. 2002) and peppers (Liu et al. 2012). The main trinucleotide repeats in Z. striolatum Diels are AGG/CCT and GGA/TCC/AGG (21.82%), which is in contrast to the dominant trinucleotide repeats in pepper (AAC/GTT), corn (CCG/GGC), rice (AGG/TCC), sorghum and soybean (AAG/TTC), tomato (AAT/ATT), and banana (AAG/CTT). These differences may be related to variations in EST-SSR features, and EST data sources and quantity.
A total of 5623 SSR-specific primer pairs were designed according to the 2392 unigene sequences. Of the 30 randomly selected primer pairs, 25 amplified products were of the expected size. These primers may be useful for identifying and improving Z. striolatum Diels varieties. They may also have applications related to resource analyses, construction of genetic maps, and the functional characterization of genes.
Acknowledgements
Professor William Yajima is gratefully acknowledged for correction to the manuscript. This research was supported by the Guizhou innovation talent base construction of potato industry technology ([2016]22), the technical innovation fund for small and medium-sized enterprises funded projects (13C26215205306) and Guizhou science and technology project ([2016]2554).
References
- Aggarwal RK, Hendre PS, Varshney RK, Bhat PR, Krishnakumar V, et al. Identification, characterization and utilization of EST-derived genic microsatellite markers for genome analyses of coffee and related species. Theor Appl Genet. 2007;114(2):359–372. doi: 10.1007/s00122-006-0440-x. [DOI] [PubMed] [Google Scholar]
- An editorial committee of flora of China . Zingiber striolatum Diels. Flora of China. Beijing: Science Press; 1981. p. 146. [Google Scholar]
- Cardle L, Ramsay L, Milbourne D, Macaulay M, Marshall D, et al. Computational and experimental characterization of physically clustered simple sequence repeats in plants. Genetics. 2000;156(2):847–854. doi: 10.1093/genetics/156.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Song Y, Li LT, Khan MA, Li XG, et al. Construction of a high-density simple sequence repeat consensus genetic map for pear (Pyrus spp.) Plant Mol Biol Rep. 2014;33(2):1–10. [Google Scholar]
- Cheng JW, Zhao ZC, Li B, Qin C, Wu ZM, et al. A comprehensive characterization of simple sequence repeats in pepper genomes provides valuable resources for marker development in Capsicum. Sci Rep. 2016;6:189–190. doi: 10.1038/srep18919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro GM, Casu R, McIntyre CL, Manners JM, Henry RJ. Microsatellite markers from sugarcane (Saccharum spp.) ESTs cross transferable to erianthus and sorghum. Plant Sci. 2001;160(6):1115–1123. doi: 10.1016/S0168-9452(01)00365-X. [DOI] [PubMed] [Google Scholar]
- Ge J, Xie H, Chui CS, Hong JM, Ma RC. Analysis of expressed sequence tags (ESTs) derived SSR markers in Chinese cabbage (Brassica campestris L. ssp. pekinensis) J Agric Biotechnol. 2005;13(4):423–428. [Google Scholar]
- Goldstein DB, Linares AR, Cavalli-Sforza LL, Feldman MW. An evaluation of genetic distances for use with microsatellite loci. Genetics. 1996;139(1):463–471. doi: 10.1093/genetics/139.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JQ, Cui HR, Chen WY, Lu MZ, Yao YL, et al. Data mining for SSRs in ESTs and development of EST-SSR marker in tea plant (Camellia sinensis) J Tea Sci. 2006;26(1):17–23. [Google Scholar]
- Kantety RV, La Rota M, Matthews DE, Sorrells ME. Data mining for simple sequence repeats in expressed sequence tags from barley, maize, rice, sorghum and wheat. Plant Mol Biol. 2002;48:501–510. doi: 10.1023/A:1014875206165. [DOI] [PubMed] [Google Scholar]
- Kelkar YD, Tyekucheva S, Chiaromonte F, Makova KD. The genome-wide determinants of human and chimpanzee microsatellite evolution. Genome Res. 2008;18(1):30–38. doi: 10.1101/gr.7113408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpatla SP, Mukhopadhyay S. Mining and survey of simple sequence repeats in expressed sequence tags of dicotyledonous species. Genome. 2005;48:985–998. doi: 10.1139/g05-060. [DOI] [PubMed] [Google Scholar]
- Li HS, Fan SL, Sheng FF. Screening of microsatellite markers from cotton ESTs. Cotton Sci. 2005;17(4):211–216. [Google Scholar]
- Liang X, Chen X, Hong Y, Liu H, Zhou G, et al. Utility of EST-derived SSR in cultivated peanut (Arachis hypogaea L.) and Arachis wild species. BMC Plant Biol. 2009;9:35–48. doi: 10.1186/1471-2229-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wang YS, Tian XL, Mao ZC, Zou XX, et al. SSR mining in pepper(Capsicum annuum L.)transcriptome and the polymorphism analysis. Acta Hortic Sin. 2012;39(1):168–174. [Google Scholar]
- Moe AM, Weiblen GD. Development and characterization of microsatellite loci in dioecious figs (Ficus, Moraceae) Am J Bot. 2011;98(2):e25–e27. doi: 10.3732/ajb.1000412. [DOI] [PubMed] [Google Scholar]
- Portis E, Nagy I, Sasvari Z, Stagel A, Barchi L, et al. The design of Capsicum spp. SSR assays via analysis of in silico DNA sequence, and their potential utility for genetic mapping. Plant Sci. 2007;172:640–648. doi: 10.1016/j.plantsci.2006.11.016. [DOI] [Google Scholar]
- Powell W, Machray GC, Provan J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996;1(7):215–222. doi: 10.1016/S1360-1385(96)86898-0. [DOI] [Google Scholar]
- Provan J, Kumar A, Shepherd L, Powell W, Waugh R. Analysis of intra-specific somatic hybrids of potato (Solanum tuberosum) using simple sequence repeats. Plant Cell Rep. 1996;16(3–4):196–199. doi: 10.1007/BF01890866. [DOI] [PubMed] [Google Scholar]
- Qu L, Xia LS, Liu D, Feng WY. Rrogress in Zingiber striolatum Diels. Yunnan J Tradit Chin Medi Mater Med. 2015;5:111–113. [Google Scholar]
- Rongwen J, Akkaya M, Bhagwat A, Lavi U, Cregan P. The use of microsatellite DNA markers for soybean genotype identification. Theor Appl Genet. 1995;90(1):43–48. doi: 10.1007/BF00220994. [DOI] [PubMed] [Google Scholar]
- Tan S, Cheng JW, Zhang L, Qin C, Nong DG, et al. Construction of an interspecific genetic map based on InDel and SSR for mapping the QTLs affecting the initiation of flower primordia in pepper (Capsicum spp.) PLoS ONE. 2015;10:1–15. doi: 10.1371/journal.pone.0119389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney RK, Thiel T, Stein N, Langridge P, Graner A. In silico analysis on frequency and distribution of microsa tellites in ESTs of some cereal species. Cell Mol Biol Lett. 2002;7(2A):537–546. [PubMed] [Google Scholar]
- Wang JY, Chen YY, Liu WL, Wu YT. Development and application of EST-derived SSR markers for bananas (Musa nana Lour.) Hereditas. 2008;30(7):933–940. doi: 10.3724/SP.J.1005.2008.00933. [DOI] [PubMed] [Google Scholar]
- Wang HL, Yang J, Boykin LM, Zhao QY, Wang YJ, et al. Developing conversed microsatellite markers and their implications in evolutionary analysis of the Bemisia tabaci complex. Sci Rep. 2014;4:1–10. doi: 10.1038/srep06351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G, Lee JM, Lee S, Choi D, Kim BD. Exploitation of pepper EST-SSRs and an SSR-based linkage map. Theor Appl Genet. 2006;114:113–130. doi: 10.1007/s00122-006-0415-y. [DOI] [PubMed] [Google Scholar]
- Yu Y, Saghai Maroof M, Buss G, Maughan P, Tolin S. RFLP and microsatellite mapping of a gene for soybean mosaic virus resistance. Phytopathology. 1993;84(1):60–64. doi: 10.1094/Phyto-84-60. [DOI] [Google Scholar]
- Zalapa JE, Cuevas H, Zhu H, Steffan S, Senalik D, et al. Using next-generation sequencing approaches to isolate simple sequence repeat (SSR) loci in the plant sciences. Am J Bot. 2012;99:193–208. doi: 10.3732/ajb.1100394. [DOI] [PubMed] [Google Scholar]
- Zhang QT, Li XY, Yang YM, Fan ST, Ai J. Analysis on SSR information in transcriptome and development of molecular markers in Lonicera caerulea. Acta Hortic Sin. 2016;43(3):557–563. [Google Scholar]
- Zong XJ, Wang WW, Wang JW, Wei HR, Yan XR, et al. The application of SYBR Green I real-time quantitative RT-PCR in quantitative analysis of sweet cherry viruses in different tissues. Acta Phytophylacica Sin. 2012;39(6):497–502. [Google Scholar]