Abstract
Alternaria leaf blight, a disease of oilseed Brassicas is caused by a necrotrophic phytopathogenic fungus Alternaria brassicae. The details of its pathogenesis and defence responses elicited in the host upon infection have not been thoroughly investigated. Here, Arabidopsis accession Gre-0 was identified to be highly susceptible to A. brassicae. A comparative histopathological analysis for disease progression and plant responses to A. brassicae in Arabidopsis and Brassica juncea revealed significant similarities between the two compatible pathosystems. Interestingly, in both the compatible hosts, ROS accumulation, cell death and callose deposition correlated with the development of the disease. Based on our results we propose that Arabidopsis-Alternaria brassicae can be an apt model pathosystem since it emulates the dynamics of the pathogen interaction with its natural host- Brassicas. The existing genetic diversity in Arabidopsis can be a starting point to screen for variation in responses to Alternaria leaf blight. Furthermore, several tools available for Arabidopsis can facilitate the dissection of genetic and molecular basis of resistance.
Keywords: Alternaria brassicae, Brassicae juncea, Arabidopsis Gre-0, Defense, Hydrogen peroxide (H2O2), Cell death
Introduction
Brassica juncea is one of the important oilseed crop grown extensively in the Indian sub-continent, Europe, Australia, and Canada. The yield potential of B. juncea is constrained by many fungal diseases among which Alternaria blight poses a major challenge worldwide. The disease lowers the grain yield and under severe incidence of infection even the oil quality is affected (Saharan et al. 2016). Alternaria leaf blight of oilseed mustard is caused by a seed borne necrotrophic fungal pathogen of Alternaria spp. belonging to the family Pleosporaceae. Both A. brassicicola and A. brassicae have been reported to cause the disease, yet A. brassicae is known to be more virulent, causing major damage to oilseed Brassicas, while A. brassicicola an opportunist co-inhabits the infected site. The fungus is capable of infecting the aerial plant parts at all the stages of growth. The disease symptoms are manifested as black/brown necrotic lesions on the leaves, esp. older ones, stem and siliques. The characteristic feature of these lesions is the presence of concentric rings which gives it a ‘bull's eye’ appearance and are seldom surrounded by a chlorotic halo (Conn et al. 1990; Sharma et al. 2002). Commercial cultivars of B. juncea do not possess a high level of tolerance towards A. brassicae. So far, histopathological studies on A. brassicae-Brassica interactions have focused mainly on the initial stages of infection esp. spore germination and penetration processes with little emphasis on host responses upon infection, thus leading to a gap in understanding the pathogenesis of the fungus (Giri et al. 2013; Goyal et al. 2013; Sharma et al. 2014). Arabidopsis has been documented as a host for a broad range of pathogens including necrotrophic fungi. The available natural genetic diversity of Arabidopsis has been exploited to decipher the molecular mechanisms underlying resistance against multiple phytopathogens. Arabidopsis has been used for studying plant interactions with A. brassicicola, and most of the accessions tested show an immune response towards the pathogen (Mukherjee et al. 2009). Since, A. brassicae is the dominating pathogen of oilseed mustard it is imperative to investigate in depth the plant-pathogen interaction for A. brassicae. So far, there is no report of Arabidopsis being used to study A. brassicae pathogenesis and disease responses.
We have identified an Arabidopsis accession, Gre-0 (Greenville, USA), which is highly susceptible to A. brassicae and thus have attempted to develop Arabidopsis as a model host for studying plant interactions. Here, we present a comparative study of the pathobiology of A. brassicae on its natural host B. juncea and the model host A. thaliana. The fungal pathogenesis, disease development and host responses were quantitatively assessed both macroscopically and microscopically. Our results reveal significant parallels between the B. juncea and Arabidopsis pathosystems, thus making Arabidopsis a promising model to investigate the mechanism of A. brassicae pathogenesis and host resistance.
Materials and methods
Fungal strains and growth conditions
The Alternaria brassicae isolate J3 used in this study was earlier isolated from the infected B. juncea leaf from Delhi fields and single spore culture was established (Kumar 2009). For routine culture, the isolate was maintained on radish extract medium at 20–22 °C under 12 h light/12 h dark cycle.
Plant material and growth conditions
Selfed seeds of B. juncea cv. Varuna were planted into 9 inch plastic pots filled with autoclaved 1:1 mix of garden soil and soilrite and grown in PGV36 at 22 °C 14 h light/10 h dark cycle and 60–80% relative humidity (RH). Arabidopsis accession Greenville-0 (Gre-0) seeds obtained from Nottingham Arabidopsis Stock Centre (NASC) were grown in 3 inch plastic pots containing autoclaved artificial soil mix (1 soilrite: 1 vermiculite: 1 perlite), for 5–6 weeks at 22 °C in growth chambers programmed for a 14-h-light/10-h-dark cycle.
Infection assays
Conidial suspensions for drop inoculation were prepared from 15-days old sporulating plates. To collect conidia, the plates were flooded with sterile water, gently scrapped and the resulting suspension was passed through three layers of muslin cloth. The concentration of spores was adjusted to 3 × 103 conidia ml−1. Leaves of one month old Varuna plants were inoculated with four to six droplets of 5 μl of A. brassicae conidial suspension. For Arabidopsis, leaves of 6 week old plants were inoculated with four droplets (5 μl) of spore suspension. During the first 3 days post inoculation (dpi), the RH was maintained at 90% and thereafter humidity was reduced to 70% till symptoms developed i.e. 7 days of post infection (dpi). For histopathological studies, the tissue samples were collected at 6, 24, 48, 72 and 96 hpi.
Histochemical assay and microscopy
A total of at least 36 infection sites for B. juncea and Gre-0 each, from different biological replicates were examined for recording various host responses upon fungal penetration. For initial fungal growth, like spore germination, hyphal structure and spread on the leaf surface, the infected leaf samples were decolorized in ethanol: acetic acid (3:1 vol/vol) and stained with 0.25% coomassie blue solution (R250) (Qualigens) for 1 min, washed in water and mounted in 50% glycerol for observation (Göllner et al. 2008). For detection of H2O2, endogenous peroxidase-dependent in situ histochemical staining employing 3-3′-Diaminobenzidine (DAB) (Sigma-D5637-1G) was used (Thordal-Christensen et al. 1997). To visualize fungal structures like ALS (appressoria like swollen, globular structures), and dead plant cells, infected leaf samples were harvested and boiled for 2 min in lactophenol trypan blue. Stained leaves were then cleared in chloral hydrate (1 g/ml in water) for 24 h at room temperature. Cleared leaves were mounted in 50% glycerol for microscopic observation (Bartsch et al. 2006). To detect callose deposition, infected leaves were cleared in ethanol: acetic acid solution (3:1 vol/vol), stained for 30 min in 150 mM K2HPO4 (pH 9.5) containing 0.01% aniline blue (Sigma: 415049-25G) in dark, and examined for fluorescence using Olympus BX51 microscope (Olympus Corporation, Japan) DAPI filters (λexc 340–380 nm, barrier filter 435–485 nm) (Vogel and Somerville 2000).
The fungal penetration events triggering defense responses (H2O2 accumulation, cell death and callose deposition) were calculated using the formula:
Quantitative reverse transcriptase (qRT)-PCR assays
Total RNA was extracted from the leaves collected from six plants in each experiment using RNeasy plant mini kit according to manufacturer’s recommendation (Qiagen, Gaithersburg, MD, USA). First-strand cDNA was synthesized from 1 µg of total RNA using MMLV Reverse Transcriptase 1st-Strand cDNA Synthesis Kit (Epicentre Biotechnologies, Madison, USA) as per manufacturer’s protocol. PCRs were carried out using the standard setting in a QuantStudio 6 Flex Real-Time PCR System (ThermoFisher Scientific, Waltham, MA, USA) with 2X SYBR Select Master Mix (ThermoFisher Scientific, Waltham, MA, USA). Gene expression levels were standardized using UBC8 (At5g41700) as internal control. Relative expression levels were calculated and represented (Willems et al. 2008).
Results
Leaves of Arabidopsis accession Gre-0 and B. juncea cv. Varuna were challenged with A. brassicae spore (3 × 103 spores ml−1) by drop inoculations. Under conducive conditions macroscopic disease symptoms in both the hosts were visible as early as 72 hpi as water-soaked lesions and by 7 dpi prominent nectroic lesion were observed (Fig. 1a–c). A comparative disease progression and induced plant defense responses for the Gre-0 and Varuna were studied.
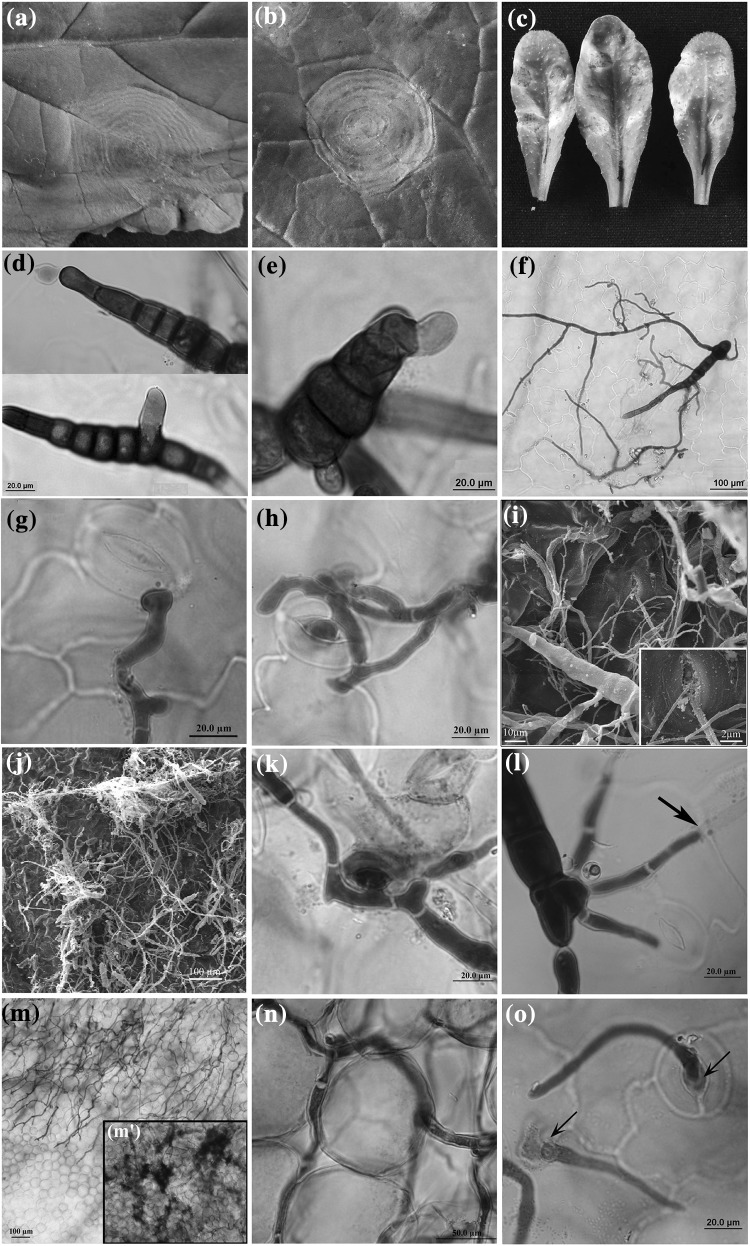
Fig. 1.
Progression of Alternaria brassicae infection on B. juncea cv. Varuna and A. thaliana accession Gre-0. Comparative analysis of macroscopic infection phenotypes 7 dpi on Varuna leaf upon artificial inoculation (a), under field conditions (b), and upon artificial inoculation on Gre-0 leaf surface (c). Initiation of spore germination at 6 hpi from terminal and intercalary cells of conidiophores on Varuna (d) and Gre-0 (e). Conidia with multiple branched hyphae were observed at 24 hpi on the surface of Varuna (f). Hyphae forming ALS near the stomatal opening on Varuna leaf surface (g). Penetration via stomata on Varuna leaf surface (h, i), Gre-0 (k) at 48 hpi. SEM image showing hyphal growth on the Varuna leaf surface at 72 hpi (j). Direct fungal penetration through epidermal cell junctions on Gre-0 leaf surface at 48 hpi (l). Fungal invasion into the healthy mesophyll cells at 96 hpi (m) and darkly stained dead cells at the site of inoculation at 96 hpi (m′). Ramified hyphal growth between the two mesophyll cells at 96 hpi (n). Re-emergence of young fungal hyphae through the stomata of Varuna 6 dpi (o)
Microscopic studies of Arabidopsis-A. brassicae interactions
The conidia were able to germinate on the Arabidopsis leaf surface by 12–24 hpi. Microscopic examination of cleared inoculated leaves revealed initiation of spore germination, within 6 hpi, as a small swelling either at the ends of the conidia or from intercalary spores (Fig. 1d, e). By 24 hpi almost 99% conidial germination was observed on both Arabidopsis and Varuna leaf with multiple branched hyphae emerging from conidiospore (Fig. 1f, Table 1). Post spore germination fungal entry into the plant is the most critical step towards successful infection. At 24 hpi, branched -primary and -secondary hyphae with several penetration attempts were observed in both the hosts. The successful penetration events increased over time from 341 sites at 24 hpi to 884 sites at 48 hpi in Gre-0 and a similar trend was observed for Varuna (Table 1). Similarly, for both the pathosystems more than 90% of the fungal penetration attempts were recorded through stomatal openings although some hyphae were seen growing over the stomata without entering them (Fig. 1h, i, k). The fungus also penetrated directly at the junctions between epidermal cells albeit at a low frequency (~ 8.3%) (Table 1; Fig. 1l). In several instances, the hyphal apices appressed to leaf surface were swollen, similar to simple appressoria like structures, and stained more intensely with trypan blue than the rest of the hyphae. In both the host plant, fungal penetration via stomata was mostly associated with the formation of Appressoria Like swollen, globular Structures (ALS) at the hyphal apices (Fig. 1g). In the colonized Varuna leaf tissue, hyphal growth within the intercellular spaces of the mesophyll cells was observed at 96 hpi (Fig. 1m, n), which was accompanied by cell death and tissue collapse (Fig. 1m′). Besides fungal proliferation within the leaf tissue, extensive mycelial growth on the leaf surface of Gre-0 and Varuna was also observed (Fig. 1j). By 6 dpi, re-emergence of fungal hyphae could be seen through natural opening like stomata and leaf margins in Varuna (Fig. 1o). Intercellular fungal growth and re-emergence of the fungus in Gre-0 could not be observed even beyond 7 dpi.
Table 1.
Germination and infection processes of Alternaria brassicae isolate J3 on Varuna and Gre-0
| Plant | No. of spores germinated/total no. of spores | Total penetration sitesd | Mode of penetratione | |||
|---|---|---|---|---|---|---|
| 6 hpia | 24 hpic | 24 hpi | 48 hpi | Stomata | Cell junction | |
| Gre-0 | 437/562 (77.8)b | 2883/2892 (99.6) | 341 | 884 | 1131 (92.3) | 94 (8.3) |
| Varuna | 260/318 (81.7) | 2235/2253 (99.2) | 577 | 1443 | 1875 (92.8) | 145 (7.2) |
For Gre-0: A total of 40 infection sites were scored from three independent experiments
For Varuna: A total of 36 infection sites were scored from two independent experiments
aTotal spores represent spore count from three leaves each collected from two experiments
bNumbers within parentheses indicate the percentage of spore germination
cTotal spores represent the spore count from all the leaves collected for host responses (H2O2, cell death and callose deposition). The experiments were repeated three times with similar results
dTotal penetration site represent the sum of all the penetration sites observed in experiments evaluating various host responses upon infection
eNumbers within parentheses indicate the percentage of penetration sites through stomata and cell junction
Disease progression during A. brassicae infection is accompanied by ROS, cell death and callose production
To further characterize and compare the defense response of the two compatible hosts to A. brassicae infection, penetration associated: (i) induction of H2O2 with 3,3′- diaminobenzidine (DAB) staining, (ii) host cell death with lactophenol trypan blue stain, and (iii) deposition of callose using aniline blue were monitored.
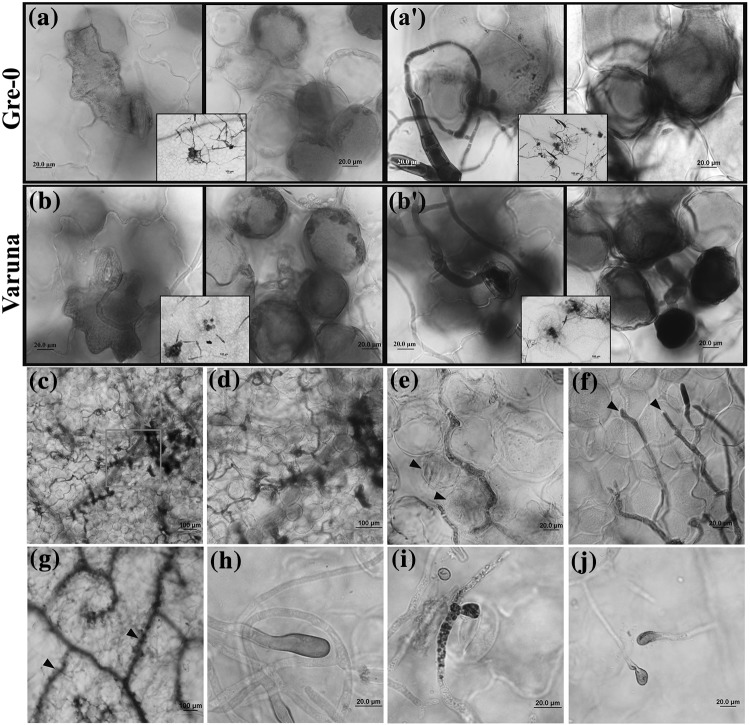
Reactive oxygen species (ROS) and callose deposition in the cell wall plays a major role in plant-pathogen interaction. ROS formation was assessed at the site of infection in a temporal fashion. At 24 hpi, only 30.5% of penetration sites had an associated DAB staining in Arabidopsis (Table 2). The staining was observed not only in the stomatal guard cells at the site of penetration but also in the adjoining epidermal cells. Additionally, H2O2 production could be detected in a discrete cluster of mesophyll cells beneath the invading hyphae (Fig. 2a, b) which spread to larger patches of mesophyll and epidermal cells by 48, 72 and 96 hpi (Fig. 3a). ROS was also detected in the growing tip of the fungal hyphae (non-penetrating) and in penetrating ALS (24 hpi) (Fig. 2h–j).
Table 2.
Quantitative analysis of H2O2 accumulation, cell death and callose deposition at the penetration sites upon A. brassicae infection
| Host | Hpi | H2Oa2 | Cell deathb | Callosec |
|---|---|---|---|---|
| Gre-0 | 24 | 27.5–32.3 | 0–9.5 | 6.2–8.8 |
| 48 | 29.5–38.8 | 6.8–10.1 | 17.3–20.7 | |
| Varuna | 24 | 26.5–26.6 | 18.2–19.3 | 16.6–18.3 |
| 48 | 56.4–57.8 | 50.4–52.1 | 46.6–48.3 |
For Gre-0: Results compiled from three independent experiments per time point
For Varuna: Results compiled from two independent experiments per time point
a, b, cRange of % penetration sites associated with H2O2 production, cell death and callose deposition respectively
Fig. 2.
Cellular responses to the Alternaria brassicae infection on Gre-0 and Varuna. Representative images of infected Gre-0 and Varuna leaves stained with 3,3'-diaminobenzidine (DAB) to detect hydrogen peroxide (H2O2) accumulation (reddish brown) and trypan blue to detect cell death (blue). At 24 hpi, H2O2 accumulates in epidermal and mesophyll cells at the site of fungal penetration, whereas cell death occurs only in mesophyll both in Gre-0 (a, a′) and Varuna (b, b′). The insets in a, b, a′ and b′ shows localization of DAB and cell death respectively around the penetrated sites in Gre-0 and Varuna at a lower magnification. Infected Varuna leaves showing cell death (CD) following invasion by the fungal hyphae into the healthy mesophyll cells-96 hpi (c, d). Slight coloration of the mesophyll cells (arrowheads) indicates the initiation of cell death around the fungus at 96 hpi (e). Leading edge of the invading hyphae grows into the intercellular space between the mesophyll cells without causing any visible cell death (arrowheads)-96 hpi (f). The extensive spread of cell death observed in Varuna leaf veins (arrowheads) at 7 dpi (g). H2O2 accumulation was observed at fungal hyphal tip and ALS on Varuna (h, i) and Gre-0 (j)
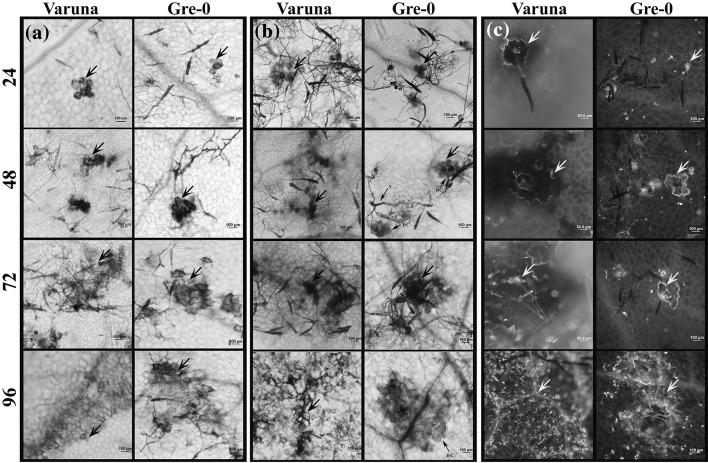
Fig. 3.
Hydrogen peroxide accumulation, cell death and callose deposition in Gre-0 and Varuna in response to A. brassicae infection. Representative pictures of inoculated leaf samples collected at 24, 48, 72 and 96 hpi were assayed for H2O2 accumulation (arrow) (a); cell death (arrow) (b) and callose deposition (blue fluorescence, yellow arrow) (c). The production of H2O2, cell death and callose deposition are restricted to a few cells during the early phases. Their spread tightly correlates with spread of the disease symptoms
Traditionally, the formation of ROS in response to pathogen invasion is associated with hypersensitive response (HR) and programmed cell death. The kinetics of cell death and its extent was assessed during the course of A. brassicae pathogenesis in both the hosts by Trypan blue staining. Only a small fraction of penetration sites were associated with cell death (5.6% in Gre-0) early during the host invasion which increased at 48 hpi (Table 2). Interestingly, during the initial phase of fungal ingress, the cell death was mostly confined to a few mesophyll cells present below the penetrating stomata while the guard cells or the adjoining epidermal cells did not show any signs of cell death despite the accumulation of ROS (Fig. 2a′, b′). These trends of H2O2 accumulation and associated cell death were also observed in the natural host Varuna. In Varuna, with the passage of time (96 hpi), the zone of dead cells extended over a larger area coincident with the ramified growth of the fungal hyphae (Figs. 2c, 3b). Microscopically it was observed that the leading edge of the invading hyphae grew into the intercellular space between the mesophyll cells without causing any visible cell death (Figs. 1n, 2f) although extensive cell death was seen in the already colonized zone (Fig. 2d, e). As expected for a necrotroph, the extent of cell death increased with the progression of the fungal growth and by 7 dpi, cell death had progressed in the veins of the infected brassica leaves as well (Fig. 2g).
Additionally, in the infected Gre-0 and Varuna leaves, callose was predominantly deposited in the epidermal cells surrounding the site of fungal penetration at 24 hpi, which extended to larger patches of cells as the fungal growth extended (Fig. 3c; Table 2).
Defense related gene expression in response to A. brassicae in Gre-0
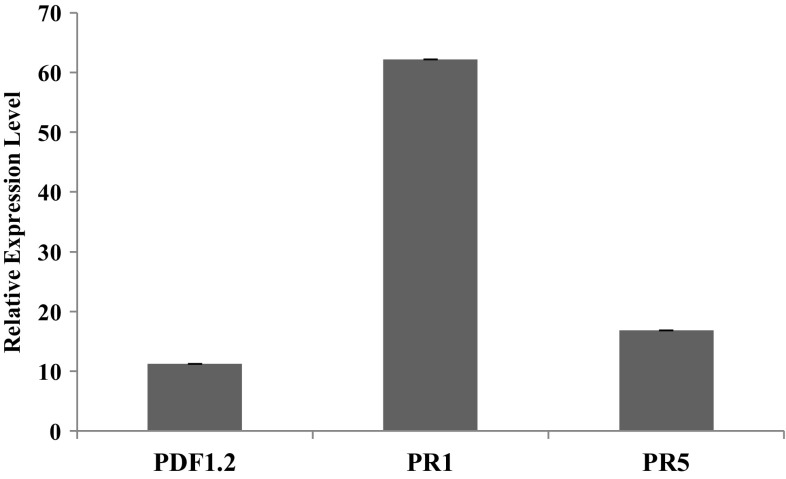
Previous studies in the susceptible host B. juncea have shown that the transcript level of the defense related gene PR1 is highly induced in response to A. brassicae and A. brassicicola infection (Mazumder et al. 2013; Nayanakantha et al. 2016). To compare if the compatible Arabidopsis accession also elicited similar defense responses, the transcription level of three representative markers for JA/Ethylene pathway-PDF1.2, and Salicylic Acid pathway- PR1, and PR5 were evaluated for infection (Fig. 4). Similar to B. juncea, the marker for the SA pathway: PR1 showed a maximum induction, while the JA pathway marker (PDF1.2) showed a comparatively moderate induction.
Fig. 4.
Expression pattern of defense related genes: PDF1.2, PR1, and PR5, at 4 days post infection (dpi) in the compatible Arabidopsis accession Gre-0. The mean values of two independent biological replicates are shown
Discussions
Compared to biotrophic phytopathogens, our understanding of the mechanisms of pathogenesis and host responses towards necrotrophic pathogens is still in infancy. This study is the first to define host–pathogen interaction involving A. brassicae and A. thaliana. Here, for the first time, we show that Arabidopsis accession Gre-0 (Greenville, USA) supports substantial A. brassicae growth. Furthermore, A. brassicae infection processes and the induced host responses are analogous between the model host Gre-0 and its natural host Varuna.
Unlike biotrophs, necrotrophs are believed to facilitate their entry into the host cells by degrading the host cell walls, thus relinquishing the need for developing specialized infection structures like appressoria (Kübicek et al. 2014). Although, appressoria like structures have been earlier reported for other Alternaria spp. e.g. A. brassicicola, A. raphanin, A. alternata, these fungi habitually penetrate directly through cell junctions (McRoberts and Lennard 1996). The appearance of unmelanised ALS associated with host penetration events in our study was unforeseen since A. brassicae predominantly (> 90%) entered the host via stomata. Formation of similar unmelanised compound appressoria for host penetration has been recently reported for several other necrotrophs viz. S. sclerotiorum, and B. cinerea (Dai et al. 2006; van Kan 2005).
The host responses to infection, like accumulation of ROS, cell death, and callose deposition, are reciprocally correlated with disease resistance in many biotrophic and hemi-biotrophic pathosystem (Koch and Slusarenko 1990; O’Connell et al. 2004; Soylu 2004). On the contrary, the successful necrotrophic infections are associated with extensive cell death and ROS production during the establishment and disease progression phase (Laluk and Mengiste 2010; Williams et al. 2011). Production of ROS and the concomitant cell death during the disease progression has been reported earlier in mustard and Arabidopsis (Col-0) upon infection with A. brassicicola-a closely related Alternaria sp. (Meur et al. 2015; Su’udi et al. 2011). In our study, restricted H2O2 accumulation and cell death during the early phase of infection and a more robust H2O2 generation accompanied by spreading cell death in the post establishment phase was observed in both the compatible hosts and it positively correlated with the disease spread. Our observations suggest that for a successful compatible interaction, a fine balance must be reached between the suppression of ROS and cell death at the initial stage of infection followed by extensive triggering of the same, post establishment. Besides the host induced ROS, A. brassicae also produces ROS at the hyphal tips during the compatible interaction (Fig. 2i, j). A close association of ROS generation and fungal aggressiveness has been explicitly recorded for various other necrotrophic fungi and bacteria. ROS-deficient mutants of S. sclerotiorum (Ssnox1), B. cinerea (bcnoxA and bcnoxB) and A. alternata (AaNoxA) have compromised virulence and have defective development (Kim et al. 2011; Segmuller et al. 2008; Takemoto et al. 2007).
It is generally believed that toxins secreted by necrotrophs induce host cell death ahead of extensive colonization which usually appears as a chlorotic halo surrounding the advancing necrotic zone (Glazebrook 2005). A. brassicae is known to secrete Destruxin B both in vitro and in planta that has phytotoxic properties (Parada et al. 2007). Surprisingly, in B. juncea at the advancing infection front, intercellular fungal hyphal growth without any associated cell death was observed. No chlorotic zone surrounding the necrotic lesion was seen in these infected samples. Additionally, earlier observation that A. brassicae suppress plant ROS and cell death during penetration, indicates that there might be a short biotrophic-like phase where the fungus suppresses plant defense responses before triggering massive cell death. Similar observations have been reported for the broad spectrum necrotroph S. sclerotiorum infecting tomato (Kabbage et al. 2013, 2015). However, from our results, it is not clear if the cell death observed is necrosis or programmed cell death. Furthermore, activation of Salicylic acid mediated pathway in the Arabidopsis accession (susceptible) is in agreement with a similar observation reported in B. juncea (Mazumder et al. 2013; Nayanakantha et al. 2016).
Callose deposition at the penetration site to stall the hyphal entry is a hallmark of plant response to infection especially against biotrophic fungi. Although in the present study, callose deposition does not appear to contribute to resistance as generalized callose deposits were observed at the sites of successful penetration and establishment in both the compatible hosts. Rather, callose deposition appears to be a default plant response to infection. Nevertheless, we cannot rule out the role of callose in basal resistance against A. brassicae as the callose synthase mutant gsl5/pmr4 showed enhanced susceptibility to the fungus (data not shown).
In this study, the comparative analysis of the processes of pathogenesis, infection and the host defense responses in the natural host B. juncea and a model host Arabidopsis Gre-0 reveal significant parallels between the two pathosystems. The extent of ROS, cell death and callose accumulation show a spatial and temporal correlation with the developing symptom, thus hinting at their putative role in susceptibility. Here we provide the foundation for future studies to investigate the plant defense mechanism and associated regulatory pathways that impart resistance against Alternaria leaf blight using the Arabidopsis model.
Acknowledgements
We thank Nottingham Arabidopsis Stock Center (NASC) for distribution of Arabidopsis accession. We acknowledge Central Instrument Facility-University of Delhi, South Campus and Centre for Genetically Modifies Crop Plants, University of Delhi for sharing plant growth space. This work was financially supported by the grants from Science and engineering Research Board (SB/FT/LS-327/2012) and Department Of Biotechnology (BT/PR13379/GBD/27/263/2009) and research fellowship to Sayanti Mandal and Sivasubramanian Rajarammohan from University Grants Commission (UGC), Government of India.
Contributor Information
Sayanti Mandal, Email: sayanti_mandal@yahoo.com.
Sivasubramanian Rajarammohan, Email: siva.r@south.du.ac.in.
Jagreet Kaur, Phone: +919958766190, Email: jagreet@south.du.ac.in.
References
- Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE. Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell. 2006;18:1038–1051. doi: 10.1105/tpc.105.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn KL, Tewari JP, Awasthi RP. A disease assessment key of Alternaria blackspot in rapseed and mustard. Can Plant Dis Surv. 1990;70:19–22. [Google Scholar]
- Dai F-M, Xu T, Wolf GA, He ZH. Physiological and molecular features of the pathosystem Arabidopsis thaliana L.-Sclerotinia sclerotiorum Libert. J Integr Plant Biol. 2006;48:44–52. doi: 10.1111/j.1744-7909.2006.00181.x-i1. [DOI] [Google Scholar]
- Giri P, Taj G, Meena PD, Kumar A. Microscopic study of Alternaria brassicae infection processes in Brassica juncea cultivars by drop plus agarose method. Afr J Microbiol Res. 2013;7:4284–4290. [Google Scholar]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Göllner K, Schweizer P, Bai Y, Panstruga R. Natural genetic resources of Arabidopsis thaliana reveal a high prevalence and unexpected phenotypic plasticity of RPW8-mediated powdery mildew resistance. New Phytol. 2008;177:725–742. doi: 10.1111/j.1469-8137.2007.02339.x. [DOI] [PubMed] [Google Scholar]
- Goyal P, Mathur AP, Chattopadhyay C. Hiopathology in Brassica juncea-Alternaria brassicae interaction and localization of histochemicals in Alternaria blight-infected B.juncea leaves. Ann Plant Protect Sci. 2013;21:322–328. [Google Scholar]
- Kabbage M, Williams B, Dickman MB. Cell death control: the interplay of apoptosis and autophagy in the pathogenicity of Sclerotinia sclerotiorum. PLoS Pathog. 2013;9:e1003287. doi: 10.1371/journal.ppat.1003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbage M, Yarden O, Dickman MB. Pathogenic attributes of Sclerotinia sclerotiorum: switching from a biotrophic to necrotrophic lifestyle. Plant Sci. 2015;233:53–60. doi: 10.1016/j.plantsci.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chen C, Kabbage M, Dickman MB. Identification and characterization of Sclerotinia sclerotiorum NADPH oxidases. Appl Environ Microbiol. 2011;77:7721–7729. doi: 10.1128/AEM.05472-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E, Slusarenko A. Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell. 1990;2:437–445. doi: 10.1105/tpc.2.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübicek CP, Starr TL, Glass NL. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol. 2014;52:427–451. doi: 10.1146/annurev-phyto-102313-045831. [DOI] [PubMed] [Google Scholar]
- Kumar A. Molecular mapping of gene(s) conferring resistance to Alternaria blight (Alternaria brassicae) in Arabidopsis thaliana. New Delhi: University of Delhi; 2009. [Google Scholar]
- Laluk K, Mengiste T. Necrotroph attacks on plants: Wanton destruction or covert extortion? Arabidopsis Book Am Soc Plant Biol. 2010;8:e0136. doi: 10.1199/tab.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder M, Das S, Saha U, Chatterjee M, Bannerjee K, Basu D. Salicylic acid-mediated establishment of the compatibility between Alternaria brassicicola and Brassica juncea is mitigated by abscisic acid in Sinapis alba. Plant Physiol Biochem. 2013;70:43–51. doi: 10.1016/j.plaphy.2013.04.025. [DOI] [PubMed] [Google Scholar]
- McRoberts N, Lennard JH. Pathogen behaviour and plant cell reactions in interactions between Alternaria species and leaves of host and nonhost plants. Plant Pathol. 1996;45:742–752. doi: 10.1046/j.1365-3059.1996.d01-4.x. [DOI] [Google Scholar]
- Meur G, Shukla P, Dutta-Gupta A, Kirti PB. Characterization of Brassica juncea–Alternaria brassicicola interaction and jasmonic acid carboxyl methyl transferase expression. Plant Gene. 2015;3:1–10. doi: 10.1016/j.plgene.2015.06.001. [DOI] [Google Scholar]
- Mukherjee AK, Lev S, Gepstein S, Horwitz BA. A compatible interaction of Alternaria brassicicola with Arabidopsis thaliana ecotype DiG: evidence for a specific transcriptional signature. BMC Plant Biol. 2009;9:31. doi: 10.1186/1471-2229-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayanakantha NMC, Rawat S, Ali S, Grover A. Differential expression of defense-related genes in Sinapis alba and Brassica juncea upon the infection of Alternaria brassicae. Trop Agric Res. 2016;27(2):123–136. doi: 10.4038/tar.v27i2.8161. [DOI] [Google Scholar]
- O’Connell R, Herbert C, Sreenivasaprasad S, Khatib M, Esquerre-Tugaye MT, Dumas B. A novel Arabidopsis-Colletotrichum pathosystem for the molecular dissection of plant-fungal interactions. Mol Plant Microbe Interact. 2004;17:272–282. doi: 10.1094/MPMI.2004.17.3.272. [DOI] [PubMed] [Google Scholar]
- Parada RY, Okab K, Yamagishib D, Kodamab M, Otanib H. Destruxin B produced by Alternaria brassicae does not induce accessibility of host plants to fungal invasion. Physiol Mol Plant Path. 2007;71:48–54. doi: 10.1016/j.pmpp.2007.10.003. [DOI] [Google Scholar]
- Saharan GS, Mehta N, Meena PD. Alternaria diseases of crucifers: biology, ecology and disease management. Singapore: Springer; 2016. [Google Scholar]
- Segmuller N, Kokkelink L, Giesbert S, Odinius D, van Kan J, Tudzynski P. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol Plant Microbe Interact. 2008;21:808–819. doi: 10.1094/MPMI-21-6-0808. [DOI] [PubMed] [Google Scholar]
- Sharma G, Kumar VD, Haque A, Bhat SR, Prakash P, Chopra VL. Brassica coenospecies: a rich reservoir for genetic resistance to leaf spot caused by Alternaria brassicae. Euphytica. 2002;125:411–417. doi: 10.1023/A:1016050631673. [DOI] [Google Scholar]
- Sharma P, Deep S, Bhati DS, Sharma M, Chowdappa P. Penetration and infection processes of Alternaria brassicicola on cauliflower leaf and Alternaria brassicae on mustard leaf: a histopathological study. Plant Pathol J. 2014;13:100–111. doi: 10.3923/ppj.2014.232.245. [DOI] [Google Scholar]
- Soylu S. Ultrastructural characterisation of the host-pathogen interface in white blister-infected Arabidopsis leaves. Mycopathologia. 2004;158:457–464. doi: 10.1007/s11046-004-2453-9. [DOI] [PubMed] [Google Scholar]
- Su’udi M, Kim MG, Park SR, Hwang DJ, Bae SC, Ahn IP. Arabidopsis cell death in compatible and incompatible interactions with Alternaria brassicicola. Mol Cells. 2011;31:593–601. doi: 10.1007/s10059-011-2203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D, Tanaka A, Scott B. NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet Biol. 2007;44:1065–1076. doi: 10.1016/j.fgb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- van Kan JAL. Infection strategies of Botrytis cinerea. Acta Hortic. 2005;669:77–90. doi: 10.17660/ActaHortic.2005.669.9. [DOI] [Google Scholar]
- Vogel J, Somerville S. Isolation and characterization of powdery mildew-resistant Arabidopsis mutants. Proc Natl Acad Sci USA. 2000;97:1897–1902. doi: 10.1073/pnas.030531997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems E, Leyns L, Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal Biochem. 2008;379:127–129. doi: 10.1016/j.ab.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Williams B, Kabbage M, Kim HJ, Britt R, Dickman MB. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 2011;7:e1002107. doi: 10.1371/journal.ppat.1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]