Abstract
Pesticides are a group of chemical substances which are widely used to improve agricultural production. However, these substances could be persistent in soil and water, accumulative in sediment or bio-accumulative in biota depending on their solubility, leading to different types of environmental pollution. The present study was done to assess the impact of pesticides-mancozeb and chlorpyrifos, via morphological and physiological parameters using Allium cepa test system. Phytotoxic effects of pesticides were examined via germination percentage, survival percentage, root and shoot length, root shoot length ratio, seedling vigor index, percentage of phytotoxicity and tolerance index. Oxidative stress on Allium seedlings caused by pesticides was also assessed by investigating the activity of antioxidative enzymes viz. catalase, peroxidase and superoxide dismutase. Correlation was worked out between morphological parameters and antioxidative enzymes to bring out the alliance between them. Mancozeb and chlorpyrifos concentrations were significantly and positively correlated with the activity of antioxidative enzymes and negatively correlated with morphological parameters. Significant positive correlation between various morphological parameters showed their interdependency. However, negative correlation was obtained between activity of antioxidative enzymes and morphological parameters. The enzymes however, showed positive correlation with each other. Based on our result we can conclude that all morphological parameters were adversely affected by the two pesticides as reflected by phytotoxicity in Allium. Their negative correlation with activity of antioxidative enzymes indicates that upregulation of antioxidative enzymes is not sufficient to overcome the toxic effect, thereby signifying the threat being caused by the regular use of these pesticides.
Keywords: Antioxidative enzymes, Correlation, Oxidative stress, Phytotoxicity
Introduction
Pesticides are toxic chemicals used on arable fields to control diseases, pests and weeds so as to decrease yield losses and also sustain high productivity. The widespread use of these pesticides has led to their augmented accumulation in soil, water and air (Bolognesi and Merlo 2011; Harnpicharnchai et al. 2013), followed by bioaccumulation, sometimes leading to influx in organisms, hindering the essential metabolic functions which are similar in both target and non target plants (Jafari et al. 2012; Naksen et al. 2016; Botías et al. 2016). The concern over the indiscriminate use of pesticides has increased due to the rise in pollution and also because of the risk to exposed organisms (Moustafa et al. 2007). According to a report of FICCI (2015), major share in the Indian crop protection market is of insecticides (60%) followed by fungicides and herbicides which account for approximately 18 and 16% respectively of total pesticides. The extensive use of pesticides in agriculture causes high acute toxicity problems which can deleteriously influence plant growth and development and can cause long term damage to the environment and human lives even in trace levels (Songa and Okonkwo 2016).
Two pesticides-mancozeb and chlorpyrifos were used in the present investigation. Mancozeb (zinc;manganese(2+); N-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate, MZ) is a fungicide of carbamate family, commonly used to control fungal diseases and thus increase crop production. Its morphotoxic and physiotoxic effects have been reported by Anitha and Savitha (2013). Chlorpyrifos (O,O-diethylO-3,5,6-trichloro-2-pyridyl-phosphorothioate, CPF), is a broad spectrum insecticide and has been in use for several years for controlling insects that effects crops plants, by reducing pod damage (Wu and Laird 2003; Khan et al. 2009; Kumar et al. 2010) and also for subterranean termites (Venkateswara Rao et al. 2005). Organophosphorus insecticides and carbamate fungicides are universally used as regular pesticides for food and cash crops including vegetables and fruits (JanakiDevi et al. 2013). Thus they result in environmental contamination which as a result affect human health especially by over stimulation of neurotransmission system (Naksen et al. 2016).
Pesticides after application undergo degradation by physiochemical reactions such as autolysis, photolysis, rearrangement and inactivation upon binding to soil and macromolecules leading to regeneration of reactive oxygen species (ROS) (Mahmood et al. 2014). ROS are responsible for stress induced damages, and it is widely accepted that chemical toxicity leads to their generation and consequently in oxidative stress (Chen et al. 2010; Faize et al. 2011). Thus various strategies utilizing enzymatic and non enzymatic components have been adopted by plants to combat the oxidative damages to the cells. The enzymatic components of the antioxidant machinery include ROS scavenging enzymes like superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) (Khan and Kour 2007). The non enzymatic components of the defence system (e.g. ascorbate, glutathione, carotenoids and tocopherols) prevent cells from damage, also by scavenging ROS (Jan et al. 2012).
In the current study, we have investigated the phytotoxic and physiotoxic effects of two pesticides via some growth parameters and the activities of antioxidant enzymes viz. CAT, POD and SOD) using the same test system—Allium seedlings, in order to elucidate the stress generated by pesticide pollution. We used plant model to investigate the effect of pesticides because they are the non target recipients of these pesticides. Their application to the target organisms is not a closed application i.e. they are introduced in the environment thereby affecting non target organisms either directly or indirectly by getting accumulated in the soil and being absorbed later. Their absorption by non target organisms, whether animals or plants is of great concern as stress is imposed on the biota. Hence, we have used a non target system, Allium cepa for evaluating its response to the two pesticides.
Materials and methods
Chemicals
Mancozeb (75% W.P) and chlorpyrifos (20% EC) were purchased from a local agricultural store in Lucknow, India. The chemicals used for our study were of analytical grade.
Plant material and pesticides treatment
The Allium cepa seeds were procured from the local market of Lucknow, India. Healthy and equal sized seeds were selected and surface sterilized by thoroughly washing with distilled water. Seeds were incubated for 24 h in different concentrations of MZ viz. 10, 30, 50, 70, 90, 110, 130 and 150 ppm and CPF viz. 5, 10, 15, 20, 25 and 30 ppm and distilled water which served as control. Different concentrations had to be used on the basis of LC 50 which was determined by probit analysis (Finney 1952). After 24 h, the control and treated seeds were placed on doubled layered filter papers prewetted with distilled water in petridish. These were incubated at 25 ± 1 °C for 15 days with regular supply of distilled water. Three replicates for each concentration were maintained along with control for comparison and each replicate had 20 seeds per petridish.
Morphological analysis
Different morphological parameters of Allium seedlings were investigated like seed germination percentage (G%), survival percentage (S%), root length (RL), shoot length (SL), root shoot length ratio (RSL ratio), seedling vigor index (SVI), percentage of phytotoxicity (P%) and tolerance index (TI). The germination percentage was taken on 3rd day after sowing on filter paper by counting the number of seeds germinated out of total number of seeds treated (Scott et al. 1984; Akinci and Akinci 2010). After 7 days of germination, S% was calculated as the ratio between the total number of seedlings survived and total number of seed treated. On 15th day the root and shoot length was measured and their ratio was also calculated. The SVI is a property of the seed, assayed by multiplying germination (%) and seedling length. It was calculated on 15th day as described by Abdul Baki and Anderson (1973). The percentage of phytotoxicity and TI was calculated on 15th day according to modified method of Chou et al. (1978) and Turner and Marshal (1972) respectively.
Extraction and assay of antioxidative enzymes
Allium seedlings were used on day 15 after sowing to analyze the effects of MZ and CPF treatment on activities of enzymatic antioxidants like catalase (CAT; EC. 1.11.1.6), peroxidase (POD; EC. 1.11.1.7) and superoxide dismutase (SOD; EC. 1.15.1.1).
CAT activity
Fresh leaf material (100 mg) was ground in 4 ml of extraction buffer (pH 7.0), in pre-chilled pestle and mortar under cold condition and was then centrifuged at 12,000 rpm for 15 min. The supernatant was mixed with 0.005 M H2O2 in potassium phosphate buffer (pH 7.0). 2 N H2SO4 was added to stop the reaction after 5 min. The reactants were standardized against 0.1 N KMnO4. CAT activity was determined by the method of Euler and Josephson (1927).
POD activity
Fresh leaf material (50 mg) was ground in 10 ml of extraction buffer in pre-chilled pestle and mortar under cold condition and was then centrifuged at 12,000 rpm for 15 min. 5 ml of 0.1 M phosphate buffer (pH 6.0), 1 ml of 0.01% H2O2 and 1 ml of 0.5% (w/v) p-phenylenediamine was added to 1 ml extract. 5 N H2SO4 was added to stop the reaction. Absorbance was read at 485 nm (Luck 1963).
SOD activity
Fresh leaf material (100 mg) was homogenized in extraction mixture containing phosphate buffer (pH 7.0), EDTA and PVP in pre-chilled mortar and pestle under cold condition and was then centrifuged for 15 min. Enzyme extract was added to the test tubes containing phosphate buffer (pH 7.8), nitro blue tetrazolium (NBT), methionine, riboflavin and EDTA and then samples were kept in sunlight for 15 min to complete the reaction. Absorbance was read at 560 nm, by a modified method of Beauchamp and Fridovich (1971), in which one unit of SOD activity was expressed as the amount of enzyme required to cause 50% inhibition of photochemical reduction of NBT.
Statistical analysis
All the data of the study was analyzed by SPSS version 15 statistical software for windows by applying one way analysis of variance (ANOVA) and Duncan’s multiple range test (DMRT). The correlation between the parameters was assessed by Pearson Correlation Coefficient analysis (Zou et al. 2003).
Results
Morphological parameters of seedlings
The present study reveals that G% and S% of Allium seeds treated with different concentrations of the two pesticides showed a significant dose dependent decrease i.e. with increasing pesticides concentrations there was a decrease in G% and S% (Tables 1 and 2). In case of MZ treated Allium seeds the G% decreased from 100% in control to 91.66% at 10 ppm and further decreased to 41.66% at 150 ppm. Similar results were found for CPF treated seeds, where the G% decreased from 100% in control to 71.66% in 5 ppm and further decreased to 18.33% at 30 ppm. In case of MZ, the LC 50 was recorded at 88 ppm but in CPF, it was at 9 ppm. The S% of treated Allium seedlings was 100% in control for both MZ and CPF. In MZ, it decreased to 86.66% at 10 ppm and further decreased to 23.33% at highest concentration of MZ used. For CPF it was 48.33% at 5 ppm and further decreased to 13.33% at the highest concentration (30 ppm) used.
Table 1.
Effect of mancozeb on the morphological parameters of Allium seedlings
| Mancozeb conc. (ppm) | Germination (%) | Survival (%) | Root length (cm) | Shoot length (cm) | Root shoot length ratio | Phytotoxicity (%) | Tolerance index | Seedling vigor index |
|---|---|---|---|---|---|---|---|---|
| 0 | 100.00 ± 0.00f | 100.00 ± 0.00g | 16.66 ± 0.67g | 18.00 ± 0.29f | 0.93 ± 0.05d | 0.00 ± 0.00a | 0.00 ± 0.00a | 3466.66 ± 44.15g |
| 10 | 91.67 ± 1.67e | 86.67 ± 1.66f | 10.11 ± 0.06f | 12.33 ± 1.20e | 0.84 ± 0.08d | 39.31 ± 0.38b | 0.60 ± 0.03h | 2053.23 ± 80.12f |
| 30 | 88.33 ± 1.66e | 83.33 ± 1.66f | 7.24 ± 0.14e | 12.66 ± 0.88e | 0.58 ± 0.05c | 56.54 ± 0.86c | 0.43 ± 0.08g | 1760.77 ± 99.44e |
| 50 | 78.33 ± 1.66d | 61.67 ± 1.67e | 6.58 ± 0.32d,e | 12.00 ± 0.57e | 0.54 ± 0.02b,c | 60.50 ± 1.92d | 0.39 ± 0.01f | 1455.90 ± 80.95d |
| 70 | 66.67 ± 1.67c | 55.00 ± 2.89d | 6.18 ± 0.09c,d | 11.20 ± 0.38d,e | 0.55 ± 0.02b,c | 62.88 ± 0.59e | 0.37 ± 0.05e | 1157.77 ± 16.87c |
| 90 | 51.67 ± 4.41b | 46.66 ± 1.68c | 6.14 ± 0.07c,d | 9.80 ± 0.26c,d | 0.63 ± 0.02c | 63.14 ± 0.42e | 0.37 ± 0.04e | 821.80 ± 59.42b |
| 110 | 48.33 ± 4.42a,b | 38.33 ± 3.34b | 5.52 ± 0.01c | 8.50 ± 0.40b,c | 0.65 ± 0.03c | 66.88 ± 0.10f | 0.33 ± 0.01d | 678.75 ± 69.61b |
| 130 | 43.33 ± 3.33a | 26.67 ± 1.67a | 3.32 ± 0.03b | 7.83 ± 0.44a,b | 0.43 ± 0.02a,b | 80.05 ± 0.02g | 0.20 ± 0.02c | 484.00 ± 44.60a |
| 150 | 41.66 ± 1.67a | 23.33 ± 3.34a | 2.03 ± 0.03a | 6.13 ± 0.13a | 0.83 ± 0.01d | 87.57 ± 0.21h | 0.12 ± 0.02b | 340.66 ± 18.72a |
Superscripts (a, b, c, d, e, f, g and h) represent groups which are significantly different at P < 0.05, as determined by ANOVA followed by Duncan’s multiple range test amongst the mean ± S.E. of 3 replicates at different treatment concentration when compared to control
Table 2.
Effect of chlorpyrifos on the morphological parameters of Allium seedlings
| Chlorpyrifos conc. (ppm) | Germination (%) | Survival (%) | Root length (cm) | Shoot length (cm) | Root shoot length ratio | Phytotoxicity (%) | Tolerance index | Seedling vigor index |
|---|---|---|---|---|---|---|---|---|
| 0 | 100.00 ± 0.00f | 100.00 ± 0.00f | 12.90 ± 0.38e | 20.67 ± 0.88g | 0.62 ± 0.01c | 0.00 ± 0.00a | 0.00 ± 0.00a | 3357.00 ± 119.35f |
| 5 | 71.66 ± 1.67e | 48.33 ± 4.41e | 4.90 ± 0.26d | 17.33 ± 0.17f | 0.28 ± 0.01b | 62.01 ± 2.05b | 0.37 ± 0.02d | 1593.66 ± 44.38e |
| 10 | 45.00 ± 2.89d | 40.00 ± 5.05d,e | 2.30 ± 0.15c | 14.43 ± 0.23e | 0.15 ± 0.00a | 82.17 ± 1.18c | 0.17 ± 0.01c | 753.33 ± 54.63d |
| 15 | 43.33 ± 3.38c,d | 31.66 ± 3.34c,d | 2.13 ± 0.33b,c | 10.83 ± 0.12d | 0.19 ± 0.02a | 83.46 ± 2.58c | 0.16 ± 0.02c | 564.66 ± 62.83c |
| 20 | 36.67 ± 1.66c | 28.33 ± 3.33b,c | 1.43 ± 0.18a,b | 8.73 ± 0.12c | 0.16 ± 0.01a | 88.88 ± 1.44d | 0.11 ± 0.04b | 373.66 ± 15.46b |
| 25 | 28.33 ± 1.67b | 18.33 ± 3.33a,b | 1.30 ± 0.10a | 6.33 ± 0.03b | 0.20 ± 0.10a | 89.92 ± 0.77d | 0.10 ± 0.07b | 216.00 ± 10.54a,b |
| 30 | 18.33 ± 4.41a | 13.33 ± 3.33a | 0.90 ± 0.05a | 3.40 ± 0.10a | 0.26 ± 0.01b | 93.02 ± 0.44d | 0.06 ± 0.04b | 77.83 ± 17.11a |
Superscripts (a, b, c, d, e and f) represent groups which are significantly different at P < 0.05, as determined by ANOVA followed by Duncan’s multiple range test amongst the mean ± S.E. of 3 replicates at different treatment concentration when compared to control
The effect of different concentrations of MZ and CPF on shoot and root length was found to be statistically significant and there was dose depended decrease with treatment at various concentrations of pesticides as shown in Tables 1 and 2. For both the pesticide, maximum SL and RL were found in control. In MZ there was a slight decrease in SL and RL compared to control at 10 ppm, but in CPF treated seedlings there was a drastic decline in the shoot and root length in 5 ppm. The RSL ratio, being important for functional balance between photosynthesis and water absorption by the roots showed a significant dose dependent decrease for pesticide treatment. The RSL ratio was highest in control and at 10 ppm gradually decreased as the concentration of MZ was increased. The RSL ratio for CPF was again highest for control and was reduced with increase in concentration of CPF.
The P% for both pesticides was 0% for control, but there was a gradual and statistically significant increase at higher concentrations. In MZ at 10 ppm, P% was 39.31% and increased to 87.5% at 150 ppm. For CPF treated Allium seeds it increased from 62.01% at 5 ppm to 93.02% at 30 ppm. The increased pesticide level adversely influenced the SVI of Allium. SVI gradually decreased with increasing concentration of pesticides i.e. a significant dose dependent decrease was observed in both (MZ and CPF) treatments. Similar trend was found in TI of treated Allium seedlings for MZ and CPF treatments.
Antioxidative enzymes
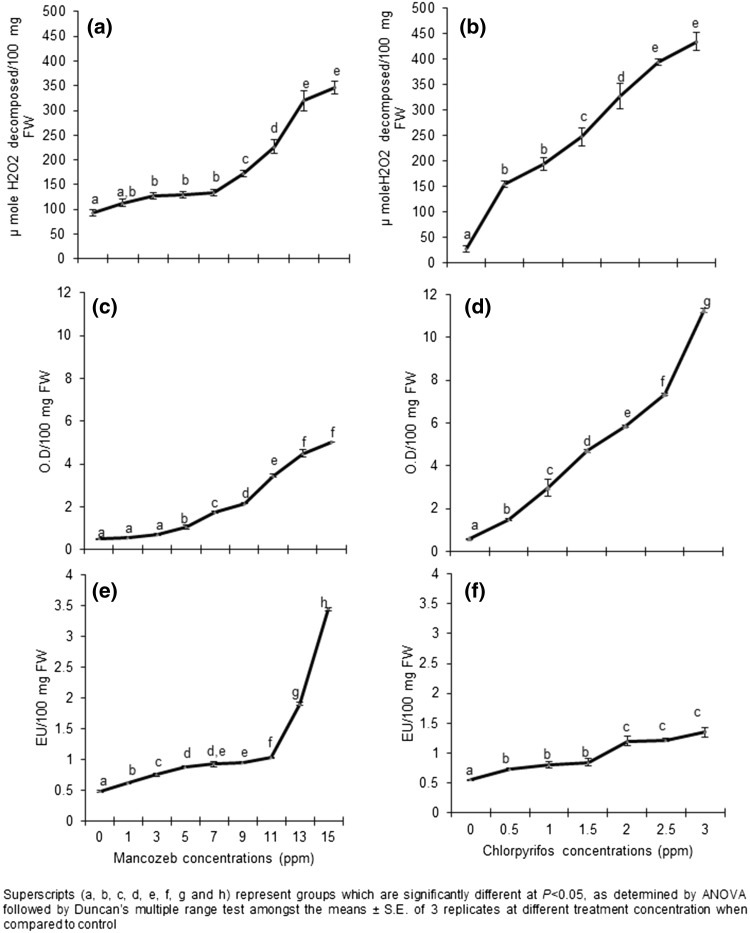
In the present study both, control Allium seedlings and those exposed to pesticides showed statistically significant increase in values for activity of all analyzed enzymes such as CAT, POD and SOD i.e. enzyme activity increased as pesticide concentration was increased (Fig. 1). The activity of CAT in treated Allium seedlings exhibited a significant dose dependent increase. In MZ treatment, CAT activity was minimum at control which then increased slightly at 10 ppm, and then became maximum at 150 ppm (Fig. 1a). In CPF treated seedlings, minimum CAT activity was observed in control which was gradually increased from 5 ppm of CPF to 30 ppm (Fig. 1b).
Fig. 1.
Effect of mancozeb and chlorpyrifos on the activity of antioxidative enzymes of Allium: a, b Catalase (CAT); c, d Peroxidase (POD); e, f Superoxide dismutase (SOD). Data represent mean ± S.E. of 3 replicates and data with different superscripts (a, b, c, d, e, f, g and h) are significantly different at P < 0.05, as determined by ANOVA followed by Duncan’s multiple range test
POD activity of Allium showed a gradual significant increase from control to treated ones. The POD activity recorded for control of MZ and CPF was 0.49 ± 0.04 and 0.58 ± 0.02 ΔOD/100 mg FW which gradually increased in 10 ppm and was maximum for 150 ppm concentration of MZ (Fig. 1c). In CPF treated Allium the POD activity significantly increased from 5–30 ppm as shown in Fig. 1d.
The pesticide treated Allium seedlings showed a significant enhancement in SOD activity as the concentration of the pesticide was increased. The minimum SOD activity was observed in control. For lower MZ concentration i.e. 10 ppm minimum SOD activity was recorded which increased for the higher concentration of MZ (150 ppm) as shown in Fig. 1e. Among CPF treated Allium, SOD activity was minimum in 5 ppm which increased to maximum for the higher concentration of CPF i.e. 30 ppm (Fig. 1f).
Significant positive correlation was observed between various morphological parameters, negative correlation was found between different morphological parameters and activity of antioxidative enzymes (CAT, POD, and SOD) while significant positive correlation in between activity of antioxidative enzymes was observed at P < 0.05, 0.01 and 0.001 as shown in Tables 3 and 4.
Table 3.
Correlation between different parameters of Allium treated with mancozeb
| Parameters | Germination (%) | Survival (%) | Root length | Shoot length | Root shoot length ratio | Catalase | Peroxidase | Superoxide dismutase |
|---|---|---|---|---|---|---|---|---|
| Germination (%) | 1 | |||||||
| Survival (%) | 0.98*** | 1 | ||||||
| Root length | 0.85** | 0.89*** | 1 | |||||
| Shoot length | 0.92*** | 0.93*** | 0.95*** | 1 | ||||
| Root shoot length ratio | 0.40 | 0.45 | 0.59 | 0.39 | 1 | |||
| Catalase | − 0.87** | − 0.89** | − 0.76* | − 0.86** | − 0.19 | 1 | ||
| Peroxidase | − 0.93*** | − 0.93** | − 0.76* | − 0.87** | − 0.22 | 0.98*** | 1 | |
| Superoxide dismutase | − 0.71 | − 0.75 | − 0.69* | − 0.75* | 0.03 | 0.90*** | 0.86** | 1 |
Significant correlation between the parameters indicated by * P < 0.05, ** P < 0.01, *** P < 0.001
Table 4.
Correlation between different parameters of Allium treated with chlorpyrifos
| Parameters | Germination (%) | Survival (%) | Root length | Shoot length | Root shoot length ratio | Catalase | Peroxidase | Superoxide dismutase |
|---|---|---|---|---|---|---|---|---|
| Germination (%) | 1 | |||||||
| Survival (%) | 0.97*** | 1 | ||||||
| Root length | 0.94*** | 0.98*** | 1 | |||||
| Shoot length | 0.98*** | 0.97*** | 0.92** | 1 | ||||
| Root shoot length ratio | 0.78* | 0.82* | 0.92** | 0.72 | 1 | |||
| Catalase | − 0.96*** | − 0.92** | − 0.85* | − 0.98** | − 0.62 | 1 | ||
| Peroxidase | − 0.88** | − 0.81* | − 0.71* | − 0.91** | − 0.42 | 0.94** | 1 | |
| Superoxide dismutase | − 0.90** | − 0.85* | − 0.78* | − 0.93** | − 0.54 | 0.97*** | 0.94** | 1 |
Significant correlation between the parameters was indicated by * P < 0.05, ** P < 0.01, *** P < 0.001
An interesting observation is that, for the two pesticides at their respective LC 50, the activity of the three enzymes evaluated for their activity were almost similar, indicating that a optimum level of the enzyme was required by the non-target plants to show tolerance. CAT activity was approximately 165 and 160 µmole H2O2 decomposed/100 mg FW for MZ and CPF at their respective LC 50. Similarly SOD activity was 0.9 EU/100 mg FW at LC 50 for MZ and 0.85 EU/100 mg FW at LC 50 for CPF. POD activity for MZ and CPF was 3.5 and 3.2 O.D/100 mg FW respectively at their LC 50.
Discussion
Extensive use of pesticides nowadays for agriculture and domestic purpose is reported to pose a potential unwanted threat to environment and other life forms existing in the ecosystem (Mrema et al. 2013; Tsaboula et al. 2016). The treatment of seeds with pesticides, helps in protecting them and the seedlings from pests and diseases but their rate of germination and growth is affected (Dhanamanjuri et al. 2013). Chlorpyrifos is a frequently used organophosphate insecticide in world for controlling insects in agricultural and non agricultural fields. According to Parween et al. (2011) exposure of Vigna radiata L. to chlorpyrifos resulted in depressed plant growth and nitrogen metabolism.
Our results indicate a significant lowering effect of pesticides on seed germination, and the effects were enhanced with dosage elevation. Thus germination of seed, a primary physiological process of plant growth is greatly influenced by environmental stress (Koornneef et al. 2002), and is very sensitive, to contaminants like pesticides (Goswami et al. 2013). Similar findings were reported by Calvelo et al. (2010) during their investigation of phytotoxic effect of hexachlorocyclohexane on germination and seedling development of Hordeum vulgare L., Brassica sp., and Phaseolus vulgaris L. In our investigation, the S%, RL, SL and RSL ratio of treated Allium seeds with different concentrations of pesticides were lower than control showing a significant dose dependent reduction. This indicated a significant phytotoxic nature of the pesticides used in the current study. Growth parameters such as RL, SL and RSL ratio can be used to estimate the toxicity of pollutants such as pesticides (Nielson and Rank 1994; Amin 2002). Growth retardation of plants at higher concentration of applied pesticides indicates reduction in cell division, cell elongation and conversion of indole-3 acetic acid (IAA) into various photo-oxidative products as these compounds function as strong auxin antagonists (Tevini and Teramura 1989). The difference in SVI was also found to be significant between treatments for both pesticides. Similar result was reported in the experiment on seed germination and seedling growth of haricot bean cultivars which showed significant dose dependent decrease of SL and RL at higher concentration of copper sulphate (Habtamu et al. 2013). The phytotoxicity significantly increased and is indicative of the toxic nature of the pesticide used in experiment.
The use of pesticides indiscriminately for better yield of crops has led to an increase in the level of pollution. Consequently the plants undergo molecular damage (Abdollahi et al. 2004) whereby reactive oxygen species are released which may even affect the genomic DNA (Moller et al. 2014). They defend themselves from the oxidative stress using their antioxidative response system (Dey and De 2012). The pesticides under investigation seem to have imposed oxidative stress on treated Allium seedlings, which is a rapid and sensitive response of plants to environmental stress (Jiang and Yang 2009; Jan et al. 2012) as revealed by the activity of the antioxidative enzymes. Plants have a complex antioxidant system comprising of enzymes such as CAT, POD, and SOD (Pandey and Rizvi 2010) to mitigate and repair the damage caused by ROS. There are various evidence of pesticide degradation by elevated activity of oxidoreductase enzymes which reflects the level of toxicity and also the ability to combat the stress (Wu and Von Tiedemann 2002; Peixoto et al. 2006; Song et al. 2007; Yildiztekin et al. 2015; Singh and Kaur 2016).
In our results, the CAT activity of treated Allium seedlings showed significant dose dependent enhancement. CAT is an oxidoreductase enzyme involved in the removal of toxic H2O2 by converting H2O2 to H2O and O2. Similar result was also observed by Zhang et al. (2009) in wheat and rice plants by application of 1,2,4-trichlorobenzene and in bitter gourd by application of dimethoate (Mishra et al. 2009). However, the activity of CAT in fresh tea plants was slightly increased by phenanthrene stress (Mei et al. 2009).
Another indicator of oxidative damage in plants is POD, an antioxidative enzymes involved in the elimination of ROS. The breakdown of H2O2 and lignin biosynthesis in the presence of H2O2 is done by POD (Bowler et al. 1992). Compared to the control, a dose dependent increase was observed in POD activity. According to Morimura et al. (1996) the role of POD in the detoxification of H2O2 under insecticide induced oxidative stress, is suggested by its marked increase in the activity. Similar results were also observed under the treatments of fungicide and herbicides (Gopi et al. 2007; Jianga et al. 2010).
Mancozeb and chlorpyrifos treated Allium seedlings in this study have shown significant enhancement in SOD activity, an essential component of a plants antioxidative defense system. SOD enzyme has a potential to convert free radicals to H2O2 by dismutation. A dose dependent increase in the level of SOD was observed. Similar augmentation in SOD activity was reported in Glycine max L. under stress generated by insecticide (Bashir et al. 2007), herbicide (Jianga et al. 2010; Wu et al. 2010) in wheat and rice, respectively, thus indicating that elevated SOD enzymatic activity was stimulated by scavenging of O−,2 therefore protected Allium seedling from MZ and CPF toxicity.
The result revealed positive correlation between different morphological parameters which showed that they are dependent on each other and the decrease in one morphological parameter appears to be related with the remaining parameters. Significant negative correlation between antioxidative enzymes and morphological parameters indicated that the morphological toxicity caused by pesticides, could be correlated to the increase into overcome the stress generated by the pesticides.
In conclusion, it can be said that Allium seedlings treated with MZ and CPF, besides showing significant dose dependent inhibitory effect on various growth parameter, also explicated the providence of pesticide metabolizing antioxidative enzyme system. The increase in antioxidative enzyme can circumvent the effect of pesticides upto a certain extent, which can vary in different plant systems and also for different pesticides. Since, the upregulation of the antioxidative enzymes viz. CAT, POD and SOD is not able to reduce the morphotoxicity of the pesticides, the matter is of great concern and needs the focus of scientists worldwide. The growing use of pesticides over the years, their persistence in the environment and the gradual accumulation of their breakdown compounds necessitates the evaluation of extent of phytotoxicity of pesticides individually and the ability of the plant system to circumvent the effect.
Acknowledgements
Authors thankfully acknowledge the University Grant Commission (UGC), India for financial assistance. FF and AK also acknowledge the Communication cell, Integral University for reviewing the manuscript and allotting manuscript communication number (IU/R&D/2017-MCN00050).
Compliance with ethical standards
Conflict of interest
All the authors contributed equally and declared that there is no conflict of interest.
Contributor Information
Firdos Fatma, Email: firdosfatma@live.com.
Sonam Verma, Email: sonamv529@gmail.com.
Aisha Kamal, Email: aisha@iul.ac.in.
Alka Srivastava, Email: alkasrivastava@hotmail.com.
References
- Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10:141–147. [PubMed] [Google Scholar]
- Abdul-Baki AA, Anderson JO. Vigor determination in soyabean application of dairy manure on germination and emergence of some selected crops. Crop Sci. 1973;13:630–633. doi: 10.2135/cropsci1973.0011183X001300060013x. [DOI] [Google Scholar]
- Akinci IE, Akinci S. Effect of chromium toxicity on germination and early seedling growth in melon (Cucumis melo L.) Afr J Biotechnol. 2010;9(29):4589–4594. [Google Scholar]
- Amin AW. Cytotoxicity testing of sewage water treatment using Allium cepa chromosome aberration assay. Pak J Biol Sci. 2002;5:184–188. doi: 10.3923/pjbs.2002.184.188. [DOI] [Google Scholar]
- Anitha SR, Savitha G. Impact of mancozeb stress on seedling growth, seed germination, chlorophyll and phenolic contents of rice cultivars. IJSR. 2013;4(7):292–296. [Google Scholar]
- Bashir F, Siddiqi TO, Mahmooduzzafar Iqbal M. The antioxidative response system in Glycine max (L.) Merr. exposed to deltamethrin, a synthetic pyrethroid insecticide. Environ Pollut. 2007;147:94–100. doi: 10.1016/j.envpol.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: imporved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bolognesi C, Merlo FD. Pesticides: human health effects. In: Nriagu JO, editor. Encyclopedia of environmental health. Burlington, USA: Elsevier; 2011. pp. 438–453. [Google Scholar]
- Botías C, David A, Hill EM, Goulson D. Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci Total Environ. 2016;566–567:269–278. doi: 10.1016/j.scitotenv.2016.05.065. [DOI] [PubMed] [Google Scholar]
- Bowler C, Anmontagu M, Inze D. Superoxide dismutase and stress tolerance: annual review plant physiology. Plant Mol Biol. 1992;43:83–116. [Google Scholar]
- Calvelo PR, Monterroso C, Macias F. Phytotoxicity of hexachlorocyclohexane: effect on germination and early growth of different plant species. Chemosphere. 2010;79:326. doi: 10.1016/j.chemosphere.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang M, Shen S. Effect of salt on malondialdehyde and antioxidant enzymes in seedling roots of Jerusalem artichoke (Helianthus tuberosus L.) Acta Physiol Plant. 2010;33:273–278. doi: 10.1007/s11738-010-0543-5. [DOI] [Google Scholar]
- Chou CH, Chiang YC, Khan CI. Impact of water pollution on crop growth in Taiwan. Bot Bull Acad Sinica. 1978;19:107–124. [Google Scholar]
- Dey A, De JN. Antioxidative potential of bryophytes: stress tolerance and commercial perspectives: a review. Pharmacologia. 2012;3:151–159. doi: 10.5567/pharmacologia.2012.151.159. [DOI] [Google Scholar]
- Dhanamanjuri W, Thoudam R, Dutta BK. Effect of some pesticides (Fungicides) on the germination and growth of seeds/seedlings of some crop plants (i.e. Cicer arietinum and Zea Mays) Middle East J Sci Res. 2013;17(5):627–632. [Google Scholar]
- Euler HV, Josephson K (1927) Uber Katalase. I. Justus Liebigs Annalen Der. Chemie 452:158–181
- Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba- Espin G, Clemente-Moreno MJ, Alcobendas R, Artlip T, Hernandez JA. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62:2599–2613. doi: 10.1093/jxb/erq432. [DOI] [PubMed] [Google Scholar]
- FICCI (2015) Ushering in the 2nd green revolution: role of crop protection chemicals. A report on Indian Agrochemical Industry, p 22. http://ficci.in/spdocument/20662/Agrochemicals-Knowledge-report.pdf
- Finney DJ. Probit analysis. 2. Cambridge: Cambridge University Press; 1952. [Google Scholar]
- Gopi R, Jaleel CA, Sairam R, Lakshmanan GMA, Gomithinayagam M, Pannerselvem R. Differential effects of hexaconazole and paclobutrazol on biomass, electrolyte leakage, lipid peroxidation and antioxidant potential of Daucus carota L. Colloids Surf B Biointerfaces. 2007;60:180–186. doi: 10.1016/j.colsurfb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Goswami MR, Banerjee P, Swarnakar S, Mukhopadhyay A. Carbaryl mediated biochemical alterations in Eggplant (Solanum melongena L.) Int J Res Environ Sci Technol. 2013;3(2):51. [Google Scholar]
- Habtamu A, Shelema M, Kedar R, Ebsa S. Seed germination and seedling growth of Haricot bean (Phaseolus vulgaris) cultivars as influenced by copper sulphate. World Sci Res J. 2013;10:312–317. [Google Scholar]
- Harnpicharnchai K, Chaiear N, Charerntanyarak L. Residues of organophosphate pesticides used in vegetable cultivation in ambient air, surface water and soil in Bueng Niam Subdistrict, Khon Kaen, Thailand. Southeast Asian J Trop Med Public Health. 2013;44(6):1088. [PubMed] [Google Scholar]
- Jafari M, Salehi M, Ahmadi S, Asgari A, Abasnezhad M, Hajigholamali M. The role of oxidative stress in diazinon-induced tissues toxicity in Wistar and Norway rats. Toxicol Mech Methods. 2012;22(8):638. doi: 10.3109/15376516.2012.716090. [DOI] [PubMed] [Google Scholar]
- Jan S, Parween T, Siddiqi TO, Mahmooduzzafar Effect of gamma radiation on morphological, biochemical and physiological aspects of plants and plant products. Environ Rev. 2012;20:7–39. doi: 10.1139/a11-021. [DOI] [Google Scholar]
- JanakiDevi V, Nagarani N, YokeshBabu M, Kumaraguru AK, Ramakritinan CM. A study of proteotoxicity and genotoxicity induced by the pesticide and fungicide on marine invertebrate (Donax faba) Chemosphere. 2013;90(3):1158–1166. doi: 10.1016/j.chemosphere.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Jiang L, Yang H. Prometryne-induced oxidative stress and impact on antioxidant enzymes in wheat. Ecotoxicol Environ Saf. 2009;72:1687–1693. doi: 10.1016/j.ecoenv.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Jianga L, Maa L, Suia Y, Hna SQ, Wua ZY, Fenga YX, Yanga H. Effect of manure compost on the herbicide prometryne bioavailability to wheat plants. J Hazard Mater. 2010;184:337–344. doi: 10.1016/j.jhazmat.2010.08.041. [DOI] [PubMed] [Google Scholar]
- Khan SM, Kour G. Sub acute oral toxicity of chlorpyriphos and protective effect of green tea extract. Pestic Biochem Physiol. 2007;89:118–123. doi: 10.1016/j.pestbp.2007.04.005. [DOI] [Google Scholar]
- Khan H, Zeb A, Ali Z, Shah SM. Impact of five insecticides on chickpea (Cicer arietinum L.) nodulation, yield and nitrogen fixing rhizospheric bacteria. Soil Environ. 2009;28:56–59. [Google Scholar]
- Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Curr Opin Plant Biol. 2002;5(1):33. doi: 10.1016/S1369-5266(01)00219-9. [DOI] [PubMed] [Google Scholar]
- Kumar NG, Nirmala P, Jayappa AH. Effect of various methods of application of insecticides on the incidence of serpentine leaf miner, Liriomyza trifolii (burgess) and other pests in soybean. Karnataka J Agric Sci. 2010;23:130–132. [Google Scholar]
- Luck H. Peroxidase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press Inc; 1963. pp. 895–897. [Google Scholar]
- Mahmood Q, Bilal M, Jan S. Herbicides, pesticides, and plant tolerance: an overview. Emerg Technol Manag Crop Stress Toleran. 2014;17:423–448. doi: 10.1016/B978-0-12-800876-8.00017-5. [DOI] [Google Scholar]
- Mei X, Lin DH, Xu Y, Wu YY, Tu YY. Effects of phenanthrene on chemical composition and enzyme activity in fresh tea leaves. Food Chem. 2009;115:569–573. doi: 10.1016/j.foodchem.2008.12.053. [DOI] [Google Scholar]
- Mishra V, Srivastava G, Prasad SM. Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UVB irradiation. Sci Hortic. 2009;120:373–378. doi: 10.1016/j.scienta.2008.11.024. [DOI] [Google Scholar]
- Moller P, Danielsen PH, Karottki DG, Jantzen K, Roursgaard M, Klingberg H, Jensen DM, Christophersen DV, Hemmingsen JG, Cao Y, Loft S. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat Res, Rev Mutat Res. 2014;762:133–166. doi: 10.1016/j.mrrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Morimura T, Ohya T, Ikawa T. Presence of ascorbate-peroxidizing enzymes in roots of Brassica campestris L. cv Komatsuna. Plant Sci. 1996;117:55–63. doi: 10.1016/0168-9452(96)04413-5. [DOI] [Google Scholar]
- Moustafa GG, Ibrahim ZS, Hashimoto Y, Alkelch AM, Sakamoto KQ, Ishizuka M, Fujita S. Testicular toxicity of profenofos in matured male rats. Arch Toxicol. 2007;81:875–881. doi: 10.1007/s00204-007-0217-2. [DOI] [PubMed] [Google Scholar]
- Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307:74–88. doi: 10.1016/j.tox.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Naksen W, Prapamontol T, Mangklabruks A, Chantara S, Thavornyutikarn P, Robson MG, Ryan PB, Barr DB, Panuwet P. A single method for detecting 11 organophosphate pesticides in human plasma and breastmilk using GC-FPD. J Chromatogr B. 2016;1025:92–104. doi: 10.1016/j.jchromb.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson MH, Rank J. Screening of toxicity and genotoxicity in waste water by the use of Allium test. Hereditas. 1994;121:249–254. doi: 10.1111/j.1601-5223.1994.00249.x. [DOI] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010;3:2–12. doi: 10.4161/oxim.3.1.10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parween T, Jan S, Mahmooduzzafar Fatma T. Alteration in nitrogen metabolism and plant growth during different developmental stages of green gram (Vigna radiata L.) in response to chlorpyrifos. Acta Physiol Plant. 2011;33:2321–2328. doi: 10.1007/s11738-011-0772-2. [DOI] [Google Scholar]
- Peixoto F, Alves-Fernandes D, Santos D, Fontaınhas-Fernandes A. Toxicological effects of oxyfluorfen on oxidative stress enzymes in tilapia Oreochromis niloticus. Pestic Biochem Physiol. 2006;85:91–96. doi: 10.1016/j.pestbp.2005.10.007. [DOI] [Google Scholar]
- Scott SJ, Jones RA, William WA. Review of data analysis methods for seed germination. Crop Sci. 1984;24(6):1192–1199. doi: 10.2135/cropsci1984.0011183X002400060043x. [DOI] [Google Scholar]
- Singh G, Kaur D. Studies on the antioxidative stress responses of fungicides carbendazim and mancozeb in seedlings of brassica (Brassica campestris L.) Int Res J Environ Sci. 2016;5(2):57–62. [Google Scholar]
- Song NH, Yin X, Chen GF, Yang H. Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere. 2007;69:1779–1787. doi: 10.1016/j.chemosphere.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Songa EA, Okonkwo JO. Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: a review. Talanta. 2016;155:289–304. doi: 10.1016/j.talanta.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Tevini M, Teramura AH. UV-B effects on terrestrial plants. Photochem Photobiol. 1989;50:479–487. doi: 10.1111/j.1751-1097.1989.tb05552.x. [DOI] [Google Scholar]
- Tsaboula A, Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Mourkidou EP. Environmental and human risk hierarchy of pesticides: a prioritization method, based on monitoring, hazard assessment and environmental fate. Environ Int. 2016;91:78–93. doi: 10.1016/j.envint.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Turner RG, Marshal C. Accumulation of zinc by subcellular root of Agrostis tannis Sibth. in relation of zinc tolerance. New Phytol. 1972;71:671–676. doi: 10.1111/j.1469-8137.1972.tb01277.x. [DOI] [Google Scholar]
- Venkateswara Rao J, Parvati K, Kavitha P, Jakka NM, Pallela R. Effect of chlorpyrifos and monocrotophos on loco motor behavior and acetyl cholinesterase activity of subterranean termites, Odontotermes obesus. Pest Manag Sci. 2005;61:417–421. doi: 10.1002/ps.986. [DOI] [PubMed] [Google Scholar]
- Wu J, Laird DA. Abiotic transformation of chlorpyrifos oxon in chlorinated water. Environ Toxicol Chem. 2003;22:261–264. doi: 10.1002/etc.5620220204. [DOI] [PubMed] [Google Scholar]
- Wu XY, Von Tiedemann A. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Pollut. 2002;116:37–47. doi: 10.1016/S0269-7491(01)00174-9. [DOI] [PubMed] [Google Scholar]
- Wu GL, Cui EJ, Tao EL, Yang EH. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa) Ecotoxicology. 2010;19:24–132. doi: 10.1007/s10646-009-0402-6. [DOI] [PubMed] [Google Scholar]
- Yildiztekin M, Kaya C, Tuna AL, Ashraf M. Oxidative stress and antioxidative mechanisms in tomato (Solanum lycopersicum L.) plants sprayed with different pesticides. Pak J Bot. 2015;47(2):717–721. [Google Scholar]
- Zhang GL, Chen WJ, Qiu LM, Sun GR, Dai QG, Zhang HC. Physiological response to 124-trichlorobenzene stress of different rice genotypes. Acta Agron Sinica. 2009;35:733–740. doi: 10.3724/SP.J.1006.2009.00733. [DOI] [Google Scholar]
- Zou KH, Tuncali K, Silverman SG. Correlation and simple linear regression. Radiology. 2003;227:617–622. doi: 10.1148/radiol.2273011499. [DOI] [PubMed] [Google Scholar]