Abstract
Background
Right ventricular (RV) dysfunction independently predicts outcomes in human myxomatous mitral valve disease (MMVD). There is limited information regarding RV systolic function in dogs with MMVD.
Hypothesis
Right ventricular systolic function differs among stages of disease, decreasing in decompensated MMVD.
Animals
Thirty‐sixclient‐owned dogs with MMVD not receiving oral cardiovascular medications.
Methods
Prospective clinical study. Dogs were categorized according to disease severity as ACVIM Stage B1, B2, or C. Seven echocardiographic indices of RV systolic function were measured. Groups were compared by 1‐way ANOVA and Tukey's HSD test. Frequencies of cases with cardiac remodeling falling outside previously established reference intervals were compared using Fisher's exact test. Intra‐ and interobserver measurement variability was calculated for each RV function index.
Results
The indices TAPSE (P = 0.029), RV StL (P = 0.012), and RV StRL (P = 0.041) were significantly different between groups. A greater proportion of B2 dogs (7 of 12) had TAPSE values above reference intervals compared with B1 (2 of 12) or C (2 of 12) dogs (P = 0.027). Measurement variability of TAPSE, RV S', and RV StG was clinically acceptable.
Conclusions and Clinical Importance
Right ventricular systolic function differs between stages of MMVD, increasing in stage B2, and declining in stage C. The prognostic importance of RV function indices, particularly TAPSE, might be worth evaluating in dogs with MMVD.
Keywords: Canine, Congestive heart failure, Right ventricle, Strain, Strain rate, TAPSE
Abbreviations
- Ao
aorta
- CHF
congestive heart failure
- CV
coefficient of variation
- HR
heart rate
- IVS
interventricular septum
- LA
left atrium
- LVIDDN
left ventricular end‐diastolic internal dimension normalized to body weight
- LV
left ventricle
- MMVD
myxomatous mitral valve disease
- MR
mitral regurgitation
- RV FAC
fractional area change in the right ventricular inlet
- RV S'
systolic myocardial velocity of the lateral tricuspid annulus
- RV StG
global longitudinal strain of the right ventricle
- RV StL
longitudinal strain of the right ventricular free wall
- RV StRG
global longitudinal strain rate of the right ventricle
- RV StRL
longitudinal strain rate of the right ventricular free wall
- RVAd
right ventricular area in diastole
- RVAs
right ventricular area in systole
- RV
right ventricle
- STE
speckle‐tracking echocardiography
- TAPSE
tricuspid annular plane systolic excursion
- TDI
tissue Doppler imaging
- TR
tricuspid regurgitation
Myxomatous mitral valve disease (MMVD) is the most common cause of heart disease and mitral regurgitation (MR) in dogs and represents the leading cause of congestive heart failure (CHF) in this species.1, 2 The progression from subclinical disease to CHF is variable and difficult to predict.3 Routine analysis of left heart size and function is typically emphasized as these chambers are directly affected by MR. Previous studies evaluating echocardiography in MMVD have focused on the left atrium (LA), left ventricle (LV), and transmitral inflow patterns to stage disease severity and gain prognostic information.4, 5, 6, 7, 8, 9 While gross measures of left heart size seemingly offer prognostic value in MMVD,5, 6, 7 the prognostic value of routine echocardiographic measures of LV systolic function has not been completely demonstrated.8, 9 Most studies have reported that LV ejection fraction, fractional shortening, and global deformation (strain) increase with advanced stages of MR in smaller breeds of dogs. This finding is attributed to the influence of enhanced ventricular preload and reduced ventricular afterload on echocardiographic measures of LV systolic function.10 While controversial, the assessment of LV systolic function is considered unreliable for estimating contractile function or prognosis in the setting of severe mitral regurgitation.10, 11
The LV and RV exhibit interdependence and interaction related to a shared ventricular septum and the influence of LA pressure on RV afterload.12 Both ventricles are also subject to neurohormonal influences activated in advanced disease.13 In people with left heart failure from primary MR, RV systolic dysfunction is present in approximately 30% of patients and represents a prognostic criterion used to stratify patient risk.14, 15, 16 Echocardiographic measures and derived variables of RV systolic function in people offer prognostic information in the setting of MR. Useful functional variables include tricuspid annular plane systolic excursion (TAPSE),14, 15 RV ejection fraction,16 and tissue Doppler imaging (TDI)‐derived systolic myocardial velocity of the lateral tricuspid annulus (RV S').15 Additionally, RV longitudinal strain (RV StL) and strain rate (RV StRL) have predicted mortality in human patients with left heart failure.17
There are limited studies investigating RV function and structural changes in dogs with MMVD. Radionucleotide studies in dogs have shown that the RV enlarges, the interventricular septum (IVS) flattens or shifts to the right, and RV systolic function decreases in CHF.18 In one canine study, RV Tei index, an indicator of global systolic and diastolic function, was higher in dogs with advanced MMVD (indicating impaired RV performance); this index independently predicted cardiac‐related death within 1 year.19 In contrast, RV S' is reportedly not different between healthy dogs and dogs with various stages of MMVD20 and TASPE has not been shown to differ between dogs with various stages of MMVD.21 Whether RV systolic function deteriorates in advanced MMVD or RV systolic function differs in dogs over progressive stages of this disease is unresolved.
The aim of this study was to analyze several echocardiographic indices of RV systolic function in dogs with MR caused by MMVD not yet receiving medical therapy. We hypothesized that RV systolic function differs across stages of MMVD and that dogs in CHF exhibit lethargic RV systolic function.
Materials and Methods
Animals
All procedures in this study were approved by the Veterinary Medical Center Clinical Research and Teaching Advisory Committee at The Ohio State University. Written consent authorizing participation of dogs in the study was obtained from all dog owners.
Dogs presenting to The Ohio State University Veterinary Medical Center for evaluation of a murmur or clinical signs of left‐sided CHF were eligible for enrollment. Dogs were further considered if they fulfilled each of the following clinical and echocardiographic criteria: (1) body weight (BW) ≤15 kg, (2) age ≥5 years, (3) systolic heart murmur with a point of maximum intensity over the left cardiac apex, (4) systolic arterial blood pressure measured by the Doppler method between 100 and 160 mmHg, (5) echocardiographic appearance of mitral valve thickening or prolapse, and (6) color and spectral Doppler evidence of MR. Body weight has a significant effect on RV function indices,22 therefore to avoid large variability between groups, a BW cut‐off ≤15 kg was chosen. Exclusion criteria included as follows: (1) prior or concurrent oral administration of cardiac medications, (2) a nonsinus heart rhythm, and (3) cardiac disease other than MMVD. Concurrent tricuspid regurgitation (TR) was neither a specific entry nor exclusion criterion. Dogs with radiographically‐confirmed CHF could receive a single parenteral dose of furosemide (2 mg/kg, IM or IV) and supplemental oxygen therapy prior to echocardiography. Dogs with persistent signs of respiratory distress after sedation and furosemide were excluded from the study, at which point any treatment deemed necessary by the attending clinician was administered.
Clinical and Radiographic Examinations
All dogs underwent complete physical examination and Doppler arterial blood pressure measurement by the same investigator (EHC). Three‐view thoracic radiographs were obtained within 3 hours of echocardiographic evaluation. Vertebral heart size was measured as previously described.23 Left‐sided CHF was defined as the presence of clinical signs of tachypnea or respiratory distress in addition to radiographic evidence of pulmonary venous congestion and infiltrates compatible with cardiogenic pulmonary edema. The presence of CHF was confirmed by at least 2 investigators.
Conventional Echocardiography and Doppler Examination
All dogs received 0.25 mg/kg butorphanol by intramuscular (IM) injection 10–15 minutes before echocardiography that was performed by the same sonographer (EHC) with an ultrasound unit1 equipped with 5‐, 6‐, and 12‐MHz phased‐array transducers. Echocardiographic recordings were captured with a simultaneous electrocardiogram (ECG) in proprietary, raw DICOM format for off‐line analysis at a digital workstation.2 This storage format offers off‐line adjustments of image gain, compression, reject, and grayscale processing.
Complete transthoracic echocardiography was performed according to standard techniques24 in dogs manually restrained in right and left lateral recumbency.
Specific measurements of interest included left ventricular end‐diastolic internal dimension, maximal end‐systolic left atrial dimension (from right parasternal long‐axis 4 chamber view), aortic dimension (right parasternal long‐axis outflow tract view, measured between maximally opened aortic leaflets), and the peak TR velocity when present. Calculated variables included left ventricular end‐diastolic internal dimension normalized to body weight (LVIDDN)25 and the ratio of LA dimension to aortic dimension (LA:Ao).3
Echocardiographic Indices of Right Ventricular Systolic Function
All indices of RV systolic function were acquired from the left apical 4‐chamber view optimized for the right heart as previously described (Fig 1).22 TAPSE was generated from M‐mode recordings with the cursor as parallel as possible to the majority of the RV free wall. Measurements needed for calculation of RV fractional area change (RV FAC) were obtained by tracing the endocardial border of the RV inflow region in end‐diastole and end‐systole to measure RV end‐diastolic area (RVAd) and RV end‐systolic area (RVAs), respectively. When visualized, papillary muscles were excluded from the tracing. The RV FAC was calculated using the formula: FAC = ([RVAd–RVAs]/RVAd) × 100. The index TDI‐derived RV S' was recorded with a 3‐mm pulse wave sample volume positioned at the level of the lateral tricuspid annulus and the cursor parallel to the longitudinal motion of the RV free wall. Peak systolic modal velocity in cm/s was measured using digital calipers. The lateral tricuspid annulus was chosen as this variable has been evaluated by the authors' laboratory and reference intervals were created in dogs.22 Strain and strain rate measurements were obtained from proprietary 2D speckle‐tracking echocardiography (STE) software2 using the LV 2‐ and 4‐chamber algorithms,22 as no defined RV STE algorithm was available at the time of this study. The region of interest for STE was defined by manually tracing the RV endocardial border from the level of the lateral tricuspid valve annulus to the RV apex for 3‐segment longitudinal strain (RV StL) and strain rate (RV StRL) and continuing to the septal tricuspid annulus (including IVS) for 6‐segment global strain (RV StG) and strain rate (RV StRG). Manual adjustments using the available software tools were made to incorporate the entire myocardial thickness and ensure adequate myocardial tracking over the cardiac cycle. When automated software approval of tracking was not obtained, the regions of interest were retraced and recalculated. Occasionally, it was necessary to manually approve the automated tracking so long as visual inspection of myocardial tracking was considered appropriate; this was required in less than 10% of the studies.
Figure 1.
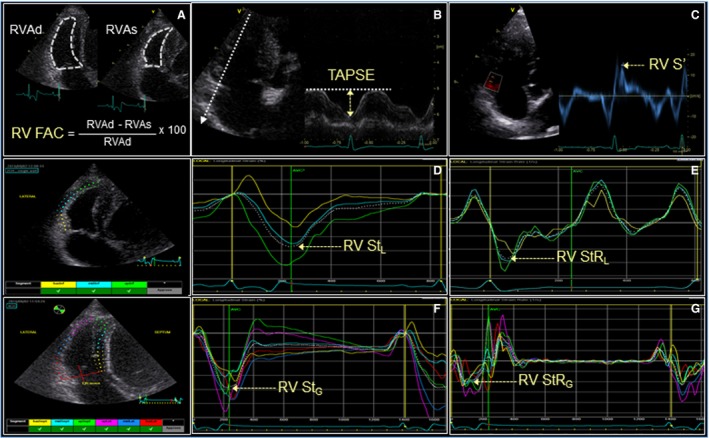
Echocardiographic right ventricular systolic function indices measured in dogs with MMVD: fractional area change (A), tricuspid annular plane systolic excursion (B), pulsed‐wave tissue Doppler imaging derived peak systolic longitudinal myocardial motion velocity at the lateral tricuspid annulus (C), 3‐segment global longitudinal strain (D) and strain rate (E), and 6‐segment global longitudinal strain (F) and strain rate (G).
The value recorded for each RV function index was determined from an average of 5 representative cardiac cycles. Heart rate (HR) recorded for each patient was calculated as the average of 5 consecutive R‐R intervals measured on the simultaneous ECG during RV imaging.
Classification of Study Groups
Dogs were divided into 3 groups based on the ACVIM classification of valvular heart disease.26 The ACVIM staging does not specify a specific method for determining remodeling, and both radiographic and echocardiographic criteria were used in this study. Specifically, asymptomatic dogs with a VHS ≤ 10.5, LVIDDN ≤ 1.6, and absence of left atrial dilatation (long‐axis LA:Ao ≤ 2.4)3 were classified as stage/group B1 (MR without remodeling); asymptomatic dogs with a VHS > 10.5, LVIDDN > 1.6, and LA:Ao > 2.4 were classified as stage/group B2; and dogs with VHS > 10.5, LVIDDN > 1.6, and LA:Ao > 2.4 and with radiographic and clinical evidence of left‐sided CHF were classified as stage/group C. No dogs in stage D were enrolled in this study.
Intra‐ and Interobserver Measurement Variability
Intra‐observer measurement variability was determined by a single observer (EHC). Using the same echocardiogram, each of the RV variables from 12 randomly selected studies was measured on 2 different days. For interobserver measurement variability, after a period of training on different images, a second observer (JDB) measured each of the RV variables from the same 12 randomly selected studies, using the same cardiac cycles independently from the first observer. The initial set of measurements obtained from observer one was compared to the measurements obtained by observer two. Image quality was subjectively assessed by each observer and recorded as marginal, satisfactory, or good.
Statistical Analysis
All statistical analyses were performed using commercial software.4 , 5
Descriptive statistics were calculated, and the distribution of data for patient characteristics and echocardiographic variables were assessed for normality using the D'Agostino–Pearson test and visual inspection of dot plots. Variables of RV function were compared across the 3 stages of MMVD by 1‐way ANOVA for normally distributed data and by the Kruskal–Wallis test for non‐normally distributed data (that could not be transformed to normality). Tukey's honest significant difference test or Bonferroni corrected Mann–Whitney–Wilcoxon tests were performed for post hoc multiple comparisons when significant differences were identified by ANOVA. Additionally, the number of dogs with values outside of a published weight‐based reference interval25 were tabulated for each variable and for each study group. Fischer's exact test was used to compare proportions between study groups. Results for normally distributed data are presented as mean ± standard deviation (SD) or as median and interquartile range (IQR) for non‐normal data. Linear regression analysis was used to evaluate the relationship between HR and each RV function index. Statistical significance for null hypothesis significant tests was set at P < 0.05.
Intra‐ and interobserver measurement variability was quantified as the coefficient of variation (CV) for 2 replicates.27 The CV was expressed as a percent value, calculated as CV = SD/Mean × 100, where SD was the square root of the variance of 12 replicated measurements and mean was the average value of the 24 measurements obtained for each variable.27 Repeatability was also evaluated using Bland–Altman difference plots with calculation of the bias between observers and their 95% limits of agreement.
Results
Thirty‐six client‐owned dogs with MMVD were prospectively enrolled in the study (Table 1). Age, BW, and sex were not significantly different between groups. The LVIDDN, VHS, and LA:Ao were significantly different between groups (Table 1). The VHS increased with stage of disease (P = 0.002) while LA:Ao and LVIDDN were significantly higher in groups B2 and C compared with B1 (P = 0.006 and P = 0.007, respectively). Tricuspid regurgitation was present in 27 of 36 (75%) of the dogs, and the proportion of dogs with TR was not different between groups (P = 0.37). The dogs in CHF (group C) had significantly higher HR (P < 0.001) and TR velocities (P = 0.013) than dogs with compensated disease (groups B1 and B2).
Table 1.
Clinical and select echocardiographic characteristics in 36 dogs with MMVD
| Variable | Group | P † | ||
|---|---|---|---|---|
| B1 (n = 12) | B2 (n = 12) | C (n = 12) | ||
| Age (mo.) | 108 ± 32 | 111 ± 29 | 109 ± 53 | 0.64 |
| Sex (M/F) | 8/4 | 7/5 | 6/6 | 0.71 |
| Body weight (kg) | 8.5 ± 3.9 | 7.5 ± 3.3 | 7.1 ± 3.3 | 0.61 |
| Heart rate (bpm) | 92 ± 20a | 93 ± 16a | 127 ± 21b | <0.001 |
| TR (present/absent) | 7/5 | 10/2 | 10/2 | 0.37 |
| TR velocity (m/s)* | 2.4 ± 0.3a | 2.6 ± 0.4a | 3.3 ± 0.7b | 0.013 |
| VHS (v) | 10.2 ± 0.2a | 11.5 ± 0.9b | 12.5 ± 1.0c | 0.002 |
| LA:Ao* | 2.3 ± 0.1a | 3.1 ± 0.2b | 4.5 ± 1.7b | 0.006 |
| LVIDDN | 1.5 ± 0.1a | 1.9 ± 0.2b | 2.1 ± 0.3b | 0.007 |
TR, tricuspid regurgitation; VHS, vertebral heart score; LA:Ao, long‐axis left atrium to aortic annulus ratio; LVIDDN, left ventricular end‐diastolic internal dimension normalized to body weight.
Data are expressed as mean ± standard deviation.
Significant group differences are italicized.
*Data not normally distributed. Median TR velocity (IQR): C = 3.3 (2.8–4.1), B2 = 2.6 (2.4–2.8), B1 = 2.4 (2.2–2.6); Median LA:Ao (IQR): C = 3.9 (3.5–4.9), B2 = 2.9 (3.1–2.8), B1 = 2.4 (2.3–2.4).
Within each row, superscripts with different letters represent a statistical difference in post hoc tests (P < 0.05) between groups.
†ANOVA or Kruskal–Wallis derived P‐value.
The indices TAPSE, RV StL, and RV StRL were significantly different between groups (Table 2, Fig 2). The index TAPSE was significantly greater in group B2 than group B1 (P = 0.029). The indices RV S', RV FAC, RV StG, and RV StRG were not different between groups. A greater proportion of B2 dogs had measures of TAPSE above published reference intervals (P = 0.027). More negative values of RV StL, reflecting increased strain, were identified in group B2 than in group B1 (P = 0.012). Measured RV StRL was significantly more negative in group C than in B1 (P = 0.041). The indices RV StL and RV StRL were unable to be measured in 3 of 36 dogs (B2 n = 1, C n = 2) because of inadequate automated myocardial tracking.
Table 2.
Echocardiographic measures of right ventricular systolic function in 36 dogs with MMVD
| RV function index | Group | P * | ||
|---|---|---|---|---|
| B1 (n = 12) | B2 (n = 12) | C (n = 12) | ||
| TAPSE (mm) | 12.0 ± 3.3a | 14.8 ± 2.7b | 12.2 ± 2.2a | 0.029 |
| RV S' (cm/s) | 12.6 ± 3.8 | 16.1 ± 4.8 | 12.4 ± 3.3 | 0.054 |
| RV FAC (%) | 53 ± 10 | 53 ± 10 | 48 ± 12 | 0.49 |
| RV StL (%) | −25.0 ± 4.8a | −32.3 ± 5.8b | −30.2 ± 6.5a | 0.012 |
| RV StRL (s−1) | −3.0 ± 0.9a | −3.6 ± 0.8a | −3.9 ± 0.9b | 0.041 |
| RV StG (%) | −24.6 ± 5.5 | −24.7 ± 4.7 | −24.8 ± 4.2 | 0.99 |
| RV StRG (s−1)* | −2.6 ± 0.9 | −2.4 ± 0.5 | −3.0 ± 0.9 | 0.050 |
| RVAd (cm2) | 3.5 ± 1.3 | 4.0 ± 1.1 | 2.9 ± 0.9 | 0.059 |
| RVAs (cm2) | 1.7 ± 0.8 | 1.9 ± 0.7 | 1.5 ± 0.7 | 0.41 |
TAPSE, tricuspid annular plane systolic excursion; RV S', systolic myocardial velocity of the lateral tricuspid annulus; RV FAC, fractional area change in the right ventricular inlet; RV StL, longitudinal strain of the right ventricular free wall; RV StRL, longitudinal strain rate of the right ventricular free wall; RV StG, global longitudinal strain of the right ventricle; RV StRG, global longitudinal strain rate of the right ventricle; RVAd, right ventricular area in diastole; RVAs, right ventricular area in systole.
Significant group differences are italicized.
Data are expressed as mean ± standard deviation.
*Data not normally distributed. Median RV StRG B1 (IQR) = −2.4 (−2.0 to −2.9), B2 = −2.5 (−2.7 to −2.1), C = −2.8 (−2.3 to −3.6).
Within each row, superscripts with different letters represent a statistical difference in post hoc tests (P < 0.05) between groups.
†ANOVA or Kruskal–Wallis derived P‐value.
Figure 2.
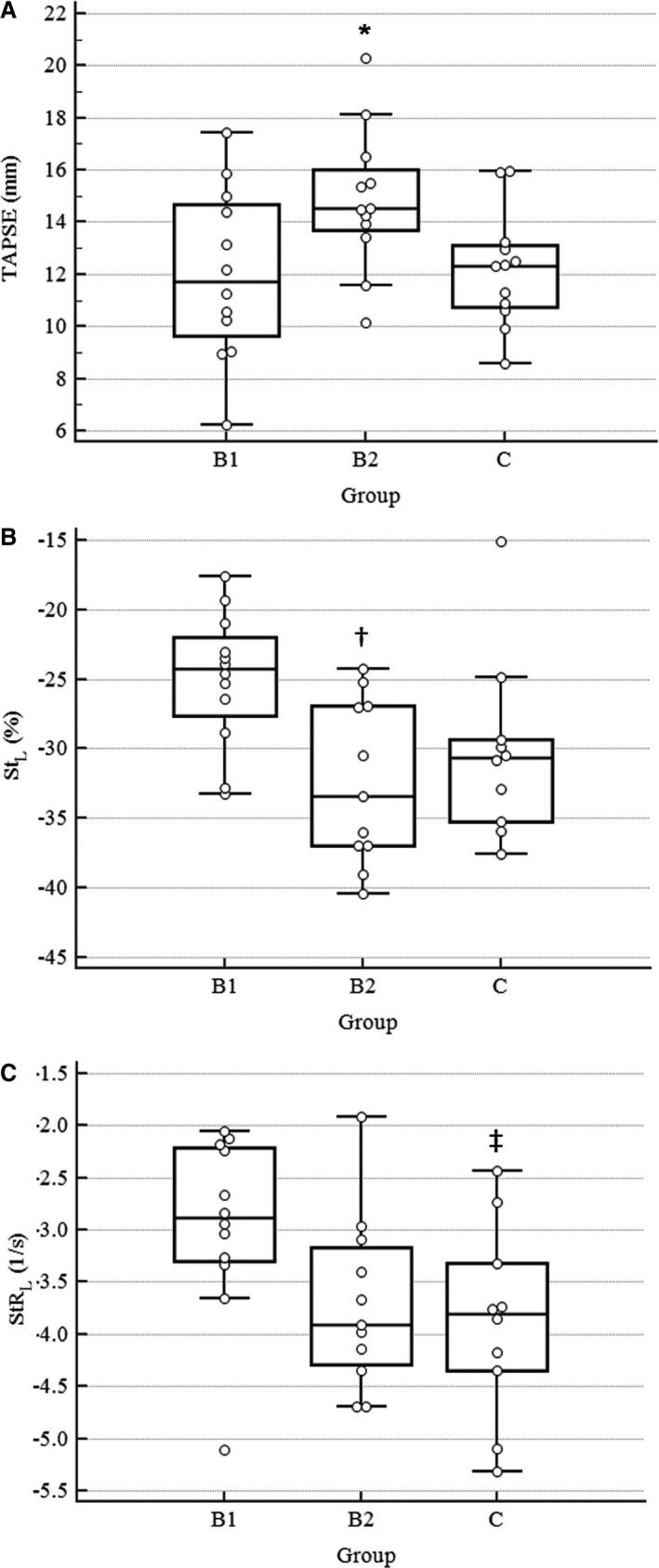
Dot plots for each of the right ventricular function indices with statistically significant differences between groups of dogs with MMVD. Individual data points are shown. Solid bars represent group means and 95% CI for the mean. *, TAPSE significantly higher in group B2 dogs compared with B1, P = 0.029; †, StL significantly increased in group B2 dogs compared with B1, P = 0.012; ‡StRL significantly increased in group C dogs compared with B1, P = 0.041.
Regression analysis demonstrated a significant, weak, positive correlation between HR and RV StRG (R 2 = 0.155, P = 0.018). No other indices were significantly correlated with HR.
Repeatability
Intra‐observer and interobserver measurement variabilities are summarized in Table 3. The coefficient of variation for intra‐observer repeatability ranged from 0.5 to 17.9% for the 7 RV function indices. Interobserver variability was greater for the 7 function indices with CV ranging from 3.6 to 20.1%. The lowest degrees of variability were recorded for linear measurements of longitudinal RV wall excursion (TAPSE) and peak systolic velocity (RV S'). In contrast, repeatability of area measurements (RV FAC) was relatively poor between observers. It was noted that 42% of all images were graded as marginal in quality for measurement. High variability was also observed with the algorithm‐generated measures of strain and strain rate. Of the STE variables, only RV StG showed clinically acceptable reproducibility.
Table 3.
Intra‐ and interobserver repeatability for RV function indices in 12 dogs with MMVD
| Intra‐observer repeatability | Bland–Altman | CV (%) | Interobserver repeatability | Bland–Altman | CV (%) | ||
|---|---|---|---|---|---|---|---|
| Bias | LOA* | Bias | LOA* | ||||
| TAPSE (mm) | 0.0 | −0.2, 0.2 | 0.5 | TAPSE (mm) | −0.3 | −1.5, 1.0 | 3.6 |
| RV S' (cm/s) | −0.2 | −1.4, 0.9 | 3.6 | RV S' (cm/s) | 0.4 | −1.7, 2.5 | 6.4 |
| RV FAC (%) | 3.0 | −9.0, 15.0 | 8.4 | RV FAC (%) | 4.7 | −23.5, 32.9 | 17.3 |
| RV StL (%) | 0.6 | −8.2, 9.3 | 11.4 | RV StL (%) | 10.1 | −30.0, 50.2 | 12.3 |
| RV StRL (s−1) | 0.0 | −1.5, 1.6 | 17.9 | RV StRL (s−1) | 3.8 | −2.9, 10.4 | 20.1 |
| RV StG (%) | 0.0 | −4.5, 4.6 | 6.5 | RV StG (%) | −0.2 | −6.0, 5.7 | 8.4 |
| RV StRG (s−1) | 0.1 | −0.6, 0.8 | 9.1 | RV StRG (s−1) | 0.5 | −1.0, 2.0 | 16.9 |
TAPSE, tricuspid annular plane systolic excursion; RV S', systolic myocardial velocity of the lateral tricuspid annulus; RV FAC, fractional area change in the right ventricular inlet; RV StL, longitudinal strain of the right ventricular free wall; RV StRL, longitudinal strain rate of the right ventricular free wall; RV StG, global longitudinal strain of the right ventricle; RV StRG, global longitudinal strain rate of the right ventricle.
*95% limits of agreement, CV, coefficient of variation.
Discussion
The principle finding of this study was that echocardiographic estimates of RV systolic function differ between ACVIM stages of dogs with MMVD. The indices TAPSE, RV S', and RV StL demonstrated a graphical “U‐shaped” pattern of hyperdynamic RV systolic function in advanced, compensated MMVD (Stage B2) with normalization in acute CHF (Stage C). Of the RV systolic function indices evaluated, TAPSE proved to be a significant and clinically useful marker of RV systolic function associated with low measurement variability in dogs with MMVD.
The results of this study suggest that RV systolic function mirrors that of the LV in dogs with MMVD. Because of alterations in LV loading secondary to chronic MR,1, 3, 28, 29 advanced MMVD is associated with hyperdynamic LV systolic function when assessed by conventional echocardiographic measures.10, 11, 30, 31 In the present study, dogs with advanced, compensated MMVD (stage B2) had measures of TAPSE above published reference intervals,25 demonstrating a hyperdynamic state. While the RV is not directly affected by mitral regurgitation, its shared structures with the LV could explain these similarities.
In both naturally occurring MMVD and an experimental model of MR in dogs, the IVS has substantial excursion throughout the cardiac cycle, with displacement into the RV via rightward shifting during diastole and a greater leftward systolic excursion.11, 30, 31 The IVS plays a pivotal role in RV function as evidenced by experimental canine studies demonstrating normal cardiac output and central venous pressure after complete cauterization of the RV free wall,32 complete occlusion of the right coronary artery,33 or when the RV free wall was removed and replaced with a pericardial patch.34 Creation of RV free wall ischemia by right coronary artery occlusion does not depress RV function until pulmonary hypertension is induced or septal ischemia is created.33 While the IVS was not specifically evaluated in this study, the increased RV systolic function in dogs with stage B2 MMVD might be related to increased septal motion known to occur in dogs with advanced MMVD.
An alternative explanation for enhanced RV systolic function in stage B2 MMVD could be enhanced Frank‐Starling forces related to an increased preload from neurohormonal activation and tricuspid regurgitation.13 While the current study did not find significant increases in RVAd or proportion of dogs with TR in stage B2, the marginal near‐field image resolution in some dogs, high inter‐ and intra‐observer variabilities, and inability to image the complex shape of the RV in its entirety using 2‐dimensional echocardiography limited our ability to exclude increased preload as a possible explanation for enhanced RV function in this group.
The tendency for normalization of RV systolic function noted in stage C dogs could relate to differences in RV afterload. Dogs suffering from CHF in the current study, similar to previous reports,3, 20 had higher peak TR velocities, suggesting higher systolic pulmonary arterial pressures. The RV is sensitive to changes in afterload34, 35 that could explain the tendency for lower TAPSE, RV StL, and RV S' in CHF. This is further supported by previous reports in dogs, many with MMVD, where TAPSE and RV S' were reduced in the presence of suspected pulmonary hypertension (peak TR velocity >3 m/s) compared with healthy controls.36, 37 Direct comparison is complicated by the fact that prior studies36, 37 of RV function in dogs with pulmonary hypertension included dogs with precapillary causes of pulmonary hypertension, whereas this study focused on dogs that would be assumed to only have post‐capillary causes of pulmonary hypertension. Different etiologies of pulmonary hypertension might impact the RV disparately.
A recent study demonstrated that IM sedation with butorphanol increases peak TR velocities in dogs with degenerative valve disease.38 All dogs in the current study received the same dose of butorphanol at a similar time before echocardiographic examination of the RV, which should have minimized the effect of sedation on TR velocity between groups. It is possible that butorphanol had an effect on the peak TR velocity values obtained in each dog; however, the average peak TR velocities within the different stages of MMVD in our study are similar to those previously reported.20
A single index of RV systolic function, RV StRL, was greatest in dogs in CHF. Although not repeated in this study, a modest positive correlation between HR and StRL was previously reported.25 The index RV StRL had the highest measurement variability of all the indices, and therefore, it is possible that the data used for statistical analysis of this index was related to random variation. It might also be that the application of an LV algorithm developed for human patients to the RV of a small‐breed dog is simply inappropriate. Alternatively, it is possible that RV StRL is a less load‐dependent marker and a truer reflection of RV systolic function.
The conclusions of this study are different from previous reports, where no difference in RV systolic function was measured between ACVIM stages of MMVD.20, 21 This discrepant result might be because of the administration of load‐altering and positive inotropic agents in some dogs in the previous studies.20, 21 Positive inotropic medications are known to positively influence RV systolic function in healthy dogs39 and would likely have a similar effect in dogs with MMVD.
The authors are unaware of other studies of RV systolic function in dogs with MMVD who have not previously received oral therapy for heart disease. With the exception of a single injectable dose of furosemide and oxygen supplementation provided to dogs presenting with acute CHF, cardiac therapy was not administered to dogs in any group. Therefore, the change in RV systolic function in the current study likely reflects the native pathophysiologic state, including type III pulmonary hypertension, rather than the effect of medications. Many of the indices measured in this study have been referred to as indices of ventricular contractility, but each are sensitive to changes in preload or afterload, as well as inotropic state and remodeling.11 Therefore, the true inotropic state of the RV in different stages of MMVD cannot be determined from this study.
Our study demonstrated high intra‐ and interobserver measurement variability for RV FAC, RV StL, RV StRL, and RV StRG. Visser et al.25 reported clinically acceptable measurement variability (CV < 10%) for TAPSE, RV S', RV FAC, RV StL, and RV StRL in 80 healthy dogs of varying body weights. Differences in study population and investigators might explain this difference in measurement variability. Dogs in the previous study did not have a displaced RV caused by left‐sided cardiomegaly; additionally, many of the dogs in that study were much larger than for the present study. The higher measurement variability presented in the current study likely reflects the difficulty in imaging the RV of dogs with substantial cardiac remodeling. With progressive LV enlargement and remodeling, the RV appeared smaller and foreshortened. Therefore, nonstandard imaging windows were often needed to better visualize the RV free wall. The near‐field image resolution, as in people,40, 41 was often poor and led to difficulty with endocardial border detection when measuring RV FAC and defining regions of interest for STE. In cases of advanced cardiac remodeling, a greater portion of the RV free wall becomes a near‐field structure whereas the basilar segment remains well visualized in these cases. This could explain why far‐field targets involving the basilar segment sampled for TAPSE and RV S' were feasible in all cases and highly repeatable, whereas RV StL and RV StRL measurements were not feasible in 3 dogs because of poor speckle tracking.
There are several limitations of this study. First, RV imaging was challenging in dogs with advanced cardiac remodeling and this could affect the accuracy or precision of operator measurements. Poor imaging of the RV per se was not considered a limitation of this study; rather, it demonstrates the challenges clinicians can expect when imaging the RV in advanced MMVD. It is possible that image quality will continue to improve with advances in ultrasound technology. Another study limitation was the relatively small sample size. The variables RV S' and global RV (6‐segment) strain, indices with low measurement variability, might have achieved statistical significance with larger samples and higher statistical power. This study did not include healthy dogs; whether RV function is significantly different between dogs with and without MMVD cannot be determined from these data. However, we used previously published reference intervals obtained from 80 healthy dogs in our laboratory as our comparison group and noted that a substantial percentage of measurements in B2 dogs fell outside those reference intervals.25 The reference intervals did need to be extrapolated because 3 of the dogs in this study weighed less than the smallest dog used to calculate the normal reference intervals. Whether or not the established reference intervals are appropriate for the smallest dogs in this study is unknown. Last, the dogs in Stage C were allowed to receive inhaled oxygen supplementation, which could have impacted RV afterload through oxygen‐dependent pulmonary artery vasodilation and therefore altered the measured indices of RV systolic function compared to dogs that did not receive oxygen therapy.
In conclusion, this study identified that TAPSE, RV StL, and RV StRL, are significantly different between stages of MMVD. Dogs in ACVIM stage B2 exhibited increased systolic function as estimated by TAPSE relative to published references intervals. The indices TAPSE, RV S', and RV StG demonstrated clinically acceptable measurement variability, even in the presence of advanced left heart remodeling. However, given the high variability in RV StL and RV StRL, these indices might be of limited use in future studies. While not observed for all, there was a general tendency for most indices of RV systolic function to increase in compensated remodeling (stage B2) but decline in dogs with congestive heart failure (stage C). Therefore, longitudinal investigations of RV systolic function indices, particularly TAPSE, are warranted to determine their prognostic importance in dogs with MMVD.
Acknowledgments
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
This work was completed at the College of Veterinary Medicine, The Ohio State University, Columbus, OH, USA.
Funding for this study was provided by the ACVIM Cardiology Resident Grant and The Ohio State University College of Veterinary Medicine Canine Research Funds.
Footnotes
GE E9 with XD Clear echocardiographic system and EchoPac software package, version BT13, GE Medical Systems, Waukesha, WI
EchoPAC 2D Strain software, Q‐Analysis (strain module), version 6.1, GE Medical Systems, Waukesha, WI
Strohm LE, Visser LC, Drost WT, Bonagura JD. 2‐dimensional long‐axis echocardiographic ratios for assessment of left atrial and ventricular size in healthy dogs and dogs with mitral regurgitation. Abstract. Vet Radiol Ultrasound 2016;57:670–684
IBM SPSS Statistics, version 24, IBM Corp, Armonk, NY
MedCalc for Windows, version 15.0, MedCalc Software, Ostend, Belgium
References
- 1. Borgarelli M, Buchanan JW. Historical review, epidemiology and natural history of degenerative mitral valve disease. J Vet Cardiol 2012;14:93–101. [DOI] [PubMed] [Google Scholar]
- 2. Häggström J, Höglund K, Borgarelli M. An update on treatment and prognostic indicators in canine myxomatous mitral valve disease. J Small Anim Pract 2009;50:25–33. [DOI] [PubMed] [Google Scholar]
- 3. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 4. Sargent J, Muzzi R, Mukherjee R, et al. Echocardiographic predictors of survival in dogs with myxomatous mitral valve disease. J Vet Cardiol 2015;17:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Hezzell MJ, Boswood A, Chang YM, et al. The combined prognostic potential of serum high‐sensitivity cardiac troponin I and N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med 2012;26:302–311. [DOI] [PubMed] [Google Scholar]
- 6. Reynolds CA, Brown DC, Rush JE, et al. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: The PREDICT cohort study. J Vet Cardiol 2012;14:193–202. [DOI] [PubMed] [Google Scholar]
- 7. Hezzell MJ, Boswood A, Moonarmart W, Elliott J. Selected echocardiographic variables change more rapidly in dogs that die from myxomatous mitral valve disease. J Vet Cardiol 2012;14:269–279. [DOI] [PubMed] [Google Scholar]
- 8. Kim JH, Park HM. Usefulness of conventional and tissue Doppler echocardiography to predict congestive heart failure in dogs with myxomatous mitral valve disease. J Vet Intern Med 2015;29:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tidholm A, Ljungvall I, Höglund K, et al. Tissue Doppler and strain imaging in dogs with myxomatous mitral valve disease in different stages of congestive heart failure. J Vet Intern Med 2009;23:1197–1207. [DOI] [PubMed] [Google Scholar]
- 10. O'Gara P, Sugeng L, Lang R, et al. The role of imaging in chronic degenerative mitral regurgitation. JACC Cardiovasc Imaging 2008;1:221–237. [DOI] [PubMed] [Google Scholar]
- 11. Bonagura JD, Schober KE. Can ventricular function be assessed by echocardiography in chronic canine mitral valve disease? J Small Anim Pract 2009;50(Suppl. 1):12–24. [DOI] [PubMed] [Google Scholar]
- 12. Santamore W, Dell'Italia L. Ventricular interdependence: Significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis 1998;40:289–308. [DOI] [PubMed] [Google Scholar]
- 13. Kittleson MD. Physiology of heart failure In: Kittleson MD, Klienle RD, eds. Small Animal Cardiovascular Medicine. St. Louis: Mosby; 1998:136–148. [Google Scholar]
- 14. Dini FL, Conti U, Fontanive P, et al. Right ventricular dysfunction is a major predictor of outcome in patients with moderate to severe mitral regurgitation and left ventricular dysfunction. Am Heart J 2007;154:172–179. [DOI] [PubMed] [Google Scholar]
- 15. Chrustowicz A, Gackowski A, El‐Massri N, et al. Preoperative right ventricular function in patients with organic mitral regurgitation. Echocardiography 2010;27:282–285. [DOI] [PubMed] [Google Scholar]
- 16. Le Tourneau T, Deswarte G, Lamblin N, et al. Right ventricular systolic function in organic mitral regurgitation: Impact of biventricular impairment. Circulation 2013;127:1597–1608. [DOI] [PubMed] [Google Scholar]
- 17. Motoki H, Borowski AG, Shrestha K, et al. Right ventricular global longitudinal strain provides prognostic value incremental to left ventricular ejection fraction in patients with heart failure. J Am Soc Echocardiogr 2014;27:726–732. [DOI] [PubMed] [Google Scholar]
- 18. Carlsson C, Haggstrom J, Eriksson A, et al. Size and shape of right heart chambers in mitral valve regurgitation in small‐breed dogs. J Vet Intern Med 2009;23:1007–1013. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura K, Morita T, Osuga T, et al. Prognostic value of right ventricular Tei index in dogs with myxomatous mitral valvular heart disease. J Vet Intern Med 2016;30:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baron Toaldo M, Poser H, Menciotti G, et al. Utility of tissue Doppler imaging in the echocardiographic evaluation of left and right ventricular function in dogs with myxomatous mitral valve disease with or without pulmonary hypertension. J Vet Intern Med 2016;30:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poser H, Berlanda M, Monacolli M, et al. Tricuspid annular plane systolic excursion in dogs with myxomatous mitral valve disease with and without pulmonary hypertension. J Vet Cardiol 2017;19:228–239. [DOI] [PubMed] [Google Scholar]
- 22. Visser LC, Scansen BA, Schober KE, Bonagura JD. Echocardiographic assessment of right ventricular systolic function in conscious healthy dogs: Repeatability and reference intervals. J Vet Cardiol 2015;17:83–96. [DOI] [PubMed] [Google Scholar]
- 23. Buchanan JW, Bücheler J. Vertebral scale system to measure heart size in radiographs. J Am Vet Med Assoc 1995;206:194–199. [PubMed] [Google Scholar]
- 24. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in dogs and cats. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 25. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 26. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 27. Synek V. Evaluation of the standard deviation from duplicate results. Accred Qual Assur 2008;13:335–337. [Google Scholar]
- 28. Lord PF, Hansson K, Carnabuci C, et al. Radiographic heart size and its rate of increase as tests for onset of congestive heart failure in Cavalier King Charles Spaniels with mitral valve regurgitation. J Vet Intern Med 2011;25:1312–1319. [DOI] [PubMed] [Google Scholar]
- 29. Kittleson MD. Myxomatous atrioventricular valve degeneration In: Kittleson MD, Klienle RD, eds. Small Animal Cardiovascular Medicine. St. Louis: Mosby; 1998:297–318. [Google Scholar]
- 30. Young A, Orr R, Smaill BH, Dell'Italia LJ. Three‐dimensional changes in left and right ventricular geometry in chronic mitral regurgitation. Am J Physiol 1996;271:H2689–H2700. [DOI] [PubMed] [Google Scholar]
- 31. Serres F, Chetboul V, Tissier R, et al. Comparison of 3 ultrasound methods for quantifying left ventricular systolic function: Correlation with disease severity and prognostic value in dogs with mitral valve disease. J Vet Intern Med 2008;22:566–577. [DOI] [PubMed] [Google Scholar]
- 32. Starr I, Jeffers WA, Meade R Jr. The absence of conspicuous increments of venous pressure after severe damage to the right ventricle of the dog, with a discussion of the relation between clinical congestive failure and heart disease. Am Heart J 1943;26:291–301. [Google Scholar]
- 33. Donald DE, Essex HE. Pressure studies after inactivation of the major portion of the canine right ventricle. Am J Physiol 1954;176:155–161. [DOI] [PubMed] [Google Scholar]
- 34. Hoffman D, Sisto D, Frater RW, Nikolic SD. Left‐to‐right ventricular interaction with a noncontracting right ventricle. J Thorac Cardiovasc Surg 1994;107:1496–1502. [PubMed] [Google Scholar]
- 35. Haddad F, Doyle R, Murphy DJ. Right ventricular function in cardiovascular disease, part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008;117:1717–1731. [DOI] [PubMed] [Google Scholar]
- 36. Serres F, Chetboul V, Gouni V, et al. Diagnostic value of echo‐Doppler and tissue Doppler imaging in dogs with pulmonary arterial hypertension. J Vet Intern Med 2007;21:1280–1289. [DOI] [PubMed] [Google Scholar]
- 37. Pariaut R, Saelinger C, Strickland KN, et al. Tricuspid annular plane systolic excursion (TAPSE) in dogs: Reference values and impact of pulmonary hypertension. J Vet Intern Med 2012;26:1148–1154. [DOI] [PubMed] [Google Scholar]
- 38. Rhinehart J, Schober KE, Scansen BA, et al. Effect of body position, exercise, and sedation on estimation of pulmonary artery pressure in dogs with degenerative atrioventricular valve disease. J Vet Intern Med 2017; Early Publication. https://doi.org/10.1111/jvim.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Visser LC, Scansen BA, Brown NV, et al. Echocardiographic assessment of right ventricular systolic function in conscious healthy dogs following a single dose of pimobendan versus atenolol. J Vet Cardiol 2015;17:161–172. [DOI] [PubMed] [Google Scholar]
- 40. Bleeker GB, Steendijk P, Holman ER, et al. Assessing right ventricular function: The role of echocardiography and complementary technologies. Heart 2006;92:i19–i26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rudski LG, Lai WW, Afilalo J, et al. Guidelines of the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. [DOI] [PubMed] [Google Scholar]


