Abstract
Background
Monitoring urine protein:creatinine ratios (UPC) in dogs with protein‐losing nephropathy (PLN) is challenging because of day‐to‐day variation in UPC results.
Hypothesis/Objectives
Determine whether single, averaged, or pooled samples from PLN dogs receiving medical treatment yield comparable UPCs, regardless of degree of proteinuria.
Animals
Twenty‐five client‐owned PLN dogs receiving medical treatment.
Methods
UPC ratios were prospectively measured in each dog utilizing 3 methods: single in‐hospital sample (day 3), average sample (days 1–3), and pooled sample (equal pooling of urine from days 1–3). Bland‐Altman analysis was performed to evaluate agreement between methods for all dogs, as well as in subgroups of dogs (UPC ≤4 or UPC >4).
Results
For all dogs, Bland‐Altman log‐transformed 95% limits of agreement were −0.07–0.18 (single versus pooled UPC), −0.06–0.16 (single versus average UPC), and −0.06–0.04 (pooled versus average UPC). For dogs with UPC ≤4, Bland‐Altman 95% limits of agreement were −0.42–0.82 (single versus pooled UPC), −0.38–0.76 (single versus average UPC), and −0.27–0.25 (pooled versus average UPC). For dogs with UPC >4, Bland‐Altman 95% limits of agreement were −0.17–2.4 (single versus pooled UPC), −0.40–2.2 (single versus average UPC), and −0.85–0.43 (pooled versus average UPC).
Conclusions and Clinical Importance
UPC ratios from all methods were comparable in PLN dogs receiving medical treatment. In PLN dogs with UPC >4, more variability between methods exists likely because of higher in‐hospital results, but whether this finding is clinically relevant is unknown.
Keywords: Protein‐losing nephropathy, Proteinuria, Variability
Abbreviations
- UPC
urine protein:creatinine ratio
- PLN
protein‐losing nephropathy
- ACE
angiotensin‐converting enzyme
- ARB
angiotensin receptor blocker
Proteinuria is a characteristic of protein‐losing nephropathy (PLN) that can lead to kidney damage, increase the risk of uremic crisis, and is associated with a higher rate of mortality in dogs with chronic kidney disease (CKD).1, 2 Dogs with PLN require lifelong management including monitoring of the magnitude of proteinuria.3 The urine protein:creatinine ratio (UPC) is a reliable and accurate means for the detection and quantification of proteinuria.4 The quantification of proteinuria allows for appropriate staging of renal disease, determination of appropriate therapeutic interventions, and assessment of response to treatment.
Monitoring dogs with proteinuria can be challenging because of day‐to‐day variation in their UPC results, making it difficult to know what changes are clinically relevant. One study of a colony of female research dogs found that a single measurement reliably estimated the UPC when UPC was <4 but indicated that 2–5 measurements are necessary when the UPC is higher.5 This study evaluated a homogenous research colony in a controlled laboratory setting and did not evaluate the biologic variability in the UPC in client‐owned dogs, which could be affected by a variety of factors in an uncontrolled natural setting. Another study in dogs showed that pooling 3 urine samples of equal volume to determine a single UPC was a reliable alternative to assessing the average of 3 separate UPC measurements.4 This study found minimal variability in samples when UPC was <4, so it is unlikely that serial samples are required. However, very few dogs with more severe proteinuria (UPC >4) were evaluated, and it is unclear whether pooled or serial urine samples are preferable for monitoring. Additionally, the biologic variability in UPC has not been assessed in dogs undergoing antiproteinuric medical treatment with angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), or both which could have important implications on the clinical management of these patients.
Many veterinary institutions use single urine samples to determine UPC whereas others use serial urine samples to determine an average UPC when managing and treating dogs with proteinuria. Although pooling urine samples instead of using serial samples would decrease the expense for clients, it is unclear whether serial urine samples are indicated in all dogs with various degrees of proteinuria. Serial urine samples also are less convenient for clients, which potentially could lead to less compliance and reluctance to return for re‐evaluations. Although the previous study4 determined that a UPC from pooled urine samples was comparable to a UPC obtained from an average of 3 single measurements, it is not known how comparable single UPC results are to pooled UPC results or how comparable single UPC results are to average UPC results. Additionally, there is no universally accepted method that is recommended for the determination of UPC when monitoring proteinuric dogs after initiation of treatment. Therefore, it is important to determine the comparability of different available methods so that therapeutic recommendations and management are consistent in veterinary patients. For example, it is not currently known whether a clinician can compare UPC results in an individual patient if the results were obtained by 2 different methods (eg, single versus pooled) during the course of treatment. Therefore, the purpose of our study was to determine whether a single sample, average sample, or pooled urine sample would be comparable to one other for determination of UPC, regardless of the degree of proteinuria in PLN dogs receiving medical treatment.
Materials and Methods
Animals
Client‐owned dogs presented to the Colorado State University (CSU) Veterinary Teaching Hospital between July 2014 and April 2016 were prospectively enrolled in this observational study. The study was approved by the Institutional Animal Care and Use Committee at Colorado State University, and all owners gave informed consent at the time of enrollment. To be eligible for inclusion, a diagnosis of PLN was required, which was defined as persistent proteinuria with inactive sediment, negative urine culture, and no known concurrent morbidities that could result in proteinuria (eg, hyperadrenocorticism). Renal biopsy was not required for enrollment. Dogs entered into the study were required to be on continuous medical treatment for proteinuria for at least 1 month. Treatment could include an ACE inhibitor, angiotensin receptor blocker (ARB), or both. Other medical therapies for proteinuria such as renal diet, thromboprophylaxis (eg, aspirin, clopidogrel), and fish oil were not required for eligibility. However, no changes could be made to the medical treatment in the 2 weeks before enrollment in order to provide consistent results. Additionally, all enrolled dogs were required to have a CBC, serum biochemistry, urinalysis, UPC, and blood pressure measurement performed in addition to a negative urine culture. Other diagnostic tests such as tick‐borne disease testing and abdominal ultrasound examination were encouraged but not required for the study. Dogs that were critically ill, had signs of nephrotic syndrome (eg, edema formation, moderate hypoalbuminemia [serum albumin concentration <2.0 g/dL]), evidence of urinary tract infection, or those receiving medications that could exacerbate proteinuria (eg, corticosteroids) were not eligible for the study.
Sample Collection
For each dog, 3 urine samples were collected at 24‐hour intervals at the same time of day (within 10 minutes) as determined by the owner; 2 urine samples were collected by voiding in a provided sterile urine collection cupa by the owner once daily (day 1 and day 2). The third urine sample (day 3) was collected by voiding or cystocentesis performed by a veterinary nurse or a single investigator (SS) in the hospital on the day of the appointment at the CSU Veterinary Teaching Hospital. Owners were instructed to collect the voided urine samples during mid‐stream and to avoid contact of urine with any hair or skin. Each owner was shown how to perform the collection and also practiced how to collect a sample from their dog before obtaining samples for the study. Urine samples from day 1 and day 2 were immediately placed in the owner's refrigerator (approximately 4°C) and were transported on a cool pack to avoid premature warming of the samples, and samples were all processed within 3 days of collection and stored at 4°C as previously recommended.6 Specifically, day 1 samples were processed within 48–52 hours of collection and stored at 4°C and day 2 samples were processed within 24–28 hours of collection and stored at 4°C. Urine samples from day 3 were processed within 4 hours of submission. If the sample could be evaluated within 30 minutes of receipt, it was kept at room temperature. If the sample could not be evaluated within 30 minutes of receipt, it was refrigerated at 2–8°C for ≤4 hours. The sample would then be allowed to warm back to room temperature before analysis.b
Sample Analysis
Before analysis, a 1.0‐mL aliquot was taken from each of the 3 separate urine samples (days 1–3) and pooled into a single sample (pooled sample) for UPC measurement. The day 1, day 2, and day 3 urine samples also were submitted for UPC measurements to determine single sample UPC, and these 3 results then were averaged to obtain the average UPC. Specifically for determination of the UPC, the benzethonium chloride methodc was used to determine the urine protein concentration from undiluted urine whereas the compensated Jaffe method (1 : 10 dilution)3 was used to determine the UPC. Routine urinalysis with sediment examination was performed by the Colorado State University clinical pathology service with 5 mL of urine from the day 3 sample as previously described.7 The sediments were considered active if bacteria were observed, if there were >30 erythrocytes/high‐powered field (hpf), or 3–6 leukocytes/hpf. An aliquot of urine from the day 3 sample also was submitted for quantitative aerobic bacterial culture.
Statistical Methods
All statistical analyses were performed with GraphPad Prism,d and statistical significance was set at P < 0.05. Three separate Bland‐Altman analyses were performed on all of the study dogs: single (day 3) UPC versus pooled UPC, single (day 3) UPC versus average UPC, and pooled UPC versus average UPC. Data were checked for normality using the Shapiro‐Wilk test and were log‐transformed (log base 10) because they did not follow a Gaussian distribution. Any transformed results then were back‐transformed to the original scale. The correlation coefficient (Spearman or Pearson r) for each of the 3 analyses also was calculated, and simple linear regression was performed to determine the regression line between the corresponding methods. Additional Bland‐Altman analyses were performed on the dogs after they were divided into 2 subgroups (UPC ≤4 or UPC >4) based on the UPC result from day 3. The subgroup analyses were checked for normality using the Shapiro‐Wilk test and were found to be normally distributed. Statistical comparison between single in‐hospital (day 3) and home (days 1 and 2) UPC data for all dogs was performed by a Friedman test with Dunn's posthoc analysis. Statistical comparison between single in‐hospital (day 3) and home (days 1 and 2) UPC data for dog subgroups (UPC ≤4 or UPC >4) was performed by a repeated‐measures analysis of variance (ANOVA) test with Dunn's posthoc analysis (data were normally distributed).
Results
Animals
Twenty‐five dogs were enrolled into the study. Sixteen were spayed females, and 9 were castrated males. No animals were sexually intact. The median age for the female dogs was 10 years (range, 2–14 years), and the median age for the male dogs was 10 years (range, 8–14 years). Twenty‐two of the 25 dogs (88%) were purebred and represented 15 breeds. Breeds with >1 representative included Pembroke Welsh Corgis and Beagles (both 3/25, 12%) and Yorkshire terriers, Miniature Schnauzers, and Labrador Retrievers (each 2/25, 8%), but there were 3 (3/25, 12%) additional Labrador Retriever mixes. Twenty‐one dogs (84%) were on an ACE inhibitor alone (19/25 were on benazepril and 2/25 were on enalapril), 2 dogs (8.0%) were on an ARB alone (telmisartan), and 2 dogs (8.0%) were on dual treatment (benazepril/losartan or enalapril/losartan). Ten dogs (40%) were azotemic as determined by International Renal Interest Society (IRIS) staging (3/25 dogs were IRIS stage 2 with serum creatinine concentration 1.7–2.0 mg/dL and 7/25 dogs were IRIS stage 3 with serum creatinine concentration 2.3–5.0 mg/dL). Fifteen dogs (15/25) were nonazotemic as determined by IRIS staging (all dogs were IRIS stage 1 with serum creatinine concentration 0.6–1.2 mg/dL).
Urine Protein:Creatinine Ratios
The mean ± SD, median, and range of UPC for the 3 collection days are presented in Table 1. The log10 transformation Bland‐Altman 95% limits of agreement, mean difference, back‐transformed Bland‐Altman 95% limits of agreement, and correlation coefficients for all dogs for each of the 3 comparisons are summarized in Table 2. For single UPC versus pooled UPC, the 95% limits of agreement were −0.07–0.18 (Fig 1A). Back transformation using the equations 10−0.07 and 100.18 resulted in 95% limits of agreement of 0.85–1.51. For single UPC versus average UPC, the 95% limits of agreement were −0.06–0.16 (Fig 1B). Back transformation using the equations 10−0.06 and 100.16 resulted in 95% limits of agreement of 0.87–1.45. For pooled UPC versus average UPC, the 95% limits of agreement were −0.06–0.04 (Fig 1C). Back transformation using the equations 10−0.06 and 100.04 resulted in 95% limits of agreement of 0.87–1.10.
Table 1.
Urine protein:creatinine ratio (UPC) results for urine samples collected at home on days 1–2 and in hospital on day 3 for all dogs and for both subgroups of dogs. UPC of samples collected in hospital was significantly higher compared to samples collected in the home environment
| Day 1 at Home | Day 2 at Home | Day 3 in Hospital | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | |
| All dogs | 4.2 ± 3.2 | 3.2 (0.9–15.8) | 3.2 ± 4.0 | 2.8 (0.7–13.3) | 4.8 ± 3.3b | 3.8 (1.2–13.8) |
| Dogs UPC ≤4 | 2.4 ± 1.0 | 2.3 (0.9–4.6) | 2.0 ± 0.8 | 1.9 (0.7–3.8) | 2.5 ± 0.9b | 2.3 (1.2–3.9) |
| Dogs UPC >4 | 6.4 ± 3.7 | 4.8 (3.2–15.8) | 6.4 ± 3.5 | 5.7 (2.7–13.3) | 7.7 ± 2.8a , b | 6.6 (4.8–13.8) |
Day 3 significantly higher than day 1.
Day 3 significantly higher than day 2.
Table 2.
Bland‐Altman 95% limits of agreement, mean difference, and correlation coefficient between urine sample handling groups and with dogs divided into 2 subgroups; UPC ≤4 and UPC >4
| Single versus Pooled | Single versus Average | Pooled versus Average | |
|---|---|---|---|
| All dogs log10‐transformed UPC 95% limits of agreement | −0.07 to 0.18 | −0.06 to 0.16 | −0.06 to 0.04 |
| Mean difference | 0.06 ± 0.06 | 0.05 ± 0.06 | −0.01 ± 0.03 |
| All dogs back‐transformed UPC 95% limits of agreement | 0.85–1.51 | 0.87–1.45 | 0.87–1.10 |
| Correlation coefficient | 0.96 | 0.98 | 0.99 |
| UPC ≤4 95% limits of agreement | −0.42 to 0.82 | −0.38 to 0.76 | −0.27 to 0.25 |
| Mean difference UPC ≤4 | 0.20 ± 0.32 | 0.19 ± 0.29 | −0.01 ± 0.13 |
| UPC >4 95% limits of agreement | −0.17 to 2.4 | −0.40 to 2.2 | −0.85 to 0.43 |
| Mean difference UPC >4 | 1.11 ± 0.66 | 0.9 ± 0.66 | −0.21 ± 0.33 |
Figure 1.
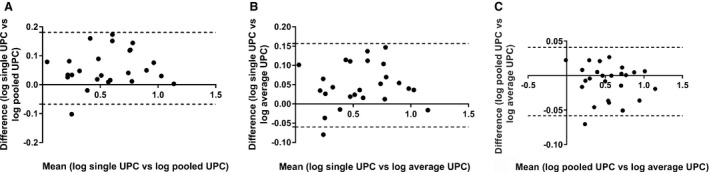
Log‐transformed Bland‐Altman plots for all study dogs illustrating the difference in urine protein:creatinine ratio (UPC) between urine sample handling groups (A) single versus pooled UPC (B) single versus average UPC, and (C) pooled versus average UPC ratio. The solid horizontal reference line at 0 represents no difference between the methods. Dogs represented by a dot above the solid line had higher UPC value on the single sample collected in hospital (graphs A, B). The dotted lines represent the 95% limits of agreement (mean ± 1.96 SD).
For all dogs, the UPC of the urine sample collected in‐hospital (day 3) was significantly higher than the UPC of samples collected at home (day 1 versus day 3, P = 0.02; day 2 versus day 3, P < 0.0001). No significant difference was found between samples collected at home on days 1 and 2. For all dogs, there was a strong correlation between a single UPC and a pooled UPC (r = 0.96, P < 0.0001), a single UPC and an average UPC (r = 0.98, P < 0.0001), and a pooled UPC and an average UPC (r = 0.99, P < 0.0001).
When the dogs were divided into subgroups (UPC ≤4 and UPC >4), based on the UPC of the day 3 sample, 14 dogs (56%) had UPC ≤4 and 11 dogs (44%) had UPC >4. The mean ± SD, median, and range of UPC for the 3 collection days for dog subgroups are presented in Table 1. Bland‐Altman 95% limits of agreement and mean difference of the values for each of the 3 methods are summarized in Table 2 for both subgroups of dogs. Bland‐Altman graphs for the comparison between the 3 methods in each subgroup are presented in Figures 2 and 3. For dogs with UPC ≤4, the UPC of the urine sample collected in‐hospital (day 3) was significantly higher than that of the sample collected at home on day 2 (P = 0.03). No significant difference was found between the sample collected in‐hospital on day 3 versus the sample collected at home on day 1 or between samples collected at home on days 1 and 2. For dogs with UPC >4, the UPC of the urine sample collected in‐hospital (day 3) was significantly higher than that of samples collected at home (day 1 versus day 3, P = 0.03; day 2 versus day 3, P = 0.01). No significant difference was found between samples collected at home on days 1 and 2.
Figure 2.
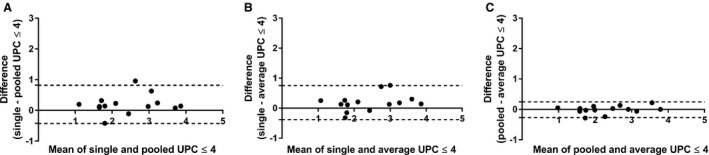
Bland‐Altman plots for dogs with urine protein:creatinine ratio (UPC) ≤4 illustrating the difference in UPC between urine sample handling groups (A) single versus pooled UPC, (B) single versus average UPC, and (C) pooled versus average UPC ratio. The solid horizontal reference line at 0 represents no difference between the methods. Dogs represented by a dot above the solid line had higher UPC value on the single sample collected in hospital (graphs A, B). The dotted lines represent the 95% limits of agreement (mean ± 1.96 SD).
Figure 3.
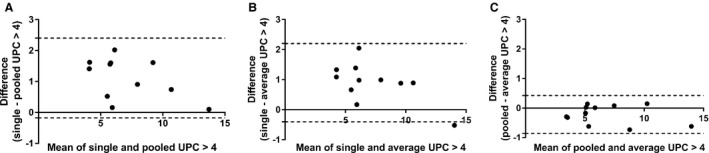
Bland‐Altman plots for dogs with urine protein:creatinine ratio (UPC) >4 illustrating the difference in UPC between urine sample handling groups (A) single versus pooled UPC, (B) single versus average UPC, and (C) pooled versus average UPC ratio. Dogs represented by a dot above the solid line had higher UPC value on the single sample collected in hospital (graphs A, B). More variability between methods is seen in dogs with UPC >4 due to higher UPC from the in‐hospital sample, but this but may not be clinically significant.
Discussion
The monitoring of PLN dogs on antiproteinuric treatment can be challenging because of day‐to‐day variation in UPC, which makes it difficult to discern what changes represent a true decrease in UPC and thus a treatment effect.2 When 3 methods for monitoring proteinuria were evaluated in our study to further inform this decision‐making process, a strong correlation was found between a single UPC compared to a pooled UPC, between a single UPC compared to an average UPC, and an even stronger correlation was found between the pooled UPC and average UPC. However, correlation does not equate with agreement and many methods designed to measure the same quantity often are strongly correlated.8 To investigate the agreements between the methods, Bland‐Altman analyses were performed.
When monitoring dogs with proteinuria, it is recommended that the UPC must change by 80% at lower UPC (near 0.5) and by at least 35% at higher UPC (near 12) to be considered clinically relevant.5 When assessing agreement between single UPC and pooled UPC in our study, we found that single UPC could differ from the pooled UPC by <15 or >51%. Although differing by <15% would be acceptable for low or high UPC, differing by >than 51% would only be acceptable at lower UPC. Therefore, utilizing either method is comparable in dogs with low‐magnitude proteinuria but may not be interchangeable in dogs with higher‐magnitude proteinuria.
To further investigate these findings, the dogs were separated into 2 subgroups: dogs with UPC ≤4 and dogs with UPC >4. Bland‐Altman analysis was performed on both groups to determine whether findings changed with different UPC magnitudes. In dogs with UPC ≤4, 95% of cases may have a single UPC between 0.42 below and 0.82 above the pooled UPC result. In dogs with UPC >4, 95% of cases may have a single UPC between 0.17 below and 2.4 above the pooled UPC result. For example, if a dog had a baseline UPC of 4.0, then depending on the method used, the subsequent UPC could be between 3.58 and 4.82, which would not be considered clinically relevant because the results only differ by 10.5–20.5%, respectively. If the baseline UPC was 8.0, then depending on the method used, the subsequent UPC could be between 7.83 and 10.4. According to a previous study,5 this result also would not be considered clinically relevant because the results only differ by 2–30%, respectively. Therefore, the difference in UPC between the 2 methods would be acceptable in dogs with lower or higher UPC results.
When comparing a single UPC to an average UPC, it was found that, for 95% of cases, the single UPC may differ by <13 or >45% from the average UPC. Thus, the difference in UPC ratios between the 2 methods at low or high UPC would be considered clinically acceptable when monitoring dogs with stable proteinuria. To further verify these findings, the analysis was repeated for the 2 subgroups of dogs (UPC ≤4 or UPC >4). In dogs with UPC ≤4, 95% of cases may have a single UPC between 0.38 below and 0.76 above the average UPC result. In dogs with UPC >4, 95% of cases may have a single UPC between 0.40 below and 2.2 above the average UPC result. For example, if a patient's baseline UPC was 4.0, then depending on the method used, the subsequent UPC could be between 3.6 and 4.76, which would not be considered clinically relevant (results only differ by 10–19%). If a patient's baseline UPC was 8.0, then depending on the method used, the subsequent UPC could be between 7.6 and 10.2 (results only differ by 5–27.5%). Similarly, this difference would not be considered clinically relevant. These findings show that the method of obtaining a single UPC result is comparable to the average UPC method in dogs at low and high magnitudes of proteinuria.
When comparing the pooled UPC to the average UPC, the pooled UPC may differ from the average UPC by <13 and >10%. Based on these findings, these methods have excellent agreement and are interchangeable for dogs with low or high magnitudes of proteinuria. To further confirm these findings, the analysis was repeated for the 2 subgroups of dogs (UPC ≤4 or UPC >4). In dogs with UPC ≤4, 95% of cases may have a pooled UPC between 0.27 below and 0.25 above the average UPC result. Thus, the difference between the 2 methods would not be clinically relevant and showed strong agreement. For dogs with UPC >4, 95% of cases may have a pooled UPC between 0.85 below and 0.43 above the average UPC, and the difference between the 2 methods also would not be considered clinically relevant. Therefore, regardless of the magnitude of proteinuria, the pooled UPC and average UPC results were very comparable.
In general, our results show that the 3 methods for measuring UPC had good agreement, regardless of the magnitude of proteinuria. Although for the majority of cases, the difference between the 2 methods would not be clinically relevant, there are a few instances in which the method chosen could impact interpretation of the results. When using the single UPC compared to the pooled UPC or average UPC, at very low magnitudes of proteinuria, the difference between the 2 methods could be considered clinically relevant. For example, if a patient had a baseline UPC of 0.5, then depending on the method used, the subsequent UPC could be between 0.08 and 1.32. According to a previous study,5 both results would be interpreted as a clinically relevant decrease or increase, respectively. However, based on the results of our study, this does not occur at very low magnitudes of proteinuria when comparing the pooled UPC to the average UPC.
In addition to the sampling method, another factor that has the potential to affect UPC interpretation is whether or not the samples are collected at home or in the hospital. Although there was excellent agreement between pooled and average samples in our study, the agreement was less strong when the single sample collected in‐hospital was compared to pooled and averaged samples. The most apparent explanation for this observation is that UPC results were significantly higher on the samples collected in‐hospital than in those collected at home, particularly for dogs with UPC >4. A previous study9 showed that the UPC of a sample obtained in the hospital typically is higher than the UPC of a sample obtained at home. Our study had evaluated the UPC from dogs presented to their hospital for a variety of reasons, and thus was not specifically designed to evaluate proteinuric dogs. However, the difference between the UPC of a sample obtained at home or in the hospital was most noticeable with UPC >0.5 and less apparent in nonproteinuric dogs. A second prospective study10 also compared UPC on samples obtained in home and hospital settings. Similar to the first study, it was not designed to evaluate proteinuric dogs alone and, as a result, the majority of dogs were classified as nonproteinuric. In contrast to the first study however, UPC measurements of samples obtained at home and in hospital were not significantly different. Based on the cumulative findings of these 3 studies, it appears reasonable to collect a urine sample for UPC evaluation in nonproteinuric dogs at home or in the hospital setting. However, during therapeutic monitoring for proteinuria or in dogs known to be proteinuric, a single sample obtained in the hospital may yield higher results that could change clinical interpretation and thus management of the patient. Therefore, for dogs that are proteinuric, it is reasonable to determine a pooled or average UPC measurement or a single UPC measurement using a sample obtained at home rather than a sample obtained in the hospital setting.
Another factor that can affect UPC measurements is the time frame in which the sample is processed after collection. A previous study showed that UPC significantly increased after 12 hours at room temperature and after 1 week of storage at 4°C.6 A transient but significant increase in UPC at 12 hours was seen in samples stored at 4°C, but the etiology and clinical relevance of this observation were unclear. Based on the findings of this study, it was recommended to process room temperature urine samples within 4 hours or to store urine samples at 4°C or frozen and analyze them within 3 days of collection. All of the urine samples in our study were processed as recommended by the previous study with regard to timing and temperature. Additionally, the day 1, day 2, and day 3 samples all were processed within the same time frames for each day. Therefore, the timing of processing is unlikely to substantially affect the variability and results of our study.
There were several limitations to our study. The findings from a previous study5 were used to determine whether a change in UPC would be considered clinically relevant or not. However, as mentioned before, the previous study was performed only in female research dogs with a particular type of PLN and whether this situation is comparable to dogs with other causes of PLN (eg, immune‐complex glomerulonephritis, glomerulosclerosis) is unknown. Renal tissues for histopathology were evaluated in a small number of dogs in our study, and none of them were affected by X‐linked hereditary nephropathy, and thus, comparing the 2 studies is challenging. Further studies investigating the day‐to‐day variation of UPC in dogs with different types of PLN (determined by renal biopsy) while on medical treatment should be pursued to determine whether more variability exists in dogs with different types of PLN.
When monitoring UPC in dogs with proteinuria, no individual method has been shown to be most accurate. As a result, there are several ways to monitor UPC in dogs, which include collecting a single sample, collecting multiple samples to obtain an average UPC, or collecting multiple samples to obtain a pooled UPC. It was previously unknown whether these 3 methods were comparable in an individual patient receiving medical treatment for monitoring purposes. Considering what is currently known regarding the variability in proteinuria in dogs, the results of our study show that, for the majority of patients, the UPC from a single sample, average sample, and pooled sample can be compared in dogs regardless of the magnitude of proteinuria. However, the UPC may be higher when obtained in the hospital and this information should be taken into account when interpreting results.
Acknowledgments
We thank the Center for Companion Animal Studies at Colorado State University for providing the funding for this study. We also thank Dr. Ann Hess for providing guidance for the statistical analysis.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The study was performed at Colorado State University Veterinary Teaching Hospital.
The study was funded through a Young Investigator Grant through the Center for Companion Animal Studies at Colorado State University.
The findings were presented at the 2017 ACVIM Annual Forum as a research abstract.
Footnotes
S/P Specimen Containers by Cardinal Health, 4 oz., Mc Gaw Hill, IL
Roche Diagnostics Chemstrip 10 UA, Indianapolis, IN
Roche Diagnostics Cobas C501, Holliston, MA
GraphPad Prism 5.0, San Diego, CA
References
- 1. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc 2005;226:393–400. [DOI] [PubMed] [Google Scholar]
- 2. Lees GE, Brown SA, Elliott J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (small animal). J Vet Intern Med 2005;19:377–385. [DOI] [PubMed] [Google Scholar]
- 3. IRIS Canine GN Study Group Standard Therapy Subgroup , Brown S, Elliot J, et al. Consensus recommendations for standard therapy of glomerular disease in dogs. J Vet Intern Med 2013;27:S27–S43. [DOI] [PubMed] [Google Scholar]
- 4. LeVine DN, Zhang D, Harris T, Vaden SL. The use of pooled vs serial urine samples to measure urine protein:creatinine ratios. Vet Clin Pathol 2010;39:53–56. [DOI] [PubMed] [Google Scholar]
- 5. Nabity MB, Boggess MM, Kashtan CE, Lees GE. Day‐to‐Day variation of the urine protein:creatinine ratio in female dogs with stable glomerular proteinuria caused by X‐linked hereditary nephropathy. J Vet Intern Med 2007;21:425–430. [DOI] [PubMed] [Google Scholar]
- 6. Rossi G, Giori L, Campagnola S, Zatelli A, et al. Evaluation of factors that affect analytic variability of urine protein‐to‐creatine ratio determination in dogs. Am J Vet Res 2012;73:779–788. [DOI] [PubMed] [Google Scholar]
- 7. Vap LM, Shropshire SB. Urine cytology: Collection, film preparation, and evaluation. Vet Clin North Am Small Anim Pract 2017;47:135–149. [DOI] [PubMed] [Google Scholar]
- 8. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 9. Duffy ME, Specht A, Hill RC. Comparison between Urine Protein: Creatinine Ratios of Samples Obtained from Dogs in Home and Hospital Settings. J Vet Intern Med 2015;29:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Citron LE, Weinstein NM, Littman MP, Foster JD. Correlation between Canine Urine Cortisol Creatinine and Protein‐Creatinine Ratios Collected At Home and In Hospital. Abstract NU05. National Harbor, MD: ACVIM Forum; June 2017. [Google Scholar]


