Abstract
Background
The Janus Kinase (JAK) and Signal Transducer and Activator of Transcription (STAT) pathways play important roles in the pathogenesis of diffuse large B cell lymphoma (DLBCL) in humans, and up‐regulated STAT3 expression and activity are associated with worse clinical outcome in humans. No studies have evaluated the JAK‐STAT signaling pathway in DLBCL of dogs.
Hypothesis
STAT3 pathway is deregulated in DLBCL in dogs. We aim to assess the expression, activation, and cellular localization of STAT3 and mitogen‐activated protein kinase ERK1/2 in DLBCL of dogs.
Animals
Forty‐three client‐owned dogs diagnosed with DLBCL by histopathology
Methods
Retrospective analysis of DLBCL in dogs, including patient characteristics and treatment, immunohistochemistry, and protein expressions by Western blot.
Results
A higher percentage of STAT3 and p‐STAT3 immunolabelled cells were observed in DLBCL of dogs when compared to normal canine lymph nodes. In STAT3 immunolabelled cells, STAT3 has higher nuclear expression in lymphoma samples than in normal or reactive lymph nodes. In addition to up‐regulated STAT3 expression and activation, mitogen‐activated kinase ERK1/2 activation is up‐regulated in DLBCL of dogs.
Conclusion and Clinical Importance
Compared with the normal canine lymph node, DLBCL of dogs has up‐regulated STAT3 pathway. Our results support future investigation of JAK inhibitors in the treatment of DLBCL in dogs.
Keywords: DLBCL, p‐ERK1/2, p‐STAT3
Abbreviations
- ABC
active B cell‐like
- AE
adverse event
- BCA
bicinchoninic acid assay
- CHOP
cyclophosphamide‐doxorubicin‐vincristine‐prednisone
- DAB
3, 3′‐diaminobenzidine
- DLBCL
diffuse large B cell lymphoma
- ECG
electrocardiogram
- HEPES
4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid
- MDR
multidrug resistant
- MPN
myeloproliferative neoplasia
- NP‐40
nonyl phenoxypolyethoxylethanol
- PBS
phosphate‐buffered saline
- PMSF
phenylmethylsulfonyl fluoride
- PT
prothrombin time
- PTT
partial thromboplastin time
- RBC
red blood cell
- RPMI 1640
Roswell Park Memorial Institute 1640
- RPM
revolutions per minute
- SDS‐PAGE
sodium dodecyl sulfate‐polyacrylamide gel electrophoresis
- Tris‐EDTA
tris‐ethylene diamine tetraacetic acid
- TYK2
tyrosine kinase 2
- VCOG
Veterinary Comparative Oncology Group
- WHO
World Health Organization
Lymphoma comprises 83% of all hematopoietic malignancies in dogs and approximately 24% of all cancers in dogs. Similar to non‐Hodgkin lymphoma (NHL) in humans, most lymphomas in dogs arise from a malignant B cell lineage.1 The most common form of lymphoma in dogs is diffuse large B cell lymphoma (DLBCL). Dogs with DLBCL typically present with generalized peripheral lymphadenopathy, with stage III to V disease as classified by the World Health Organization (WHO) staging system for domestic animals.1 Also, similar to NHL in humans, the drugs most effective for treating DLBCL in dogs are the drugs comprising cyclophosphamide‐doxorubicin‐vincristine‐prednisone (CHOP)‐based chemotherapy protocols.2 Although >80% of canine patients with DLBCL initially respond to CHOP‐based chemotherapy, the duration of response is relatively short, with a median survival time of approximately 10 months.3, 4 Although many CHOP variations and other chemotherapy protocols have been assessed, overall clinical outcome remains unchanged.2, 3 Hence, CHOP‐based chemotherapy remains the standard of care for dogs with DLBCL, and there is a substantial need for identifying new therapeutic targets for treating canine patients with DLBCL.
The Janus Kinase (JAK) and Signal Transducer and Activator of Transcription (STAT) pathways (JAK‐STAT) are critical in both normal hematopoiesis and hematologic malignancies.5, 6, 7 There are 4 members of the JAK family of cytoplasmic tyrosine kinases in vertebrates: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2).8 Each JAK protein is constitutively associated with a cytokine receptor that lacks intrinsic tyrosine kinase activity and activates specific members of the STAT family as downstream effectors. The ligand binding activates the JAK kinases, leading to JAK autophosphorylation, which subsequently phosphorylates intracellular receptor tyrosines and creates binding sites for STAT phosphorylation. The phosphorylated STAT then translocates into the nucleus to initiate transcription of effector genes involved in apoptosis, cell cycle progression, and proteasomal degradation.8 Among the JAK family of kinases, JAK2 is the most extensively studied because of its critical pathogenic role in myeloproliferative neoplasia (MPN) of humans.9, 10 Deregulated JAK/STAT signaling also is implicated in lymphomagenesis,11 and JAK2‐mediated activation of STAT3 promotes growth and survival in a variety of lymphomas in humans.5, 6, 7 Constitutively activated STAT3 promotes cell proliferation and survival in the active B cell‐like (ABC) subtype of DLBCL in humans.7 In addition, constitutively activated STAT3 is correlated with more advanced clinical stage and overall poor survival of DLBCL in humans.12, 13
Although the JAK/STAT pathway plays a critical role in DLBCL of humans, no studies have evaluated this pathway in DLBCL of dogs. We aimed to evaluate STAT3 expression, activation, cellular localization, and its interacting target ERK1/2 in DLBCL of dogs.
Materials and methods
Patient samples
Lymph node samples from 43 client‐owned dogs with a histologic diagnosis of naïve multicentric DLBCL were collected from the archives at UW Veterinary Care, the teaching hospital of the University of Wisconsin School of Veterinary Medicine, from 2008 to 2013. Archived lymph node samples from patients meeting the eligibility criteria for the study were gathered for immunohistochemical staining. Information regarding staging diagnostic tests (CBC, biochemistry profile, urinalysis, thoracic radiographs, abdominal ultrasound findings, and bone marrow aspiration) was abstracted from patient medical records. Ten normal and 10 reactive canine lymph node samples were used as controls.
Immunohistochemical (IHC) staining for STAT3 and p‐STAT3
Immunohistochemical evaluation was performed on consecutive sections using the indirect immunoperoxidase staining method. The slides first were deparaffinized in xylene and rehydrated in ethanol. Antigen retrieval was performed by water bath treatment of slides immersed in Tris‐ethylene diamine tetraacetic acid (Tris‐EDTA) buffer (for p‐STAT3) or in citrate buffer (for STAT3) for 25 minutes at 95°C. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 10 minutes at room temperature. Samples were blocked with 10% goat serum for 60 minutes at room temperature before the primary antibody incubation. Samples were incubated with mouse anti‐STAT3 (1:600, Cell Signaling Technology1, 124H6) or rabbit anti‐p‐STAT3 (1:400, Cell Signaling Technology,1 Y705 D3A7) in a humidified chamber overnight at 4°C. Signal Stain1 Boost IHC Reagents (horseradish peroxidase, mouse or rabbit) were used as secondary antibodies. Slides then were rinsed in phosphate‐buffered saline (PBS) and stained with 3, 3′‐diaminobenzidine (DAB, Cell Signaling Technology1) for 1 minute at room temperature. Slides were rinsed with distilled water and dehydrated through graded alcohols and xylene. Sections were counterstained with Mayer's hematoxylin solution. A simple canine mammary carcinoma was used as a positive control for STAT3 and p‐STAT3 (Fig S1) as previously described.14 Negative control was achieved by incubating samples with 1% goat serum without anti‐STAT3 or anti‐p‐STAT3 antibody (Fig S1). Immunolabeling for STAT3 and p‐STAT3 was evaluated by light microscopy to determine the percentage of positive immunolabelled cells. Five representative fields of each sample were evaluated at 60× high power field. The mean percentage of positive cells per sample was calculated by averaging the percentage of positive cells/total cells counted in each of the 5 representative fields.
To evaluate cytoplasmic versus nuclear localizations of STAT3 and p‐STAT3, a minimum of 100 STAT3 or p‐STAT3 positively stained cells were evaluated per 1 60× high power field, and a total of 5 representative fields of each sample was assessed. Staining patterns were classified as nuclear, cytoplasmic, or both. All immunohistochemical samples were evaluated and quantified by a board‐certified veterinary pathologist with ImageJ software.2
Fine needle aspirates, cell lysate preparation and Western blot
Two normal canine peripheral lymph nodes, 1 reactive lymph node and 6 canine DLBCLs, were aspirated by standard trephination technique using 20 gauge needles. The aspirated cells were flushed into C10 medium containing Roswell Park Memorial Institute (RMPI)1640 with 10% fetal bovine serum, 1× penicillin/streptomycin, 1× nonessential amino acids, 1× L‐glutamine, 1 mM sodium pyruvate, and 1× 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid (HEPES). Cells were centrifuged at 1.5 × 103 revolutions per minute (RPM) for 5 minutes at 4°C and were resuspended in ammonium‐chloride‐potassium lysing buffer for red blood cell (RBC) lysis. After RBC lysis, cells were washed once with 1× PBS and lysed in prechilled radioimmunoprecipitation assay (RIPA) buffer (Tris‐HCL 25 mM, NaCL 150 mM, nonyl phenoxypolyethoxylethanol (NP)‐40 1%, sodium dodecyl sulfate 0.1% and sodium deoxycholate 1%) with 1× phenylmethylsulfonyl fluoride (PMSF), 1× Halt protease inhibitor cocktail (Thermo Fisher4) and 1 × Halt™ phosphatase inhibitor cocktail (Thermo Fisher3) and incubated on ice for 20 minutes. The supernatant was collected after full‐speed centrifugation for 15 minutes at 4°C. The protein concentrations were checked by a standard bicinchoninic acid assay (BCA) method (Pierce™ BCA Protein Assay Kit4). Total cell lysates were mixed with 4× Laemmli sample buffer (Bio‐rad5) and 30 μg protein was loaded for each sample. Sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE), Western blotting, and peroxidase‐based chemiluminescent detection were performed according to standard laboratory protocols. Antibodies used for blotting were as follows: mouse anti‐STAT3 (1:1,000, Cell Signaling Technology1, 124H6), rabbit anti‐p‐STAT3 (1:500, Cell Signaling Technology1, Y705 D3A7), rabbit anti‐ERK1/2 (1:2,000, Cell Signaling Technology1, #9102), rabbit anti‐p‐ERK1/2 (1:2,000, Cell Signaling Technology1, #9101), and mouse anti‐β‐actin (1:2,000, Sigma‐Aldrich6, AC‐15).
Statistical analysis
All Cox proportional hazard survival models were fit using the coxph function, and included only main effects of stage (III, IV, V), substage (a or b), and dichotomized STAT3 or p‐STAT3. High values of STAT3 and p‐STAT3 were defined as ≥0.76 for STAT3, or ≥0.15 for p‐STAT3. Statistical analyses in IHC staining were conducted by using GraphPad Prism v6.05 (GraphPad Software7). One‐way analysis of variance (ANOVA) followed by Tukey's posthoc test was used to compare STAT3 and p‐STAT3 expression in the different canine tissues. P values ≤0.05 were considered significant.
Results
Patient demographics
All 43 canine patients with DLBCL were followed at the University of Wisconsin‐Madison Veterinary Teaching Hospital. The median age of patients was 8.1 years (range, 0.6–13.3 years). The mean weight of the patients was 27.8 kg (range, 3.9 kg–50.5 kg). The majority of patients were either female spayed or male castrated (Table 1). Forty‐two of 43 patients had complete or partial lymphoma staging performed at the initial diagnosis. Of the 42 patients, all had CBC and biochemistry profile performed, 27 had urinalyses, 37 had thoracic radiographs, 10 had abdominal ultrasound examination, and 15 had bone marrow aspirates collected (Table 2). Other staging tests included multidrug‐resistant (MDR) mutation screening (2 patients), PCR of antigen rearrangement (1 patient), electrocardiogram (ECG) (1 patient) and prothrombin time (PT) and partial thromboplastin time (PTT) (1 patient). Most laboratory test results were normal. The most common abnormal CBC findings were as follows: low‐grade anemia (5/42), thrombocytopenia (4/42), and atypical mononuclear cells (6/42). The most common abnormal biochemistry findings were increased ALT and ALP activity (5/42) and low serum albumin concentration (3/42). Of the 37 patients that had thoracic radiographs, 10 had normal results. Nineteen had sternal, mediastinal or tracheobronchial lymphadenopathy, 3 patients had radiographic evidence of lymphoma infiltration into the lung, and 2 patients had pleural effusion. Other abnormal thoracic radiographic findings included hepatomegaly (4/37), pulmonary fibrosis (1/37), chronic rib fractures (3/37), cardiomegaly (2/37), and peritoneal effusion (1/37). Of the 10 patients that had abdominal ultrasound examination, 7 had abdominal lymphadenopathy and 5 had infiltrative lesions in the spleen. Other abnormal findings on abdominal ultrasound examination included hepatomegaly (2/10), adrenal mass (1/10), and nephrolithiasis (1/10). Of the 15 patients that had bone marrow aspirates performed, 13 had normal results, 1 patient had lymphoma infiltration into the bone marrow, and 1 patient had mild plasma cell hyperplasia. Twenty‐seven dogs were classified as stage III, 12 dogs as stage IV, and 4 dogs as stage V. Of the 43 dogs, 38 were substage a, and remaining 5 were substage b. Three patients had 1 other type of tumor histology in addition to lymphoma and those included mixed mammary adenoma, jejunal adenocarcinoma, and splenic leiomyosarcoma (Table 1).
Table 1.
Demographic characteristics for the overall 43 canine patients with DLBCL
| Age at Diagnosis (years) | |
| Mean | 8.2 |
| Median | 8.1 |
| Range | 0.6–13.3 |
| Sex | |
| MN | 19 |
| FS | 19 |
| MI | 5 |
| Breed | |
| Golden retriever | 5 |
| Labrador retriever | 6 |
| Other pure breda | 12 |
| Mixed breed | 9 |
| Weight (KG) | |
| Mean | 27.8 |
| Median | 29 |
| Range | 3.9–50.5 |
| Stage | |
| III | 27 |
| IV | 12 |
| V | 4 |
| Substage | |
| a | 38 |
| b | 5 |
Other pure‐bred dogs included 3 beagles, 2 border collies, 2 standard poodles, 2 vizslas, 2 West Highland white terriers, and 1 each American foxhound, Boston terrier, Briard, Brittany, Bernese mountain dog, dalmatian, doberman pinscher, German shepherd, Irish setter, papillon, and pit bull terrier
Table 2.
Summary of staging diagnostic tests for high STAT3, low STAT3, high p‐STAT3 and low p‐STAT3 and all canine patients with DLBCL
| Parameters | High STAT3 (n = 26) | Low STAT3 (n = 17) | High p‐STAT3 (n = 18) | Low p‐STAT3 (n = 25) | Total |
|---|---|---|---|---|---|
| Thoracic radiograph | 88% (23/26) | 82% (14/17) | 78% (14/18) | 92% (23/25) | 86% (37/43) |
| Abdominal ultrasound | 23% (6/26) | 23% (4/17) | 5% (1/18) | 36% (9/25) | 23% (10/43) |
| Bone marrow aspiration | 38% (10/26) | 29% (5/17) | 22% (4/18) | 44% (11/25) | 35% (15/43) |
| CBC | 100% (26/26) | 94% (16/17) | 94% (17/18) | 100% (25/25) | 97% (42/43) |
| Chemistry profile | 100% (26/26) | 94% (16/17) | 94% (17/18) | 100% (25/25) | 97% (42/43) |
| Urinalysis | 69% (18/26) | 52% (9/17) | 61% (11/18) | 64% (16/25) | 63% (27/43) |
STAT 3 and p‐STAT3 are up‐regulated in DLBCL of dogs
To determine STAT3 and its active form p‐STAT3 expressions, 43 naïve canine DLBCL primary tumors from 38 popliteal, 2 submandibular, 2 prescapular, and 1 inguinal lymph nodes were evaluated by immunohistochemistry. Ten normal canine lymph nodes and 10 reactive lymph nodes were used as controls. For internal staining control, simple mammary carcinoma was used as a positive control as previously described,14 and normal canine lymph node tissue stained without STAT3 or p‐STAT3 primary antibodies was used as negative control (Fig S1). In canine DLBCL, 76% and 15% of cells stained positive for STAT3 and p‐STAT3, respectively. In contrast, only 49% and 3.9% of cells stained positive for STAT3 and p‐STAT3 in normal canine lymph nodes (Table 3). In reactive lymph nodes, 64% and 11.5% of cells stained positive for STAT3 and p‐STAT3, respectively. A statistically significant increase of both STAT3 (P = 0.0057) and p‐STAT3 (P = 0.006)‐positive cells was identified in DLBCL when compared with normal canine lymph nodes. No statistically significant difference was observed between the DLBCL and reactive lymph nodes (STAT3, P = 0.3142; p‐STAT3, P = 0.5373), although the absolute percentages of positively stained cells were higher in DLBCLs than in reactive lymph nodes (Fig 1). The 43 lymphoma patients were further divided into 4 cohorts based on high STAT3, low STAT3, high p‐STAT3, or low p‐STAT3 expression. In the canine DLBCL samples tested, STAT3 was expressed in a mean of 76% of the cells, and p‐STAT3 was expressed in a mean of 15% of the cells. The cut‐off of high versus low expression was based on the mean percentage of cells expressing STAT3 (76%) and p‐STAT3 (15%) in canine DLBCL samples (Table 3). No significant differences in age, sex, breed, weight, stage, or substage were found between high versus low STAT3 or p‐STAT3 cohorts (Tables 4, 5). Our data showed a higher percentage of STAT3 and p‐STAT3 immunolabelled cells in canine DLBCL compared with normal canine lymph nodes, and the mean percentage of STAT3 or p‐STAT3 was not associated with age, sex, breed, clinical stage, or substage.
Table 3.
The mean percentage of STAT3 or p‐STAT3 immunolabelled cells in canine DLBCL, normal canine lymph nodes, or reactive lymph nodes
| Sample | STAT3 | p‐STAT3 |
|---|---|---|
| Normal lymph node (n = 10) | 49.16% | 3.91% |
| Reactive lymph node (n = 10) | 63.97% | 11.58% |
| DLBCL (n = 43) | 76.31% | 15.42% |
Figure 1.
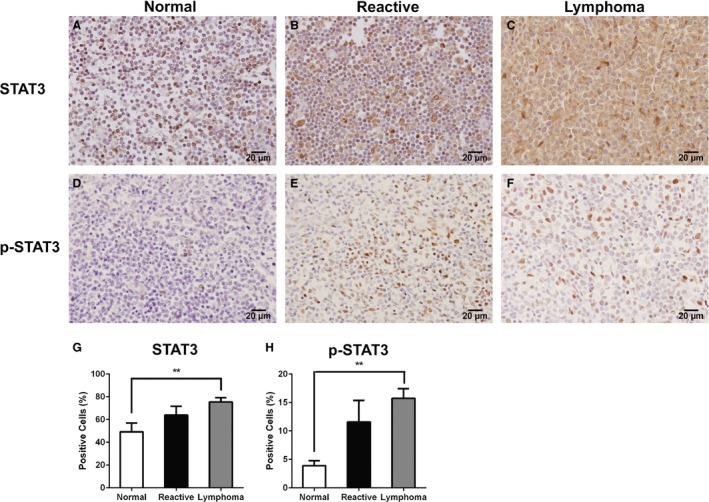
STAT3 and phosphorylated STAT3 (p‐STAT3) are up‐regulated in canine DLBCL. Immunohistochemistry was conducted to evaluate expression of STAT3 (A‐C) and p‐STAT3 (D‐F) proteins in canine normal lymph nodes (A, D), canine reactive lymph nodes (B, E), and canine DLBCL (C, F). Scale bars correspond to 20 μm. (G, H) Mean (±SD) percentage of STAT3 and p‐STAT3 immunolabelled cells. Compared with normal canine lymph nodes, there was a statistical increase in STAT3 and p‐STAT3 immunolabelled cells in canine DLBCL. Normal lymph nodes (n = 10), reactive lymph nodes (n = 10) and DLBCL (n = 43) (**P < 0.01).
Table 4.
Demographic characteristics of canine DLBCL patients with high versus low percentage of STAT3
| Variables | High STAT3 (n = 26) | Low STAT3 (n = 17) | P value |
|---|---|---|---|
| Age at diagnosis (years) | |||
| Mean | 8.4 | 7.9 | 0.5944 |
| Median | 8.5 | 8.1 | |
| Range | 3.4–13.3 | 0.6–12.2 | |
| Sex | |||
| MN | 11 | 8 | 0.6366 |
| FS | 11 | 8 | |
| MI | 4 | 1 | |
| Breed | |||
| Golden retriever | 1 | 4 | 0.1873 |
| Labrador retriever | 6 | 0 | |
| Beagle | 2 | 1 | |
| Other pure breda | 13 | 7 | |
| Mixed breed | 4 | 5 | |
| Weight (KG) | |||
| Mean | 28.8 | 26.3 | 0.5039 |
| Median | 30.7 | 29 | |
| Range | 6.8–43 | 3.9–50.5 | |
| Stage | |||
| III | 16 | 11 | |
| IV | 9 | 3 | 0.1603 |
| V | 1 | 3 | |
| Substage | |||
| a | 25 | 14 | 0.2862 |
| b | 1 | 3 | |
Other pure‐bred dogs included 3 beagles, 2 border collies, 2 standard poodles, 2 vizslas, 2 West Highland white terriers, and 1 each American foxhound, Boston terrier, Briard, Brittany, Bernese mountain dog, dalmatian, doberman pinscher, German shepherd, Irish setter, papillon, and pit bull terrier
Table 5.
Demographic characteristics of canine DLBCL patients with high versus low percentage of p‐STAT3
| Variables | High p‐STAT3 (n = 18) | Low p‐STAT3 (n = 25) | P value |
|---|---|---|---|
| Age at biopsy (years) | |||
| Mean | 8.6 | 7.9 | 0.4241 |
| Median | 9.1 | 8.1 | |
| Range | 3.8–12.0 | 0.6–13.3 | |
| Sex | |||
| MN | 6 | 13 | 0.2008 |
| FS | 8 | 9 | |
| MI | 0 | 3 | |
| Breed | |||
| Golden retriever | 1 | 3 | 0.6414 |
| Labrador retriever | 5 | 4 | |
| Other pure breda | 8 | 15 | |
| Mixed breed | 4 | 3 | |
| Weight (KG) | |||
| Mean | 26.5 | 28.4 | 0.5944 |
| Median | 29 | 31.2 | |
| Range | 7.8–50.5 | 3.9–46.8 | |
| Stage | |||
| I‐III | 11 | 16 | 0.9304 |
| IV | 5 | 7 | |
| V | 2 | 2 | |
| Substage | |||
| a | 16 | 23 | 0.2829 |
| b | 2 | 2 | |
Other pure‐bred dogs included 3 beagles, 2 border collies, 2 standard poodles, 2 vizslas, 2 West Highland white terriers, and 1 each American foxhound, Boston terrier, Briard, Brittany, Bernese mountain dog, dalmatian, doberman pinscher, German shepherd, Irish setter, papillon, and pit bull terrier
Canine DLBCL has higher STAT3 nuclear expression compared with normal or reactive lymph nodes
Because STAT3 is activated by phosphorylation at Tyr705, which induces STAT3 dimerization, nuclear translocation and DNA binding, nuclear localization of STAT3 is an indicator of activated STAT3 pathway. We further evaluated STAT3 and p‐STAT3 nuclear versus cytoplasmic expression in canine DLBCL. Although p‐STAT3 expression was exclusively nuclear in all canine DLBCL, reactive lymph node and normal lymph node samples, total STAT3 was expressed either exclusively in the cytoplasm or within both the cytoplasm and nucleus (Fig 2). Furthermore, canine DLBCL had significantly higher nuclear STAT3 staining compared with normal or reactive canine lymph node (Fig 2 G). In canine DLBCL, 98% of STAT3 immunolabelled cells showed both a cytoplasmic and nuclear staining pattern and 2% of cells only showed cytoplasmic staining. In contrast, 70% of STAT3 immunolabelled cells in normal canine lymph node showed both cytoplasmic and nuclear staining, and 30% of cells were stained exclusively in the cytoplasm (Table 6). Canine DLBCL had significantly higher STAT3 nuclear expression compared with normal lymph node (P < 0.0001) or reactive lymph node (P < 0.0001; Fig 2 G).
Figure 2.
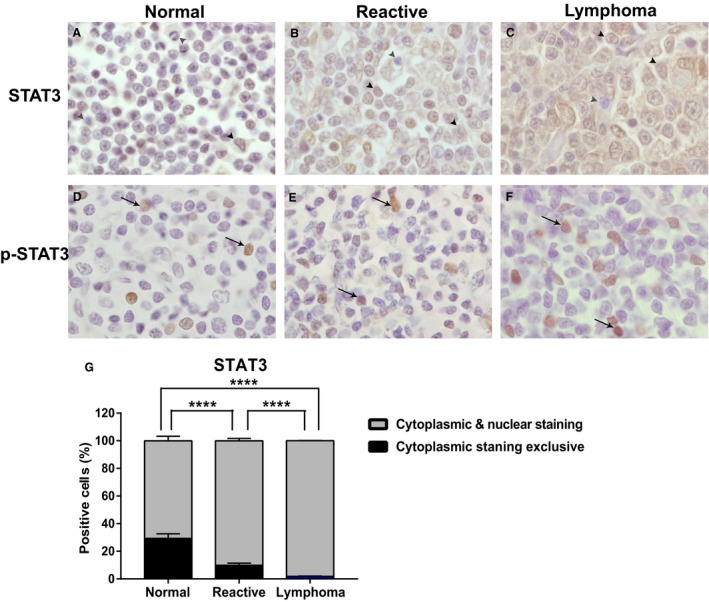
Total STAT3 has an increased nuclear expression in canine DLBCL. Immunohistochemistry was conducted to evaluate nuclear versus cytoplasmic expression of STAT3 (A‐C) and p‐STAT3 (D‐F) in canine normal lymph nodes (A, D), canine reactive lymph nodes (B, E), and canine DLBCL (C, F). (G) Mean (±SD) percentage of cytoplasmic staining versus both cytoplasmic and nuclear staining in STAT3 immunolabelled cells. Red arrow head: cytoplasmic staining, black arrow: nuclear staining, black arrow head: both cytoplasmic and nuclear staining. Normal lymph nodes (n = 10), reactive lymph nodes (n = 10) and DLBCL (n = 42) (***P < 0.0001).
Table 6.
The mean percentage of STAT3 immunolabelled cells with cytoplasm exclusive staining, or with both cytoplasm and nucleus staining
| Sample | Cytoplasm exclusive | Cytoplasm and nucleus |
|---|---|---|
| Normal lymph node (n = 10) | 29.4% | 70.6% |
| Reactive lymph node (n = 10) | 9.8% | 90.2% |
| DLBCL (n = 42) | 1.9% | 98.1% |
Mitogen‐activated protein kinase ERK1/2 is up‐regulated in canine DLBCL
Because STAT3 transcriptional activation also can be regulated by phosphorylation at Ser727 by the mitogen‐activated protein kinase (MAPK) pathway, we evaluated the p44/42 MAPK (Erk1/2) signaling pathway in primary canine DLBCL. Cells from 6 cytologically diagnosed canine DLBCL (Fig 3, samples 4–9), 2 normal canine lymph nodes (Fig 3, samples 1 and 2), and 1 reactive lymph node (Fig 3, sample 3) were obtained by fine needle aspiration. Immunophenotype of B cell origin was confirmed either by flow cytometry or immunocytochemistry using CD3 and CD20 markers. Western blot analyses were conducted with primary antibodies against total STAT3, p‐STAT3, total ERK1/2, or p‐ERK1/2. Phosphorylated ERK1/2 (p‐ERK1/2) was up‐regulated in 5 of 6 canine lymphoma samples (Fig 3, samples 4–7 and 9) compared with normal lymph node (samples 1–2) or reactive lymph node controls (sample 3). In addition, 4 of 6 lymphoma samples (Fig 3, samples 4–6 and 9) showed higher total ERK expression compared with normal lymph nodes (samples 1–2). Consistent with the IHC results (Fig 1), p‐STAT3 was expressed at a higher level in most of the lymphoma samples (Fig 3, samples 4,6, 7 and 9) when compared with the normal lymph nodes (samples 1–2), but not when compared with the reactive lymph node (sample 3).
Figure 3.
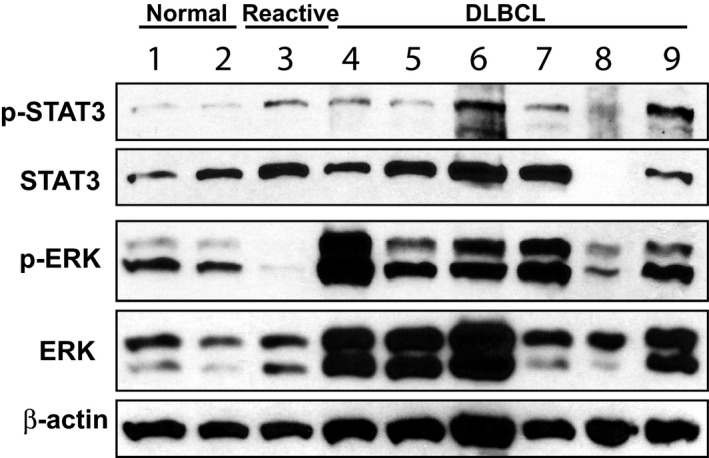
p‐ERK1/2 and p‐STAT3 are up‐regulated in canine diffuse large B cell lymphoma (DLBCL). Immunoblots for p‐STAT3, total STAT3, p‐ERK1/2 and total ERK1/2 in normal canine lymph node (1–2), reactive lymph node (3), and canine DLBCLs (4–9). Beta‐actin was used as a loading control.
Discussion
Although the JAK/STAT pathway has been shown to play a critical role in cancer biology in humans, this pathway has not been well studied in veterinary patients with naturally occurring cancers. Earlier veterinary studies have evaluated the expression of STAT3 and p‐STAT3 in malignant neoplasia of dogs.15, 16, 17 p‐STAT3 expression is significantly higher in canine metastatic mammary tumors compared with nonmetastatic tumors.15 STAT3 activation contributes to survival and proliferation of canine osteosarcoma cell lines in vitro.16 STAT3 also is overexpressed in canine hemangiosarcomas compared with canine hemangiomas.17 Our study shows that the percentage of total STAT3 and p‐STAT3 immunolabelled cells was significantly higher in canine DLBCL patients compared with normal lymph nodes, and that canine DLBCL had more nuclear expression of total STAT3 compared with normal lymph nodes. In addition to increased p‐STAT3 protein expression, canine DLBCL had higher total ERK and p‐ERK expression. Therefore, the JAK‐STAT pathway is up‐regulated in canine DLBCL.
In our study, STAT3 expression and activation were higher in canine reactive lymph nodes compared with normal lymph node control (Figs 1, 3). This observation can be explained by the active role of the STAT3 pathway in inflammation, infection, and immune‐mediated processes.18, 19 Tumor‐promoting inflammation is a fundamental hallmark of cancer.20 The correlation among inflammation, immunity, and cancer development is evident in many solid tumors and hematopoietic malignancies.18, 19, 21 The activated STAT3 pathway is critical for T cell differentiation and memory.19 In addition, STAT3 can induce a procarcinogenic inflammatory microenvironment.18 Taken together, strong evidence suggests that the STAT3 pathway may be crucial in producing a cancer inflammatory microenvironment.
Avoiding immune destruction is another essential hallmark of cancer.20 A key feature in the interaction of cancer cells with the tumor microenvironment is their ability to suppress an antitumor immune response. STAT3 signaling can suppress antitumor immunity by decreasing the activity of dendritic cells, T cells and natural killer cells, but increasing the function of tumor‐infiltrating regulatory T cells (Treg).22 Therapies targeting STAT3 in tumor‐infiltrating immune cells may block expression of many tumor‐associated factors, neutralize the tumor‐induced immunosuppressive microenvironment and thereby contribute to antitumor immunity. Simultaneous usage of small molecule drugs such as JAK inhibitors in combination with other pathway inhibitors or standard cytotoxic chemotherapies has promising therapeutic potential. For example, concomitant administration of the JAK1/2 inhibitor, AZD1480 with cediranib (vascular endothelial growth factor inhibitor) was demonstrated to significantly decrease glioma tumor volume and microvascular density in people.23 Blockage of the STAT3 pathway in a controlled manner may reverse immune suppression, activate immune response, and improve the efficacy of immune‐therapeutic approaches.
Studies of people with DLBCL show that high nuclear expression of STAT3 and p‐STAT3 is correlated with overall shorter survival and poor prognosis.13, 24 This observation may be explained in part by the multiple cellular functions of STAT3 in cell survival, differentiation, proliferation, invasion, angiogenesis, and metastasis.12 A correlation has been observed between p‐STAT3 and the antiapoptotic protein survivin in DLBCL of humans, and p‐STAT3 and survivin expression both are important prognostic factors. In a cohort of patients negative for p‐STAT3, median survival time (MST) was 163.5 months compared with a MST of 22.2 months in patients positive for p‐STAT3.24 p‐STAT3 expression also is more commonly observed in ABC‐DLBCL and is associated with more advanced clinical stage, involvement of multiple extranodal sites, and unfavorable prognosis.13 Our study shows that STAT3 and p‐STAT3 expression in canine DLBCL is up‐regulated and can be variable. It will be informative to further evaluate the impact of STAT3 and p‐STAT3 expression on clinical outcome. High STAT3 and p‐STAT3 expression may be associated with poor prognosis and shorter median survival time. A prospective analysis of larger patient cohorts with standardized treatment protocols will be necessary to answer this question.
Multiple small molecule JAK inhibitors currently are under investigation in clinical trials for treating myeloproliferative disease and leukemia in humans.25, 26, 27, 28 In veterinary medicine, the JAK1/2 inhibitor oclacitinib (Apoquel8) was approved by the Food and Drug Administration in 2013 for treating dogs with atopic dermatitis.25, 29, 30, 31 Our finding that the STAT3 pathway is up‐regulated in canine DLBCL is encouraging because it supports further exploration of the potential therapeutic effect of JAK2 inhibitors in canine patients with DLBCL. Like most small molecule inhibitors, JAK inhibitors may achieve maximum therapeutic effects when combined with cytotoxic chemotherapy drugs. Future phase I/II clinical trials for cytotoxic chemotherapy combined with JAK2 inhibitors are warranted.
Conclusions
Our results show that a higher percentage of STAT3 and p‐STAT3 immunolabelled cells in canine DLBCL compared with canine normal lymph node. Although p‐STAT3 expression is exclusively nuclear, canine DLBCL has higher nuclear expression of total STAT3 than normal or reactive lymph nodes. In addition to up‐regulated p‐STAT3 expression, canine DLBCL expresses higher amounts of p‐ERK1/2. Therefore, we conclude that the JAK/STAT pathway is up‐regulated in dogs with DLBCL. Our data support further investigations into the use of JAK inhibitors to treat dogs with DLBCL and may provide a new therapeutic direction for treating this common hematologic malignancy of dogs.
Supporting information
Figure S1. Immunohistochemistry was conducted to evaluate the expression of STAT3 (A, B) and p‐STAT3 (C, D) in canine simple mammary carcinoma. Scale bars correspond to 20 μm. Simple canine mammary carcinoma was used as a positive control for STAT3 and p‐STAT3. Negative control was achieved by incubating samples with 1% goat serum without anti‐STAT3 or anti‐p‐STAT3 antibody.
Acknowledgments
The work was conducted at the University of Wisconsin‐Madison, and was supported by start‐up funds from UW‐Madison, and NIH grants K01OD020153‐01A1 and T35OD011078. We thank the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) for use of its Shared Services (Histology Lab and Biostatistics center) to complete this research. The data were presented in part as a research abstract at the 2016 ACVIM Forum, Denver, CO.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Footnotes
Cell Signaling Technology, Danvers, MA
NIH Image, Bethesda, MA
Thermo Scientific Pierce Protein Biology, Madison, WI
Thermo Fisher Scientific, Madison, WI
Bio‐Rad Laboratories, Hercules, CA
Sigma‐Aldrich Corporation, St. Louis, Missouri
GraphPad Software, La Jolla, CA
Zoetis, Parsippany‐Troy Hills, NJ
References
- 1. Valli VE, San Myint M, Barthel A, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol 2011;48:198–211. [DOI] [PubMed] [Google Scholar]
- 2. Hosoya K, Kisseberth WC, Lord LK, et al. Comparison of COAP and UW‐19 protocols for dogs with multicentric lymphoma. J Vet Intern Med 2007;21:1355–1363. [DOI] [PubMed] [Google Scholar]
- 3. Valerius KD, Ogilvie GK, Mallinckrodt CH, et al. Doxorubicin alone or in combination with asparaginase, followed by cyclophosphamide, vincristine, and prednisone for treatment of multicentric lymphoma in dogs: 121 cases (1987‐1995). J Am Vet Med Assoc 1997;210:512–516. [PubMed] [Google Scholar]
- 4. Keller ET, MacEwen EG, Rosenthal RC, et al. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. J Vet Intern Med 1993;7:289–295. [DOI] [PubMed] [Google Scholar]
- 5. Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non‐Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug‐mediated apoptosis. Clin Cancer Res 2003;9:316–326. [PubMed] [Google Scholar]
- 6. Amin HM, McDonnell TJ, Ma Y, et al. Selective inhibition of STAT3 induces apoptosis and G(1) cell cycle arrest in ALK‐positive anaplastic large cell lymphoma. Oncogene 2004;23:5426–5434. [DOI] [PubMed] [Google Scholar]
- 7. Ding BB, Yu JJ, Yu RY, et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B‐cell subtype of diffuse large B‐cell lymphomas. Blood 2008;111:1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinberg RA. Cytoplasmic Signaling Circuitry Programs Many of the Traits of Cancer. Biology of Cancer, 2nd ed 2014:175–229. [Google Scholar]
- 9. Kralovics R, Passamonti F, Buser AS, et al. A gain‐of‐function mutation of JAK2 in myeloproliferative disorders. New Engl J Med 2005;352:1779–1790. [DOI] [PubMed] [Google Scholar]
- 10. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–1061. [DOI] [PubMed] [Google Scholar]
- 11. Scott LM, Gandhi MK. Deregulated JAK/STAT signalling in lymphomagenesis, and its implications for the development of new targeted therapies. Blood Rev 2015;29:405–415. [DOI] [PubMed] [Google Scholar]
- 12. Wu ZL, Song YQ, Shi YF, et al. High nuclear expression of STAT3 is associated with unfavorable prognosis in diffuse large B‐cell lymphoma. J Hematol Oncol 2011;4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ok CY, Chen J, Xu‐Monette ZY, et al. Clinical implications of phosphorylated STAT3 expression in De Novo diffuse large B‐cell lymphoma. Clin Cancer Res 2014;20:5113–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teng SP, Hsu WL, Chiu CY, et al. Overexpression of P‐glycoprotein, STAT3, phospho‐STAT3 and KIT in spontaneous canine cutaneous mast cell tumours before and after prednisolone treatment. Vet J 2012;193:551–556. [DOI] [PubMed] [Google Scholar]
- 15. Krol M, Pawlowski KM, Dolka I, et al. Density of Gr1‐positive myeloid precursor cells, p‐STAT3 expression and gene expression pattern in canine mammary cancer metastasis. Vet Res Commun 2011;35:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fossey SL, Bear MD, Kisseberth WC, et al. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer 2011;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petterino C, Rossetti E, Drigo M. Immunodetection of the signal transducer and activator of transcription‐3 in canine haemangioma and haemangiosarcoma. Res Vet Sci 2006;80:186–188. [DOI] [PubMed] [Google Scholar]
- 18. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009;9:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 2013;368:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 21. He G, Karin M. NF‐kappaB and STAT3 ‐ key players in liver inflammation and cancer. Cell Res 2011;21:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 2005;11:1314–1321. [DOI] [PubMed] [Google Scholar]
- 23. de Groot J, Liang J, Kong LY, et al. Modulating antiangiogenic resistance by inhibiting the signal transducer and activator of transcription 3 pathway in glioblastoma. Oncotarget 2012;3:1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sung J‐Y, Lim S‐J, Kim YW, et al. Prognostic significance of pSTAT3 and Survivin expression in diffuse large B‐cell lymphoma. Basic Appl Pathol 2010;3:7–13. [Google Scholar]
- 25. Cook AM, Li L, Ho Y, et al. Role of altered growth factor receptor‐mediated JAK2 signaling in growth and maintenance of human acute myeloid leukemia stem cells. Blood 2014;123:2826–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rutgen BC, Hammer SE, Gerner W, et al. Establishment and characterization of a novel canine B‐cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk Res 2010;34:932–938. [DOI] [PubMed] [Google Scholar]
- 27. Tefferi A, Pardanani A. JAK inhibitors in myeloproliferative neoplasms: rationale, current data and perspective. Blood Rev 2011;25:229–237. [DOI] [PubMed] [Google Scholar]
- 28. Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 2013;27:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gadeyne C, Little P, King VL, et al. Efficacy of oclacitinib (Apoquel(R)) compared with prednisolone for the control of pruritus and clinical signs associated with allergic dermatitis in client‐owned dogs in Australia. Vet Dermatol 2014;25:512–518, e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cosgrove SB, Wren JA, Cleaver DM, et al. A blinded, randomized, placebo‐controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel(R)) in client‐owned dogs with atopic dermatitis. Vet Dermatol 2013;24:587–597, e141–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonzales AJ, Bowman JW, Fici GJ, et al. Oclacitinib (APOQUEL((R))) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J Vet Pharmacol Ther 2014;37:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Immunohistochemistry was conducted to evaluate the expression of STAT3 (A, B) and p‐STAT3 (C, D) in canine simple mammary carcinoma. Scale bars correspond to 20 μm. Simple canine mammary carcinoma was used as a positive control for STAT3 and p‐STAT3. Negative control was achieved by incubating samples with 1% goat serum without anti‐STAT3 or anti‐p‐STAT3 antibody.


