Abstract
Background
Hepatic circulatory disturbances have been associated with obesity and fatty liver in humans. In the veterinary literature, however, there is limited information regarding the effects of different body condition scores (BCS) on liver hemodynamic indices in dogs.
Objectives
To investigate the influence of BCS on liver hemodynamic indices.
Animals
Fifty‐three client‐owned dogs of various breeds were included.
Methods
Prospective observational study. Dogs were divided into 3 BCS groups using a 5‐point scale: G1 – 12 ideal dogs, G2 – 21 overweight dogs, G3 – 20 obese dogs. Mean portal velocity (MPV), portal blood flow volume (PBFV), portal congestion index (PCI), hepatic artery resistivity index (HARI), and hepatic vein (HV) spectral wave were obtained by pulsed Doppler sonography. Alkaline phosphatase (ALP), gamma‐glutamyl transferase (GGT), and alanine aminotransferase (ALT) activities were determined. Liver enzymes activities and liver hemodynamic indices were compared among groups.
Results
Obese dogs had lower MPV, higher percentage of abnormal hepatic vein spectral wave and higher median ALP activity than did ideal dogs (P < 0.05). Overweight and obese dogs had lower PBFV than ideal dogs (P < 0.01). Overweight dogs had higher median GGT activity than ideal dogs (P < 0.05). No difference was observed for PCI, HARI and median ALT activity among the groups.
Conclusions and Clinical Importance
Obesity was associated with changes in portal vein indices and in HV spectral wave. These changes were accompanied by significant differences in some liver enzymes activities and could be a sign of early liver disease.
Keywords: Canine, Doppler, Hepatic disease, Obesity
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AO
aorta
- BCS
body condition score
- CVC
caudal vena cava
- GGT
gamma‐glutamyl transferase
- HARI
hepatic artery resistivity index
- HV
hepatic vein
- MPV
mean portal velocity
- PBFV
portal blood flow volume
- PCI
portal congestion index
- PVA
portal vein area
- PVD
portal vein diameter
- PV
portal vein
- W
weight
Obesity in dogs has increased considerably and is considered the major nutritional disease in companion animals.1 An overweight dog is considered clinically obese when its weight is 15% above the ideal weight,1 and it is estimated that 22–40% of dogs presented to veterinary clinics are overweight or obese.2 These high rates also reflect the rise in the obesity incidence of the worldwide human population.3 Obesity is not only associated with the accumulation of excessive body fat, but also with the development of many other diseases.3 In addition to orthopedic, urogenital and oncological disorders,4 excessive weight gain may lead to dysfunction of the heart5 and lungs,6 and to metabolic and hormonal changes in dogs.3
Visceral obesity and metabolic syndrome are closely associated with progressive fatty liver infiltration.7 Diffuse fatty liver disease, previously reported as a benign condition, is the most prevalent chronic liver disease in human patients and may progress to more severe forms of liver disease.7 Fatty liver also can affect overweight and obese dogs.8 Recently, the role of adipose tissue accumulation in metabolic syndrome and insulin resistance has been investigated in veterinary medicine.9
In veterinary clinical practice, abdominal sonography is a reliable method for assessment of liver diseases.10 In human patients, abdominal sonography has high sensitivity and specificity in the diagnosis of fatty liver in asymptomatic overweight individuals who present with incidentally found abnormal liver enzyme activity.11 Doppler modality has been used widely for characterization and monitoring of fatty liver and other obesity‐related liver lesions in human patients.12, 13, 14 Previous studies showed that mean portal velocity (MPV) was statistically lower and abnormal pattern of the hepatic vein (HV) waveform was statistically higher in human patients with fatty liver infiltration compared to healthy individuals.13 Other studies found a significant inverse correlation between grade of fatty liver based on histology and hepatic artery resistivity index (HARI)14 and between grade of fatty liver and MPV.15 Some authors suggest that impaired hepatic blood flow in obese patients occurs as a result of decreased vascular compliance.12
An ultrasonographic study in overweight and obese cats showed that liver was hyperechoic compared to fat of the falciform ligament.16 However, hyperechogenicity of hepatic parenchyma is not specific for fatty infiltration.17 Other liver conditions that may be associated with obesity, including steroid hepatopathy, can cause increased liver echogenicity.18 Furthermore, ultrasonography has limited ability to detect steatosis if <15 to 30% of the hepatocytes are affected. Therefore, patients with mild fatty liver infiltration can have normal attenuation of liver parenchyma on ultrasound examination.19 In addition, assessment of liver parenchyma by means of B‐mode sonography is a subjective method, which may vary according to the examiner.20 On the other hand, Doppler spectral analysis and quantification of hemodynamic indices is associated with greater objectivity and smaller interobserver and interequipment variability.21 Pulsed Doppler sonography may provide additional information and may aid in the final diagnosis when combined with B‐mode sonography and supplementary diagnostic tests.22, 23
Although obese dogs also are predisposed to fatty liver development because of dietary excess,24 and the incidence of obesity is rapidly increasing in small animals,1 there is limited information about obesity‐related liver disease and its sonographic assessment in small animals.16, 25
The aim of our study was to determine whether overweight and obesity influence Doppler liver hemodynamic indices. We hypothesized that obesity in dogs leads to significant changes in MPV (mean portal velocity), PBFV (portal blood flow volume), PCI (portal congestion index), HARI (hepatic artery resistivity index), and hepatic vein (HV) spectral wave.
Materials and Methods
Animals
This prospective observational study was performed at the School of Veterinary Medicine and Animal Science, Sao Paulo State University, Botucatu, Sao Paulo State, Brazil. Recruitment and data collection were performed during 1 year. The base population comprised client‐owned dogs presented to the veterinary hospital. Written informed consent was obtained from the owners. Dogs were assigned a body condition score (BCS) and were allocated into 1 of 3 groups, according to a 1‐to 5‐point scale, previously proposed:26
Group 1 (G1)—ideal dogs (control group) with a BCS equal to 3.
Group 2 (G2)—overweight dogs with BCS equal to 4;
Group 3 (G3)—obese dogs with BCS equal to 5.
Animals included in G1 were considered healthy based on physical examination, hematological and biochemical tests, thoracic radiographs, Doppler echocardiography, and abdominal sonography. Dogs included in G2 and G3 were submitted to the same diagnostic tests as those performed in dogs of G1. Inclusion was based on the presence of overweight or obesity without other comorbidities that could influence the study. Exclusion criteria consisted of previous treatment with hepatotoxic drugs, previous diagnosis of liver disease, and the presence of endocrinopathies. Hyperglycemic dogs and those with radiographic pulmonary changes or cardiac valve regurgitation also were excluded.
Before blood collection and sonographic assessment, dogs were fasted for 12 hours with free access to water. This study was performed according to the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA) and was approved by the Ethics Committee on Animal Use (CEUA) of School of Veterinary Medicine and Animal Science, Sao Paulo State University (protocol 98/2013).
Blood Sample Collection
After weighing the dogs, blood was collected from the jugular vein into 4 mL Z Serum Clot Activator tubes.a The samples were centrifuged at 1500 × g for 10 minutes. Serum was separated and transferred into polypropylene tubes for immediate processing. Enzymatic activities of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma‐glutamyl transferase (GGT) were measured using colorimetric commercial kits.b Reading was carried out using an automatic biochemistry analyzer Cobas Mira Plus Chemestry System.c
Pulsed Doppler Sonography
Pulsed Doppler sonography was performed by a single investigator with a Logic3d device and a convex (2–5 MHz) multifrequency transducer. Dogs were manually restrained in left lateral or dorsal recumbency for liver vessel assessment. Portal vein morphometry was evaluated at the 11th right intercostal space, and aorta, caudal vena cava, and portal vein were concomitantly visualized in cross‐sectional plane. After obtaining portal vein diameter (PVD), the portal vein area (PVA) was calculated, as previously described.22
Color Doppler was used to visualize and identify liver vasculature. Mean portal velocity was measured in the right branch of portal vein when an ideal insonation angle could not be obtained in the main vessel, as previously reported.27 In all dogs, measurement of MPV was performed at angles of insonation <60°. Doppler sample volume size was adjusted to incorporate the entire diameter of the vessel, according to uniform insonation method.28 The pulse repetition frequency was manually adjusted to the lowest value without aliasing. Mean portal velocity was obtained by the average of 3 consecutive spectral waves. Portal congestion index and PBFV then were calculated according to formulas previously described as follows:29, 30
Hepatic vein spectral wave was obtained in caudate and right lateral branches, in right sagittal plane. These 2 branches were selected because of their close orientation to the Doppler signal, allowing use of the correct insonation angle (<60°). The sample volume was placed at a distance of approximately 2 cm from the CVC to avoid any influence of this vessel, with Doppler sample volume size of 2 or 3 mm. The spectral analysis was recorded for 3 cycles, preferably at the end of inspiration, and the waveform pattern was classified as triphasic, biphasic or monophasic. The triphasic pattern consisted of 2 anterograde peaks and a short reverse flow, the biphasic pattern consisted of loss of the reverse flow, and the monophasic pattern consisted of a flat continuous flow.
At the level of the porta hepatis, the main branch of the hepatic artery was identified in most dogs using color Doppler, between the PV and the CVC. In some animals, however, the hepatic artery was not visualized with color Doppler, and the sample volume was placed in the liver parenchyma, between the PV and CVC, dorsal to the PV, until an arterial spectral pattern was obtained. The sample volume was adjusted, avoiding aquisition of Doppler signs from the adjacent PV.31 Hepatic artery resistivity index was automatically calculated by the software of the ultrasound device, after manual measurement of the systolic peak velocity and the end‐diastolic velocity. A minimum of 3 consecutive and similar Doppler velocity waveforms within a consistent series were required and the average HARI was calculated.
Statistical Analysis
The Kolmogorov‐Smirnov test indicated that quantitative sonographic variables had parametric distribution (were compatible with normal distribution), and these data were analyzed using analysis of variance, with age included in the analysis as a covariate, followed by the Tukey posthoc test. Associations between qualitative sonographic variables and BCS were determined using Goodman's test for contrasts between and within multinomial populations. Biochemical quantitative data had nonparametric distribution, and these data were analyzed using the Kruskal‐Wallis test (for data with non‐normal distribution) followed by Dunn's posthoc test for multiple comparisons. Statistical tests were evaluated at a significance level of P < 0.05.
Results
Classification and Grouping
Fifty‐three dogs of various breeds, from 2 to 16 years of age (mean age, 8.1 years), 18 males and 35 females, intact or neutered, were included in the study. Based on BCS, 12 dogs were included in the control group (G1), 21 overweight dogs in G2, and 20 obese dogs in G3. Most of the overweight and obese animals recruited were middle‐aged dogs (18/41, 43.9%), with ages ranging from 6 to 10 years, females (28/41, 68.3%) and neutered (35/41, 85.4%). The most common overweight and obese breeds represented were mixed breed (17/41, 41.5%), Lhasa Apso (5/41, 12.2%), Dachshund (4/41, 9.8%), Poodle (3/41, 7.3%) and Miniature Pinscher (2/41, 4.9%).
Pulsed Doppler Sonography
Mean liver hemodynamic indices are summarized in Table 1. Portal vein diameter and PVA did not differ among the groups. Although PCI and HARI did not differ among the groups, a gradual increase in PCI was observed from G1 to G3 and a decrease in HARI from G1 to G2 and G3. Significant differences were found in MPV between G1 and G3 dogs (P < 0.05) and in PBFV between G1 and the other groups of dogs (P < 0.05). Mean portal velocity and PBFV also gradually decreased from G1 to G3 (Fig 1). None of the quantitative Doppler hemodynamic variables was significantly associated with age. Abnormal HV Doppler waveform or biphasic pattern was more frequent in G3 than in G1 (P < 0.05), and the triphasic pattern was more frequent in G1 than in G3 (P < 0.05; Fig 2). None of the dogs had a monophasic pattern (Table 2).
Table 1.
Comparison of portal vein diameter (PVD), portal vein area (PVA), and hemodynamic hepatic indices among dogs of G1 (ideal), G2 (overweight) and G3 (obese) (mean ± SD), and age‐adjusted mean
| Groups | |||
|---|---|---|---|
| G1 (n = 12) | G2 (n = 21) | G3 (n = 20) | |
| PVD (cm) | 0.62 ± 0.17 | 0.59 ± 0.14 | 0.64 ± 0.15 |
| (0.63)a | (0.59) | (0.64) | |
| PVA (cm2) | 0.32 ± 0.17 | 0.29 ± 0.14 | 0.33 ± 0.16 |
| (0.32) | (0.29) | (0.33) | |
| MPV (cm/s) | 17.23 ± 3.7b | 15.01 ± 3.34 | 13.66 ± 2.56b |
| (16.12) | (15.5) | (13.8) | |
| PCI (cm × s) | 0.018 ± 0.008 | 0.022 ± 0.014 | 0.024 ± 0.011 |
| (0.02) | (0.021) | (0.024) | |
| PBFV (mL/min/kg) | 32.69 ± 11.86b , c | 19.58 ± 7.68c | 15.22 ± 8b |
| (30.36) | (20.59) | (15.53) | |
| HARI | 0.63 ± 0.06 | 0.61 ± 0.16 | 0.61 ± 0.09 |
| (0.63) | (0.62) | (0.62) | |
Age‐adjusted mean.
P < 0.05 between G1 and G3.
P < 0.05 between G1 and G2.
Figure 1.
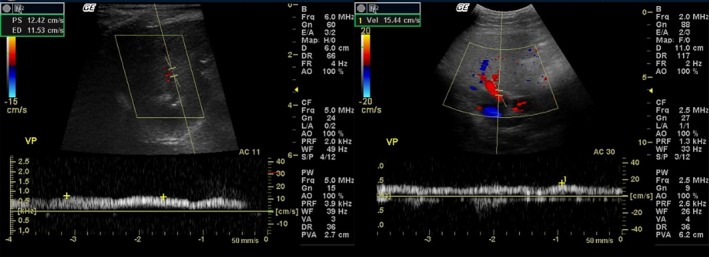
Pulsed Doppler ultrasound images. The portal mean velocity (PMV) of an obese dog (left) is lower (12.42 cm/s) than the PMV of an overweight dog (right) (15.44 cm/s). VP: portal vein.
Figure 2.
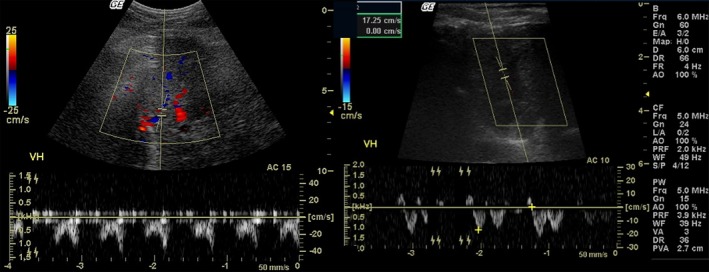
Pulsed Doppler sonographic images show biphasic morphology of the hepatic vein (HV) waveform in an obese dog (left) and triphasic morphology of the HV waveform in an ideal dog (right). VH: hepatic vein.
Table 2.
Distribution of hepatic vein Doppler waveform frequency in dogs of G1 (ideal), G2 (overweight), and G3 (obese)
| Groups | Hepatic vein wave morphology | ||
|---|---|---|---|
| Monophasic | Biphasic | Triphasic | |
| G1 (n = 12) | 0 | 1 (8.3%)a | 11 (91.7%)a |
| G2 (n = 21) | 0 | 6 (28.6%) | 15 (71.4%) |
| G3 (n = 20) | 0 | 12 (60%)a | 8 (40%)a |
P < 0.05 between G1 and G3.
Liver Enzymes Activities
Median liver enzyme activities for each group are summarized in Table 3. Median biochemical results were within reference ranges in all groups. No statistical difference was observed for ALT among the groups, although a gradual increase was noted from G1 to G3. Median ALP was higher in G3 compared to G1, and GGT was higher in G2 compared to G1 (P < 0.05).
Table 3.
Median (minimum‐maximum) values of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma‐glutamyl transferase (GGT) enzymes obtained in dogs of G1 (ideal), G2 (overweight), and G3 (obese)
| Liver enzymes | Groups | ||
|---|---|---|---|
| G1 (n = 12) | G2 (n = 21) | G3 (n = 20) | |
| ALT (U/L) | 38.1 (26–60.7) | 40 (22–94) | 45 (4.7–213) |
| ALP (U/L) | 26.95 (8–81)a | 54.2 (9.7–199.8) | 63 (12.7–2910)a |
| GGT (U/L) | 1.2 (0.4–4.5)b | 2.7 (1–7.7)b | 2 (0.6–34.8) |
Reference values: ALT – 21 a 102U/L; ALP – 20 a 156U/L; GGT – 1.2 a 6.4U/L.43
P < 0.05 between G1 and G3.
P < 0.05 between G1 and G2.
Discussion
Effects of obesity on hepatic hemodynamic indices are well described in human patients.12 However, screening for liver disease for overweight and obesity has not been well described in animals.16, 32 To the authors’ knowledge, ours is the first study to evaluate the liver of overweight and obese dogs using pulsed Doppler sonography.
B‐mode sonographic signs including liver enlargement, increase in liver parenchyma echogenicity, deep attenuation, and vessel blurring in the overweight or obese patient, are strongly suggestive of diffuse fatty liver.33 However, evaluation of liver parenchyma echogenicity can be subject to interobserver variability20 and may underestimate the presence of disease in cases of mild fatty infiltration.19 Therefore, sonographic features of the liver using B‐mode sonography were not compared among groups in our study. Because of the absence of clinical signs in the client‐owned dogs included in our study, fine needle aspiration of the liver and liver biopsy were not performed, and cytological and histological diagnoses were not obtained.
In human obese patients, hepatic steatosis may cause Doppler sonographic changes in the liver because of compression of the vessels by enlarged hepatocytes containing lipid droplets.20 In addition, a correlation between Doppler hemodynamic indices and grade of fatty infiltration of the liver parenchyma has been reported.14, 15, 31 The main hypothesis of our study was that obesity in dogs leads to hepatic circulatory changes, identified by Doppler sonography, as occurs in humans. The results obtained showed that BCS influenced MPV, PBFV, and HV spectral wave morphology.
Some diseases can lead to changes in PVD. Smaller PVD was reported in dogs with an extrahepatic portosystemic shunt as compared to healthy dogs, whereas those with an intrahepatic portosystemic shunt had larger PVD.23 Portal hypertension in dogs, as opposed to human patients, was not related to an increase in PVD.22 In our study, overweight and obesity did not influence PVD mean values. In obese human patients with fatty liver disease, changes in PVD also were not detected before and after 6 months of therapy, when ALT activity and liver volume significantly decreased.34
Portal hemodynamic changes in dogs with naturally occurring hepatic diseases rarely have been evaluated.23 Nevertheless, dogs with experimental cirrhosis had lower MPV and PBFV than did control dogs.22 In our study, overweight and obese dogs had MPV close to those previously reported for healthy dogs,28 whereas ideal BCS dogs had MPV close to those of the control group in a study of experimentally induced liver cirrhosis.22 However, statistical difference was found in MPV between ideal BSC and obese dogs and a decrease was observed with obesity. This result corroborates previous studies published in the medical literature.13, 15 The reason for a decrease in MPV may be decreased vascular compliance, because of fatty deposition.
Portal blood flow volume was significantly different between ideal BSC dogs (G1) and the other groups. In addition, it was below results previously reported for healthy dogs28 in the overweight and obese dogs. Lower than normal portal blood flow volume also was seen in obese human patients with fatty liver.34 Impaired compliance of the vessels and increased vascular resistance in the overweight and obese dogs probably led to a decrease in PBFV, as observed for MPV, in our study. The significant decrease in PBFV suggests impairment in liver circulation because this vessel is the main contributor to the blood supply of the organ. This decrease can lead to impairment in metabolic functions as well as in hepatocyte nutrition, arising from hepatotrophic factors contained in splenic and cranial and caudal mesenteric venous blood.30 However, this is a reversible condition and significant improvement in PBFV and MPV was observed after 6 months of dietary and pharmacological treatment in obese human patients.34
Portal congestion index is an important index in hepatic hemodynamics evaluation.29 This index increases with chronic liver diseases and can be useful in the early diagnosis of those diseases.22 No difference was found in PCI among the groups in our study, although the highest mean value was noted in obese dogs and the lowest mean value in ideal BCS dogs. Calculation of PCI is based on the ratio between PVA and MPV.29 Despite a significant decrease in MPV in G3 compared to G1, a significant increase was not observed in PCI, because PVA was not different between the groups. In human patients with cirrhosis and portal hypertension, an increase in PCI was observed because of a significant decrease in MPV and significant increase in PVA.29 In dogs with cirrhosis, no difference was noted in PVA, despite a significant decrease in MPV and PCI.22 Further studies are required to better elucidate the response of PVA to chronic liver diseases in dogs. However, duration of the abnormal hemodynamics affecting the liver and portal venous pressure also are important factors that can influence PCI29 and could have influenced the results obtained in our study.
Clinical utility of HARI is not well defined in dogs.35 In human patients, HARI can change as a consequence of diseases that lead to liver dysfunction or malignancy. Nevertheless, even in the human medical literature, the hemodynamic effects of fatty liver on this index are not clearly defined.31 Previous studies performed in rabbits with fatty liver showed that moderate fatty infiltration led to a significant decrease in portal and total blood flow, and, consequently, an increase in arterial flow.36 This response, known as the “liver buffer mechanism”, occurs to maintain stable hepatic blood supply.35 An increase in arterial flow, in turn, leads to a decrease in HARI, according to results obtained in a study that included overweight and obese human patients with presumptive fatty liver.31 Although mean HARI decreased in the overweight and obese dogs in our study, no statistical differences were found among the groups. A significant decrease in HARI would be expected because of increased arterial flow. On the other hand, fat deposition in liver parenchyma could lead to increased vascular resistance and increased HARI. This may explain why significant changes in this index were not observed among the different groups in our study. Controversial HARI results also were obtained in human patients with cirrhosis, and may be related to opposite effects of this index.36 The results differed from a study in obese human patients that showed decreases in these values31 as well as in a study that used a rabbit model of hepatic steatosis.37 On the other hand, obese human patients with fatty liver had HARI constant before and after treatment of the disease.34
In healthy individuals, the spectral Doppler waveform of the HV is triphasic.38 Recently, some authors have introduced the term “tetraphasic” to refer to the HV waveform in healthy dogs, because the “V” wave, which is transitional, may peak above the baseline in some animals.39 Hepatic and cardiac disorders are the most common causes of HV waveform abnormality.38, 39 Accordingly, in our study, echocardiographic evidence of regurgitant valves was 1 of the exclusion criteria. In addition, detailed medical history, clinical examination and supplementary diagnostic tests provided support for the exclusion of other liver disorders. Because respiratory conditions also may influence the HV Doppler waveform,38 spectral analysis preferably was recorded at the end of inspiration. Previous studies showed that, at the end of expiration, there is insufficient blood return to the heart through the HV, attenuating the anterograde waveforms and causing a flattened monophasic pattern.38
The higher frequency of abnormal biphasic HV wave pattern detected in G3 in comparison with G1 corroborates findings reported in obese human patients.12, 14 This result is also consistent with previous studies that showed a significantly higher percentage of abnormal HV wave patterns in obese dogs.40 This finding was attributed to an increased volume of hepatocytes in the fatty liver, which leads to compression and decreased intrahepatic venous compliance, decreasing HV pulsatility, that changes from a triphasic pattern to a biphasic or even monophasic pattern.12 In our study, a histopathological diagnosis was not obtained. However, the abnormal HV wave morphology could be explained by the compressive effect of fat deposition in the hepatocytes over the HV, because other factors that could lead to HV waveform changes were excluded. Positive correlation was observed between the HV monophasic pattern and severe fatty liver, HV biphasic pattern and moderate fatty liver, and HV triphasic pattern and mild fatty liver, based on histological features of fatty liver disease in humans.14 Thus, the predominance of biphasic pattern in obese dogs may indicate the presence of moderate fatty liver.
Median biochemical results of the 3 groups remained within normal ranges.41 However, statistical difference occurred in ALP between ideal BCS and obese dogs, and in GGT between ideal BCS and overweight dogs. These findings were similar to results obtained in other studies of obese dogs.42, 43 Alkaline phosphatase and GGT are closely related to cholestasis and biliary retention.44 Obesity in the humans has led to development of biliary diseases, because of excessive hepatic secretion of cholesterol, subsequent supersaturation of bile, an increase in gallbladder volume, and impairment in gallbladder contraction.45 Therefore, in our study, biliary dysfunction could explain the significantly higher activity of GGT in the overweight dogs as compared to the ideal BCS dogs, and the significantly higher activity of ALP in the obese as compared to the ideal BCS dogs. Although ALP is present in several other tissues and its increase can be associated with other causes, such as bone disorders, endocrine diseases, neoplasias and drugs,46 detailed clinical history, physical examination and laboratory tests performed in the dogs of our study were not consistent with these causes. Alanine aminotransferase, on the other hand, is diagnostic marker for hepatocellular injury.47 Overweight and obese dogs in our study did not have significant increases in ALT. However, a slight increase in ALT was observed with increase of obesity, from G1 to G3. Although increased ALT has been related to obesity associated with the fat content of the liver in humans,48 another study showed the presence of liver injury even with low ALT activity.49 In addition, in human patients abdominal height and abdominal visceral fat better correlated with ALT activity than did body mass index.50 In our study, liver parenchymal damage probably existed in the overweight and obese dogs because of the hemodynamic changes, despite normal ALT. Furthermore, dogs with other liver diseases, including chronic active hepatitis (confirmed by histology) had ALT activity within normal ranges.51 One study showed a significant increase in ALT activity in obese dogs,42 whereas in another study, no difference was observed between lean and overweight dogs.43 Further studies with larger population size are needed to better elucidate ALT activity in overweight and obese dogs.
Conclusions
Obesity in our study was associated with changes in portal vein hemodynamic indices and in HV spectral wave. These changes were accompanied by significant differences in some liver enzyme activities and could be a sign of early liver disease, highlighting the importance of preventing or treating weight gain in dogs.
Acknowledgment
The study was supported by FAPESP (“Fundação de Amparo à Pesquisa do Estado de São Paulo”), under protocol 2013/15457‐6.
Conflict of Interest Declaration
Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The study was performed at Sao Paulo State University, Botucatu ‐ Sao Paulo, Brazil.
Footnotes
VACUETTE®, Grainer Bio‐One, Americana, Sao Paulo, Brazil
Ebram® Produtos Laboratoriais Ltd, Sao Paulo, SP, Brazil
Roche Diagnostic System Incorporated
GE Healthcare
References
- 1. Grosselin J, Wren JA, Sunderland SL. Canine obesity: An overview. J Vet Pharmacol Ther 2007;30:1–10. [DOI] [PubMed] [Google Scholar]
- 2. McGreevy PD, Thomsom PC, Pride C, et al. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet Rec 2005;156:695–707. [DOI] [PubMed] [Google Scholar]
- 3. Zoran DL. Obesity in dogs and cats: A metabolic and endocrine disorder. Vet Clin North Am Small Anim Pract 2010;40:221–239. [DOI] [PubMed] [Google Scholar]
- 4. German AJ. Obesity in companion animals. Companion Anim Pract 2010;32:42–50. [Google Scholar]
- 5. Mehlman E, Bright JM, Jeckel K, et al. Echocardiographic evidence of left ventricular hypertrophy in obese dogs. J Vet Intern Med 2013;27:62–68. [DOI] [PubMed] [Google Scholar]
- 6. Manens J, Bolognin M, Bernaerts F, et al. Effects of obesity on lung function and airway reactivity in healthy dogs. Vet J 2012;193:217–221. [DOI] [PubMed] [Google Scholar]
- 7. Despreux JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887. [DOI] [PubMed] [Google Scholar]
- 8. Jones TC, Hunt RD, King NW. Veterinary Pathology, 6th ed Baltimore: Maryland: Williams and Wilkins; 1997. [Google Scholar]
- 9. Verkest KR. Is the metabolic syndrome a useful clinical concept in dogs? A review of the evidence. Vet J 2014;199:24–30. [DOI] [PubMed] [Google Scholar]
- 10. Nyland TG, Matoon JS. Small Animal Diagnostic Ultrasound, 2nd ed Philadelphia, Pennsylvania: WB Saunders; 2005. [Google Scholar]
- 11. Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non‐alcoholic steatohepatitis. Clin Liver Dis 2007;11:37–54. [DOI] [PubMed] [Google Scholar]
- 12. Karabulut N, Kazil S, Yagci B, et al. Doppler waveform of the hepatic veins in an obese population. Eur Radiol 2004;14:2268–2272. [DOI] [PubMed] [Google Scholar]
- 13. Solhjoo E, Mansour‐Ghanaei F, Moulaei‐Langorudi R, et al. Comparison of portal vein Doppler indices and hepatic vein Doppler waveform in patients with nonalcoholic fatty liver disease with healthy control. Hepat Mon 2011;11:740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohammadinia AR, Bakhtavar K, Ebrahimi‐Daryani N, et al. Correlation of hepatic vein Doppler waveform and hepatic artery resistance index with the severity of nonalcoholic fatty liver disease. J Clin Ultrasound 2010;38:346–352. [DOI] [PubMed] [Google Scholar]
- 15. Erdogmus B, Tamer A, Buyukkaya R, et al. Portal vein hemodynamics in patients with non‐alcoholic fatty liver disease. Tohoku J Exp Med 2008;215:89–93. [DOI] [PubMed] [Google Scholar]
- 16. Nicoll RG, O'Brien RT, Jackson MW. Qualitative ultrasonography of the liver in obese cats. Vet Radiol Ultrasound 1998;39:47–50. [DOI] [PubMed] [Google Scholar]
- 17. Larson MM. Liver and spleen In: Thrall DE, eds. Textbook of Veterinary Radiology, 6th ed Saunders: Elsevier; 2013:667–692. [Google Scholar]
- 18. Larson MM. Ultrasound imaging of the hepatobiliary system and pancreas. Vet Clin Small Anim 2016;46:1–28. [DOI] [PubMed] [Google Scholar]
- 19. Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- 20. Strauss S, Gavish E, Gottlieb P, et al. Interobserver and intraobserver variability in the assessment of fatty liver. Am J Roentgenol 2007;189:320–323. [DOI] [PubMed] [Google Scholar]
- 21. Sacerdoti D, Gaiani S, Buonamico P, et al. Interobserver and interequipment variability of hepatic, splenic, and renal arterial Doppler resistance indices in normal subjects and patients with cirrhosis. J Hepatol 1997;27:986–992. [DOI] [PubMed] [Google Scholar]
- 22. Nyland TG, Fischer PE. Evaluation of experimentally induced canine hepatic cirrhosis using duplex Doppler ultrasound. J Vet Radiol 1990;31:189–194. [Google Scholar]
- 23. d'Anjou MA, Penninck DP, Cornejo L, et al. Ultrasonographic diagnosis of portosystemic shunting in dogs and cats. Vet Radiol Ultrasound 2004;45:424–437. [DOI] [PubMed] [Google Scholar]
- 24. Kabir M, Catalano KJ, Ananthnarayan S, et al. Molecular evidence supporting the portal theory: A causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab 2005;288:454–461. [DOI] [PubMed] [Google Scholar]
- 25. Nicoll RG, Jackson MW, Knipp BS, et al. Quantitative ultrasonography of the liver in cats during obesity induction and dietary restriction. Res Vet Sci 1998;64:1–6. [DOI] [PubMed] [Google Scholar]
- 26. Laflamme DP. Development and validation of a body condition score system for dogs. Canine Pract 1997;22:10–15. [Google Scholar]
- 27. Sartor R, Mamprim MJ, Takahira RF, et al. Hemodynamic evaluation of the right portal vein in healthy dogs of different body weights. Acta Vet Scand 2010;52:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lamb C, Mahoney PN. Comparison of three methods for calculation portal blood flow velocity in dogs using duplex‐Doppler ultrasonography. Vet Radiol Ultrasound 1994;35:190–194. [Google Scholar]
- 29. Moriyasu F, Nishida O, Ban N, et al. “Congestion index” of the portal vein. Am J Roentgenol 1986;46:735–739. [DOI] [PubMed] [Google Scholar]
- 30. Kantrowitz BM, Nyland TG, Fischer RP. Estimation of portal blood flow using duplex real‐time and pulsed Doppler ultrasound imaging in the dog. Vet Radiol 1989;30:222–226. [Google Scholar]
- 31. Mihmanli I, Kantarci F, Yilmaz MH, et al. Effect of diffuse fatty infiltration of the liver on hepatic artery resistency index. J Clin Ultrasound 2005;33:95–99. [DOI] [PubMed] [Google Scholar]
- 32. Kawata R, Sakata K, Kunieda T, et al. Quantitative evaluation of fatty liver by computed tomography in rabbits. Am J Roentgenol 1984;142:741–746. [DOI] [PubMed] [Google Scholar]
- 33. Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007;102:2708–2715. [DOI] [PubMed] [Google Scholar]
- 34. Magalotti D, Marchesini G, Ramilli S, et al. Splanchnic haemodynamics in non‐alcoholic fatty liver disease: Effect of a dietary/pharmacological treatment. A pilot study. Dig Liver Dis 2004;36:406–411. [DOI] [PubMed] [Google Scholar]
- 35. Lamb C, Burton CA, Carlisle CH. Doppler measurement of hepatic arterial flow in dogs: Technique and preliminary findings. Vet Radiol Ultrasound 1999;40:77–81. [DOI] [PubMed] [Google Scholar]
- 36. Seifalian AM, El‐Desoky A, Davidson BR. Hepatic indocyanine green uptake and excretion in a rabbit model of steatosis. Eur Sur Res 2001;33:193–201. [DOI] [PubMed] [Google Scholar]
- 37. Taourel P, Blanc P, Dauzat M, et al. Doppler study of mesenteric, hepatic and portal circulation in alcoholic cirrhosis: Relationship between quantitative Doppler measurements and the severity of portal hypertension and hepatic failure. Hepatology 1998;28:932–935. [DOI] [PubMed] [Google Scholar]
- 38. Scheinfeld MH, Bilali A, Koenigsberg M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics 2009;29:2081–2098. [DOI] [PubMed] [Google Scholar]
- 39. Kim J, Kim S, Eom K. Pulsed‐wave Doppler ultrasonographic evaluation of hepatic vein in dogs with tricuspid regurgitation. J Vet Sci 2017;18:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carvalho CF, Jerico MM, Cogliati B, et al. Association of Doppler wave pattern of hepatic veins and fatty liver disease degree. J Liver 2015;4:1–5. [Google Scholar]
- 41. Kaneko JJ, Harvey JW, Bruss ML. Clinical Biochemistry of Domestic Animals, 6th ed San Diego, California: Academic Press; 2008. [Google Scholar]
- 42. Tribuddharatana T, Kongpiromchean Y, Sribhen K, et al. Biochemical alterations and their relationships with the metabolic syndrome components in canine obesity. Natural Science 2011;45:622–628. [Google Scholar]
- 43. Yamka RM, Friesen KG, Frantz NZ. Identification of canine markers related to obesity and the effects of weight loss on the markers of interest. Int J Appl Res Vet M 2006;4:282–292. [Google Scholar]
- 44. Center SA. Interpretation of liver enzymes. Vet Clin North Am Small Anim Pract 2007;37:297–333. [DOI] [PubMed] [Google Scholar]
- 45. Dittrick GW, Thompson JS, Campos D, et al. Gallbladder pathology in morbid obesity. Obes Surg 2005;15:238–242. [DOI] [PubMed] [Google Scholar]
- 46. Fernandez NJ, Kidney BA. Alkaline phosphatase: Beyond the liver. Vet Clin Path 2007;36:223–233. [DOI] [PubMed] [Google Scholar]
- 47. Schindhelm RK, Diamant M, Dekker JM, et al. Alanine aminotransferase as a marker of nonalcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev 2006;22:437–443. [DOI] [PubMed] [Google Scholar]
- 48. Miyake T, Kumagi T, Hirooka M, et al. Metabolic markers and ALT cutoff level for diagnosing nonalcoholic fatty liver disease: A community‐based cross‐sectional study. J Gastroenterol 2012;47:696–703. [DOI] [PubMed] [Google Scholar]
- 49. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286–1292. [DOI] [PubMed] [Google Scholar]
- 50. Stranges S, Dorn JM, Muti P, et al. Body fat distribution, relative weight, and liver enzyme levels: A population‐based study. Hepatology 2004;39:754–763. [DOI] [PubMed] [Google Scholar]
- 51. Doige CE, Lester S. Chronic active hepatitis in dogs – a review of fourteen cases. J Am Anim Hosp Assoc 1981;17:725–730. [Google Scholar]


