Abstract
Background
Cats with hypertrophic cardiomyopathy (HCM) and congestive heart failure (CHF) can have resolution of both left ventricular hypertrophy and CHF.
Objectives
To describe the clinical characteristics of cats with transient myocardial thickening (TMT) and CHF compared with a control population of cats without resolution of HCM.
Animals
A total of 21 cats with TMT, 21 cats with HCM.
Methods
Retrospective study. Clinical records at 4 veterinary centers were searched for TMT cases and a control group of cats with HCM and CHF. TMT was defined as initial maximal left ventricular wall thickness (LVWT) ≥6 mm with left‐sided CHF, with subsequent resolution of CHF, reduction in left atrium/aorta (LA/Ao), and LVWT<5.5 mm. HCM was defined as persistent LVWT ≥6 mm.
Results
Cats with TMT were younger (2 [0.4–11.4] years) than cats with HCM (8 [1.6–14] years) (P < 0.0001), and antecedent events were more common (15/21 versus 6/21, respectively) (P = 0.01). In cats with TMT, LVWT normalized from 6.8 [6.0–9.7] mm to 4.8 [2.8–5.3] mm and LA/Ao decreased from 1.8 [1.6–2.3] to 1.45 [1.2–1.7] after a mean interval of 3.3 (95% CI: 1.8–4.7) months. CHF recurred in 1 of 21 TMT and 15 of 21 cats with HCM. Cardiac treatment was discontinued in 20 of 21 cats with TMT and 0 of 21 HCM cats. All cats with TMT survived, whereas 8 of 19 cats with HCM died during the study period.
Conclusions and Clinical Importance
TMT occurs in younger cats, and antecedent events are common. The prognosis is better in cats with CHF associated with TMT than HCM.
Keywords: HCM phenocopy, Hypertrophic cardiomyopathy, Myocarditis, Takotsubo cardiomyopathy
Abbreviations
- 95% CI
95% confidence intervals
- CHF
congestive heart failure
- CMR
cardiac magnetic resonance
- cTnI
cardiac troponin I
- HCM
hypertrophic cardiomyopathy
- LA/Ao
left atrium to aorta ratio
- LAFS%
left atrial fractional shortening
- LVFS%
left ventricle fractional shortening
- LVIDd
left ventricular internal dimension in diastole
- LVWT
left ventricular wall thickness
- RPLA
right parasternal long‐axis view
- RPSA
right parasternal short‐axis view
- SAM
systolic cranial motion of the mitral valve
- TMT
transient myocardial thickening
A hypertrophic cardiomyopathy (HCM) phenotype is characterized by increased left ventricular wall thickness (LVWT) in the absence of abnormal loading conditions capable of producing a similar degree of ventricular thickening.1, 2 Myocardial wall thickness depends on 3 elements: the number of myocytes, myocyte size, and the volume of the interstitial space.3, 4 In HCM, the increased LVWT is mediated by an increase in mass of individual myocytes and interstitial fibrous connective tissue,5 with the former being the most important component.3, 4, 6 Other diseases can mimic HCM by causing an increase in LVWT that is not due to myocyte hypertrophy or hyperplasia, such as intracellular accumulation of metabolic products (storage diseases) or interstitial infiltration of proteins, cells or fluid, such as in amyloidosis or myocarditis.2, 7 These conditions represent up to 10% of all adult human cases with an HCM phenotype.2 In cats, an HCM phenotype has been described with endocrine disorders, such as hyperthyroidism or acromegaly, dehydration (pseudohypertrophy), and infiltrative disease (cardiac lymphoma).8, 9, 10, 11 It is not known whether metabolic diseases or inflammatory disease are responsible for the abnormal phenotype seen in some cats diagnosed with HCM.
Cats with HCM and congestive heart failure (CHF) are generally reported to have a poor prognosis.12, 13 We were aware of anecdotal reports of cats presenting with HCM and CHF that appeared to have a good prognosis, and a longitudinal echocardiographic study conducted by one of the authors (LW) confirmed that in some cats, apparent left ventricular hypertrophy had resolved by subsequent echocardiographic examinations (unpublished data). Furthermore, it has been suggested that cats with this clinical course are often young and have a history of an antecedent event, such as general anesthesia for neutering within days of presentation with CHF.14 As reverse remodeling with normalization of cardiac structure and function is not an expected outcome in HCM,15, 16 these clinical observations describe a transient form of myocardial thickening associated with CHF in cats. Transient myocardial thickening (TMT) mimicking HCM has been described in some forms of acute/fulminant myocarditis in humans7, 17, 18, 19 and in atypical cases of stress‐induced (Takotsubo) cardiomyopathy.20, 21, 22, 23 In both situations, the increased LVWT is associated with myocardial edema.7, 20, 22, 23, 24, 25, 26, 27
The aims of this study were to identify case details of cats with TMT and CHF from multiple veterinary cardiologists and describe their clinical and echocardiographic characteristics. Additionally, the clinical and echocardiographic features of cats with TMT were compared with a control population of cats with HCM and CHF, to identify characteristics at presentation that might distinguish cats with TMT from HCM.
Materials and Methods
This study was approved by the Clinical Research Ethical Review Board of the Royal Veterinary College (URN 2015–1397).
The medical records and echocardiographic database of 4 referral centers (Royal Veterinary College, Vetsuisse Faculty, Highcroft Veterinary Referrals, and North Downs Specialists Referrals) were searched for cats diagnosed as HCM associated with CHF that showed normalization of LVWT over time. TMT cases were defined as cats with at least 2 echocardiographic examinations that had increased LVWT (LVWTd ≥ 6 mm)28, 29 and left atrial dilatation (left atrium to aorta ratio [LA/Ao] ≥1.6)30 with left‐sided CHF at presentation but showed subsequent normalization of LVWT (LVWTd < 5.5 mm) and left atrial size.31 Additionally, cats with left ventricular fractional shortening <40% or with evidence of focal left ventricular wall thinning with hypokinetic or dyskinetic myocardial segments at their final echocardiographic examination were excluded from the TMT group.15, 16 A control group of cats with HCM was selected from the medical records and echocardiographic database at each center involved in the study. Cats with at least 2 echocardiographic examinations at least 5 months apart with left atrial dilatation (LA/Ao ≥ 1.6) and left‐sided CHF on presentation and persistently increased LVWT (LVWTd ≥ 6 mm) were selected as control HCM cats. All cats had CHF documented on thoracic radiographs or echocardiography (pleural effusion).
Echocardiographic Data
All echocardiographic examinations were reviewed and remeasured at each center by an ECVIM (3 centers) or ACVIM (1 center) board‐certified veterinary cardiologist. Echocardiographic video loops from all cats with TMT submitted to the study (before and after ventricular thickening resolution) were further reviewed by 1 observer (JNM) for quality control and to ensure the inclusion criteria were met. Left ventricular free wall and interventricular septal thicknesses were measured by a leading edge to leading edge technique from a two‐dimensional (2D) right parasternal long‐axis 4‐ or 5‐chambered view (RPLA) and a short‐axis view at the papillary muscle level (RPSA), as the average of the thickest end‐diastolic segment on 3 different cardiac cycles in each view (RPLA and RPSA). End‐diastolic frames were defined as the first frame after mitral valve closure in RPLA and as the time point in the cardiac cycle of greatest left ventricular internal diameter in RPSA.32 The maximal averaged end‐diastolic wall thickness from both the interventricular septum and left ventricular free wall on these 2 views was recorded, and the highest value was used for final data analysis, defined as LVWT. All 2D measurements of cardiac chambers were made by an inner edge to inner edge technique on the interface between the blood pool and myocardial wall.33 Left ventricular internal diameter in diastole (LVIDd) was measured in 2D from a RPLA and RPSA view at the level of the chordae tendineae, in an end‐diastolic frame, on 3 different cardiac cycles in each view. The ratio of the left atrium to aorta (LA/Ao) was measured in 2D from a short‐axis view at the heart base, in the frame after aortic valve closure (end‐ventricular systole).30 Left atrial (LAFS%) and left ventricular (LVFS%) fractional shortening were measured by M‐Mode from a right parasternal short‐axis at the heart base30 and right parasternal short‐axis at the papillary muscle, respectively. The presence of systolic cranial motion of the mitral valve (SAM) was assessed on 2D and color Doppler from a right parasternal long‐axis 5‐chambered view, as a systolic motion of the tip of the cranial mitral valve leaflet toward the interventricular septum producing turbulent flow in the left ventricular outflow tract and mitral regurgitation.34 The presence of spontaneous echocardiographic contrast (SEC) or a thrombus was assessed from a RPLA and short‐axis views at the heart base.
Exclusion criteria included normal left atrial size (LA/Ao < 1.6) and the presence of systemic diseases known to affect the cardiovascular system, such as hyperthyroidism (all cats >8 years of age had T4 measured), systemic hypertension (systolic blood pressure ≥160 mmHg),35 diabetes mellitus, and chronic kidney disease (IRIS Stage >II).
Clinical Data
Additional data collected from the medical and echocardiographic records included age at diagnosis, sex, breed, presenting clinical signs, heart rate, respiratory rate, temperature, presence of a murmur, gallop or arrhythmia (and ECG diagnosis when available), and systolic blood pressure at presentation. In addition, thoracic radiographic findings (ie pulmonary edema, pleural effusion, or both), number of echocardiographic examinations performed, treatment, and any clinical pathology results available at presentation, such as cardiac biomarkers, complete blood count, serum chemistry, or serology for Toxoplasma gondii, Bartonella henselae, or FIV/FeLV, were recorded. The medical history of each cat was carefully evaluated for the presence of antecedent events, defined as any new medication, general anesthesia, and/or traumatic incident occurring within 14 days of presentation with CHF.
Outcome Data
Medical records were additionally reviewed for relapses of CHF, aortic thromboembolism, and cardiac death (death or euthanasia because of signs of CHF and/or aortic thromboembolism, or sudden death). In cases where treatment was discontinued, the duration of treatment was determined from the discharge date from hospital until all cardiac treatment was stopped. At the time of data collection (September 2015 to September 2016), owners of cats identified with TMT were contacted and invited for follow‐up echocardiographic examinations. All echocardiographic parameters in cats with TMT at the time of normalization of LVWT (“normal echo”) were compared with the last available echo (“final echo”).
Statistical Analysis
Data were tested for normality graphically and by Shapiro‐Wilk test and for homogeneity of variances by Levene's test. Between‐group (TMT versus HCM) comparisons were carried out by Fisher's exact for categorical variables and a Mann‐Whitney U‐test for continuous variables. A Wilcoxon signed‐rank test was used for within‐group comparisons of continuous variables. Data are reported as median [range], mean (95% confidence interval 95% CI), frequency, and percentage. P values <0.05 were considered statistically significant. Statistical analysis was performed by commercially available software.1
Results
Twenty‐one TMT cases were identified in the 4 centers, and 21 HCM cases were selected as control group. The frequency of TMT cases seen at the 4 centers ranged between 1 and 4 cases every 2 years. Cats with TMT were younger (2 [0.4–11.4] years) than cats with HCM (8 [1.6–14] years), P < 0.0001. Males were over‐represented in the HCM group (18 of 21 cats were male versus 10 of 21 males in the TMT group, P = 0.02). Cats with HCM were also heavier than cats with TMT (P = 0.04) Several breeds were represented in both groups with equal distribution. Domestic shorthair (DSH) was the most common breed (10 DSH with TMT and 14 DSH with HCM). The TMT population also included 4 British shorthair 2 Ragdoll, and 2 Domestic Long Hair. The HCM population also included 2 British Shorthair and 2 Maine Coon. Other breeds included 1 Birman, 1 Russian Blue and 1 Sphynx in the TMT group and 1 Domestic Long Hair and 1 Exotic Shorthair in the HCM group. Antecedent events were identified in 71% of cats with TMT (15/21) and 29% of cats with HCM (6/21, P = 0.01). In the TMT group, the most frequent antecedent event was general anesthesia (7 cats) followed by road traffic accident (2 cats). Anesthesia was performed for ovariohysterectomy (3 cats), and placement of indwelling urinary catheter, extraction of intestinal foreign body, tooth extraction, and draining abscess (1 cat each). Other antecedent events included vaccination, bite wound, pneumonia, fever of unknown origin, fever with thoracolumbar pain, and abdominal pain with vomiting (1 cat each). A range of drug combinations were administered during these events, including antibiotics (n = 12), NSAIDs (n = 7), opioids (n = 5), fluids IV (n = 4), corticosteroids (n = 3), bronchodilators (n = 2), and antacids (n = 1). In the HCM group, antecedent events included general anesthesia, eye prolapse, vaccination, abscess, and anorexia (1 cat each). Medications administered to the HCM group included antibiotics (n = 4), corticosteroids (n = 2), antiemetics (n = 2), and bronchodilators (n = 1). There were no differences between groups in the other clinical examination variables evaluated (Table 1).
Table 1.
Summary of additional clinical characteristics and laboratory parameters at initial presentation in cats with TMT and HCM
| TMT | HCM | P Value | |
|---|---|---|---|
| Body weight (kg) | 4.1 (1.9–6.2) | 4.8 (2.7–7.2) | 0.04 |
| Heart rate (bpm) | 180 (90–250) | 190 (155–220) | 0.8 |
| Respiratory rate (breaths per minute) | 64 (40–104) | 58 (40–130) | 0.8 |
| Murmur (% yes) | 7/21 (33%) | 9/15 (60%) | 0.18 |
| Arrhythmia (% yes) | 2/21 (10%) (VPCs, transient 3AVB) | 3/17 (18%) (1 AF, 1 FAT, 1 VPCs) | 0.64 |
| Pulmonary edema/pleural effusion/both | 6/2/13 | 11/1/9 | 0.28 |
| Hematocrit (%) | 33% (15–54) | 35% (24–51) | 1.0 |
| Urea (mmol/L) | 12.8 (3.9–34) | 11.4 (6.6–20.5) | 0.27 |
| Furosemide dose at discharge (mg/kg/d) | 2.1 (0.9–5.4) | 2.0 (1.4–4.0) | 0.99 |
AF, atrial fibrillation; 3AVB, third‐degree atrioventricular block; FAT, focal atrial tachycardia; HCM, hypertrophic cardiomyopathy; TMT, transient myocardial thickening; VPCs, ventricular premature complexes.
Echocardiographic Data
A total of 174 echocardiographic scans were evaluated from the 42 cats enrolled in the study. Cats with TMT had a median of 3 [2–7] scans and cats with HCM 4 [2–9] scans performed during the study period. The time elapsed between the first and last echo was 8 [1.0–57] months for the whole population and 6 [1–57] months for cats with TMT and 10 [5–48] months for cats with HCM. At presentation, cats with TMT had thinner left ventricular walls than cats with HCM (P = 0.001) (Table 2, Fig 1). Similarly, at presentation, cats with TMT had smaller left atria than cats with HCM (P < 0.0001) (Table 2, Fig 1). In cats with TMT, after a median period of 3.3 (95% CI: 1.8–4.7) months, the LVWT normalized (Fig 2). Left atrial size decreased over time in the TMT group, while it remained severely dilated in the HCM group (Table 3 , Fig 1). Left atrial fractional shortening was reduced in both groups at presentation but improved significantly in the TMT group over time (P = 0.001), while it remained markedly reduced in the HCM group (P = 0.67) (Table 3, Fig 3).
Table 2.
Echocardiographic variables at initial presentation in cats with TMT and HCM
| TMT | HCM | P Value | |
|---|---|---|---|
| LVWT (mm) | 6.8 (6.0–9.7) | 8.1 (6.0–12.3) | 0.001 |
| LA/Ao | 1.8 (1.6–2.3) | 2.4 (1.6–3.4) | <0.0001 |
| Papillary muscle hypertrophy (% yes) | 13/21 (62%) | 14/21 (67%) | .75 |
| Symmetric wall thickening (%yes) | 20/21 (95%) | 16/21 (76%) | .18 |
| SAM (% yes) | 6/21 (29%) | 6/21 (29%) | 1.0 |
| Pericardial effusion (% yes) | 9/21 (43%) | 8/21 (38%) | 0.5 |
| SEC (% yes) | 4/21 (19%) | 8/21 (38%) | 0.3 |
LVWT, left ventricular wall thickness; LA/Ao, left atrium to aorta ratio; SAM, systolic cranial motion; SEC, spontaneous echocardiographic contrast.
Figure 1.
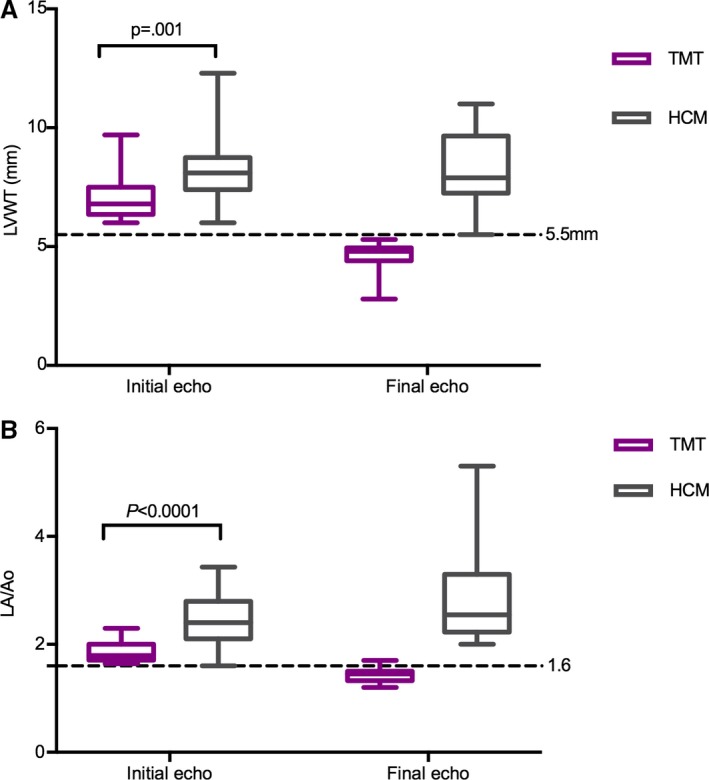
Left ventricular wall thickness (LVWT) (A) and left atrial size (LA/Ao) (B) in cats with TMT and HCM at presentation and final echocardiographic examination. At presentation, left ventricular walls were thicker in cats with HCM. The left atrium was larger in cats with HCM at presentation and remained dilated over time, while it decreased over time in the TMT population. By definition, the LVWT and LA/Ao decreased between the initial and the final echo in the TMT population, and so those two datasets were not subjected to statistical analysis. Echo, echocardiogram; TMT, transient myocardial thickening; HCM, hypertrophic cardiomyopathy.
Figure 2.
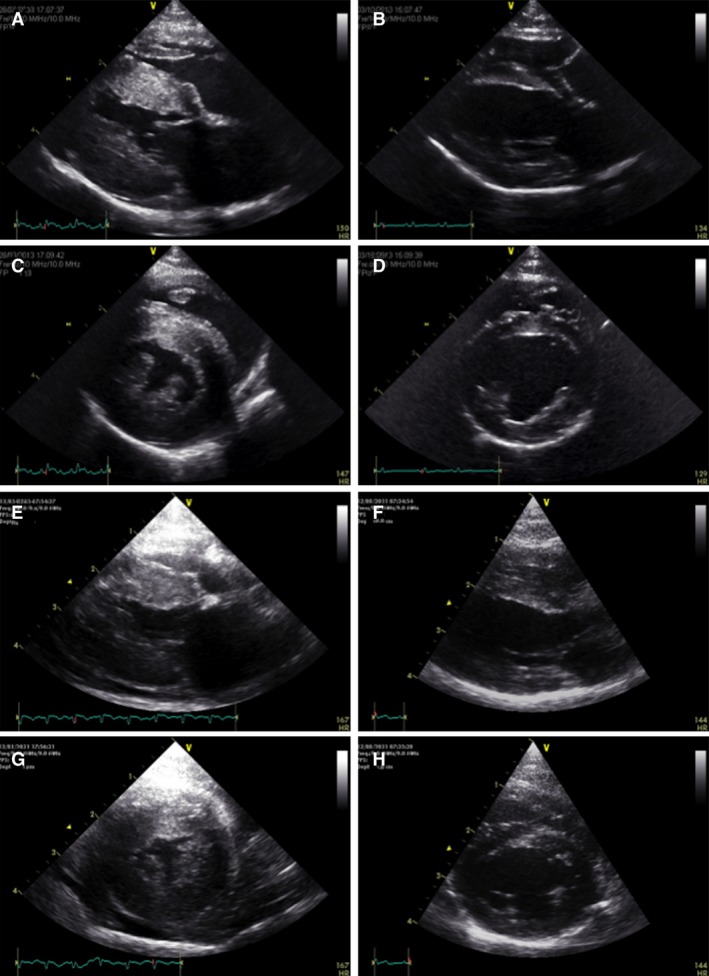
Right parasternal long‐axis (A, B, E, F) and short‐axis views (C, D, G, H) at end‐diastolic frame from 2 TMT cases at initial presentation (A, C and E, G) and 7 months later (B, D and F, H). The initial severely increased left ventricular wall thickness (A, C, E, G) and mild pericardial effusion (E, G) resolved completely, with a morphologically normal heart 7 months later.
Table 3.
Selective echocardiographic variables at presentation and final echocardiographic examination in cats with TMT and HCM
| TMT | HCM | |||
|---|---|---|---|---|
| Initial Echo | Final Echo | Initial Echo | Final Echo | |
| LVWT (mm) | 6.8 (6.0–9.7) | 4.8 (2.8–5.3) | 8.1 (6.0–12.3) | 7.9 (5.5–11) |
| LA/Ao | 1.8 (1.6–2.3) | 1.45 (1.2–1.7) | 2.4 (1.6–3.4) | 2.6 (2.0–5.3) |
| LAFS% | 17 (4.3–21) | 27 (15–49.6) | 8.6 (0.9–17) | 9 (1.7–16) |
Cats with TMT showed a marked and clinically significant decrease in LVWT and LA size over time. LA/Ao, left atrium to aorta ratio; LVWT, left ventricular wall thickness; LAFS%, left atrial fractional shortening; LVWT, left ventricular wall thickness.
Figure 3.
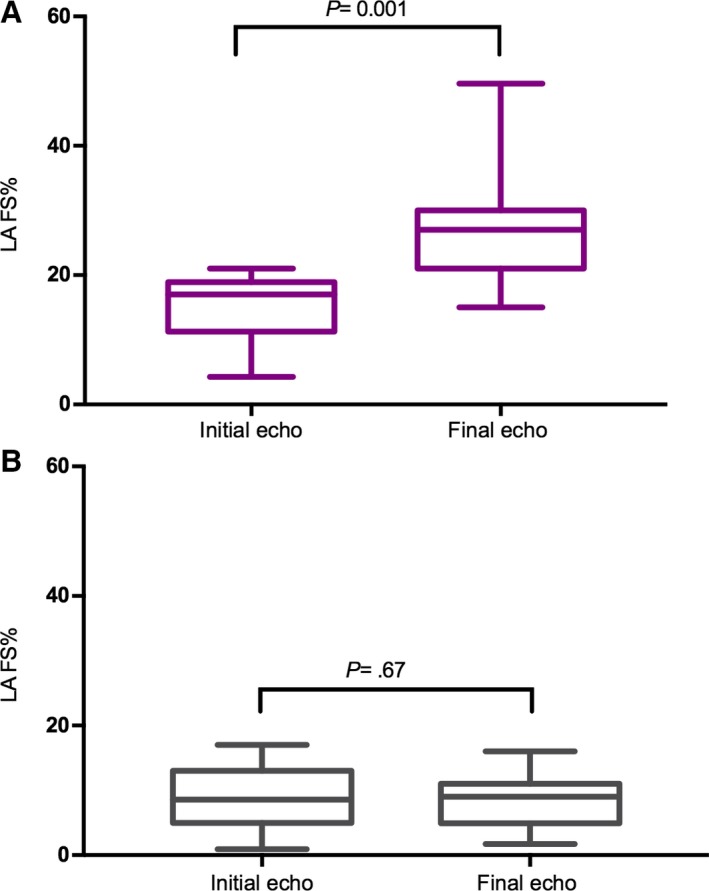
Left atrial fractional shortening (LA%FS) in cats with TMT (A) and HCM (B) at presentation and final echocardiographic examination. LA%FS was reduced in both groups at presentation but improved in the TMT group over time. Echo, echocardiogram.
Cardiac troponin I (cTnI) was available in 13 of 21 cats with TMT and 6 of 21 cats with HCM at presentation. Median cTnI was 2.1 [0.05–63.8] ng/mL in cats with TMT and 1.2 [0.3–6.4] ng/mL in HCM (P = 0.2). In 7 of 13 cats with TMT, cTnI was repeated at the time of LVWT normalization. Median cTnI was elevated at presentation and normalized once myocardial thickening resolved (2.1 [0.05–63.8] ng/mL versus 0.01 [0.0–0.3] ng/mL, P = .018) (reference <0.16 ng/mL).36 IgM and IgG antibodies for T. gondii and B. henselae were measured in 4 cats with TMT. Toxoplasma was negative in 4 of 4 and Bartonella was positive in 3 of 4 cats (IgM 1:160–640, reference <1:20; IgG 80–640, reference <1:20), although only 1 of 3 cats was treated for Bartonella.
Cats from both groups received various combinations of cardiac medications at the time of CHF diagnosis. All cats received furosemide but a greater proportion of cats with HCM (19/21) than cats with TMT (12/21) received medications in addition to furosemide (eg diltiazem, atenolol, spironolactone, pimobendan, and ACE inhibitors, P = 0.03). Additional characteristics of the study population are presented in Table 1.
Long‐term Follow‐up in Cats with TMT
After normalization of LVWT to <5.5 mm 15 of 21 cats with TMT had a further follow‐up scan up to 5 [1–57] months later. In all cases, there was a further decrease in LVWT from a median LVWT of 5.1 [3.8–5.4] mm to 4.8 [2.8–5.3] mm (P = 0.02, Fig 4), while left ventricular systolic function and left atrial size remained normal.
Figure 4.
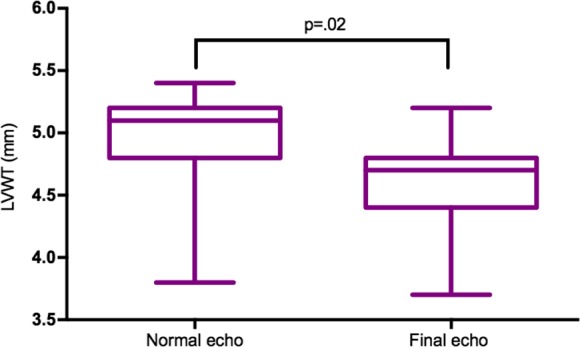
Long‐term follow‐up in 15 cats with TMT. Normal left ventricular wall thickness was defined as <5.5 mm (normal echo), but all cats with serial echos showed a further decreased in wall thickness over time. Echo, echocardiogram.
Outcome Data
Congestive heart failure recurred in 1 of 21 cats with TMT shortly after discharge, and all cats were alive at the time of writing. CHF recurred in 15 of 21 cats with HCM; 2 of 21 had an aortic thromboembolus; and 8 of 19 died during the study period (2 cats were lost to follow‐up). In 20 of 21 cats with TMT, all cardiac drugs were discontinued at 4.6 (95% CI: 2.3–6.9) months after discharge, as the heart was morphologically normal on repeated echocardiographic examinations (ie normal LVWT and LA size), and none of the cats showed clinical signs thereafter. Conversely, all cats with HCM (21/21) received medications for heart disease during the duration of the study or until death. In 1 cat with TMT, administration of clopidogrel and aspirin were not stopped, even though the heart was normal on the last echocardiographic examination, as the cat had a previous suspicion of a thrombus on the mitral valve. The cat was lost to follow‐up.
Discussion
In this study, we describe a transient form of myocardial disease in cats that mimics HCM and is associated with CHF but has an excellent long‐term prognosis. TMT is a relatively uncommon condition that seems to affect young cats, often with a history of an antecedent event.
The underlying pathophysiology causing the transient ventricular wall thickening is unknown, but in view of the fast reverse remodeling seen in our cases, myocardial edema and/or transient cellular infiltration might be responsible. Acute myocarditis in humans can cause TMT that resembles HCM at initial presentation.2, 7, 17, 18, 19, 25, 26, 37, 38, 39, 40, 41, 42 In these cases, the increased ventricular wall thickness was shown to be associated with severe myocardial interstitial edema.7, 24, 25, 26 Myocardial edema is one of the main features of the inflammatory response in acute myocarditis in humans.41 Myocarditis is poorly described in small animals but increased LVWT can occur with toxoplasma myocarditis, myocarditis caused by FIV, and in eosinophilic myocarditis.42, 43, 44 The increased LVWT can normalize if the patient survives the acute CHF episode, as described in a cat with toxoplasma myocarditis.42
Cats with TMT were younger than cats with HCM (median age 1.7 versus 8 years). In people, acute myocarditis is most common in young individuals,45 with an average age of 10 years reported in 43 patients from 9 different publications (95% CI: 0.45–19.6).7, 17, 18, 19, 38, 39, 40, 46, 47 It is possible that TMT is a form of myocarditis as described in people. In our study, very few cats had serological testing for Bartonella and Toxoplasma done which hinders any meaningful conclusion, especially considering the high prevalence of Bartonella seropositivity in healthy cats.48 In humans, a cause for myocarditis is not identified in the majority of cases, although various infectious agents, drugs (eg hypersensitivity reactions), and toxins have all been reported to cause myocarditis. Viral infections are considered to be the most common underlying cause.7, 40, 45, 49
The majority of cats with TMT in our study had events preceding their presentation in CHF, and these were more common in cats with TMT than HCM (71% of TMT versus 29% of HCM cases). Stalis et al50described very similar antecedent stressful events to those observed in our TMT population in 75% of young cats with endomyocarditis. Many cats received drugs before presenting with TMT, and hypersensitivity drug reactions are a known cause of myocarditis in humans.45, 49 Alternatively, the myocardial changes observed could potentially be a consequence of a catecholamine surge caused by emotional or physical triggers. In humans, catecholamines may cause toxic myocarditis in cases of phaeocromocytoma45, 51 or stress‐induced (Takotsubo) cardiomyopathy due to an exaggerated sympathetic stimulation.52 Takotsubo cardiomyopathy is a transient left ventricular dysfunction most frequently characterized by an apical “ballooning” phenotype in people,27, 52, 53 but myocardial edema mimicking HCM has also been described in Takotsubo cases, and differentiation from true HCM requires advanced cross‐sectional imaging.20, 21, 22, 23, 27, 54 TMT could then potentially be caused by an emotional or physical stress associated with the reported antecedent events.
Cardiac troponin I was markedly elevated in most cats with TMT where it was assessed. Blood concentration of cardiac troponins is highly sensitive and specific for cardiac myocyte injury, and highly suggestive of acute myocarditis when other causes of severe myocardial injury have been excluded.45, 55, 56 However, serum concentration of cardiac troponins is elevated in Takotsubo patients,53 and the differentiation between these 2 entities might require cardiac magnetic resonance (CMR) imaging.57 cTnI might potentially be useful to differentiate TMT from HCM cases, although there is likely to be overlap between groups. Cats with HCM can also have substantially elevated serum cTnI concentrations, and some cats with TMT have normal to mildly increased cTnI.
At presentation, cats with TMT had thinner left ventricular walls than cats with HCM. If myocardial edema is indeed the mechanism behind increased wall thickness in TMT, it makes sense that the degree of wall thickening is limited and potentially not as extreme as seen with left ventricular hypertrophy in HCM cases. There was marked overlap in LVWT between the 2 groups, and this feature cannot be used to differentiate cats with TMT from HCM.
The cats with TMT had mild increases in left atrial size and decreased atrial fractional shortening that normalized over time. These findings suggest an acute myocardial insult without time for the left atrium to adapt to the increased filling pressures (ie dilate), which caused acute increase in left atrial and pulmonary capillary pressure resulting in acute pulmonary edema.
Cats with an HCM phenotype and CHF following corticosteroid administration can show normalization of the morphological cardiac changes over time and can have an excellent long‐term prognosis.58 This has been postulated to be a unique form of CHF induced by corticosteroids. The clinical findings and clinical course of cats with corticosteroid‐associated CHF were very similar to our population, although older (9.3 [5.6–12.4]) than the cats in this study (2 [0.4–11.4] years).58 In our study, there was no difference in the proportion of cats receiving corticosteroids between the TMT and HCM populations. The cats with corticosteroid‐associated CHF received these drugs for a variety of medical reasons, including events that would have been classified as antecedent stressful events in our study.
Based on our results, there were no clinical or echocardiographic characteristics at presentation differentiating TMT from HCM, besides the younger age of cats with TMT and the presence of antecedent events. The different progression over time may be the only way of differentiating TMT from HCM, where cats with TMT show reverse remodeling with normalization of LVWT and left atrial size, while cats with HCM show a progressive deterioration of their cardiac function. CMR imaging and endomyocardial biopsy are both used in humans to make the diagnosis of myocarditis, with biopsy being the gold standard.45
Transient myocardial thickening should be considered as a differential diagnosis in cats with an HCM phenotype, especially in young cats with a history of an antecedent event. Additionally, the perception of a poor prognosis in symptomatic HCM (specifically with CHF) and the need for lifelong treatment may discourage clinicians and owners from attempting treatment and might result in premature euthanasia. The identification and description of a transient form of heart failure in cats associated with an excellent prognosis may have an important impact on clinical decisions made by veterinarians when faced with a young cat with severe CHF. Furthermore, lifelong treatment may not be appropriate for all cats with an initial diagnosis of decompensated HCM.
This was a retrospective study and, as such, has some inherent limitations. More advanced echocardiographic parameters, such as tissue Doppler imaging and diastolic function evaluation, were not systematically assessed in our population of cats. These might have helped distinguishing TMT from HCM cats, although the echocardiographic variables assessed in our study are the ones most commonly used in routine clinical practice. Another limitation is that echocardiography was performed at different time points after presentation, and some cats received diuretics before the initial echocardiographic assessment. These were cats in critical condition presented in acute left‐sided CHF that hindered detailed echocardiographic assessment. Myocardial thickening could have therefore been caused by pseudohypertrophy due to hypovolemia secondary to diuretic treatment or as a result of some of the reported antecedent events. However, we believe it is unlikely that the increased LVWT in the cats with TMT was pseudohypertrophy, as left atrial enlargement was one of the inclusion criteria, and all cats showed complete resolution of clinical signs with diuretic treatment.
Conclusions
We describe a transient form of myocardial disease in cats that causes CHF but is associated with a better prognosis than expected for cats with HCM and CHF. TMT appears to preferentially affect young cats and often follows an antecedent event but is difficult to differentiate from HCM at presentation. The recognition of TMT could have an important impact in daily clinical cardiology, leading to clinicians and owners attempting treatment in cases classically thought to have a poor prognosis, and potentially avoiding premature euthanasia. Additionally, TMT might influence the definition of HCM and design of studies of HCM in cats.
Acknowledgment
JNM's PhD was funded by Everts Luff Feline Endowment.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
This study was not supported by any grant.
This work was done at the Royal Veterinary College, Vetsuisse Faculty, Highcroft Veterinary Referrals and North Downs Specialist Referrals.
The results of this study were presented as an abstract at the 26th ECVIM‐CA Annual Congress 2016, Gothenburg, Sweden.
Footnote
SPSS 22.0.1, IBM Company, Chicago, IL
References
- 1. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270–276. [DOI] [PubMed] [Google Scholar]
- 2. Authors/Task Force members , Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 3. Fujiwara H, Hoshino T, Yamana K, et al. Number and size of myocytes and amount of interstitial space in the ventricular septum and in the left ventricular free wall in hypertrophic cardiomyopathy. Am J Cardiol 1983;52:818–823. [DOI] [PubMed] [Google Scholar]
- 4. Kaltenbach M, Hopf R, Kunkel B. New Aspects of Hypertrophic Cardiomyopathy: Morphology, Mechanisms and Therapie. Berlin, Germany: Springer Science & Business Media; 2012:421. [Google Scholar]
- 5. Unverferth DV, Baker PB, Pearce LI, et al. Regional myocyte hypertrophy and increased interstitial myocardial fibrosis in hypertrophic cardiomyopathy. Am J Cardiol 1987;59:932–936. [DOI] [PubMed] [Google Scholar]
- 6. Frenzel H, Schwartzkopff B, Reinecke P, et al. Evidence for muscle fiber hyperplasia in the septum of patients with hypertrophic obstructive cardiomyopathy (HOCM). Quantitative examination of endomyocardial biopsies (EMCB) and myectomy specimens. Z Kardiol 1987;76(Suppl 3):14–19. [PubMed] [Google Scholar]
- 7. Hiramitsu S, Morimoto S, Kato S, et al. Transient ventricular wall thickening in acute myocarditis: A serial echocardiographic and histopathologic study. Jpn Circ J 2001;65:863–866. [DOI] [PubMed] [Google Scholar]
- 8. Bond BR, Fox PR, Peterson ME, et al. Echocardiographic findings in 103 cats with hyperthyroidism. J Am Vet Med Assoc 1988;192:1546–1549. [PubMed] [Google Scholar]
- 9. Campbell FE, Kittleson MD. The effect of hydration status on the echocardiographic measurements of normal cats. J Vet Intern Med 2007;21:1008–1015. [DOI] [PubMed] [Google Scholar]
- 10. Carter TD, Pariaut R, Snook E, et al. Multicentric lymphoma mimicking decompensated hypertrophic cardiomyopathy in a cat. J Vet Intern Med 2008;22:1345–1347. [DOI] [PubMed] [Google Scholar]
- 11. Myers JA, Lunn KF, Bright JM. Echocardiographic findings in 11 cats with acromegaly. J Vet Intern Med 2014;28:1235–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Payne J, Luis Fuentes V, Boswood A, et al. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract 2010;51:540–547. [DOI] [PubMed] [Google Scholar]
- 13. Rush JE, Freeman LM, Fenollosa NK, et al. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc 2002;220:202–207. [DOI] [PubMed] [Google Scholar]
- 14. Glaus T, Wess G. [Left ventricular hypertrophy in the cat ‐ “when hypertrophic cardiomyopathy is not hypertrophic cardiomyopathy”]. Schweiz Arch Für Tierheilkd 2010;152:325–330. [DOI] [PubMed] [Google Scholar]
- 15. Harris KM, Spirito P, Maron MS, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end‐stage phase of hypertrophic cardiomyopathy. Circulation 2006;114:216–225. [DOI] [PubMed] [Google Scholar]
- 16. Baty CJ, Malarkey DE, Atkins CE, et al. Natural history of hypertrophic cardiomyopathy and aortic thromboembolism in a family of domestic shorthair cats. J Vet Intern Med 2001;15:595–599. [DOI] [PubMed] [Google Scholar]
- 17. Kosutic J. Severe transient left ventricular hypertrophy in an infant with acute myocarditis and heart failure. Pediatr Cardiol 2004;25:677–680. [DOI] [PubMed] [Google Scholar]
- 18. Riera Sagrera M, Fiol Sala M, Pérez Bárcena J, et al. Acute myocarditis and left ventricular “hypertrophy”. Echocardiogr 2000;17:567–570. [DOI] [PubMed] [Google Scholar]
- 19. Hauser AM, Gordon S, Cieszkowski J, et al. Severe transient left ventricular “hypertrophy” occurring during acute myocarditis. Chest 1983;83:275–277. [DOI] [PubMed] [Google Scholar]
- 20. Kudo K, Funabashi N, Uehara M, et al. Reversible left ventricular wall thickening during recovery procedure in takotsubo cardiomyopathy demonstrated by multislice computed tomography. Int J Cardiol 2011;149:e97–e99. [DOI] [PubMed] [Google Scholar]
- 21. Madias JE. Two cases of reversible left ventricular hypertrophy during recovery from takotsubo cardiomyopathy: Hypertrophy or myocardial edema after an attack of takotsubo syndrome? Echocardiogr 2013;30:989. [DOI] [PubMed] [Google Scholar]
- 22. Hwang H‐J, Lee H‐M, Yang I‐H, et al. Evolutionary change mimicking apical hypertrophic cardiomyopathy in a patient with takotsubo cardiomyopathy. Echocardiogr 2014;31:E293–E295. [DOI] [PubMed] [Google Scholar]
- 23. Madias JE. Transient apical pseudohypertophy due to myocardial edema in patients with Takotsubo syndrome. Heart Lung J Crit Care 2016;45:81. [DOI] [PubMed] [Google Scholar]
- 24. Zagrosek A, Wassmuth R, Abdel‐Aty H, et al. Relation between myocardial edema and myocardial mass during the acute and convalescent phase of myocarditis–a CMR study. J Cardiovasc Magn Reson 2008;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsiamis E, Panagopoulou V, Aggeli C, et al. Segmental myocardial hypokinesis and hypertrophy as initial echocardiographic presentation of myocarditis. Int J Cardiol 2014;176:1460–1461. [DOI] [PubMed] [Google Scholar]
- 26. Wittlieb‐Weber CA, Harris MA, Rossano JW. Transient left ventricular wall thickening in a 14‐year‐old girl with influenza A myocarditis. Cardiol Young 2015;25:187–190. [DOI] [PubMed] [Google Scholar]
- 27. Izgi C, Ray S, Nyktari E, et al. Myocardial edema in Takotsubo syndrome mimicking apical hypertrophic cardiomyopathy: An insight into diagnosis by cardiovascular magnetic resonance. Heart Lung J Crit Care 2015;44:481–485. [DOI] [PubMed] [Google Scholar]
- 28. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation 1995;92:2645–2651. [DOI] [PubMed] [Google Scholar]
- 29. Wagner T, Fuentes VL, Payne JR, et al. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol 2010;12:171–182. [DOI] [PubMed] [Google Scholar]
- 30. Abbott JA, MacLean HN. Two‐dimensional echocardiographic assessment of the feline left atrium. J Vet Intern Med 2006;20:111–119. [DOI] [PubMed] [Google Scholar]
- 31. Langhorn R, Tarnow I, Willesen JL, et al. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014;28:1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol 2015;17(Suppl 1):S244–S257. [DOI] [PubMed] [Google Scholar]
- 33. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 34. Schober K, Todd A. Echocardiographic assessment of left ventricular geometry and the mitral valve apparatus in cats with hypertrophic cardiomyopathy. J Vet Cardiol 2010;12:1–16. [DOI] [PubMed] [Google Scholar]
- 35. Taylor SS, Sparkes AH, Briscoe K, et al. ISFM Consensus Guidelines on the Diagnosis and Management of Hypertension in Cats. J Feline Med Surg 2017;19:288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sleeper MM, Clifford CA, Laster LL. Cardiac troponin I in the normal dog and cat. J Vet Intern Med 2001. Oct;15:501–503. [DOI] [PubMed] [Google Scholar]
- 37. Pinamonti B, Alberti E, Cigalotto A, et al. Echocardiographic findings in myocarditis. Am J Cardiol 1988;62:285–291. [DOI] [PubMed] [Google Scholar]
- 38. Nakagawa M, Hamaoka K. Myocardial thickening in children with acute myocarditis. Chest 1993;104:1676–1678. [DOI] [PubMed] [Google Scholar]
- 39. Kondo M, Takahashi M, Shimono Y, et al. Reversible asymmetric septal hypertrophy in acute myocarditis. Serial findings of two‐dimensional echocardiogram and thallium‐201 scintigram. Jpn Circ J 1985;49:589–593. [DOI] [PubMed] [Google Scholar]
- 40. Liao PK, Seward JB, Hagler DJ, et al. Acute myocarditis associated with transient marked myocardial thickening and complete atrioventricular block. Clin Cardiol 1984;7:356–362. [DOI] [PubMed] [Google Scholar]
- 41. Sekiguchi M, Yu ZX, Hasumi M, et al. Histopathologic and ultrastructural observations of acute and convalescent myocarditis: A serial endomyocardial biopsy study. Heart Vessels Suppl 1985;1:143–153. [DOI] [PubMed] [Google Scholar]
- 42. Simpson KE, Devine BC, Gunn‐Moore D. Suspected toxoplasma‐associated myocarditis in a cat. J Feline Med Surg 2005;7:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rolim VM, Casagrande RA, Wouters ATB, et al. Myocarditis caused by feline immunodeficiency virus in five cats with hypertrophic cardiomyopathy. J Comp Pathol 2016;154:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keeshen TP, Chalkley M, Stauthammer C. A case of an unexplained eosinophilic myocarditis in a dog. J Vet Cardiol 2016;18:278–283. [DOI] [PubMed] [Google Scholar]
- 45. Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 46. Matsuoka Y, Kitamura K, Nishiguchi T, et al. Transient myocardial thickening in acute myocarditis–serial study by two‐dimensional echocardiography. Acta Paediatr 1989;31:216–221. [DOI] [PubMed] [Google Scholar]
- 47. Morimoto S‐I, Kato S, Hiramitsu S, et al. Narrowing of the left ventricular cavity associated with transient ventricular wall thickening reduces stroke volume in patients with acute myocarditis. Circ J 2003;67:490–494. [DOI] [PubMed] [Google Scholar]
- 48. Pennisi MG, Marsilio F, Hartmann K, et al. Bartonella species infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013;15:563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuchynka P, Palecek T, Masek M, et al. Current diagnostic and therapeutic aspects of Eosinophilic myocarditis. Biomed Res Int 2016;2016:2829583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stalis IH, Bossbaly MJ, Van Winkle TJ. Feline endomyocarditis and left ventricular endocardial fibrosis. Vet Pathol 1995;32:122–126. [DOI] [PubMed] [Google Scholar]
- 51. Ferreira VM, Marcelino M, Piechnik SK, et al. Pheochromocytoma is characterized by catecholamine‐mediated myocarditis, focal and diffuse myocardial fibrosis, and myocardial dysfunction. J Am Coll Cardiol 2016;67:2364–2374. [DOI] [PubMed] [Google Scholar]
- 52. Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–548. [DOI] [PubMed] [Google Scholar]
- 53. Templin C, Ghadri JR, Diekmann J, et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 54. Kato T, Ban Y, Kuruma S, et al. Two cases of reversible left ventricular hypertrophy during recovery from takotsubo cardiomyopathy. Echocardiogr 2013;30:E92–E94. [DOI] [PubMed] [Google Scholar]
- 55. Smith SC, Ladenson JH, Mason JW, et al. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997;95:163–168. [PubMed] [Google Scholar]
- 56. Ukena C, Kindermann M, Mahfoud F, et al. Diagnostic and prognostic validity of different biomarkers in patients with suspected myocarditis. Clin Res Cardiol 2014;103:743–751. [DOI] [PubMed] [Google Scholar]
- 57. Jorge C, Sargento L, Gato Varela M, et al. Takotsubo syndrome or acute myocarditis? The role of cardiac magnetic resonance imaging. Rev Port Cardiol 2012;31:609–613. [DOI] [PubMed] [Google Scholar]
- 58. Smith S, Tobias A, Fine D, et al. Corticosteroid‐associated congestive heart failure in 12 cats. Intern J Appl Res Vet Med 2004;2:159–170. [Google Scholar]


