Abstract
Background
Immune‐mediated hemolytic anemia (IMHA) is the most common hematologic immune‐mediated disease in dogs. Complement fixation on erythrocytes causes hemolysis. Complement inhibition decreases hemolysis in people with the hemolytic disease and also may prove effective in treating IMHA in dogs.
Hypothesis/Objectives
Evaluate the in vitro efficacy of 2 complement inhibitors used in humans against canine complement.
Methods
The inhibitory activity of the C3‐inhibitor compstatin and recombinant human C1‐esterase inhibitor (C1‐INH) was evaluated using an in vitro hemolytic assay and spectrophotometric measurement of released hemoglobin. Dose‐response curves for each inhibitor were generated.
Results
Compstatin decreased approximately 50% of canine complement‐mediated hemolysis in initial experiments. This inhibition largely was lost when a new lot of drug was purchased. C1‐INH showed a dose‐dependent inhibition. The highest concentration of C1‐INH tested (500 μg/mL) decreased >80% of canine complement‐mediated hemolysis, and the lowest concentration tested (31.25 μg/mL) decreased hemolysis >60%.
Conclusions and Clinical Importance
Human C1‐INH is a robust inhibitor of canine complement‐mediated hemolysis, whereas compstatin was minimally and variably effective. Human C1‐INH may substantially decrease complement‐mediated hemolysis in dogs with IMHA and warrants further investigation.
Keywords: Anemia, Dogs, Immune‐mediated hemolytic anemia, Membrane attack complex
Abbreviations
- Ab‐SRBCs
antibody‐coated sheep erythrocytes
- CH50
total hemolytic complement assay
- CUHA
Cornell University Hospital for Animals
- C1‐INH
human recombinant C1‐esterase inhibitor
- DRBCs
erythrocytes from a healthy dog
- FcγR
immunoglobulin receptors
- HI
heat‐inactivated canine complement serum
- hIVIG
human intravenous immunoglobulin
- IMHA
immune‐mediated hemolytic anemia
- MAC
membrane attack complex
- MASP‐1
mannose‐binding lectin‐associated serine proteinase‐1
- MASP‐2
mannose‐binding lectin‐associated serine proteinase‐2
- MBL
mannose‐binding lectins
- PNH
paroxysmal nocturnal hemoglobinuria
- PBS
phosphate‐buffered saline
Immune‐mediated hemolytic anemia (IMHA) is the most common hematologic immune‐mediated disease in dogs.1 It is considered a type II hypersensitivity reaction in which antibodies (IgG, IgM, and IgA) directly or indirectly bind cell surface antigens on erythrocyte membranes.2 Antibody binding leads to accelerated hemolysis and clearance of erythrocytes primarily by macrophages in the spleen and liver, or to complement fixation on the membrane of erythrocytes. Complement fixation can lead to the formation of the membrane attack complex (MAC) and subsequent intravascular hemolysis.2, 3, 4 Intravascular hemolysis is associated with a more acute and severe clinical presentation that likely contributes to IMHA's high mortality rate.5 Some of the standard immunomodulatory treatments for IMHA inhibit complement‐mediated hemolysis to a limited extent. Glucocorticoids alter the function and expression of immunoglobulin receptors (FcγR) on macrophages and decrease antibody affinity for erythrocyte antigens. Blockade of FcγR impairs clearance of antibody‐ and complement‐coated erythrocytes by macrophages and decreases extravascular hemolysis,6 although complement fixation and subsequent intravascular hemolysis still can occur. Several studies report the use of human intravenous immunoglobulin (hIVIG) administered IV as an immunomodulatory treatment for IMHA in dogs.7, 8 It is thought that hIVIG blocks FcγR on macrophages and inhibits the active forms of complement factors C3 and C4. Given blockade of FcγR by hIVIG is immediate, and inhibition of C3 and C4 prevents MAC formation, some authors have proposed the use of hIVIG for initial stabilization of dogs with IMHA.6, 8 Unfortunately, evidence for the efficacy of hIVIG is lacking,7 and its use in IMHA also is limited by the risk of adverse drug reactions, high cost, and a lack of consensus regarding treatment protocols.8 Other immunosuppressive therapies take days to weeks to become effective, do not inhibit complement, and may cause adverse effects, such as myelosuppression, hepatotoxicity, and gastrointestinal upset.2, 3, 9
Complement inhibition in humans with paroxysmal nocturnal hemoglobinuria (PNH) has been shown to stop complement‐mediated hemolysis, decrease the need for blood transfusions, and decrease the risk of thrombosis.10 Paroxysmal nocturnal hemoglobinuria is a clonal hematopoietic stem cell disorder characterized by uncontrolled complement activation and complement‐mediated hemolysis.10, 11 Studies in vitro using erythrocytes from patients with PNH demonstrated the ability of complement inhibitors to effectively decrease complement‐mediated hemolysis in a dose‐dependent manner.10, 11 Given the effectiveness of complement inhibition in PNH and the role complement plays in the pathogenesis of IMHA, it was reasoned that complement inhibition might prove effective in treating dogs with IMHA. In particular, complement inhibition may be most beneficial in dogs with high levels of complement activation. Our objectives therefore were to evaluate the effectiveness of 2 complement inhibitors used in humans for the inhibition of canine complement‐mediated hemolysis in vitro.
Material and Methods
Inhibition of Complement‐Mediated Hemolysis
The standard total hemolytic complement assay (CH50)12 was modified and used to detect canine complement activation. Commercial (rabbit) antibody‐coated sheep erythrocytesa (Ab‐SRBCs; 5 × 108 cells/mL) were washed once by centrifugation at 1,000 × g for 3 minutes at 4 °C with 4 mL of phosphate‐buffered saline (PBS; 137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH 7.4). The resulting supernatant was removed, and the Ab‐SRBCs were resuspended to the original concentration with PBS. Commercial canine complement serumb was serially diluted (1 : 8 to 1 : 128) with PBS. Washed Ab‐SRBCs were incubated with equal volumes (100 μL each) of diluted canine complement serum, PBS (blank), or water (total lysis). Each sample was run in duplicate. After 30‐minute incubation at 37 °C in a water bath, samples were centrifuged at 1,500 × g for 5 minutes at 4 °C. Then, 50 μL of supernatant from each sample was transferred to a designated well on a 96‐well flat‐bottom microtiter plate. Each well was prefilled with 50 μL of distilled water for a final volume of 100 μL. Absorbance at 540 nm (OD540) was read with a plate spectrophotometer.c The mean absorbance of each duplicate was calculated, and the percentage of hemolysis relative to water‐induced hemolysis (total lysis) was determined by the following formula:
To confirm that hemolysis was mediated by complement, canine complement‐intact serum was heat‐inactivated (HI) for 30 minutes at 56 °C.13 A 50 : 50 mixture of HI serum and canine complement‐intact serum was prepared. Then, 100 μL of Ab‐SRBCs was incubated with equal volumes of HI serum or the 50 : 50 serum mixture. The percentage of hemolysis for each reaction then was determined.
Compstatind was evaluated in the modified CH50 assay at concentrations ranging from 0.001 to 100 μM, and C1‐INHe was evaluated at concentrations ranging from 31.25 to 500 μg/mL. Based on optimization experiments, canine complement‐intact serum was used at 1 : 8 and 1 : 16 dilutions. Compstatin was reconstituted with PBS (400 μM), and stock solutions were prepared by serial dilution (400–0.004 μM) and stored frozen until used. Stock solutions were further diluted (1 : 2) with canine complement serum (1 : 8 and 1 : 16, dilution) and prewashed Ab‐SRBCs to achieve a final concentration of 0.002–200 μM in a total volume of 100 μL. Reactions were run in duplicate along with the following controls: PBS (blank); distilled water (total lysis). After incubation in a water bath (37 °C) for 1 hour, tubes were centrifuged at 1,500 × g for 5 minutes at 4 °C. Then, absorbance (OD540) of supernatants (50 μL) was read. Mean absorbance was calculated, and the percentage of hemolysis relative to water‐induced hemolysis was determined.
Vials of C1‐INH were reconstituted with PBS (500 μg/mL), and serial dilutions were prepared (31.25–500 μg/mL). Then, 50 μL of each dilution was transferred to a vial containing 25 μL of diluted canine complement serum (1 : 8 and 1 : 16) and 25 μL of Ab‐SRBCs (100 μL, final volume). The hemolysis inhibition assay then was run as described above. Dose‐response curves for each drug were obtained by plotting the percentage of hemolysis relative to water (total lysis; ordinate), against the inhibitor concentration (abscissa).
Data Analysis
Dose‐response curves for each drug (compstatin and C1‐INH) were plotted by a best‐fit model and commercial software.f , g The median percentage of hemolysis was compared across doses by analysis of variance (ANOVA) with a Kruskal‐Wallis post‐test.
Results
Inhibition of Complement‐Mediated Hemolysis
Canine complement serum caused hemolysis of Ab‐SRBCs in a concentration‐dependent manner. The lowest dilutions of serum tested (1 : 16 and 1 : 8) caused a higher percentage of hemolysis than water (Fig 1). Heat inactivation of canine complement serum resulted in complete loss of the hemolytic activity and 1 : 1 mixing of intact and HI canine complement serum led to a proportionate decrease in hemolysis (Fig 1).
Figure 1.
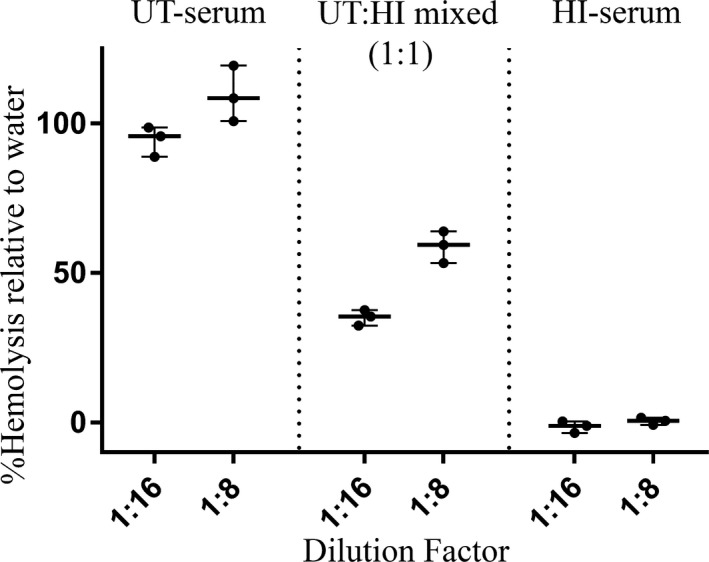
Whisker plot representing the hemolytic activity of untreated (UT) and heat‐inactivated (HI) canine complement serum on antibody‐coated sheep erythrocytes (Ab‐SRBCs). The hemolytic activity of 1 : 1 mixture of UT and HI canine complement serum is also shown. Lines represent the median, and dots are individual experimental replicates. Hemolysis was measured by quantifying the release of hemoglobin using spectrophotometry (OD540). Percentages (%) of hemolysis of canine complement serum compared to lysis in distilled water (ordinate) are plotted against the serum dilution factor (abscissa).
The C3‐inhibitor compstatin5 showed a relatively consistent degree of hemolysis inhibition, regardless of the concentration. All evaluated concentrations (0.001–100 μM) of compstatin decreased canine complement‐induced hemolysis (1 : 8 serum dilution, data not shown) by <8% and by <40% at a 1 : 16 serum dilution (Fig 2A). Two different lots of the drug were tested. The first lot tested showed a greater decrease in complement‐mediated hemolysis compared to the second lot (Fig 2A).
Figure 2.
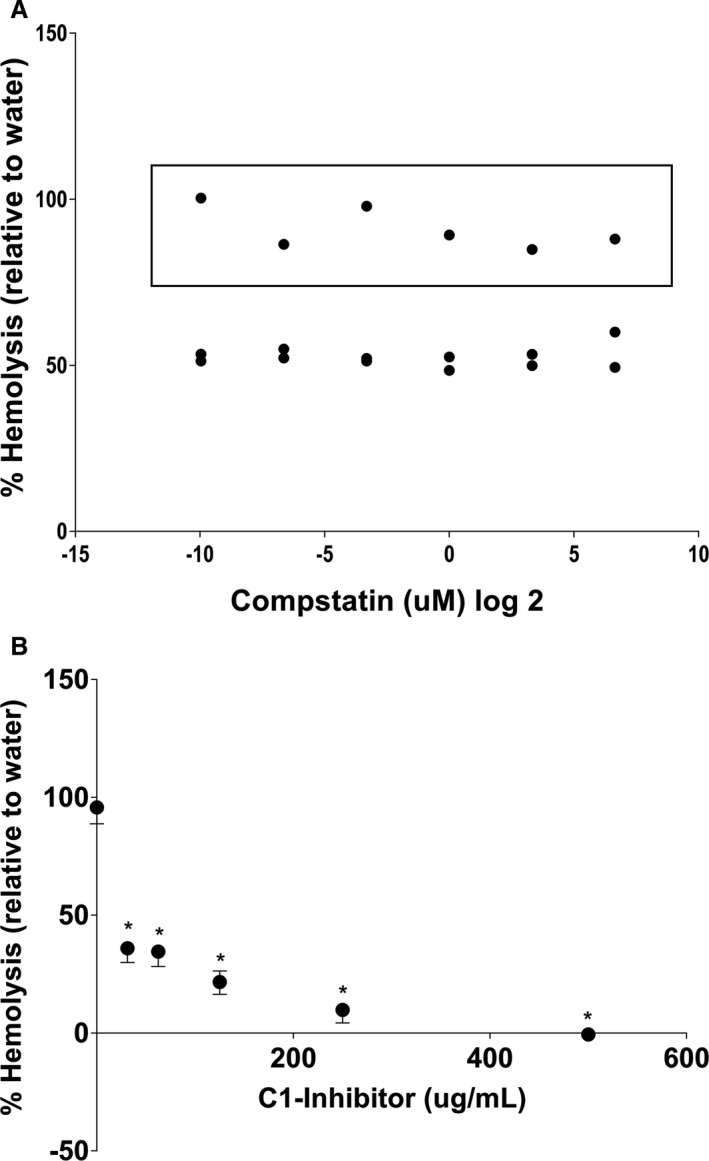
Inhibition of canine complement‐mediated hemolysis of antibody‐coated sheep erythrocytes (Ab‐SRBCs) by two human complement inhibitors. Percentages (%) of hemolysis of canine complement serum compared to lysis in distilled water (ordinate) are plotted against the inhibitor concentration (abscissa). Hemolysis was measured by quantifying the release of hemoglobin by spectrophotometry (OD540). Data represent individual replicate experiments. (A) Antibody‐coated sheep erythrocytes were incubated with canine complement serum and increasing concentrations of C3‐inhibitor (Compstatin, 0.001–100 μM, shown in the log2 transformation to avoid compression of data points on the axis). The data points within the box are derived from an experiment after a new lot of drug was purchased. (B) Antibody‐coated sheep erythrocytes were incubated with canine complement serum and increasing concentrations of human recombinant C1‐Inhibitor (C1‐INH, 31.25–500 μg/mL). Data represent the median and range of three independent experiments. *P < 0.05 compared to no inhibitor (ANOVA, Kruskal‐Wallis).
Human recombinant C1‐INH6 caused a dose‐dependent and robust decrease in canine complement‐induced hemolysis in vitro (Fig 2B). The highest concentration of C1‐INH evaluated (500 μg/mL) nearly abolished all canine complement‐mediated hemolysis, whereas the lowest concentration (31.25 μg/mL) decreased hemolysis >60%.
Discussion
The complement inhibitor C1‐INH dose‐dependently inhibited canine complement‐mediated hemolysis. These results are comparable to those reported for in vitro studies in humans with PNH, in which C1‐INH (from 810 to 3,243 μg/mL) dose‐dependently inhibited complement‐mediated hemolysis.11 Because of solubility limitations of the C1‐INH lyophilized powder available, lower concentrations (31.25–500 μg/mL) were evaluated. It was determined, however, that C1‐INH at the concentrations used was effective at decreasing complement‐mediated hemolysis in vitro. It remains to be determined if similar complement inhibition can be achieved in vivo, particularly in dogs with IMHA with high levels of complement activation. Notably, previous studies investigating other complement‐mediated pathologies in the dog suggest that C1‐INH has cross‐species bioactivity.14, 15 Documentation provided by the Food and Drug Administration (FDA) register for a commercially available form of human C1‐INHh support a wide margin of safety for the administration of this protein to dogs relative to organ damage. Experimentally in dogs, parenteral C1‐INH administration prevents early ischemia‐reperfusion injury associated hypoxemia in an allogenic lung transplant rejection model15 and decreases endotoxin‐induced pulmonary dysfunction and coagulation activation.14 In addition to limiting hemolysis, proximal inhibition of complement activation is anticipated to decrease generation of complement‐derived chemotactic and anaphylactic factors and the generation of opsonins C3b, C4b, and C5b.16 Decreases in the concentrations of these factors could decrease inflammation associated with IMHA, and perhaps its downstream consequences.16 Previous data from various animal models and studies in humans also support the potential role of C1‐INH in decreasing coagulation activation, clotting, and fibrinolysis in dogs.11, 14, 15, 17 This is clinically relevant in IMHA dogs because coagulation abnormalities are common and have been associated with an overall worse prognosis.2, 18, 19, 20
Compstatin was only moderately effective at inhibiting canine complement‐mediated hemolysis in vitro. This result is consistent with previous studies that suggest the activity of compstatin is species specific.21, 22 In 1 such study, hemolysis assays showed that compstatin had a similar inhibitory effect against human and cynomologous monkey complement, but the dose required to achieve equal efficacy against canine complement was 2 orders of magnitude higher.21 Similarly, compstatin is a less effective inhibitor of complement‐mediated hemolysis in rodents and various other nonprimate mammalians species.22 In assays evaluating binding of purified human, baboon, mouse, and rabbit C3 to immobilized compstatin, only human and baboon C3 binding was evident.22 It has thus been suggested that low‐affinity binding to nonprimate complement may be responsible for the decreased efficacy in these species.22 Binding of canine C3 to compstatin has not been directly evaluated, but may underlie some of the decreased efficacy documented in our study. Additionally, C1‐INH and compstatin act at different levels of the complement cascade. C1‐INH acts at a proximal level by binding to C1 and to mannose‐binding lectin (MBL)‐associated serine proteinases‐1 (MASP‐1) and MASP‐2 of the MBL pathway.17, 23, 24 In contrast, compstatin prevents the cleavage of C3 by C3‐convertases by binding of native and activated C3 fragments.21, 25, 26 Therefore, C1‐INH prevents complement activation in a more global fashion than does compstatin or other compounds targeted at the more distal C3.17, 23, 24
The cause of the decrease in biological efficacy among drug lots of compstatin is unclear. The drug was prepared and stored in identical fashion, and the protocol for the assay was not altered between experiments. Based on the provided product sheets, there was a minimal variation in the amino acid composition determined by high‐performance liquid chromatography among lots of drugs. It remains undetermined if this would explain the different results because the amino acid sequence was not altered. This discrepancy was not further pursued because C1‐INH emerged as a more potent inhibitor and was deemed more likely to be clinically effective. Further evaluation of compstatin may be indicated if C1‐INH does not prove useful in future studies.
In conclusion, our data suggest that C1‐INH warrants further investigation as a potential means to control complement‐mediated pathologies, specifically hemolysis in the dog. However, given that it is a human‐derived protein with a high glycosylation pattern that may increase its antigenicity, it is important to determine whether C1‐INH induces antibody formation or an inflammatory response in dogs, which may limit its long‐term use.24 Given that complement activation may be variable across patients and that this variability may affect response to this potential therapeutic approach, an investigation into a means of quantifying complement activation in dogs also is needed.
Acknowledgments
We thank Cheryl Wong for her technical assistance.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
All work was performed at the College of Veterinary Medicine, Cornell University, Ithaca, NY.
The study was undertaken using a grant provided by Frankie's Friends Charitable Pet Foundation, Tampa, FL, and a Resident Research Grant provided by the College of Veterinary Medicine, Cornell University, Ithaca, NY.
A portion of this study was presented at the 2017 American College of Veterinary Internal Medicine Forum, National Harbor, MD.
Footnotes
Antibody‐sensitized sheep erythrocytes, Complement Technology Inc, Tyler, TX
Canine (mixed breed) Complement Serum, Innovative Research Inc, Novi, MI
SpectraMax M3 Multi‐Mode Microplate Reader, Molecular Devices, Sunnyvale, CA
Compstatin, Tocris Bioscience, Pittsurgh, PA
Human recombinant C1 Inhibitor, Sigma‐Aldrich, Saint Louis, MO
Excel 2010, Microsoft Corp, Redmon, WA
GraphPad Prism version 6.01 for Windows, GraphPad Software, La Jolla, CA
Ruconest, Salix Pharmaceuticals, Raleigh, NC
References
- 1. Piek CJ. Immune‐mediated hemolytic anemias and other regenerative anemias In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 8th ed. Philadelphia, PA: Elsevier; 2017:829–837. [Google Scholar]
- 2. Giger U. Regenerative anemias caused by blood loss or hemolysis In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 6th ed. St Louis, MO: Elsevier/Saunders; 2005:1886–1907. [Google Scholar]
- 3. Michael JD. Immune‐mediated anemias in the dog In: Douglas J, Weiss KJW, eds. Schalm's Veterinary Hematology. 6th ed. Ames: Wiley‐Blackell; 2010:216–225. [Google Scholar]
- 4. McCullough S. Immune‐mediated hemolytic anemia: Understanding the nemesis. Vet Clin North Am Small Anim Pract 2003;33:1295–1315. [DOI] [PubMed] [Google Scholar]
- 5. Kelly JD, Farrow BRH. Auto‐immune haemolytic anaemia in a dog. Aust Vet J 1970;46:475–479. [DOI] [PubMed] [Google Scholar]
- 6. Ruiz P, Gomez F, King M, et al. In vivo glucocorticoid modulation of guinea pig splenic macrophage Fc gamma receptors. J Clin Invest 1991;88:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whelan MF, O'Toole TE, Chan DL, et al. Use of human immunoglobulin in addition to glucocorticoids for the initial treatment of dogs with immune‐mediated hemolytic anemia: Original study. J Vet Emerg Crit Care 2009;19:158–164. [DOI] [PubMed] [Google Scholar]
- 8. Spurlock NK, Prittie JE. A review of current indications, adverse effects, and administration recommendations for intravenous immunoglobulin. J Vet Emerg Crit Care 2011;21:471–483. [DOI] [PubMed] [Google Scholar]
- 9. Swann JW, Skelly BJ. Systematic review of evidence relating to the treatment of immune‐mediated hemolytic anemia in dogs. J Vet Intern Med 2013;27:1–9. [DOI] [PubMed] [Google Scholar]
- 10. Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood 2015;124:2804–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeZern AE, Uknis M, Yuan X, et al. Complement blockade with a C1 esterase inhibitor in paroxysmal nocturnal hemoglobinuria. Exp Hematol 2014;42:857–861.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costabile M. Measuring the 50% haemolytic complement (CH50) activity of serum. J Vis Exp 2010;37:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soltis RD, Hasz D, Morris MJ, Wilson ID. The effect of heat inactivation of serum on aggregation of immunoglobulins. Immunology 1979;36:37–45. [PMC free article] [PubMed] [Google Scholar]
- 14. Guerrero R, Velasco F, Rodriguez M, et al. Endotoxin‐induced pulmonary dysfunction is prevented by C1‐ esterase inhibitor. J Clin Invest 1993;91:2754–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salvatierra A, Velasco F, Rodriguez M, et al. C1‐esterase inhibitor prevents early pulmonary dysfunction after lung transplantation in the dog. Am J Respir Crit Care Med 1997;155:1147–1154. [DOI] [PubMed] [Google Scholar]
- 16. Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat Rev Drug Discov 2010;9:43–56. [DOI] [PubMed] [Google Scholar]
- 17. Caliezi C, Wuillemin WA, Zeerleder S, et al. C1‐Esterase inhibitor: An anti‐inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol Rev 2000;52:91–112. [PubMed] [Google Scholar]
- 18. Carr AP, Panciera DL, Kidd L. Prognostic factors for mortality and thromboembolism in canine immune‐mediated hemolytic anemia: A retrospective study of 72 dogs. J Vet Intern Med 2002;16:504–509. [DOI] [PubMed] [Google Scholar]
- 19. Kidd L, Mackman N. Prothrombotic mechanisms and anticoagulant therapy in dogs with immune‐mediated hemolytic anemia. J Vet Emerg Crit Care (San Antonio) 2013;23:3–13. [DOI] [PubMed] [Google Scholar]
- 20. Scott‐Moncrieff JC, Treadwell NG, McCullough SM, Brooks MB. Hemostatic abnormalities in dogs with primary immune‐mediated hemolytic anemia. J Am Anim Hosp Assoc 2001;37:220–227. [DOI] [PubMed] [Google Scholar]
- 21. Furlong ST, Dutta AS, Coath MM, et al. C3 activation is inhibited by analogs of compstatin but not by serine protease inhibitors or peptidyl Alpha‐ketoheterocycles. Immunopharmacology 2000;48:199–212. [DOI] [PubMed] [Google Scholar]
- 22. Sahu A, Morikis D, Lambris JD. Compstatin, a peptide inhibitor of complement, exhibits species‐specific binding to complement component C3. Mol Immunol 2003;39:557–566. [DOI] [PubMed] [Google Scholar]
- 23. Bianchino AC, Poon PH, Schumaker VN. A mechanism for the spontaneous activation of the first component of complement, C1, and its regulation by C1‐inhibitor. J Immunol 1988;141:3930–3936. [PubMed] [Google Scholar]
- 24. Bos I, Hack CE, Abrahams JP. Structural and functional aspects of C1‐inhibitor. Immunobiology 2002;205:518–533. [DOI] [PubMed] [Google Scholar]
- 25. Sahu A, Pangburn MK. Investigation of mechanism‐based inhibitors of complement targeting the activated thioester of human C3. Biochem Pharmacol 1996;51:797–804. [DOI] [PubMed] [Google Scholar]
- 26. Soulika AM, Holland MCH, Sfyroera G, et al. Compstatin inhibits complement activation by binding to the beta‐chain of complement factor 3. Mol Immunol 2006;43:2023–2029. [DOI] [PubMed] [Google Scholar]


