Abstract
Background
In veterinary medicine, congenital methemoglobinemia associated with nicotinamide adenine dinucleotide (NADH)‐cytochrome b5 reductase (b5R) deficiency is rare. It has been reported in several breeds of dogs, but little information is available about its etiology.
Objectives
To analyze the NADH‐cytochrome b5 reductase gene, CYB5R3, in a Pomeranian dog family with methemoglobinemia suspected to be caused by congenital b5R deficiency.
Animals
Three Pomeranian dogs from a family with methemoglobinemia were analyzed. Five healthy beagles and 5 nonrelated Pomeranian dogs without methemoglobinemia were used as controls.
Methods
Methemoglobin concentration, b5R activity, and reduced glutathione (GSH) concentration were measured, and a turbidity index was used to evaluate Heinz body formation. The CYB5R3 genes of the affected dog and healthy dogs were analyzed by direct sequencing.
Results
Methemoglobin concentrations in erythrocytes of the affected dogs were remarkably higher than those of the control dogs. The b5R activity of the affected dogs was notably lower than that of the control dogs. DNA sequencing indicated that this Pomeranian family carried a CYB5R3 gene missense variant (ATC→CTC at codon 194) that resulted in the replacement of isoleucine (Ile) by leucine (Leu).
Conclusions and Clinical Importance
This dog family had familial congenital methemoglobinemia caused by b5R deficiency, which resulted from a nonsynonymous variant in the CYB5R3 gene. This variation (c.580A>C) led to an amino acid substitution (p.Ile194Leu), and Ile194 was located in the proximal region of the NADH‐binding motif. Our data suggested that this variant in the canine CYB5R3 gene would affect function of the b5R in erythrocytes.
Keywords: CYB5R3 gene, Familial methemoglobinemia, Missense variant, NADH‐cytochrome b5 reductase deficiency, Nonsynonymous SNP
Abbreviations
- NADH
nicotinamide adenine dinucleotide
- b5R
NADH‐cytochrome b5 reductase
- CYB5R3
cytochrome b5 reductase
- FAD
flavin adenine dinucleotide
- GSH
reduced glutathione
Methemoglobinemia is characterized by an increase in the concentration of oxidized hemoglobin (iron in the ferric state).1 Methemoglobin impairs the normal transport of oxygen to tissues, leading to cyanosis and exercise intolerance at concentrations >20%.2, 3 The reduction in methemoglobin to hemoglobin primarily depends on nicotinamide adenine dinucleotide (NADH) methemoglobin reductase (NADH‐cytochrome b5 reductase, b5R [EC.1.6.2.2.]).4 There are 2 forms of b5R: a membrane‐bound form (the somatic form) and a soluble form (the erythrocytic form), which lacks the membrane anchor domain.5 Both types of b5R are encoded by the cytochrome b5 reductase (CYB5R3) gene, and in rats and humans, it has reported that they are generated from alternative transcripts differing in the first exon.6, 7 Two forms are composed of a flavin adenine dinucleotide (FAD) domain and an NADH‐binding domain.5 NADH‐cytochrome b5 reductase contains 4 sequence motifs that are highly conserved among several species of animals. These sequences are associated with flavin binding (92RxYT SxxS N 98), FAD/flavin mononucleotide (FMN) selectivity (125G RxxS T 128), or NADH‐binding (181GxGxxP186 and 274CGxxxM279).6 Recently, the 3‐dimensional structures of human,8 rat,9 and porcine10 b5R have been resolved by X‐ray crystallography, and they were nearly identical to each other.6
In humans, it is known that deficiency of this enzyme causes recessive congenital methemoglobinemia,2 and >40 variants in the CYB5R3 gene have been described.5 There are 2 distinct clinical forms of the condition, type I and type II.5 Type I, which is limited to defects in the soluble form of b5R found in erythrocytes, is relatively well tolerated and primarily manifests as cyanosis.11 In contrast, type II disease, which is caused by defects in the membrane‐bound form of b5R in somatic cells, is much more severe and results in cyanosis, neurological dysfunction, and premature death.12
In veterinary medicine, both congenital and acquired forms of methemoglobinemia have been reported.1, 13, 14, 15, 16, 17, 18, 19 Acquired methemoglobinemia most commonly occurs as a result of exposure to drugs or toxic oxidizing agents (eg, sulfonamides, nitrates).14 On the other hand, congenital methemoglobinemia associated with b5R deficiency has been reported rarely in animals.1, 13, 14, 15, 16, 17, 18, 19 It has been detected in several breeds of dogs, including the Pomeranian,16 Chihuahua,1, 13 Borzoi,17 English setter,17 Alaskan eskimo,18 and Miniature Poodle breeds.18 More recently, it has been reported that a mixed‐breed dog with methemoglobinemia had 2 potentially causal nonsynonymous single‐nucleotide polymorphisms (SNPs) in the CYB5R3 gene.19 In b5R‐deficient dogs, methemoglobin accounts for 13–41% of total hemoglobin, and b5R activity in these dogs is <33% that of normal dogs.1, 13, 14, 15, 16, 17, 18, 19 In addition, the previously described dogs did not exhibit clinical signs other than cyanosis.1, 13, 14, 15, 16, 17, 18, 19 Therefore, it seems likely that the b5R deficiency seen in these dogs was similar to the type I condition found in humans. The deficiency in dogs is presumed to be an inherited disorder,4 but familial studies have not been performed.
We analyzed genomic DNA from a Pomeranian family with methemoglobinemia and identified a novel missense variant in the CYB5R3 gene. In addition, we generated predicted canine b5R structures using homology modeling and estimated the effects of a single amino acid change on enzyme function.
Materials and Methods
Cases
A 2‐year‐old female Pomeranian (case 1, proband) was admitted to a private animal hospital for evaluation of anorexia and vomiting associated with intestinal ileus. The dog's oral mucous membranes, tongue, and lower abdominal skin exhibited a bluish discoloration on physical examination (Fig 1A and B). No cardiac or pulmonary abnormalities were detected on auscultation. During a preoperative examination of an inguinal hernia and intestinal obstruction, the dog's blood was found to be much darker than that of a normal dog (Fig 1C). The hematologic findings obtained at that time included a PCV of 51.4%, hemoglobin concentration of 19.6 g/dL, and total leukocyte count of 21 800/μL. There was no history of exercise intolerance or respiratory distress at home, and the dog had not been exposed to drugs or chemicals that are known to induce methemoglobinemia, including sulfonamides and acetaminophen. During surgery, the dog's percutaneous oxygen saturation (SpO2) fell to 90% (reference range, 96–100%). There were no surgical or anesthetic complications. The dog appeared clinically normal the next day, but its oral mucous membranes remained dark blue.
Figure 1.
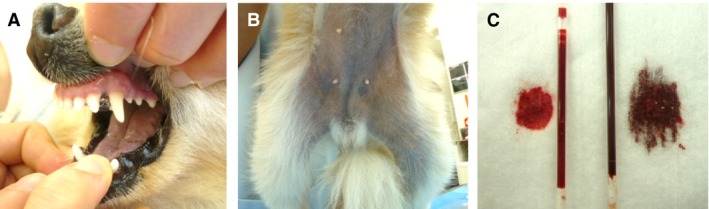
Clinical signs of cyanosis in the patient dog (Case 1). Cyanosis of the oral mucous membranes and tongue (A) and lower abdominal skin (B) as well as a spot test of venous blood from the patient (C; right) and a normal dog (C; left).
A blood sample was submitted to the Veterinary Teaching Hospital at Iwate University (IUVTH) to test for methemoglobinemia. In addition, 2 blood samples from the sire (case 2) and 1 of its siblings (case 3) were submitted to allow a family study of methemoglobinemia. These dogs also had cyanotic mucous membranes but not breathlessness or exercise intolerance. Their blood was brownish in color. The family pedigree is shown in Figure 2. Of the dog's siblings, 3 were cyanotic, 1 did not exhibit cyanosis, and the presence or absence of cyanosis could not be determined in the remaining dog. The dam was clinically normal, judging from physical examination, and did not exhibit cyanosis. Although b5R deficiency was previously reported in a Pomeranian puppy in the USA,16 the relationship between that puppy and the present dog family is unclear.
Figure 2.
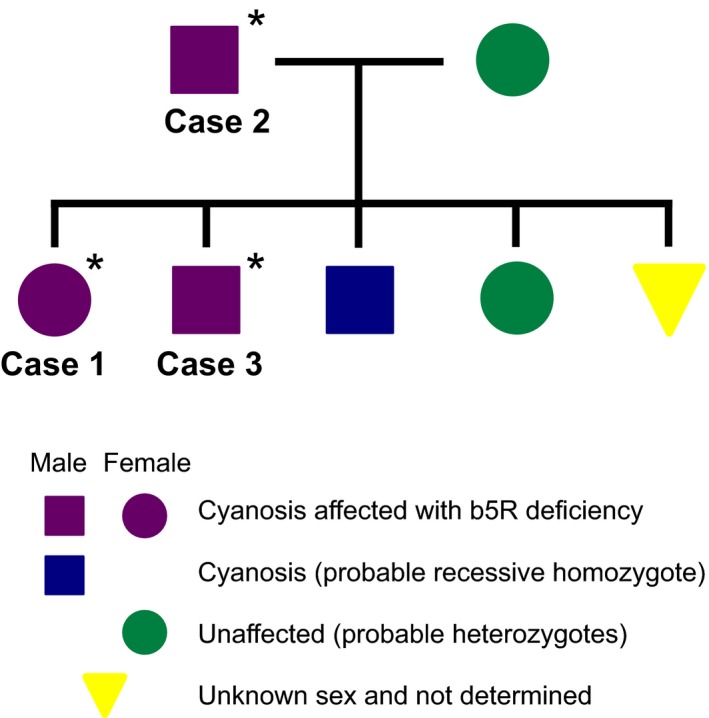
Family pedigree of the patient dog. *, Dogs were examined in the present study.
Measurement of the Concentrations of Methemoglobin and Reduced Glutathione (GSH), NADH‐Cytochrome b5 Reductase Activity, and Turbidity Index
Venous blood samples were collected from the affected dogs and healthy control dogs in ethylenediaminetetraacetic acid (EDTA) tubes and examined. Five beagles that were bred at Iwate University and 5 Pomeranian dogs that visited IUVTH for other diseases and that were free from methemoglobinemia were used as controls. Blood samples were collected with the informed consent of the dog owners. All of the procedures involving experimental animals were performed in accordance with the guidelines regulating animal use at the Department of Veterinary Medicine, Iwate University.
The methemoglobin concentration of erythrocytes was measured as previously described20 and expressed as a percentage of the total amount of hemoglobin. Erythrocyte b5R activity and reduced glutathione (GSH) concentration were evaluated as previously described.21 The b5R activity was measured by assessing NADH‐ferricyanide reductase activity. A turbidity index for evaluating Heinz body formation in erythrocytes was assayed as previously described.22
Genome Analysis
We used genomic DNA to analyze the canine CYB5R3 gene. Genomic DNA was isolated from whole blood with a commercial kit.a It is thought that the membrane‐bound form and soluble form of the enzyme are generated from alternative transcripts differing in the first exon, 1M and 1S, respectively.7 The canine CYB5R3 gene is located on chromosome 10, and exon 1M is found in the 530 bp gap (chr10: 22820169‐22820698) in the current version of the dog genome (CanFam3.1, NC_006592.3).23 DNA sequence corresponding to exon 1S exists in the CYB5R3 gene (chr10: 22822395‐22823086).24 We analyzed the predicted promoter regions (to −671 bp from the ATG in exon 1M), 10 exons (exon 1M, 1S, 2, 3, 4, 5, 6, 7, 8, and 9) with splice junctions, and the 3′‐UTR (1118 bp). The primers we used are listed in Table S1. Polymerase chain reaction (PCR) was performed in accordance with the manufacturer's protocol.b The reaction conditions for most amplicons, including exons 1S, 2, 3 to 4, 5, 6 to 7, 8, and 9–3′, were as follows: the initial denaturation was conducted at 94°C for 1 minute followed by 30 cycles of 10 seconds at 98°C and 1 minute at 68°C. The amplification of the region from the promoter to exon 1M and exon 9–5′ regions was performed with the following conditions: initial denaturation was carried out at 94°C for 1 minute followed by 30 cycles of 10 seconds at 98°C, 10 seconds at 60°C, and 1 minute at 68°C. Successful amplification was verified by visualization in a 1.5% agarose gel, and the products were purified with a commercial kit.c The PCR products were directly sequenced in an automated sequencerd with a sequencing kit.e
Homology Modeling
The predicted structure of the soluble form of canine b5R (Met24 to Phe301) was generated by the automated comparative protein modeling server SWISS‐MODEL.f The generated 3‐dimensional model was visualized by PyMOL software.g To analyze whether the detected amino acid substitutions affected protein function, the SIFT (Sorting Intolerant From Tolerant)h and PROVEAN PROTEINi programs were used.
Results
Methemoglobin and Reduced Glutathione (GSH) Concentrations, b5R Activity, and Turbidity Indices of Canine erythrocytes
The methemoglobin concentrations of the erythrocytes collected from dogs 1, 2, and 3 were remarkably higher than those of the control dogs (Table 1). The b5R activity of the affected dogs was notably lower than that of the controls (Table 1). The activity seen in the affected dogs was <33% that in the normal dogs. There was no difference between the affected and control dogs in the GSH concentrations and turbidity indices (Table 1).
Table 1.
Methemoglobin content, NADH‐ferricyanide reductase activity, GSH concentration, and a turbidity index in erythrocytes of the affected and control dogs
| Methemoglobin (%) | NADH‐ferricyanide reductase activity (IU/gHb) | GSH concentration (μmol/gHb) | Turbidity index | ||
|---|---|---|---|---|---|
| Controls | Beagles (n = 5) | 0.31 ± 0.22 | 19.1 ± 2.67 | 10.4 ± 0.73 | 0.40 ± 0.05 |
| Pomeranians (n = 5) | 0 | 16.5 ± 1.85 | 13.3 ± 1.83 | 0.35 ± 0.06 | |
| Cases | 1 | 27.1 | 5.7 | 10.8 | 0.36 |
| 2 | 11.5 | 5.7 | 12.4 | 0.39 | |
| 3 | 26.9 | 3.0 | 32.0 | 0.17 |
NADH‐cytochrome b5 reductase activity was measured by assessing NADH‐ferricyanide reductase activity. The results of the control dogs are expressed as mean ± standard deviation and mean values, respectively. Hb, hemoglobin.
Sequence analysis of the canine CYB5R3 gene
For CYB5R3 genotyping, we first attempted to fill the 530 bp gap at the CYB5R3 exon 1M locus. The start codon in exon 1M was found to be 337 bp downstream of the 5′ end of the gap, whereas the end of exon 1 was located 173 bp upstream of the 3′ end of the gap. The information about polymorphism in the canine CYB5R3 gene was compared with the previous studies.23, 24 In our study, a new SNP in the promoter region, c.1‐234 T/G, was identified, and all the tested dogs were homozygous for the G allele at this position. In addition, 1 intronic SNP, c.634‐77 C/A, was found in the amplicons of exon 8, and the SNP was far from splice junctions.
Interestingly, 1 nonsynonymous variant in exon 7, an A→C transition (c.580A>C), was found in the CYB5R3 gene of all of the affected dogs (Fig 3). The A→C base substitution identified in this region causes replacement of isoleucine by leucine at amino acid residue 194 (p.Ile194Leu) in the canine b5R protein. Direct sequencing analysis showed that all of the affected dogs were homozygous for the C allele (Fig 3). In contrast, all of the control dogs, including the nonrelated Pomeranian dogs, had AA alleles at the same locus.
Figure 3.
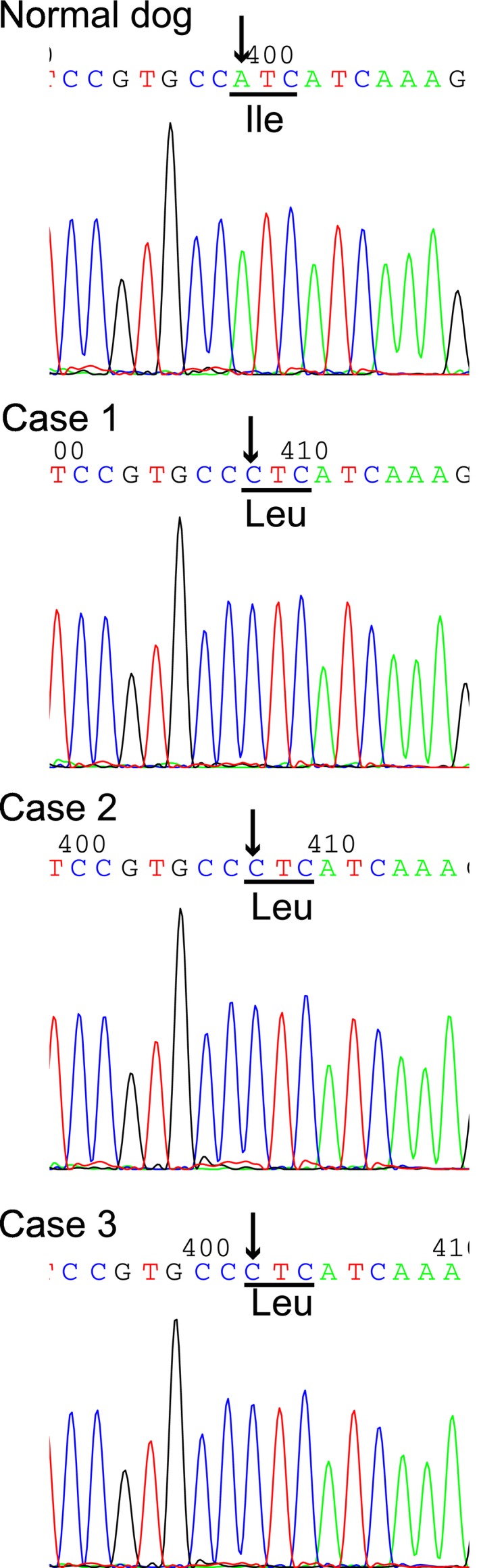
Detection by direct DNA sequencing of a homozygous single base substitution in exon 7 (c.580A>C) of the canine CYB5R3 gene in the affected dogs. This missense changes the ATC codon to a CTC codon, which results in the replacement of an isoleucine (Ile) by a leucine (Leu) at amino acid residue 194. The position of a base change is indicated by an arrow in the chromatograms. The codon with the A→C substitution is underlined.
Homology Modeling
According to the primary amino acid sequence of canine b5R (Gene ID: 474479), Ile194 is located in the caudal region of the NADH‐binding motif 181GTGITP186. We generated a predicted model of the soluble form of canine b5R by the SWISS‐MODEL server.f The query sequence (Met24 to Phe301) and the soluble forms of porcine and human b5R (PDB ID: 3W5H and 1UMK) shared 95.9 and 92.9% sequence identity, respectively. We used the 3‐dimensional structure of porcine b5R as a template for homology modeling.
The predicted structure of the soluble form of canine b5R, as visualized by the PyMOL software,g is shown in Figure 4. As shown in Figure 4, the predicted canine b5R structure consists of 2 major domains: the N‐terminal or FAD‐binding domain (Ile34 to Leu148) and the C‐terminal or NADH‐binding domain (Val172 to Phe301). These 2 domains are connected by a flexible hinge region with 3‐strand antiparallel β‐sheets (Leu149 to Thr171) (Fig 4). The modeling indicated that the Ile194 in canine b5R is located in an α‐helix extending from Gly181 to Lys196 and forms a hydrogen bond with Val190 to maintain a spiral conformation in the α‐helix.
Figure 4.
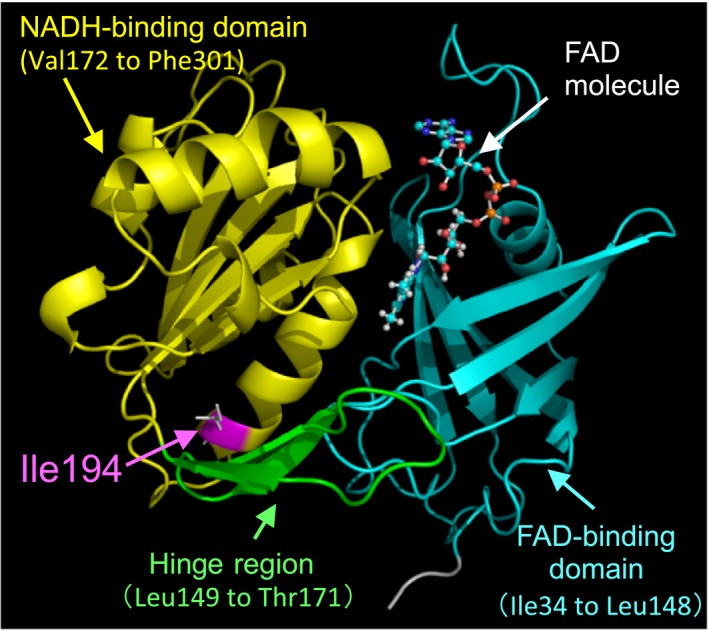
A ribbon diagram of the 3‐dimensional model of the predicted canine soluble form b5R was created from the query sequence of the SWISS‐MODELf by the PyMOLg program. The FAD‐binding domain is shown in cyan (Ile34 to Leu148), while the NADH‐binding domain is shown in yellow (Val172 to Phe301), and the hinge region is shown in green (Leu149 to Thr171). This structure was based on a previously reported porcine model.9 Ile194 is shown in pink and was replaced by leucine in the Pomeranian dogs with familial congenital methemoglobinemia. The FAD molecule is represented by a ball and stick model. X‐ray crystallography indicated that the FAD cofactor was able to noncovalently bind to the large, wide boundary cleft between the two major domains,8, 10 whereas the NADH molecule was able to fit into the interdomain cleft at the reside of the FAD isoalloxazine ring.10
Next, we analyzed whether or not the Ile→Leu substitution at amino acid residue 194 might affect protein function. SIFT analysish predicted that the Ile194Leu would likely be tolerated without functionally damaging the enzyme (SIFT score, >0.05). In addition, the PROVEAN PROTEIN programi indicated that Ile194Leu was neutral (score, −1.877).
Discussion
We analyzed blood samples from Pomeranian dogs with suspected methemoglobinemia. These dogs had higher methemoglobin concentrations than did the control dogs, and b5R activity of the affected dogs was <33% that of the control dogs. The GSH concentrations and turbidity indexes of the affected dogs were within the normal reference ranges. All of the affected dogs belonged to the same family and were not exposed to any drugs and chemicals that are known to cause methemoglobinemia, suggesting that the methemoglobinemia seen in these dogs was caused by familial congenital b5R deficiency. In humans, congenital b5R deficiency is inherited in a recessive fashion.5 We could not examine blood samples from the dam and other siblings. However, based on their clinical findings, it was suspected that the unaffected dogs were heterozygous at the relevant locus.
Methemoglobin normally forms at low rates in vivo. It is estimated that 0.5–3.0% of erythrocyte hemoglobin is oxidized to methemoglobin each day in humans and dogs.18 Three main mechanisms are responsible for reducing methemoglobin in erythrocytes: the NADH pathway, the nicotine adenine dinucleotide phosphate (NADPH) pathway, and a nonenzymatic pathway involving GSH and ascorbic acid. The NADH pathway catalyzed by b5R is most important and is responsible for reducing up to 95% of methemoglobin.25, 26, 27 The other pathways account for small amounts of methemoglobin reduction under normal conditions.2, 25, 26, 27 Therefore, congenital methemoglobinemia commonly is caused by b5R deficiency,2, 5 and in humans, more than 40 variants in the CYB5R3 gene have been described.5 We hypothesized that the methemoglobinemia identified in this Pomeranian dog family might have been caused by b5R dysfunction, and we analyzed the canine CYB5R3 gene. As a result, it was found that this Pomeranian family carries a missense variant, c.580A>C, in the CYB5R3 gene, which causes replacement of Ile→Leu at amino acid residue 194. The A→C base substitution seen in this region was not found in the control dogs, including nonrelated Pomeranian dogs. In fact, b5R activity in the affected dogs was markedly decreased. This finding indicated that the methemoglobinemia seen in this Pomeranian dog family was caused by congenital b5R deficiency due to c.580A>C in the CYB5R3 gene.
A limitation of our study was our inability to evaluate expression levels of CYB5R3 mRNAs and b5R proteins in erythrocytes from dogs. Nonsynonymous SNPs have been reported to be able to affect not only mRNA stability, and thus protein expression, but also enzymatic activity.23, 28 We tried to analyze the b5R protein concentrations in dog erythrocytes by immunoblotting using an anti‐human b5R antibody, but no b5R protein signals were detected. Recently, it has been reported that the synonymous cording SNP in the CYB5R3 gene might contribute to the risk of sulfonamide hypersensitivity in dogs.23 Dogs cannot detoxify sulfonamides by N‐acetylation because they lack N‐acetyltransferase genes.23, 29 Instead, b5R plays a major role in sulfonamide detoxification in dogs.23, 29 An A→G base change at position 729 in exon 8, which does not result in an amino acid substitution (proline→proline at codon 243), is associated with low b5R protein expression in the canine liver, and 78% of dogs with sulfonamide hypersensitivity had the GG genotype.23 As a result of genotyping, all of the affected dogs and 1 of the control dogs were 729 GG homozygotes (data not shown). The b5R activity of the healthy dog with the 729 GG genotype was within the reference range (data not shown), suggesting that this synonymous SNP at position 729 in CYB5R3 gene might not affect erythrocytic b5R activity. Although it remains unclear whether or not the nonsynonymous SNP found in our study affects CYB5R3 gene expression in erythrocytes, our data indicated that replacement of Ile→Leu at amino acid residue 194 of b5R due to c.580A>C in the CYB5R3 gene would affect the function of b5R in erythrocytes of affected dogs.
The b5R utilizes electrons from NADH to reduce ferricytochrome b5 to ferrocytochrome b5 via its FAD prosthetic group,1, 24 and the resultant ferrocytochrome b5 binds to ferriheme subunits in methemoglobin and transfers electrons to them.1, 24 This electron transfer process reduces the ferric atom in methemoglobin back to hemoglobin (which can bind to oxygen again) and regenerates ferricytochrome b5.1, 24 In congenital methemoglobinemia of humans, several genetic variants have been detected in the CYB5R3 gene,5 but most of them are located in regions not directly related to the FAD or NADH‐binding motif. In in vitro studies, most of the mutant proteins exhibited some dysfunction, including a reduction in catalytic activity and instability against heat and proteases.5, 30, 31
The 3‐dimensional structure of human b5R was resolved by X‐ray crystallography and can be used to predict the effects of amino acid changes on enzyme function.5 In the homology modeling that we performed, Ile194 in canine b5R corresponded to the residue located at the 1st α‐helix (Gly181 to Lys196) in the NADH‐binding domain. Isoleucine and leucine are isomers, and their only structural difference relates to the positions of their methyl groups. We generated an Ile194Leu prediction model by PyMOL,g but replacement of isoleucine by leucine did not affect the hydrogen bond with Val190. Moreover, 2 computerized analysesh , i showed that Ile194Leu would probably be tolerated and not cause enzyme dysfunction. These findings can be explained by the fact that the amino acid change was relatively subtle because isoleucine and leucine are both uncharged nonpolar residues. On the other hand, b5R mutations in humans affecting the proximal region of the NADH‐binding motif disrupt the tertiary structure of b5R and cause functional damage to it.30, 31 In mature erythrocytes, which have no protein‐synthesizing machinery, such mutant proteins would markedly shorten the half‐life of b5R molecules. So far, no nonsynonymous variants affecting the Ile194 residue have been reported in the human CYB5R3 gene. Additional studies are needed to confirm our findings and determine the functional relevance of the canine b5R variant detected in our study.
Supporting information
Table S1. Primers used in this study.
Acknowledgments
Conflict of Interests Declaration
The authors declare that they have no conflict of interests.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
This study was performed at Iwate University. Local veterinarians (TS and KO) contributed to the clinical assessments and sample collection, and were in contact with the dogs’ owners.
Part of this study has been presented as an oral case report at the 12th Scientific Meeting of the Japanese College of Veterinary Internal Medicine (February 19–21, 2016).
A part of this study was supported by a grant from the Organization for the Revitalization of the Sanriku Region and Regional Development, Iwate University, and by a Grant‐in‐Aid for Scientific Research C (24580442) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
DNeasy blood and tissue kit, Qiagen
Tks Gflex DNA polymerase, Takara Bio, Japan
Isospin PCR product, Nippon Gene, Japan
ABI‐PRISM 3500 genetic analyzer, Applied Biosystems, Warrington, UK
BigDye terminator cycle sequencing kit, Applied Biosystems, Warrington, UK
https://swissmodel.expasy.org/
https://www.pymol.org/
http:/sift.jcvi.org/www/SIFT_seq_submit2.html
http:/provean.jcvi.org/seq_submit.php
References
- 1. Key DM, Kelly JH. Methemoglobinemia in a dog. Vet Med Small Anim Clin 1980;75:245–246. [PubMed] [Google Scholar]
- 2. Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann Emerg Med 1999;34:646–656. [DOI] [PubMed] [Google Scholar]
- 3. Rockwood GA, Armstrong KR, Baskin SI. Species comparison of methemoglobin reductase. Exp Biol Med 2003;228:79–83. [DOI] [PubMed] [Google Scholar]
- 4. Harvey JW. Pathogenesis, laboratory diagnosis and clinical implications of erythrocyte enzyme deficiencies in dogs, cats and horses. Vet Clin Pathol 2006;35:144–156. [DOI] [PubMed] [Google Scholar]
- 5. Percy MJ, Lappin TR. Recessive congenital methaemoglobinaemia: cytochrome b(5) reductase deficiency. Br J Haematol 2008;141:298–308. [DOI] [PubMed] [Google Scholar]
- 6. Roma GW, Crowley LJ, Barber MJ. Expression and characterization of a functional canine variant of cytochrome b5 reductase. Arch Biochem Biophys 2006;452:69–82. [DOI] [PubMed] [Google Scholar]
- 7. Leroux A, Mota VL, Kahn A. Transcriptional and translational mechanisms of cytochrome b5 reductase isoenzyme generation in humans. Biochem J 2001;355:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bando S, Takano T, Yubisui T, et al. Structure of human erythrocyte NADH‐cytochrome b5 reductase. Acta Crystallogr Sect D 2004;60:1929–1934. [DOI] [PubMed] [Google Scholar]
- 9. Bewley MC, Marohnic CC, Barber MJ. The structure and biochemistry of NADH‐dependent cytochrome b5 reductase are now consistent. Biochemistry 2001;40:13574–13582. [DOI] [PubMed] [Google Scholar]
- 10. Yamada M, Tamada T, Takeda K, et al. Elucidations of the catalytic cycle of NADH‐cytochrome b5 reductase by X‐ray crystallography: New insights into regulation of efficient electron transfer. J Mol Biol 2013;425:4295–4306. [DOI] [PubMed] [Google Scholar]
- 11. Scott EM, Griffith IV. The enzymic defect of hereditary methemoglobinemia: Diaphorase. Biochim Biophys Acta 1959;34:584–586. [DOI] [PubMed] [Google Scholar]
- 12. Hsieh HS, Jaffé ER. Electrophoretic and functional variants of NADH‐methemoglobin reductase in hereditary methemoglobinemia. J Clin Invest 1971;50:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harvey JW, Ling GV, Kaneko JJ. Methemoglobin reductase deficiency in a dog. J Am Anim Hosp Assoc 1974;164:1030–1033. [PubMed] [Google Scholar]
- 14. Atkins CE, Kaneko JJ, Congdon LL. Methemoglobin reductase deficiency and methemoglobinemia in a dog. J Am Anim Hosp Assoc 1981;17:829–832. [Google Scholar]
- 15. Rahilly L, Mandell DC. Methemoglobinemia In: Silverstein DC, Hopper K, eds. Small Animals Critical Care Medicine. St. Louis, MO: Saunders; 2009:374–378. [Google Scholar]
- 16. Fine DM, Eyster GE, Anderson LK, Smitley A. Cyanosis and congenital methemoglobinemia in a puppy. J Am Anim Hosp Assoc 1999;35:33–35. [DOI] [PubMed] [Google Scholar]
- 17. Letchworth GJ, Bentinck‐Smith J, Bolton GR, et al. Cyanosis and methemoglobinemia in two dogs due to a NADH methemoglobin reductase deficiency. J Am Anim Hosp Assoc 1977;13:75–79. [Google Scholar]
- 18. Harvey JW, King RR, Berry CR, Blue JT. Methemoglobin reductase deficiency in dogs. Comp Haematol Int 1991;1:55–59. [Google Scholar]
- 19. Jaffey JA, Harmon MR, Villani NA, et al. Long‐term treatment with methylene blue in a dog with hereditary methemoglobinemia caused by cytochrome b5 reductase deficiency. J Vet Intern Med 2017;31:1860–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hegesh E, Hegesh J, Kaftory A. Congenital methemoglobinemia with a deficiency of cytochrome b5. N Engl J Med 1986;314:757–761. [DOI] [PubMed] [Google Scholar]
- 21. Beutler E. RED CELL METABOLISM: A Manual of Biochemical Methods third Edition. Orlando, FL: Grune & Stratton, Inc. 1984;81–83:131‐134. [Google Scholar]
- 22. Winterbourn CC. Protection by ascorbate against acetylphenylhydrazine‐induced Heinz body formation in glucose‐6‐phosphate dehydrogenase deficient erythrocytes. Br J Haematol 1979;41:245–252. [DOI] [PubMed] [Google Scholar]
- 23. Funk‐Keenan J, Sacco J, Wong YY, et al. Evaluation of polymorphisms in the sulfonamide detoxification genes CYB5A and CYB5R3 in dogs with sulfonamide hypersensitivity. J Vet Intern Med 2012;26:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKenna JA, Sacco J, Son TT, et al. Congenital methemoglobinemia in a dog with a promoter deletion and a nonsynonymous coding variant in the gene encoding cytochrome b5. J Vet Intern Med 2014;28:1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yubisui T, Takeshita M, Yoneyama Y. Reduction of methemoglobin through flavin at the physiological concentration by NADPH‐flavin reductase of human erythrocytes. J Biochem (Tokyo) 1980;87:1715–1720. [DOI] [PubMed] [Google Scholar]
- 26. Yubisui T, Takeshita M. Purification and properties of soluble NADH‐cytochrome b5 reductase of rabbit erythrocytes. J Biochem (Tokyo) 1982;91:1467–1477. [DOI] [PubMed] [Google Scholar]
- 27. Yubisui T, Murakami K, Shirabe K, et al. Structural analysis of NADH‐cytochrome b5 reductase in relation to hereditary methemoglobinemia. Prog Clin Biol Res 1989;319:107–121. [PubMed] [Google Scholar]
- 28. Nackley AG, Shabalina SA, Tchivileva IE, et al. Human catechol‐O‐methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science 2006;314:1930–1933. [DOI] [PubMed] [Google Scholar]
- 29. Trepanier LA, Ray K, Winand NJ, et al. Cytosolic arylamine N‐acethltransferase (NAT) deficiency in the dog and other canids sue to an absence of NAT genes. Biochem Pharmacol 1997;54:73–80. [DOI] [PubMed] [Google Scholar]
- 30. Higasa K, Manabe J, Yubisui T, et al. Molecular basis of hereditary methaemoglobinamia type I and II: Two novel mutations in the NADH‐cytochrome b5 reductase gene. Br J Haematol 1998;103:922–930. [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Wu Y, Zheng P, et al. A novel mutation in the NADH‐cytochrome b5 reductase gene of a Chinese patient with recessive congenital methemoglobinemia. Blood 2000;95:3250–3255. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used in this study.


