Abstract
Background
Prophylactic administration of calcitriol has been suggested to mitigate the risk of hypocalcemia after parathyroidectomy. The effect of calcitriol on postoperative serum ionized calcium concentrations has not been evaluated in dogs after parathyroidectomy.
Hypothesis/Objectives
To determine the effect of prophylactic calcitriol administration on postoperative serum ionized calcium (iCa) concentrations in dogs with primary hyperthyroidism (PHPTH) treated by parathyroidectomy.
Animals
Seventy‐eight dogs with primary hyperparathyroidism treated surgically.
Methods
Multi‐institutional retrospective case study. Medical records from 2005 to 2015 were evaluated. Dogs were included if they had a diagnosis of PHPTH and had surgery to remove parathyroid tissue. Serum iCa concentrations were monitored for a minimum of 2 days postoperatively. Two study groups were evaluated: calcitriol administration and no calcitriol administration.
Results
Serial postoperative iCa concentrations measured at 12‐hour time intervals for 2 days postoperatively were positively associated with preoperative iCa concentrations. This association was evident at each time interval, and the effect of preoperative iCa concentrations on postoperative iCa concentrations decreased as time elapsed (12 hours, P < 0.0001; 24 hours, P < 0.0001; 36 hours, P < 0.04; and 48 hours, P = 0.01). Prophylactic calcitriol administration was not found to be significantly associated with postoperative iCa concentrations or its rate of decrease after parathyroidectomy.
Conclusion and Clinical Importance
We found no protective value in administering calcitriol prophylactically to prevent hypocalcemia in the immediate postoperative period (48 hours) after parathyroidectomy. Preoperative iCa concentrations had a significant positive association with postoperative iCa concentrations throughout the monitoring period.
Keywords: Hypervitaminosis D, Hypocalcemia, Parathyroid glands, Primary hyperparathyroidism
Abbreviations
- iCa
ionized calcium
- PHPTH
primary hyperparathyroidism
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone‐related protein
- tCa
total calcium
- VIF
variance inflation factor
Calcium regulation is vital to normal homeostasis and is achieved primarily by secretion of parathyroid hormone (PTH) from the chief cells of the parathyroid glands in response to decreasing serum ionized calcium (iCa) concentrations.1, 2 Parathyroid hormone increases serum calcium concentrations by inducing osteoclast‐mediated bone resorption as well as stimulating intestinal absorption and renal resorption of calcium.3, 4, 5, 6, 7 In healthy dogs, normal or increased serum iCa concentrations have a strong negative feedback effect on PTH secretion. The hypercalcemia observed in primary hyperparathyroidism (PHPTH) is caused by autonomous secretion of PTH by the parathyroid chief cells in the face of normal or increased iCa concentrations.1, 2, 8, 9 Primary hyperparathyroidism most commonly is caused by a parathyroid adenoma, whereas parathyroid hyperplasia and parathyroid carcinoma are identified less frequently.1, 9, 10, 11, 12
Surgical removal or ablation of the affected parathyroid glands is the recommended treatment for dogs with PHPTH because of concern for the adverse effects of long‐term hypercalcemia.1, 3, 8, 11, 13, 14, 15 Studies in both humans and dogs have identified postoperative hypocalcemia as a risk factor for morbidity and mortality after parathyroidectomy.1, 3, 11, 15, 16 Previous studies showed a steep and linear decrease in serum PTH and serum iCa concentrations within the first 24 hours after parathyroidectomy, followed subsequently by a plateau. The largest decrease in serum iCa concentrations typically occurs in the first 24–48 hours after removal of the abnormal parathyroid gland, with most dogs becoming normocalcemic within 48 hours of surgery.1, 11, 13, 14, 15, 16, 17, 18, 19 This rapid decrease is a consequence of the short half‐life of PTH (3–5 minutes), and the plateau results from reestablishment of normal parathyroid axis and parathyroid gland function with respect to PTH production. Recent studies have failed to identify preoperative factors that can predict when a patient will develop postoperative hypocalcemia.3, 13
To prevent clinical signs of hypocalcemia in the immediate postoperative period, many dogs are treated with PO calcitriol (1,25 dihydroxycholecalciferol, 1,25 dihydroxyvitamin D3) perioperatively, to increase intestinal absorption of calcium.1, 20, 21, 22 Despite the potential to mitigate the risk of hypocalcemia, the benefit of prophylactic administration of calcitriol has not been well documented, and, in fact, its administration may not impact the rate of decrease in serum iCa concentrations.1, 3, 8, 13 Furthermore, adverse effects associated with calcitriol administration may include continued suppression of PTH production, hypercalcemia, hyperphosphatemia, mineralization of soft tissues, and acute kidney injury.16, 17 There currently are no widely accepted guidelines for perioperative calcitriol administration in dogs with PHPTH. Dogs often are treated with calcitriol prophylactically based on severity of preoperative hypercalcemia (eg, iCa >1.75 mmol/L or total calcium [tCa] concentration >15 mg/dL), chronicity of disease, institutional tradition, or simply surgeon preference.1, 3, 13 These individual recommendations require validation with evidence‐based medicine, because unnecessary treatment with calcitriol could result in persistent hypercalcemia and a delay in recovery of the previously suppressed normal parathyroid glands.1, 3, 13
The purpose of our study was to evaluate the acute effect of calcitriol administration on serum iCa concentrations by comparing populations of dogs with PHPTH: those that received no prophylactic calcitriol treatment and those that received only prophylactic calcitriol treatment. We hypothesized that there would be no significant difference in the decrease in serum iCa concentrations between the study groups postoperatively.
Materials and Methods
Case Selection
Medical records of all dogs with a diagnosis of PHPTH that underwent parathyroidectomy at 3 academic teaching hospitals from January 2005 through December 2015 were reviewed retrospectively. Dogs were included if they had an increased serum iCa concentration with a normal or increased serum PTH concentration, underwent ultrasonography of the neck, had surgery to remove parathyroid tissue, and were hospitalized with serum iCa concentrations monitored for a minimum of 2 days postoperatively. Dogs were excluded if they had increased parathyroid hormone‐related protein (PTHrP) concentration or if another cause of hypercalcemia was identified from the results of CBC, serum biochemistry, urinalysis, thoracic radiography, or abdominal ultrasound examination. Cases also were excluded if they did not respond to the initial surgery, had multiple surgeries for recurrence of hypercalcemia, had evidence of renal or gastrointestinal disease that could affect calcium and phosphorous regulation, or if they received prophylactic PO calcium supplementation.
Medical Records Review
Medical records were reviewed to collect information on each dog's breed, sex, body weight, date of surgery, and age at the time of surgery. Information on preoperative and postoperative clinical signs, preoperative and postoperative serum iCa concentrations, preoperative serum PTH concentrations, surgical technique, surgical procedure, number of glands removed, histologic analysis of the removed parathyroid tissue, days of hospitalization, and postoperative calcitriol dose administered was recorded. All patients had serum iCa, PTH, and PTHrP concentrations measured at Michigan State University's Endocrinology Diagnostic Laboratory and serial iCa concentrations determined with the individual institution's blood gas analyzer. The serum iCa concentration reference range is 1.25–1.45 mmol/L at Michigan State University's Endocrinology Diagnostic Laboratory.
Dogs were entered into 2 treatment groups, no calcitriol administration after surgery and postoperative PO prophylactic calcitriol administration that started the day of surgery. The decision to supplement was based on clinician preference, and most patients were not treated with calcitriol at institutions B (University of Pennsylvania School of Veterinary Medicine) and C (Kansas State University College of Veterinary Medicine). Most patients were treated with calcitriol at institution A (Michigan State University College of Veterinary Medicine). Dogs that received calcitriol were further stratified into 2 groups; low‐dose calcitriol (<20 ng/kg/d) and high‐dose calcitriol (≥20 ng/kg/d) to evaluate potential effects of dosage. Stratification of calcitriol dosage was based on published calcitriol treatment protocols.1, 20, 23, 24, 25
Statistical analysis
Univariate statistical analysis (chi‐squares, Kruskal‐Wallis test, and 1‐way ANOVA) was performed to evaluate for any associations among the 3 treatment groups (no calcitriol, <20 ng/kg/d calcitriol, ≥20 ng/kg/d) and the evaluated numerical, nominal, and categorical variables to assess for selection bias. Numerical variables included preoperative serum iCa concentrations, serum PTH concentrations, age, and weight. Nominal variables included institution, sex, reproductive status, surgical procedure, histopathology, 1 or >1 glands removed, and cases that had bilateral glands removed. Surgical procedures were defined as parathyroidectomy (removal of only the affected parathyroid gland), partial thyroidectomy (removal of affected parathyroid gland and adjacent thyroid tissue), and thyroidectomy (removal of entire thyroid gland and both associated parathyroid glands). Unilateral multiple affected glands were defined as dogs with both parathyroid glands affected on the same side, and bilateral multiple affected glands were defined as dogs with an affected parathyroid gland on both the left and right. No dog had >2 parathyroid glands removed at the time of surgery. Normality of the residuals (errors) was evaluated by means of a probability plot and histogram. Not all data were normally distributed, and therefore, nonparametric tests (eg, Kruskal‐Wallis) were performed for all variables.
After univariate analysis, a multiple linear regression model was developed. The response variable used for statistical analysis was postoperative serum iCa concentration measured at 12‐hour time intervals (12, 24, 36, and 48 hours postoperatively). Multiple linear regression was used to evaluate measured and binary nominal factors against the continuous response variable of serum iCa concentration at 12‐hour intervals over 2 days of hospitalization. Factors evaluated for their impact on postoperative serum iCa concentration were age, sex, reproductive status, weight, preoperative serum iCa concentration, preoperative serum PTH concentration, implementation of calcitriol administration, calcitriol dosage administered (ng/kg/d), surgical technique, and cases in which >1 parathyroid gland was removed. Preoperative serum calcium concentrations levels reviewed included both serum iCa and tCa concentrations based on the monitoring protocol of the treating teaching hospital. Calcitriol dosing was evaluated both as total dose (ng per 24‐hour period) and dosage (ng/kg/d). Factors were tested for multicollinearity (variance inflation factor, VIF), and factors with excessive multicollinearity (VIF > 2.5) were excluded. Normality of the residuals (errors) was evaluated by means of a Q‐Q plot and histogram and accepted. These factors then were entered into a multiple linear regression model to evaluate statistical significance. All factors with a P value <0.10 were retained, and P < 0.05 was considered significant. Postoperative serum iCa concentrations were plotted using the mean serum iCa concentration for each designated study group at 12‐hour time points over the 2 days of hospitalization. The slope of these trends then was calculated by determining the change in postoperative serum iCa concentration over time. All analyses were performed using commercially available statistical software.1
Results
Evaluation of medical records identified 78 dogs that had a parathyroidectomy performed to treat PHPTH and met all the inclusion criteria. Descriptive statistics performed are presented in Table 1 (55 dogs received calcitriol supplementation and 23 dogs received no calcitriol). Breeds of the dogs included in the study were mixed‐breed dogs (n = 22), Golden Retriever (5), Shih Tzu (5), Siberian Husky (4), Beagle (3), Dachshund (3), and 2 each of the following breeds: Australian Shepherd, Bichon Frise, Bull Terrier, Collie, Jack Russell Terrier, Keeshond, and Labrador Retriever. Twenty‐two other breeds were represented by a single individual.
Table 1.
Distribution of dogs and numerical and nominal variables within treatment groups (No calcitriol, <20 ng/kg/d, ≥20 ng/kg/d)
| Total | No Calcitriol | <20 ng/kg/d Calcitriol | ≥20 ng/kg/d Calcitriol | |
|---|---|---|---|---|
| Dogs | 78 | 23 | 26 | 29 |
| Dogs from Institution A | 63 | 12 | 24 | 27 |
| Dogs from Institution B | 6 | 5 | 1 | 0 |
| Dogs from Institution C | 9 | 6 | 1 | 2 |
| Median age years (Range) | 10 (3–14) | 11 (6–14) | 10 (3–13) | 10 (5–14) |
| Male neutered/intact | 37/4 | 11/1 | 11/1 | 15/2 |
| Female neutered/intact | 36/1 | 12/0 | 11/1 | 13/0 |
| Median weight kg (Range) | 23.9 (5.8–70) | 22.7 (5.8–61.8) | 33.5 (10.5–70.0) | 19.6 (6.0–38.1) |
| Mean pre‐op iCa (mmol/L ±SD) | 1.84 ± 0.24 | 1.78 ± 0.20 | 1.90 ± 0.26 | 1.83 ± 0.24 |
| Solitary gland affected (R, L) | 47 (26, 21) | 14 (7, 7) | 16 (11, 5) | 17 (8, 9) |
| Unilateral multiple glands affected | 14 | 2 | 6 | 6 |
| Bilateral multiple glands affected | 17 | 7 | 4 | 6 |
| Glands removed | 86 | 25 | 29 | 32 |
| Adenoma | 51 | 15 | 15 | 21 |
| Carcinoma | 13 | 2 | 6 | 5 |
| Hyperplasia | 18 | 7 | 5 | 6 |
| Undifferentiated | 4 | 1 | 3 | 0 |
Of the 47 dogs that had a single parathyroid mass, parathyroidectomy of a single gland was performed in 16 dogs and a partial thyroidectomy was performed in 31 dogs. Of the dogs with multiple presumed affected glands (31), multiple parathyroidectomies were performed in 9 dogs, multiple partial thyroidectomies were performed in 8 dogs, and a complete thyroidectomy was performed in 14 dogs. No dog had >2 parathyroid glands removed at the time of surgery. Of the 109 submitted glands, 23 glands were normal histopathologically and 86 had histopathologic changes. A single mass was confirmed, based on histologic findings in 90% (70/78) of the dogs. Only 87% (86/99) of abnormal glands identified ultrasonographically and 79% (86/109) of abnormal glands identified intraoperatively had histopathologic change (parathyroid adenoma, hyperplasia, or carcinoma) consistent with primary hyperparathyroidism.
Fifty‐five dogs (55/78; 71%) were treated prophylactically with calcitriol at a mean dosage of 23 ± 12 ng/kg/d (range, 6–52 ng/kg/d). When stratified based on dosage, 29 dogs received a dosage ≥20 ng/kg/d, whereas 26 dogs received a dosage <20 ng/kg/d. Dosing of dogs varied among clinicians and institutions based on clinician preference. The more commonly formulated 250‐ng capsules led to larger variations in ng/kg/day dosage as patient body weight decreased. Twenty‐three dogs (23/78; 29%) received no calcitriol after surgery. All dogs that received calcitriol were placed on the initial postoperative dosage for a minimum of 7 days. After that period, owners were instructed to slowly taper the calcitriol over the next 2–4 weeks. The exact length of treatment with calcitriol was not identified in the medical records and presumably varied among clinicians and institutions. No dogs required emergency calcium supplementation or exhibited clinical signs of hypocalcemia in the postoperative period.
Univariate analysis of the numerical variables showed that only weight had an association with treatment group (no calcitriol, <20 ng/kg/d calcitriol, ≥20 ng/kg/d calcitriol), with the <20 ng/kg/d group significantly higher than the others (P = 0.0004). Chi‐square analysis of the nominal variables showed an association between institution and postoperative treatment with calcitriol (P = 0.001). Institution A had 19% of dogs that were not treated with calcitriol, 38% with low‐dose calcitriol, and 43% with high‐dose calcitriol. Institution B had 83% of dogs that were not treated with calcitriol, 17% with low‐dose calcitriol, and 0% with high‐dose calcitriol. Institution C had 67% of dogs that were not treated with calcitriol, 11% with low‐dose calcitriol, and 12% with high‐dose calcitriol. No other variables were found to have a significant association with an individual treatment group (Table 1).
Multiple linear regression identified a significant association of higher preoperative serum iCa concentrations with higher postoperative serum iCa concentrations throughout the monitoring period (12 hours, P < 0.0001; 24 hours, P < 0.0001; 36 hours, P < 0.04; and, 48 hours, P < 0.01). Multiple affected glands were associated with higher postoperative serum iCa concentrations at 36 and 48 hours postoperatively (P = 0.05 and P = 0.002), respectively, whereas bilaterally affected glands were associated with lower postoperative serum iCa concentrations at 36 hours and 48 hours postoperatively (P = 0.005 and P = 0.0002), respectively. Age (48 hours, P = 0.004), weight (48 hours, P = 0.001), and calcitriol dosage (12 hours, P = 0.02) were significantly associated at single 12‐hour time points within 48 hours, but were not consistently associated with serum iCa concentrations postoperatively.
Postoperative serum iCa concentrations were plotted using the mean iCa concentrations for the designated study groups at each 12‐hour time point throughout the 2 days of hospitalization. Plots were created based on the study groups of no calcitriol administration and calcitriol administration as well as low‐dose and high‐dose calcitriol administration (Figs 1, 2, Table 2). The P values and the slopes (change in postoperative serum iCa concentrations over time) from the multiple linear regression model for preoperative serum iCa concentrations were significantly different at 12 hours (P < 0.0001, slope 0.548), 24 hours (P < 0.0001, slope 0.332), 36 hours (P < 0.04, slope 0.163), and 48 hours (P = 0.01, slope 0.169).
Figure 1.
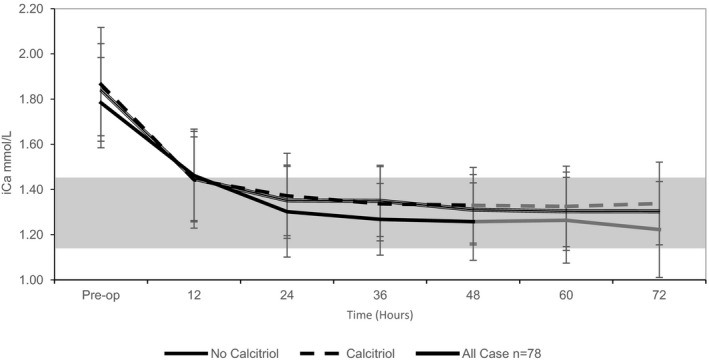
Graph plot of the mean iCa (mmol/L) over time for treatment groups (no calcitriol administration; calcitriol administration) with standard deviation bars. iCa trends between the treatment groups are similar and illustrate the association of pre‐op iCa to post‐op iCa in the immediate post‐op period. Both study groups become normocalcemic within 24 hours and then plateau through 72 hours. The first 48 hours includes the entire study group (black, n = 78), while 23 cases were lost from 48 hours through 72 hours (gray, n = 55). Normal iCa reference ranges (gray shaded).
Figure 2.
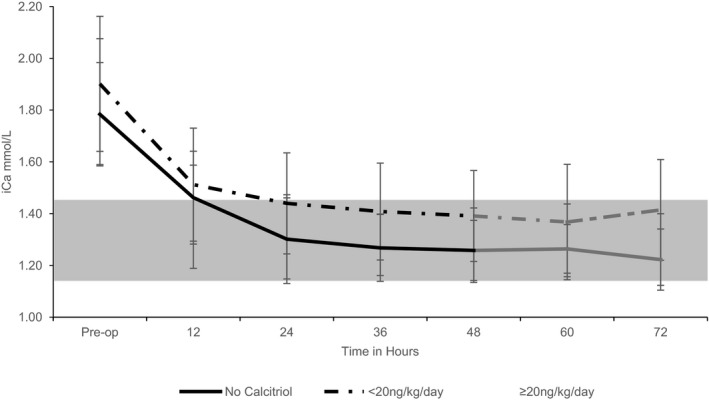
Graph of the mean iCa (mmol/L) over time for treatment groups (no calcitriol administration; stratified calcitriol administration [<20 ng/kg/d, ≥20 ng/kg/d]) with standard deviation bars. iCa trends between the treatment groups are similar and illustrate the association of pre‐op iCa to post‐op iCa in the immediate post‐op period. Both study groups become normocalcemic within 24 hours and then plateau through 72 hours. The first 48 hours includes the entire study group (black, n = 78), while 23 cases were lost from 48 hours through 72 hours (gray, n = 55). Normal iCa reference ranges (gray shaded).
Table 2.
Mean iCa (mmol/L) ± SD for treatment groups (no calcitriol supplementation; stratified calcitriol supplementation [<20 ng/kg/d, ≥20 ng/kg/d]) pre‐op and at 12 hour, 24 hour, 36 hour, and 48 hour (n = 78) and 60 hour and 72 hour (n = 55). iCa trends between the treatment groups are similar and illustrate the association of pre‐op iCa of post‐op iCa in the immediate post‐op period
| Pre‐op iCa | 12‐Hour iCa | 24‐Hour iCa | 36‐Hour iCa | 48‐Hour iCa | 60‐Hour iCa | 72‐Hour iCa | |
|---|---|---|---|---|---|---|---|
| All cases, n = 78 | 1.84 ± 0.24 | 1.45 ± 0.20 | 1.35 ± 0.19 | 1.35 ± 0.16 | 1.31 ± 0.16 | 1.30 ± 0.16 | 1.30 ± 0.17 |
| No calcitriol | 1.78 ± 0.20 | 1.46 ± 0.21 | 1.30 ± 0.20 | 1.27 ± 0.16 | 1.26 ± 0.17 | 1.26 ± 0.19 | 1.22 ± 0.21 |
| Calcitriol (all) | 1.86 ± 0.25 | 1.44 ± 0.21 | 1.37 ± 0.19 | 1.33 ± 0.16 | 1.33 ± 0.17 | 1.34 ± 0.18 | 1.34 ± 0.18 |
| <20 ng/kg/d | 1.90 ± 0.26 | 1.51 ± 0.22 | 1.44 ± 0.20 | 1.41 ± 0.19 | 1.39 ± 0.18 | 1.37 ± 0.22 | 1.41 ± 0.19 |
| ≥20 ng/kg/d | 1.83 ± 0.24 | 1.39 ± 0.20 | 1.30 ± 0.16 | 1.28 ± 0.12 | 1.28 ± 0.14 | 1.30 ± 0.14 | 1.26 ± 0.14 |
Fifty‐five dogs had additional data collected through 72 hours postoperatively. Distribution of the 23 cases lost to follow‐up included no calcitriol treatment (7/23 [30%]), low‐dose calcitriol treatment (9/26 [35%]), and high‐dose calcitriol treatment (6/29 [21%]). Among the remaining 55 cases, multiple linear regression continued to identify preoperative serum iCa concentrations as significant (60 hours, P = 0.08, slope 0.152; and 72 hours, P = 0.04, slope 0.218), and calcitriol administration continued to show no effect on serum iCa concentrations during the time period. Similar plots of serum iCa concentrations at 60 and 72 hours postoperatively also were created and showed a nadir and plateau beyond 24 hours (Figs 1, 2).
Discussion
Many clinicians advocate prophylactic calcitriol administration after parathyroidectomy to shorten monitoring and hospitalization times and to mitigate the risk of hypocalcemia.1, 3, 8, 13 Alternatively, some clinicians prefer only to implement calcitriol with or without calcium administration only if the dog shows clinical signs or experiences a marked decrease in serum iCa concentrations. The latter approach aims to minimize ongoing suppression of the remaining parathyroid glands by calcitriol‐induced gastrointestinal calcium absorption, and this approach also avoids possible adverse effects of calcitriol administration previously described.14, 15, 20, 21, 22, 23, 24, 25 Although prevention of critically low serum iCa concentrations is crucial to prevent abnormal physiology, some degree of hypocalcemia is required to stimulate the remaining parathyroid glands. Without stimulation from decreasing serum iCa concentrations, the remaining parathyroid glands will remain suppressed and fail to produce adequate amounts of PTH. Our results confirm the hypothesis that prophylactic postoperative calcitriol administration calcitriol does not blunt the acute rate of decrease in serum iCa concentration after removal of the autonomously functioning parathyroid gland. We also identified a significant positive association between preoperative and postoperative serum iCa concentrations. Unfortunately, similar to other studies, our data failed to identify a single variable that was associated with the rate of decrease in serum iCa concentrations or the prediction of hypocalcemia postoperatively. These findings support the contention that it is difficult to predict or blunt the rate of decrease in serum iCa concentrations or development of hypocalcemia based solely on serum biochemical monitoring.
Some authors have suggested that preoperative serum calcium concentrations are indicative of the potential risk for postoperative hypocalcemia.11, 16 The current veterinary literature contains conflicting information about the value of preoperative serum iCa and tCa concentrations for predicting postoperative hypocalcemia.1, 3, 11, 13, 16 In our study, a higher preoperative serum iCa concentration was not associated with a more rapid decrease in serum iCa concentration or risk hypocalcemia postoperatively, but was in fact protective. Dogs that had higher preoperative serum iCa concentrations had significantly higher postoperative serum iCa concentrations at all time points throughout the monitoring period. This association, as indicated by the slope, was greatest at the earliest time point of 12 hours, at which postoperative iCa = intercept + 0.54× (preoperative iCa). The slope decreased from 0.54 at 12 hours to 0.35, 0.17, and 0.17 at 24, 36, and 48 hours, respectively. The protective nature of preoperative serum iCa concentration against hypocalcemia therefore was most identifiable at 12 hours postoperatively, and its impact decreased as time elapsed.
It has been recommended that dogs, particularly those that meet the aforementioned preoperative serum iCa concentration risk factors, be given calcitriol starting on the day of surgery and as early as 24–36 hours before surgery to mitigate these risks.1 This protocol has been proposed to combat the anticipated trough of serum PTH concentrations associated with suppression of remaining normal parathyroid glands by the autonomously secreting diseased parathyroid gland. Previous studies have documented that serum PTH concentrations decrease in a linear fashion within the first 24 hours after surgery and reach a plateau shortly afterward.13 A similar linear decrease in serum iCa concentrations in the first 24 hours postoperatively has been documented followed by a plateau during which serum iCa concentration after removal of often remained within the reference range.13 These data indicate that the serum iCa concentration will reach its nadir within the first 48 hours postoperatively. In 1 study, all 54 dogs experienced resolution of hypercalcemia within the first 48 hours. Our results were similar, with no significant difference between dogs that were treated prophylactically with calcitriol and the untreated group during the 48‐hour monitoring period (Fig. 1, Table 1). The decision to include only dogs that had a minimum of 48 hours of in‐hospital monitoring was based on these data. Despite the fact that dogs often are discharged after 48–72 hours of in‐hospital monitoring, hypocalcemia has been documented up to 21 days postoperatively.1 Consequently, owners should be educated about common clinical signs associated with hypocalcemia, and serum iCa concentrations should be monitored weekly on an outpatient status during this time period.
All dogs in our study that received calcitriol had treatment initiated on the day of surgery. The pharmacokinetics of calcitriol indicate that peak serum concentrations occur 3–6 hours after a PO dose and that a single dose can have biological effects for as long as 3–5 days although steady state often is reached up to 7 days after dose initiation.26 Calcitriol's direct effect on intestinal receptors and programming of enterocytes occur primarily in undifferentiated cells of the intestinal crypts, and turnover of these cells takes place every 24 hours.27 Based on the pathophysiology and pharmacokinetics of calcitriol, it can be assumed that there would be some impact within the first 2 days postoperatively, even if its maximal effect may not be appreciated this quickly. Interestingly, calcitriol administration did not change the rate of decrease in serum iCa concentrations among study groups during the postoperative period in our population of dogs with PHPTH. It is unclear whether or not general anesthesia and opioid‐induced gastrointestinal ileus could have played roles in delayed absorption of the calcitriol and subsequently blunted its physiologic effects.
Autonomously functioning parathyroid glands can be difficult to identify. Despite the use of perioperative serum PTH assays to document removal of autonomously functioning parathyroid tissue, most surgeons rely on ultrasonography, clinical experience, and subjective evaluation during surgery.23, 28, 29, 30 Difficulty identifying the affected gland was highlighted in our study in which 87% (86/99) of abnormal glands identified ultrasonographically and 79% (86/109) of abnormal glands identified intraoperatively had histopathologic changes consistent with PHPTH. Difficulty identifying autonomously functioning parathyroid tissue can lead to increased unilateral or bilateral dissection. Bilateral dissection has been shown to be a strong independent risk factor for hypocalcemia in humans, which is consistent with the association that bilateral parathyroidectomy had on lower serum iCa concentrations at 36 and 48 hours postoperatively.31 Histopathologic evaluation of parathyroid glands has been subject to controversy over the past decade because of the similarity of the histopathologic appearance of adenomas and carcinomas.32 Parathyroid adenomas were most common in our study, representing 59% of the samples evaluated. Although this finding is consistent with previous veterinary literature, carcinomas were more common (15%) in our population. Although a standardized protocol for histopathologic diagnosis is available in veterinary medicine, it has not been widely adopted.6 The diagnosis of parathyroid carcinoma in humans is made by considering a combination of biological behavior, histologic appearance, and immunohistochemistry.33 Unfortunately, none of the biopsy specimens evaluated in our study were evaluated for HRPT2 mutation status or parafibromin immunostaining which is commonly performed in human medicine. As such, we may have overdiagnosed parathyroid carcinomas in our population of animals. Regardless, no association was found between histologic diagnosis and changes in postoperative serum iCa concentrations in our study groups.
Our study had several limitations, primarily associated with its retrospective design. The most noteworthy limitation is a lack of uniformity in the treatment protocol among those dogs that received calcitriol. Within the study groups, the dosage used was variable, even within the same institutions. Some animals received high dosage of calcitriol, whereas other received substantially lower dosages. Administration protocols consisted of calcitriol that was most often dosed in increments of 250 ng, because the most commonly available sources of calcitriol were 250‐ng capsule, 500‐ng capsule, and 1 μg/mL liquid for use PO. Based on medical records review, most dogs were dosed with 250‐ or 500‐ng capsules. This practice led to large dosing ranges among the 55 dogs (6–52 ng/kg/d) because dogs were dosed based on the calcitriol formulation available, affecting the dosing regimens, especially for lower weight patients. There is no current consensus for calcitriol dosing postoperatively for dogs with PHPTH with calcitriol dosages ranging from as high as 20–30 ng/kg/d to as low as 3–6 ng/kg/d.1, 34, 35, 36 Owing to the retrospective design of our study, medical record review did not permit determination of patient caloric intake, and this may have affected not only calcitriol absorption, but also calcium absorption by enterocytes.
As a consequence of the multi‐institutional and retrospective nature of our study, differences in data collection occurred. These included the blood gas machines used and their associated reference ranges, as well as differences in the discretion of clinician or institutional protocol regarding implementation of prophylactic calcitriol administration. Although preoperative measurements of serum iCa, PTH, and PTHrP concentrations were standardized by the protocol of Michigan State University Endocrine Diagnostic Laboratory, the postoperative monitoring at each institution was performed with each institution's in‐house blood gas analyzer. Some variations in the reference ranges of the different machines may have been present, but all analyzers were calibrated routinely, and only results of serum iCa concentrations measured with ion‐selective potentiometry, rather than calculated values, were used. Utilizing measured serum iCa concentrations helps minimize the error associated with calculated values, but interassay variability still may be present and was not evaluated in our study. A number of indicators for the prophylactic use of calcitriol treatment have been recommended, including severity of preoperative hypercalcemia and chronicity of disease, although many clinicians choose to treat all dogs with PHPTH as a form of risk mitigation. Signs of hypocalcemia are documented infrequently and unpredictably in dogs treated for PHPTH.3, 13 In our study, despite institutional variations in protocol, selection bias was minimized by the inherent fact that individual clinicians at selected institutions were consistent in their treatment protocols despite clinical variations among dogs with PHPTH in our study. Based on medical record review, individual clinicians consistently implemented the same postoperative treatment protocol (no calcitriol supplementation or calcitriol supplementation) independent of an individual dog's clinical or biochemical data. Univariate statistical analysis of both numerical and nominal variables confirmed no bias among the treatment groups. This finding suggests that clinicians did not select protocols based on preoperative serum iCa concentrations or the number of affected glands, but based on personal treatment preference.
Lack of long‐term follow‐up and duration of monitoring are additional limitations of our study. Although dogs were excluded if monitoring was not performed for at least the first 48 hours, our study does not provide data for long‐term outcomes of dogs with PHPTH that are treated by parathyroidectomy. Although the veterinary literature suggests that most dogs with PHPTH will have the most rapid decrease in serum iCa concentration and will become normocalcemic within the first 48 hours postoperatively, additional evidence suggests that if dogs are to become hypocalcemic it likely will happen within the first 7 days postoperatively although it has been documented as much as 21 days postoperatively.1, 3, 13, 16 The duration of calcitriol administration also was not evaluated in our study to fully assess its long‐term impact. It is not clear whether or not dogs would have developed rebound hypocalcemia after discontinuation of calcitriol. Although no dogs in our study became hypocalcemic, long‐term assessment of dogs in both treatment groups was not obtained in our study.
Our study does not support calcitriol administration of dogs in the postoperative period after parathyroidectomy for PHPTH. It also challenges previously established treatment recommendations based on speculation that patients with a higher preoperative serum iCa concentration are at higher risk for a rapid decrease in serum iCa concentration postoperatively and therefore require preemptive prophylactic calcitriol administration. These recommendations are based on empirical data and require validation with evidence‐based medicine, because the use of calcitriol may delay stimulation of remaining parathyroid tissues, impairing or delaying reestablishment of a normal parathyroid feedback system. Although use of calcitriol prophylactically showed no added benefit for protecting against the rate of decrease in serum iCa concentration in the acute postoperative period (and therefore hypocalcemia), further investigation by a controlled prospective approach is warranted to evaluate the long‐term effects of prophylactic calcitriol administration and possible adverse effects such as delayed return of native parathyroid function.
Acknowledgments
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Footnote
SAS, version 9.3, SAS Institute Inc., Cary, NC
References
- 1. Feldman EC. Hypercalcemia and primary hyperparathyroidism In: Feldman EC, Nelson RW, Reusch C, Scott‐Moncrieff JCR, eds. Canine and Feline Endocrinology, 4th ed St Louis, MO: Elsevier Saunders; 2015:579–624. [Google Scholar]
- 2. Berger B, Feldman EC. Primary hyperparathyroidism in dogs: 21 cases (1976–1986). J Am Vet Med Assoc 1987;191:350–356. [PubMed] [Google Scholar]
- 3. Milovancev M, Schmiedt CW. Preoperative factors associated with postoperative hypocalcemia in dogs with primary hyperparathyroidism that underwent parathyroidectomy: 62 cases (2004–2009). J Am Vet Med Assoc 2013;242:507–515. [DOI] [PubMed] [Google Scholar]
- 4. Sawyer ES, Northrup NC, Schmiedt CW, et al. Outcome of 19 dogs with parathyroid carcinoma after surgical excision. Vet Comp Oncol 2012;10:57–64. [DOI] [PubMed] [Google Scholar]
- 5. Mundy GR. Hypercalcemia of malignancy revisited. J Clin Invest 1988;82:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hruska KA, Teitelbaum SL. Renal osteodystrophy. N Engl J Med 1995;333:166–174. [DOI] [PubMed] [Google Scholar]
- 7. Messinger J, Windham W, Ward C. Ionized hypercalcemia in dogs: A retrospective study of 109 cases (1998–2003). J Vet Intern Med 2009;23:514–519. [DOI] [PubMed] [Google Scholar]
- 8. Feldman EC, Hoar B, Pollard R, et al. Pretreatment clinical and laboratory findings in dogs with primary hyperparathyroidism: 210 cases (1987–2004). J Am Vet Med Assoc 2005;227:756–761. [DOI] [PubMed] [Google Scholar]
- 9. Wilson JW, Harris SG, Moore WD, et al. Primary hyperparathyroidism in a dog. J Am Vet Med Assoc 1974;164:942–946. [PubMed] [Google Scholar]
- 10. van Vonderen IK, Kooistra HS, Peeters ME, et al. Parathyroid hormone immunohistochemistry in dogs with primary and secondary hyperparathyroidism: The question of adenoma and primary hyperplasia. J Comp Pathol 2003;129:61–69. [DOI] [PubMed] [Google Scholar]
- 11. Gear RN, Neiger R, Skelly BJ, et al. Primary hyperparathyroidism in 29 dogs: Diagnosis, treatment, outcome and associated renal failure. J Small Anim Pract 2005;46:10–16. [DOI] [PubMed] [Google Scholar]
- 12. DeVries SE, Feldman EC, Nelson RW, et al. Primary parathyroid gland hyperplasia in dogs: Six cases (1982–1991). J Am Vet Med Assoc 1993;202:1132–1136. [PubMed] [Google Scholar]
- 13. Arbaugh M, Smeak D, Monnet E. Evaluation of preoperative serum concentrations of ionized calcium and parathyroid hormone as predictors of hypocalcemia following parathyroidectomy in dogs with primary hyperparathyroidism: 17 cases (2001‐2009). J Am Vet Med Assoc 2012;241:233–236. [DOI] [PubMed] [Google Scholar]
- 14. Pollard RE, Long CD, Nelson RW, et al. Percutaneous ultrasonographically guided radiofrequency heat ablation for treatment of primary hyperparathyroidism in dogs. J Am Vet Med Assoc 2001;218:1106–1110. [DOI] [PubMed] [Google Scholar]
- 15. Long CD, Goldstein RE, Hornof WJ, et al. Percutaneous ultrasound‐guided chemical parathyroid ablation for treatment of primary hyperparathyroidism in dogs. J Am Vet Med Assoc 1999;215:217–221. [PubMed] [Google Scholar]
- 16. Dear JD, Kass PH, Della Maggiore AM, Feldman EC. Association of hypercalcemia before treatment with hypocalcemia after treatment in dogs with primary hyperparathyroidism. J Vet Int Med 2017;31:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brito Galvao JF, Nagode LE, Schenck PA, Chew DJ. Calcitriol, calcidiol, parathyroid hormone, and fibroblast growth factor‐23 interactions in chronic kidney disease. J Vet Emerg Crit Care 2013;23:134–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasor L, Pollard R, Feldman EC. Retrospective evaluation of three treatment methods for primary hyperparathyroidism in dogs. J Am Anim Hosp Assoc 2007;43:70–77. [DOI] [PubMed] [Google Scholar]
- 19. Torrance AG, Nachreiner R. Intact parathyroid hormone assay and total calcium concentration in the diagnosis of disorders of calcium metabolism in dogs. J Vet Intern Med 1989;3:86–89. [DOI] [PubMed] [Google Scholar]
- 20. Stewart ZA, Blackford A, Somervell H, et al. 25‐hydroxyvitamin D deficiency is risk factor for symptoms of postoperative hypocalcemia and secondary hyperparathyroidism after minimally invasive parathyroidectomy. Surgery 2005;138:1018–1026. [DOI] [PubMed] [Google Scholar]
- 21. Mittendorf E, Merlino J, McHenry C. Post‐parathyroidectomy hypocalcemia: Incidence, risk factors and management. Am Surg 2004;70:114–119. [PubMed] [Google Scholar]
- 22. Marx SJ. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med 2000;343:1863–1875. [DOI] [PubMed] [Google Scholar]
- 23. Wisner ER, Nyland TG, Feldman EC. Ultrasonographic evaluation of the parathyroid glands in hypercalcemic dogs. Vet Radiol Ultrasound 1993;34:108–111. [Google Scholar]
- 24. Silverberg SJ, Shane E, Jacobs TP, et al. A 10‐year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 1999;341:1249–1255. [DOI] [PubMed] [Google Scholar]
- 25. Scheneck PA, Chew DJ. Investigation of hypercalcemia and hypocalcemia In: Mooney CT, Peterson ME, eds. BSAVA Manual of Canine and Feline Endocrinology, 4th ed Quedgeley, Gloucester, UK: British Small Animal Veterinary Association; 2012:221–233. [Google Scholar]
- 26. Jin S, Park J, Kim C. Pharmacokinetics of oral calcitriol in healthy human based on the analysis with an enzyme immunoassay. Pharmacol Res 2009;60:57–60. [DOI] [PubMed] [Google Scholar]
- 27. Chew DJ, Nagode LA, Schenck PA. Treatment of hypoparathyroidism In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy XIV. St Louis, MO: Saunders/Elsevier; 2009:246. [Google Scholar]
- 28. Ham K, Greenfield CL, Barger A, et al. Validation of rapid parathyroid hormone assay and intraoperative measurement of parathyroid hormone in dogs with benign naturally occurring primary hyperparathyroidism. Vet Surg 2009;38:122–132. [DOI] [PubMed] [Google Scholar]
- 29. Feldman EC, Wisner ER, Nelson RW, et al. Comparison of results of hormonal analysis of samples obtained from the selected venous sites versus cervical ultrasonography for localizing parathyroid masses in dogs. J Am Vet Med Assoc 1997;211:54–57. [PubMed] [Google Scholar]
- 30. Wisner ER, Penninck D, Biller DS, et al. High‐resolution parathyroid sonography. Vet Radiol Ultrasound 1997;38:462–466. [DOI] [PubMed] [Google Scholar]
- 31. Baldassarre RL, Chang DC, Brumund KT, et al. Predictors of hypocalcemia after thyroidectomy: Results from the nationwide inpatient sample. ISRN Surg 2012;2012:838614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bailey DB, Page RL. Tumor of the endocrine system In: Withrow SJ, Vail DM, eds. Withrow and MacEwen's Small Animal Clinical Oncology, 4th ed Philadelphia, PA: Saunders; 2007:583–609. [Google Scholar]
- 33. Marcocci C, Cetani F, Rubin MR, et al. Parathyroid carcinoma. J Bone Miner Res 2008;23:1869–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chew DJ, Nagode LA. Treatment of hyperparathyroidism In: Bonagura JD, ed. Kirk's Current Veterinary Therapy, XIII ed. Philadelphia, PA: W. B. Saunders Company; 2000:340–345. [Google Scholar]
- 35. Polzin D. Chronic kidney disease In: Ettinger S, Feldman E. eds, Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 6th ed. St. Louis, MO: Elsevier; 2005:1756–1785. [Google Scholar]
- 36. Crystal M. Hypoparathyroidism In: Tilley L, Smith F. eds., Blackwell's 5‐Minute Veterinary Consult: Canine and Feline. Ames, IA: Blackwell; 2007:720–721. [Google Scholar]


