Abstract
Background
Cell‐free DNA (cfDNA) comprises short, double‐stranded circulating DNA sequences released from damaged cells. In people, cfDNA concentrations correlate well with disease severity and tissue damage. No reports are available regarding cfDNA kinetics in dogs.
Objectives/Hypothesis
Cell‐free DNA will have a short biological half‐life and would be able to stratify mild, moderate, and severe tissue injury. Our study aims were to determine the kinetics and biological half‐life of cfDNA and to contrast them with those of creatine kinase (CK).
Animals
Three groups of 10 dogs undergoing open ovariohysterectomy, surgery for cranial cruciate ligament rupture (CCLR), or hemilaminectomy.
Methods
Plasma for cfDNA and CK analysis was collected at admission, at induction of anesthesia, postsurgery (time 0) and at 6, 12, 24, 36, 48, 60, and 72 hours after surgery.
Results
The biological half‐life of plasma cfDNA and CK were 5.64 hours (95% confidence interval [CI 95], 4.36–7.98 hours) and 28.7 hours (CI95, 25.3–33.3 hours), respectively. In the hemilaminectomy group, cfDNA concentrations differed significantly from admission at 6–12 hours after surgery. Creatine kinase activity differed among the surgical groups and reached a peak 6 hours after surgery. In the ovariohysterectomy and CCLR groups, plasma CK activity 72 hours after surgery did not differ from admission activity of the ovariohysterectomy group. In contrast, in the hemilaminectomy group, plasma CK activity after 72 hours did not return to the ovariohysterectomy group admission activity.
Conclusions and Clinical Importance
Plasma CK activity has a longer biological half‐life than previously thought. In contrast to plasma CK activity, cfDNA has a short half‐life and could be a useful marker for peracute severe tissue injury.
Keywords: Biological half‐life, Cell‐free DNA, Creatine kinase, Dog, Surgery
Abbreviations
- CCLR
cranial cruciate ligament rupture
- cfDNA
cell‐free DNA
- CK
creatine kinase
- HL
hemilaminectomy
- OVH
ovariohysterectomy
- TPLO
tibial plateau leveling osteotomy
- TTA
tibial tuberosity advancement
Cell‐free DNA (cfDNA) comprises short, double‐stranded DNA sequences, circulating unbound in plasma.1 The source of cfDNA in the circulation still is largely unknown. Release of cfDNA from cells after apoptosis and necrosis,2 lysis of cells in the bloodstream,3 and spontaneous release from cells under stress4 are possible sources of cfDNA. In people, DNA hydrolysis by DNAse 1 nuclease is the predominant pathway of cfDNA clearance from the blood,5 leading to a reported biological half‐life of 157.6 minutes at 37°C.6
In 1948, the presence of cfDNA was reported in human serum.7 Since then, increased concentrations of cfDNA in blood and body fluids have been linked to systemic lupus erythematosus8 and various cancers.9 Cell‐free DNA concentrations correlated well with tumor burden and response to radiation therapy.9 Cell‐free DNA has become widely studied in human medicine and has utility as a clinical noninvasive biomarker not only in cancer research but also in prenatal diagnostics,10 organ transplantation,11 and in several emergency conditions including stroke,12 myocardial infarction,13 sepsis,14 and severe trauma.15, 16, 17, 18 Because of its short half‐life, cfDNA is a reliable approximation of the current status of tissue injury and abates as resolution occurs.6, 15
In veterinary medicine, the focus of research on cfDNA has mainly centered on its potential value in the diagnosis, prognosis, and monitoring of the response to treatment of cancer in dogs. Dogs with mammary tumors or lymphoma have high concentrations of cfDNA in the blood, and the concentration of cfDNA is inversely correlated with remission time.19, 20 The ability to detect cfDNA in canine blood led to the investigation of cfDNA in other disease states.21, 22 A significant increase in the blood concentration of cfDNA was found in diseased dogs, as compared to healthy dogs, and a positive association between cfDNA concentration and disease severity and survival was observed.21 Both dogs with sepsis and those with moderate‐to‐severe trauma had significantly increased cfDNA concentrations compared to healthy dogs,22 and cfDNA concentration was associated with death in dogs with immune‐mediated hemolytic anemia.23
A short half‐life and a good correlation with disease severity would render cfDNA a useful tool to quantify the extent of tissue injury. Based on current knowledge, we hypothesized that, in dogs, cfDNA would have a short biological half‐life and its concentration would be able to stratify mild, moderate, and severe tissue injury. Accordingly, the aims of our study were to determine the kinetics of cfDNA and its biological half‐life. To contrast with cfDNA, we estimated similar variables for creatine kinase activity. We chose to approach the hypothesis and aims using a model of controlled tissue injury in dogs that underwent 3 types of surgeries associated with mild, moderate, and severe tissue injury.
Materials and Methods
Animals
We collected plasma samples from dogs presented to the Massey University Veterinary Teaching Hospital between October 2015 and April 2017. Inclusion criteria were client‐owned dogs presented for cranial cruciate ligament rupture (CCLR) surgically managed by tibial tuberosity advancement (TTA) or tibial plateau leveling osteotomy (TPLO) surgical techniques, thoracolumbar disk disease decompressed by hemilaminectomy surgery (HL), and bitches presented for an elective, open ovariohysterectomy surgery (OVH). The surgeries were stratified according to perceived tissue injury (mild, moderate, and severe) on the basis of the extent of dissection required for each procedure. In an open OVH, the incision is primarily through the linear alba although there can be some injury to the rectus abdominis muscle and crushing and stretching of uterine muscle. In a TPLO, there is more extensive dissection with elevation of the pes anserinus, cranial tibial, and popliteal muscles in addition to an arthrotomy. The HL was considered the most severe trauma because of the need to extensively elevate and transect epaxial musculature and use Gelpi retractors for extended time periods during surgery. Enrollment of 10 patients for each category in the study gave a total of 30 patients. Table 1 presents the descriptive characteristics of the dogs included in the study. The Massey University Animal Ethics Committee approved the study (MUAEC protocol 15/50) and enrollment of patients required informed, signed client consent.
Table 1.
The descriptive characteristics of the dogs included in the study
| HL | CCLR | OVH | ||
|---|---|---|---|---|
| Age (months)a | 42 (9–128) | 40 (18–96) | 69 (5–144) | |
| Sex | M | 7 | 5 | 0 |
| F | 3 | 5 | 10 | |
| Neuter | E | 1 | 2 | 0 |
| N | 9 | 8 | 10 | |
| Weight (kg)b | 25 (7.7–43.5) | 36 (8–45.6) | 17.5 (7.7–33.4) | |
| Breed | Akita | 1 | ||
| Cocker Spaniel | 1 | |||
| Crossbreed | 1 | |||
| Dachshund | 4 | |||
| Dogue de Bordeaux | 1 | |||
| German Shorthaired Pointer | 1 | |||
| Golden Retriever | 1 | |||
| Harrier | 5 | |||
| New Zealand Heading Dog | 1 | |||
| Huntaway | 1 | |||
| Labrador Retriever | 1 | 4 | ||
| Lhasa Apso | 1 | |||
| Mastiff | 1 | |||
| Miniature Schnauzer | 1 | |||
| Pekingese | 1 | |||
| Rottweiler | 1 | |||
| Staffordshire Bull Terrier | 1 | |||
| Toy Poodle | 2 |
HL, hemilaminectomy; CCLR, surgeries for cranial cruciate ligament rupture; OVH, open ovariohysterectomy; M, male; F, female; E, entire; N, neuter.
Age and weight are presented as median (range).
There is a statistically significant difference between the CCLR and OVH groups (P < 0.01).
There were statistically significant differences between groups (P < 0.01).
Sample Collection
Each dog enrolled in the study had a 2 mL sample of whole blood in ethylenediaminetetraacetic acid (EDTA) collected by jugular venipuncture upon arrival (admission sample). A 16 G, 20 cm long indwelling IV catheter was placed in the lateral saphenous vein after induction of anesthesia for the intended surgical procedure. For each blood sample, we irrigated the indwelling IV catheter with 5 mL 0.9% sodium chloride and aspirated back 1 mL of whole blood that was discarded. Plasma was harvested from an additional 2 mL of whole blood that was placed in an EDTA tube. At the end of blood collection, the indwelling IV catheter was irrigated with 0.9% sodium chloride. Collection of blood samples took place at the time of admission, after induction of anesthesia, immediately after surgery ended and then at 6, 12, 24, 36, 48, 60, and 72 hours after surgery. At 72 hours, we also collected a sample from the bag of 0.9% sodium chloride that had been used to irrigate the catheter throughout the sampling period. This sample served as a negative control to ensure that there was no DNA contamination in the 0.9% sodium chloride before sampling.
Sample Handling
Centrifugation of whole blood samples at 3,000 × g for 15 minutes at 4°C facilitated harvesting the plasma. Equal volumes of the harvested plasma were stored at −80°C until the time of analysis.
Cell‐free DNA Analysis
We used the Qubit dsDNA HS Assay Kit and Qubit 2.0 fluorimeter1 and quantified the plasma concentration of cell‐free DNA (cfDNA) as previously described.21 Plasma samples were thawed in batches of 15–50 samples, and a volume of 20 μL was used for the analysis. The Qubit assay utilizes a dye that fluoresces with a higher intensity when bound to double‐strand DNA (dsDNA), and the recorded amount of fluorescence is proportional to the amount of dsDNA in the sample. The dilution algorithm provided by the manufacturer within the Qubit 2.0 determined the concentration of the cfDNA. Calibration of the Qubit 2.0 with the provided standards preceded each run. We assayed a single sample with a previously measured concentration of cfDNA that had been previously separated into aliquots and stored at −20°C with every batch of samples as an interassay control. The coefficient of variation determined the intra‐assay precision on 22 samples run in triplicates.
Creatine Kinase Analysis
A commercial veterinary diagnostic laboratory2 measured the activity of plasma creatine kinase (CK) on a Roche/Hitachi analyzer.3
Statistical Analysis
A priori power sample size analysis was performed by G*Power version 3.1.9.8.4 The analysis indicated that 9 dogs in each group would suffice to detect a difference of 20% in plasma CK activity on repeated measures of plasma CK activity on the same dog with a power of 0.8 and alpha probability error of 0.05, assuming that the correlation for the repeated measurements on the same dog was 0.60.
All statistical analyses were performed by R 3.3.3.24 Distribution of data was assessed by visual inspection of the data on quantile‐quantile plots, histogram plots, and by the Shapiro‐Wilk test. To balance the “age” and “weight” variables, these continuous variables were transformed into ordinal variables with approximately 3 equal parts; “weight” was subdivided into <20, 20–35, and >35 kg and “age” was subdivided into <40, 40–80, and >80 months.
Analysis of repeated measurements of cfDNA was performed by the lmer() function of the lme4 package.25 The dependent variable cfDNA was analyzed by a linear mixed model that included the fixed effects of “surgery,” “time” and the interaction between “surgery” and “time,” and the random effect of “dog.” The residuals were assumed to have expected mean zero, common variance () across times and common covariances (), where i and j denote residuals at different times on the same dog. The variance because of “dog” was assumed to have an expected zero value with a common “dog” variance (). “Age,” “weight,” and “sex” did not contribute significantly enough to the model to be included in the final model (P > 0.05).
The dependent variable CK was analyzed with the same model as cfDNA but also included the fixed effects of “age” and “weight” with the same distributional properties for the residuals of repeated measures on the same dog, as described above. “Sex” effect did not contribute significantly enough to the model to be included in the final model (P > 0.05).
The lsmeans() function of the lsmeans package26 was used to obtain the least squares means and standard errors and was used for Tukey's pairwise mean comparisons.
To calculate the biological half‐life of plasma cfDNA and CK, we used the lme4 package lm() function to perform simple linear regression on the log‐transformed down slope of the least squares means curves of plasma cfDNA and CK. The half‐life was calculated from the following formula: t(1/2) = log(1/2)/coefficient of regression slope.27
The correlation between plasma cfDNA and CK was evaluated by the Spearman's rank correlation ρ and Kendall's rank correlation τ.
Results
Plasma Cell‐free DNA
The least squares means of plasma cell‐free DNA (cfDNA) concentrations at the various time points of the study are shown in Table 2.
Table 2.
Least squares mean of plasma cell‐free DNA (μg/L) in 30 dogs, stratified by the perceived severity of tissue trauma at surgery (10 dogs per group)
| Time | Lsmean | SE | 95% CI |
|---|---|---|---|
| HL | |||
| Admission | 648 | 144 | 363–932 |
| Induction | 570 | 144 | 286–854 |
| Post‐Op | 734 | 138 | 462–1,007 |
| 6 | 1,458 | 138 | 1,185–1,730 |
| 12 | 1,446 | 138 | 1,174–1,719 |
| 24 | 1,074 | 144 | 789–1,358 |
| 36 | 770 | 144 | 485–1,055 |
| 48 | 645 | 144 | 360–929 |
| 60 | 675 | 144 | 390–960 |
| 72 | 607 | 144 | 322–892 |
| CCLR | |||
| Admission | 776 | 138 | 503–1,048 |
| Induction | 749 | 138 | 477–1,022 |
| Post‐Op | 758 | 138 | 486–1,031 |
| 6 | 756 | 138 | 483–1,028 |
| 12 | 819 | 138 | 546–1,091 |
| 24 | 856 | 138 | 583–1,128 |
| 36 | 873 | 138 | 601–1,146 |
| 48 | 859 | 138 | 587–1,132 |
| 60 | 735 | 138 | 463–1,008 |
| 72 | 835 | 138 | 563–1,108 |
| OVH | |||
| Admission | 611 | 138 | 338–883 |
| Induction | 623 | 138 | 350–895 |
| Post‐Op | 673 | 138 | 400–945 |
| 6 | 787 | 138 | 514–1,059 |
| 12 | 794 | 138 | 521–1,066 |
| 24 | 789 | 138 | 516–1,061 |
| 36 | 773 | 138 | 501–1,045 |
| 48 | 723 | 138 | 451–996 |
| 60 | 688 | 138 | 416–960 |
| 72 | 695 | 138 | 422–967 |
HL, hemilaminectomy; CCLR, surgery for cranial cruciate ligament rupture; OVH, open ovariohysterectomy; Lsmean, least squares mean; SE, standard error; CI, confidence interval.
Between‐group Comparisons
Plasma cfDNA concentrations (μg/L) were significantly higher in the hemilaminectomy (HL) group, compared to both the CCLR and open ovariohysterectomy (OVH) groups at 6 hours (1,458 ± 138 versus 756 ± 138 and 787 ± 138; P = 0.001 and P = 0.002) and 12 hours (1,446 ± 138 versus 819 ± 138 and 794 ± 138; P = 0.004 and P = 0.003; Fig 1). No significant differences were identified between the CCLR and open OVH groups at any time.
Figure 1.
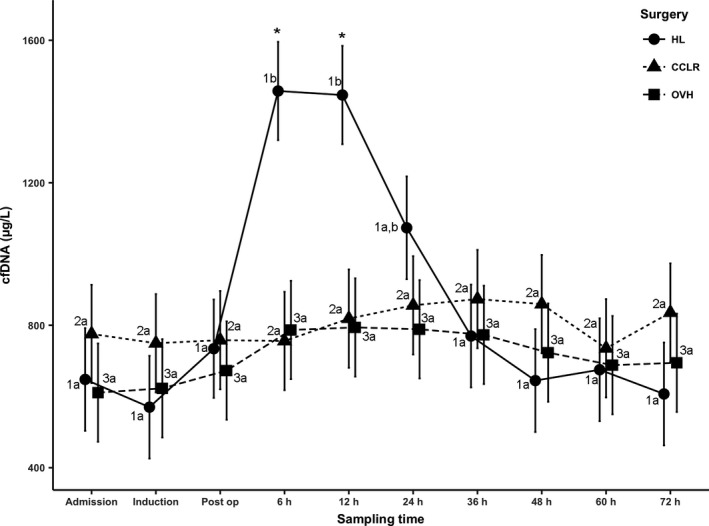
Least squares means of plasma cell‐free DNA. Different letters for the same number indicate P < 0.05 within the group. “*” indicates P < 0.05 between hemilaminectomy and the other 2 groups per time period. Post‐op, postoperative period; HL, hemilaminectomy; CCLR, surgeries for cranial cruciate ligament rupture; OVH, open ovariohysterectomy.
Within‐group Comparisons
In the HL group, plasma cfDNA reached a peak at 6 hours through 12 hours and rapidly returned to admission concentrations by 72 hours. In contrast, in the open OVH and CCLR groups, plasma cfDNA reached a shallow peak at 12 and 36 hours, respectively.
In the HL group, significant differences were observed between times 6 hours through 12 hours compared to the other time points (other than 24 hours postoperatively; Fig 1). Within the CCLR and open OVH groups, there were no differences between any of the time points.
The calculated biological half‐life of plasma cfDNA was 5.64 hours (CI 95, 4.36–7.98 hours).
Plasma CK
The geometric means are shown in Table 3, which were derived from back transformation of the least squares means of log plasma CK at the various time points of the study.
Table 3.
Geometric mean (GM) of plasma creatine kinase activity (IU/L) in 30 dogs, stratified by the perceived severity of tissue trauma at surgery (10 dogs per group)
| Time | GM | 95% CI |
|---|---|---|
| HL | ||
| Admission | 229.5 | 125.8–419 |
| Induction | 202.6 | 111–370 |
| Post‐Op | 373.5 | 207.5–672 |
| 6 | 1192.9 | 662.2–2,149 |
| 12 | 1129.7 | 627.2–2,035 |
| 24 | 1074.5 | 588–1,964 |
| 36 | 574.4 | 314.4–1,050 |
| 48 | 497.4 | 272.2–909 |
| 60 | 315.1 | 172.3–576 |
| 72 | 298.9 | 163.4–547 |
| CCLR | ||
| Admission | 108.4 | 59.8–197 |
| Induction | 147.3 | 81.5–266 |
| Post‐Op | 199.4 | 110.1–361 |
| 6 | 640.7 | 353.7–1,161 |
| 12 | 751.1 | 414.6–1,361 |
| 24 | 731 | 401.3–1,332 |
| 36 | 504.7 | 277.5–918 |
| 48 | 339.5 | 186.3–618 |
| 60 | 221.9 | 121.8–404 |
| 72 | 110.1 | 60.4–201 |
| OVH | ||
| Admission | 125.4 | 69.3–227 |
| Induction | 139.3 | 76.9–252 |
| Post‐Op | 195.8 | 108.1–355 |
| 6 | 365.8 | 202–663 |
| 12 | 347.9 | 191.6–632 |
| 24 | 231.2 | 127.4–419 |
| 36 | 159 | 87.8–288 |
| 48 | 135.3 | 74.6–245 |
| 60 | 108.8 | 59.8–198 |
| 72 | 96.7 | 53.2–176 |
HL, hemilaminectomy; CCLR, surgeries for cranial cruciate ligament rupture; OVH, open ovariohysterectomy; GM, geometric mean; CI, confidence interval.
Between‐group Comparisons
Overall, a significant difference was identified between the HL and open OVH groups (estimate 1.03, P = 0.013).
A significant difference in the log plasma CK activity was observed between the HL and open OVH groups at 6 hours through 72 hours (estimate 1.182, P = 0.017; estimate 1.178, P = 0.017; estimate 1.536, P = 0.002; estimate 1.284, P = 0.010; estimate 1.302, P = 0.008; estimate 1.063, P = 0.037; estimate 1.129, P = 0.025, respectively).
Within‐group Comparisons
In the HL and open OVH groups, plasma CK reached a peak at 6 hours in comparison with the CCLR group in which plasma CK reached a peak at 12 hours. In the HL group, plasma CK did not return to admission activity by 72 hours. In contrast, in the CCLR and open OVH groups, plasma CK reached admission activity at 72 hours.
Within the HL and CCLR groups, the period of 6 hours through 24 hours was significantly different from the periods of admission, induction, immediately postoperative, 60 and 72 hours (Fig 2). Within the open OVH group, the period of 6 hours through 12 hours was significantly different from the period of admission, induction, 48, 60, and 72 hours (Fig 2).
Figure 2.
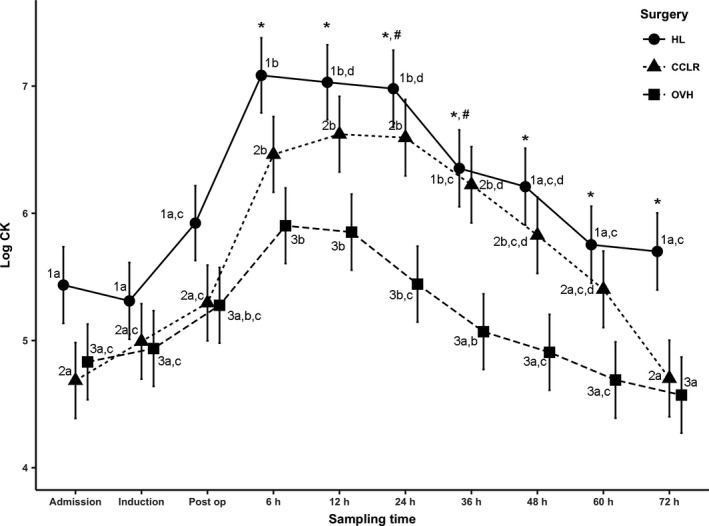
Least squares means of log plasma creatine kinase. Different letters for the same number indicate P < 0.05 within the group. “*” indicates P < 0.05 between hemilaminectomy and the ovariohysterectomy group per time period. “#” indicates P < 0.05 between hemilaminectomy and the cranial cruciate ligament rupture surgery group per time period. Post‐op, postoperative period; HL, hemilaminectomy; CCLR, surgeries for cranial cruciate ligament rupture; OVH, open ovariohysterectomy.
The calculated biological half‐life of plasma CK was 28.7 hours (CI 95, 25.3–33.3 hours).
Correlation between cfDNA and CK
Moderate‐to‐strong correlation was observed between plasma CK and cfDNA for all surgeries and time intervals, for all surgeries at the down slope from 6 to 72 hours, and for the HL surgery at the down slope from 6 to 72 hours (Table 4).
Table 4.
Correlation coefficients between plasma creatine kinase and cell‐free DNA
| Spearman's Rank Correlation ρ | P Value | Kendall's Rank Correlation τ | P Value | |
|---|---|---|---|---|
| All surgery | 0.53 | 0.003 | 0.38 | 0.003 |
| All surgery 6–72 hours | 0.47 | 0.040 | 0.35 | 0.030 |
| HL surgery 6–72 hours | 0.96 | 0.003 | 0.9 | 0.003 |
HL, hemilaminectomy.
Discussion
The aims of our study were to determine the kinetics of plasma cfDNA and its biological half‐life. The results indicate that after substantial tissue damage, plasma cfDNA concentrations increased sharply at 6 hours for a period of approximately 6 hours and then decreased abruptly to baseline concentrations. Plasma cfDNA did not increase significantly when mild or moderate tissue damage occurred. The lack of increase in cfDNA postsurgery in the CCLR and OVH groups also could be a consequence of the different trauma type experienced during these surgeries, which is mainly traction and compression of soft tissues, as opposed to HL where there is substantial blunt dissection and retraction of the muscle by Gelpi retractors to expose the surgical site. Alternatively, the differences between the CCLR and OVH groups and the HL group could be secondary to the proportion of injured tissue. The fact that cfDNA plasma concentrations were increased for only a short period of time implies that in dogs, plasma cfDNA is a marker of substantial peracute tissue injury. Also, it is less sensitive to milder forms of tissue injury, and it is less sensitive in the later phases after an acute insult, as was apparent in our results in which cfDNA plasma concentrations returned to baseline after 12 hours (Fig 1).
Our results are in agreement with the previous studies indicating that plasma cfDNA is a marker associated with peracute inflammation. In our study, plasma cfDNA did not differ among groups in the immediate postoperative period even though the times it took to complete OVH, TTA and TPLO, and HL were different. We speculate that there is a temporal association between the early phase of inflammation and the increase in plasma cfDNA concentration at 6–12 hours. In the early phase of inflammation, inflammatory cytokines increase vascular permeability because of activation of the endothelium.28 The activated endothelium contracts and the intercellular gaps become wider. Therefore, cfDNA that leaked from injured cells into the interstitial space could diffuse rapidly along its concentration gradient and its plasma concentration would increase. Thus, we argue that cfDNA is a good indicator of early inflammation and tissue damage. In contrast, we expect that in chronic inflammation, despite increased permeability of the inflamed vascular bed, there would be insufficient amounts of cfDNA to diffuse into the circulation because of fibrosis and scarring. Hence, we postulate that chronic inflammation would not be associated with high plasma cfDNA concentrations. This hypothesis is supported by a recent meta‐analysis that compared plasma cfDNA among 4 groups: healthy controls, acute inflammation, chronic inflammation, and acute infections.29 In the metanalysis, the cfDNA concentration in chronic inflammatory conditions was substantially lower than in acute inflammation and infection, yet still higher than the control. A similar trend was recently identified in dogs.21 In that study, small but statistically significant differences were observed between healthy control dogs when compared to dogs with chronic and acute disease conditions. Dogs with acute disease conditions had significantly higher plasma cfDNA concentrations than did dogs with chronic disease conditions. The authors defined chronic versus acute disease based on the history but did not report the associate conditions.
Previous studies have characterized the kinetics of plasma CK. In 1 study, the 3 canine isoforms of CK were purified and their half‐lives determined after an IV injection.30 This pharmacokinetic study indicated that the half‐life of plasma CK in dogs was 119.5 minutes.30 We calculated the time plasma CK would reach 50% of its peak activity after surgery. In marked contrast to the previously described study, we found that for all surgeries, the biological half‐life of plasma CK was 28.7 hours (CI 95, 25.3–33.3 hours). This estimated biological half‐life should be considered when monitoring trends in plasma CK activity. The estimated biological half‐life is different than the pharmacologic half‐life. The biological half‐life takes into account CK released from the primary insult and CK that further leaks from myofiber damage induced by local ischemia, thrombosis, and inflammation. Thus, in a clinical setting, the biological half‐life would better predict the decrease in serum CK activity after an isolated insult. Deviation from that prediction should alert the clinician to possible ongoing muscle damage. In dogs, plasma CK activities are assumed to be proportional to the extent of muscle injury. This assumption is based on a single pharmacokinetic study in which the supernatants of dog muscle homogenates were injected IV and IM into 6 dogs.31 In another study, serum CK activity was measured over 48 hours after HL and OVH.32 Similar to our study, clear stratification in serum CK activity was observed according to the type of surgery. In that study, serum CK activity after HL did not return to baseline at 48 hours. We followed plasma CK activity longer than in the previous study32 and found that, in the HL group, plasma CK activity did not return to baseline even after 72 hours. Both our study and the previous study32 indicate that plasma CK activity is proportional to the extent of muscle injury and that the biological half‐life is approximately 28 hours.
To our knowledge, ours is the first study to estimate the time it takes plasma cfDNA to reach 50% of its peak concentration in a clinical setting. We found that the biological half‐life of plasma cfDNA was 5.64 hours. The short biological half‐life of plasma cfDNA combined with the long half‐life of plasma CK could permit differentiation of progressive tissue injury from acute nonprogressive injury. For example, after substantial trauma, progressive tissue injury might result from thrombosis or compartment syndrome. Monitoring plasma CK activity might not be useful because of the duration of time that it would take to return to baseline. In that scenario, co‐measurement of plasma cfDNA along with CK activity would indicate if there is substantial ongoing tissue injury.
In conclusion, plasma cfDNA has a short biological half‐life, and plasma CK has a longer biological half‐life than previously thought. Plasma cfDNA could be a useful marker for peracute severe tissue injury. Combining measurement of plasma cfDNA with CK activity may allow differentiation of progressive tissue injury from acute nonprogressive injury.
Author Contributions
Gal A, Wilson IJ, and Burchell RK formulated the hypothesis, designed the study, collected samples, analyzed the data, and wrote the manuscript. Worth AJ, Burton SE, Gedye KR, Clark KJ, Crosse KR, Jack M, Odom TF, De Grey SJ, McGlade KMS, Tomlin SC, and Lopez‐Villalobos N contributed to the development of the hypothesis, design of the study, collection and processing of the samples, analysis of the data, and critically contributed to the writing of the manuscript.
Acknowledgments
This work was supported by the Institute of Veterinary, Animal and Biomedical Sciences (IVABS), College of Science, Massey University's Lewis Fitch grant. The authors thank Drs. Jon Bray and Richard Kuipers for their assistance in case recruitment.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Footnotes
Qubit dsDNA HS Assay Kit and Qubit 2.0 fluorimeter, Life Sciences, Carlsbad, CA, USA
IDEXX New Zealand, Palmerston North, New Zealand
Cobas CK Roche Diagnostics GmbH, Mannheim, Germany
G*Power Version 3.1.9.3 © Franz Faul, Edgar Erdfelder, Albert‐Georg Lang, and Axel Buchner, 2006, 2009
References
- 1. Breitbach S, Tug S, Simon P. Circulating cell‐free DNA an up‐coming molecular marker in exercise physiology. Sports Med 2012;42:565–586. [DOI] [PubMed] [Google Scholar]
- 2. Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001;61:1659–1665. [PubMed] [Google Scholar]
- 3. Gormally E, Caboux E, Vineis P, et al. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: Practical aspects and biological significance. Mutat Res 2007;635:105–117. [DOI] [PubMed] [Google Scholar]
- 4. Stroun M, Maurice P, Vasioukhin V, et al. The origin and mechanism of circulating DNA. Ann N Y Acad Sci 2000;906:161–168. [DOI] [PubMed] [Google Scholar]
- 5. Barra GB, Santa Rita TH, de Almeida Vasques J, et al. EDTA‐mediated inhibition of DNases protects circulating cell‐free DNA from ex vivo degradation in blood samples. Clin Biochem 2015;48:976–981. [DOI] [PubMed] [Google Scholar]
- 6. Yao W, Mei C, Nan X, et al. Evaluation and comparison of in vitro degradation kinetics of DNA in serum, urine and saliva: A qualitative study. Gene 2016;590:142–148. [DOI] [PubMed] [Google Scholar]
- 7. Mandel P, Metais P. Les acides nucléiques du plasma sanguin chez l'homme. C R Seances Soc Biol Fil 1948;142:241–243. [PubMed] [Google Scholar]
- 8. Koffler D, Agnello V, Winchester R, et al. The occurrence of single‐stranded DNA in the serum of patients with systemic lupus erythematosus and other diseases. J Clin Invest 1973;52:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leon SA, Shapiro B, Sklaroff DM, et al. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res 1977;37:646–650. [PubMed] [Google Scholar]
- 10. Norton ME, Jacobsson B, Swamy GK, et al. Cell‐free DNA analysis for noninvasive examination of trisomy. N Engl J Med 2015;372:1589–1597. [DOI] [PubMed] [Google Scholar]
- 11. De Vlaminck I, Valantine HA, Snyder TM, et al. Circulating cell‐free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med 2014;6:241ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rainer TH, Wong LK, Lam W, et al. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin Chem 2003;49:562–569. [DOI] [PubMed] [Google Scholar]
- 13. Chang CP, Chia RH, Wu TL, et al. Elevated cell‐free serum DNA detected in patients with myocardial infarction. Clin Chim Acta 2003;327:95–101. [DOI] [PubMed] [Google Scholar]
- 14. Martins GA, Kawamura MT, Carvalho MG. Detection of DNA in the plasma of septic patients. Ann N Y Acad Sci 2000;906:134–140. [DOI] [PubMed] [Google Scholar]
- 15. Gogenur M, Burcharth J, Gogenur I. The role of total cell‐free DNA in predicting outcomes among trauma patients in the intensive care unit: A systematic review. Crit Care 2017;21:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lo YM, Rainer TH, Chan LY, et al. Plasma DNA as a prognostic marker in trauma patients. Clin Chem 2000;46:319–323. [PubMed] [Google Scholar]
- 17. Lam NY, Rainer TH, Chan LY, et al. Time course of early and late changes in plasma DNA in trauma patients. Clin Chem 2003;49:1286–1291. [DOI] [PubMed] [Google Scholar]
- 18. Macher H, Egea‐Guerrero JJ, Revuelto‐Rey J, et al. Role of early cell‐free DNA levels decrease as a predictive marker of fatal outcome after severe traumatic brain injury. Clin Chim Acta 2012;414:12–17. [DOI] [PubMed] [Google Scholar]
- 19. Beffagna G, Sammarco A, Bedin C, et al. Circulating cell‐free DNA in dogs with mammary tumors: Short and long fragments and integrity index. PLoS One 2017;12:e0169454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schaefer DM, Forman MA, Kisseberth WC, et al. Quantification of plasma DNA as a prognostic indicator in canine lymphoid neoplasia. Vet Comp Oncol 2007;5:145–155. [DOI] [PubMed] [Google Scholar]
- 21. Burnett DL, Cave NJ, Gedye KR, et al. Investigation of cell‐free DNA in canine plasma and its relation to disease. Vet Q 2016;36:122–129. [DOI] [PubMed] [Google Scholar]
- 22. Letendre JA, Goggs R. Measurement of plasma cell‐free DNA concentrations in dogs with sepsis, trauma, and neoplasia. J Vet Emerg Crit Care (San Antonio) 2017;27:307–314. [DOI] [PubMed] [Google Scholar]
- 23. Jeffery U, Ruterbories L, Hanel R, et al. Cell‐free DNA and DNase activity in dogs with immune‐mediated hemolytic anemia. J Vet Intern Med 2017;31:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 25. Bates D, Maechler M, Bolker B, et al. Fitting linear mixed‐effects models using lme4. J Stat Softw 2015;67:1–48. [Google Scholar]
- 26. Lenth RV. Least‐squares means: The R package lsmeans. J Stat Softw 2016;69:1–33. [Google Scholar]
- 27. Leong JW, Dore ND, Shelley K, et al. The elimination half‐life of urinary cotinine in children of tobacco‐smoking mothers. Pulm Pharmacol Ther 1998;11:287–290. [DOI] [PubMed] [Google Scholar]
- 28. Ackermann MR. Inflammation and healing In: Zachary JF, ed. Pathologic Basis of Veterinary Disease, 6th ed St. Louis, MO: Elsevier; 2017:73–131. [Google Scholar]
- 29. Frank MO. Circulating cell‐free DNA differentiates severity of inflammation. Biol Res Nurs 2016;18:477–488. [DOI] [PubMed] [Google Scholar]
- 30. Rapaport E. The fractional disappearance rate of the separate isoenzymes of creatine phosphokinase in the dog. Cardiovasc Res 1975;9:473–477. [DOI] [PubMed] [Google Scholar]
- 31. Aktas M, Lefebvre HP, Toutain PL, et al. Disposition of creatine kinase activity in dog plasma following intravenous and intramuscular injection of skeletal muscle homogenates. J Vet Pharmacol Ther 1995;18:1–6. [DOI] [PubMed] [Google Scholar]
- 32. Nevill B, Leisewitz A, Goddard A, et al. An evaluation of changes over time in serum creatine kinase activity and C‐reactive protein concentration in dogs undergoing hemilaminectomy or ovariohysterectomy. J S Afr Vet Assoc 2010;81:22–26. [DOI] [PubMed] [Google Scholar]


