Abstract
Myoclonic epilepsy in Rhodesian Ridgeback (RR) dogs is characterized by myoclonic seizures occurring mainly during relaxation periods, a juvenile age of onset and generalized tonic‐clonic seizures in one‐third of patients. An 8‐month‐old female intact RR was presented for myoclonic seizures and staring episodes that both started at 10 weeks of age. Testing for the DIRAS1 variant indicated a homozygous mutant genotype. Unsedated wireless video‐electroencephalography (EEG) identified frequent, bilaterally synchronous, generalized 4 Hz spike‐and‐wave complexes (SWC) during the staring episodes in addition to the characteristic myoclonic seizures with generalized 4–5 Hz SWC or 4–5 Hz slowing. Photic stimulation did not evoke a photoparoxysmal response. Repeat video‐EEG 2 months after initiation of levetiracetam treatment disclosed a >95% decrease in frequency of myoclonic seizures, and absence seizures were no longer evident. Absence seizures represent another seizure type in juvenile myoclonic epilepsy (JME) in RR dogs, which reinforces its parallels to JME in humans.
Keywords: Canine, DIRAS1, Electroencephalography (EEG), Wireless video‐EEG
Abbreviations
- AED
antiepileptic drug
- EEG
electroencephalography
- JME
juvenile myoclonic epilepsy
- MRI
magnetic resonance imaging
- PSWC
polyspike‐wave complexes
- RR
Rhodesian Ridgeback
- SWC
spike‐and‐wave complexes
A novel genetic myoclonic epilepsy in juvenile Rhodesian Ridgeback (RR) dogs, characterized by vigorous myoclonic seizures that occur mainly during relaxation periods, recently has been described.1 More than one‐third of affected dogs develop generalized tonic‐clonic seizures in the course of the disease, and 35% are reported to be photosensitive.1 The mean age of onset is 6 months (range, 6 weeks–1.5 years).1 Wireless video‐electroencephalography (EEG) in unsedated dogs was used as a tool to investigate the spontaneous and recurrent epileptic nature and to characterize the EEG features of the electroclinical syndrome.1 Ambulatory video‐EEG confirmed the epileptic origin of the myoclonic twitches.1 Typically, affected dogs show generalized 4–5 Hz spike‐and‐wave complexes (SWC) and polyspike‐wave complexes (PSWC) with a fronto‐central maximum.1 Genetic analyses identified a fully penetrant autosomal recessive 4‐bp truncating deletion mutation in the DIRAS1 gene, which is suggested to play a role in acetylcholine release.1, 2
Myoclonic epilepsy in RRs has important parallels to juvenile myoclonic epilepsy (JME) in humans, including juvenile onset, myoclonic seizures as the predominant seizure type with propagation to generalized tonic‐clonic seizures, similar EEG characteristics and photosensitivity.3, 4, 5, 6 In humans with JME however, apart from myoclonic seizures and generalized tonic‐clonic seizures (80–95% of patients), a third seizure type, namely absence seizures (approximately 30% of patients), is reported.7, 8 In the following case report, we describe the occurrence of absence seizures in a RR dog diagnosed with JME, completing the triad of seizure types observed in humans with JME.
An 8‐month‐old female intact RR was presented for multiple episodes of unresponsiveness, staring into space without any visible purposeful movement. Additionally, the dog experienced myoclonic jerks that occurred mainly at rest. Age of onset of both entities was 10 weeks with myoclonic seizures being observed a few days earlier than the staring episodes. At the beginning, myoclonic jerks manifested as nodding movements of the head, but became more vigorous in the course of the disease. At time of presentation, myoclonic seizures were said to resemble an electric shock such that the dog would jump into the air. Photosensitivity was not reported. Prior treatment with imepitoin (12.6 mg/kg PO q12h) decreased the intensity but not the frequency of the myoclonic seizures and did not influence the frequent staring episodes. According to the owner, the dog's behavior was normal between episodes, and the dog did not show learning difficulties or any developmental delay.
Results of physical and neurologic examinations, as well as CBC, serum biochemistry profile, plasma ammonia concentrations, and abdominal ultrasound examination were unremarkable. Testing for the DIRAS1 variant identified the homozygous mutant genotype (c.564_567delAGAC). Unsedated wireless video‐EEG with synchronized video recording was performed using an ambulatory EEG recorder1 in a quiet environment in which the dog was encouraged to lie down, because the episodes in question were reportedly more likely to occur at rest. Fifteen subdermal stainless‐steel needle electrodes were used (F3, Fz, F4, F7, F8, C3, Cz, C4, O1, Pz, O2, T3, T4, Ref, Neut). Electrodes were placed as described previously and could be placed without any sedation.1 Impedance was kept <10 kOhm. Time of recording was approximately 2 hours. At the end of the EEG study, photic stimulation was performed, utilizing a lamp with circular reflector and a viewing distance of 30 cm. The following flash frequencies (in Hz) were used in this order: 1 – 6 – 11 – 18 – 7 – 12 – 16 – 4 – 25 – 10 – 17 – 9 – 14 – 3, employing a period of 10 seconds of stimulation followed by a rest of 5 seconds per flash frequency.
Frequent episodes occurred, where the dog stared into the space and did not respond to any stimulus presented by us. Video‐EEG confirmed the occurrence of generalized 4 Hz SWC associated with the staring events (Fig 1). During these episodes, the dog occasionally would lower the neck and appear as if the dog were about to buckle (Video S1; 14:12:30 hours). The dog's behavior was normal before (Video S2; 13:54:58 hours) and after the staring events (Video S3; 14:13:11 hours). The dog also showed several vigorous myoclonic seizures (213 jerks in the first hour of recording) that were characterized by twitches of the face, cervical, and proximal limb musculature or the trunk, accompanied by generalized 4–5 Hz SWC with a central maximum (Fig 2, Video S4; 14:13:52 hours) or 4–5 Hz slowing, and sporadic single spikes. During photic stimulation, some myoclonic seizures occurred, but seizure frequency did not change and the myoclonic seizures were not associated in time with the photic stimuli. Therefore, the dog was not classified as photosensitive. A diagnosis of JME in RRs with occurrence of myoclonic seizures and absence seizures was made.
Figure 1.
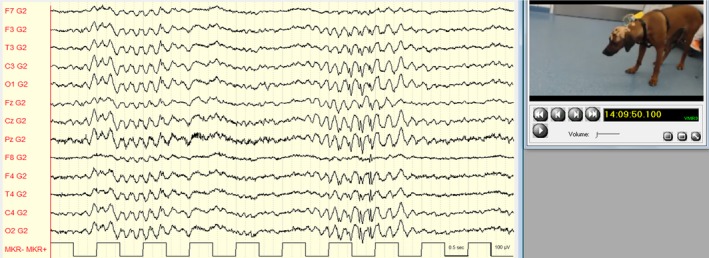
Absence seizure with generalized 4 Hz spike‐and‐wave complexes. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.
Figure 2.
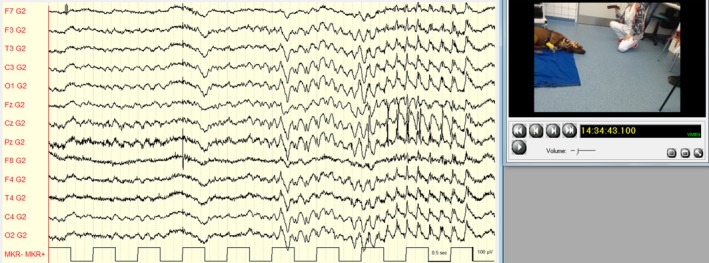
Myoclonic seizure with generalized 4–5 Hz spike‐and‐wave complexes. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.
A 20‐minute wireless video‐EEG with subsequent photic stimulation was performed on 5 healthy relatives with known genotype (mother [heterozygous], 2 sisters [both wild type], 2 brothers [1 wild type, 1 heterozygous]), and 1 affected littermate (male, homozygous for the DIRAS1 variant, 1 hour of recording). Healthy controls had normal EEG and no photosensitivity. The affected brother had myoclonic twitches that started at 9 weeks of age. Generalized tonic‐clonic seizures or absence seizures were not observed by the owner. The EEG demonstrated myoclonic seizures associated with polyspikes or PSWC, no absence seizures, and no photoparoxysmal response upon photic stimulation.
Treatment with levetiracetam (24 mg/kg PO q8h) was initiated, and imepitoin was tapered. Owners were asked to keep a seizure diary. To ensure punctual drug administration, the owners used an automated dispensing machine that was filled with the pills embedded in treats. Owners reported a substantial decrease in seizure frequency and intensity, from multiple violent myoclonic jerks per day to 1 mild myoclonic twitch per week and a complete cessation of absence seizures.
Two months after treatment onset with levetiracetam, a follow‐up EEG was performed. One‐hour unsedated video‐EEG disclosed only 6 mild and hardly visible myoclonic seizures (97.2% decrease in seizure frequency compared to the initial recording), and absence seizures were no longer recorded.
Discussion
In human medicine, JME is a common type of idiopathic generalized epilepsy, accounting for 4.1% of all epilepsies and 26.7% of idiopathic generalized epilepsies.4 Juvenile myoclonic epilepsy usually begins between 12 and 18 years of age, with a mean age of onset of approximately 14 years.9 Myoclonic jerks usually occur after awakening, are bilateral, arrhythmic, and predominate on the upper limbs, making the patients drop or throw objects.3 Most patients with JME continue to develop rare generalized tonic‐clonic seizures, which often are preceded by a cluster of myoclonic jerks, and approximately 30% experience absence seizures.7, 8 Photosensitivity is seen in another 30% of patients.10 Electroencephalography identifies generalized and irregular SWC and PSWC with a fronto‐central accentuation.4, 11 Complete seizure control is achieved in the majority of patients, but relapse rate after antiepileptic drug (AED) withdrawal may be up to 91%.12, 13 Despite a strong genetic background with positive family history in at least 40% of patients and several genetic loci associated with this syndrome, no unique pathogenic mechanism has yet been identified.9, 14
Juvenile myoclonic epilepsy in humans and dogs shares a wide range of similar characteristics such as myoclonic seizures, generalized tonic‐clonic seizures, ictal and interictal EEG features, and photosensitivity. The disease occurs in juvenile patients in both species, but onset may be much earlier in a substantial portion of dogs, with some being as young as 6 weeks of age.1 In the current case report, we describe the manifestation of absence seizures in a RR dog suffering from JME, highlighting another important parallel to JME in humans.
The International League Against Epilepsy (ILAE) defines absence seizures as “sudden cessation of activity with a brief pause and staring, sometimes with eye fluttering and head nodding or other automatic behaviors” with an obligatory EEG pattern of generalized spike waves.15 This definition holds true for the behavior and EEG pattern observed in the dog described here.
Several theories have been advanced to explain the pathophysiology of absence seizures.16 The initial hypothesis suggests that generalized SWC are created by a pacemaker in the brainstem and diencephalon (centrencephalic theory), whereas the “thalamic clock theory” assumes a pacemaker in the reticular thalamic nucleus.16, 17 The “corticoreticular theory”, proposing a thalamocortical network for the generation of generalized seizures and SWC, has attracted much attention.16, 17 Although this concept postulates that the SWC seen in absence seizures are a consequence of the transformation of sleep spindles in the hyperexcitable cortex, another study concluded that, although both spike‐wave discharges and sleep spindles arise from the cortico‐thalamo‐cortical network, the initiation site of the activity is different with sleep spindles emerging from the thalamus and spike‐wave discharges from the cortex (cortical focus theory).16, 18, 19
To our knowledge, only 1 other case report describes absence seizures with myoclonic features in a dog.20 An 8‐month‐old Chihuahua was presented for staring episodes associated with head and nose twitching.20 Diagnostic evaluation (neurologic examination and blood evaluation, magnetic resonance imaging [MRI]) was unremarkable.20 Long‐term video‐EEG identified generalized bilaterally synchronous 4 Hz SWC time‐locked to the clinical events.20 However, the prevalence of absence seizures in dogs may be highly underestimated. In 1 study, owners perceived a generalized tonic‐clonic seizure to be more harmful than a focal seizure and were less likely to report a focal seizure to their veterinarian.21 Similarly, owners and veterinarians might underestimate the impact of absence seizures or not even identify those episodes as being epileptic. Some patients with absence seizures may go on to develop generalized tonic‐clonic seizures. Therefore, the clinician should specifically ask about the occurrence of absence seizures and educate the owner about the semiology of this seizure type. Moreover, a definitive diagnosis of absence seizures requires confirmation by EEG. In the majority of studies in which EEG was used as a diagnostic tool, dogs were sedated or even anesthetized.22, 23, 24, 25, 26, 27 This technique makes the diagnosis of absence seizures challenging, because level of alertness or consciousness, a key factor for the diagnosis of absence seizures, cannot be assessed. Consequently, EEG should be performed in unsedated dogs to enable the diagnosis of absence seizures.28
The dog in our study exhibited typical absence seizures characterized by impaired consciousness in association with generalized SWC with a frequency of 4 Hz. In human medicine, absence seizures are considered as transient impairment of consciousness time‐locked to generalized 3 Hz spike‐wave discharges.29 In the current understanding, however, typical absence seizures that occur in association with idiopathic generalized epilepsies are characterized by generalized spike‐ or polyspike‐wave discharges with a frequency of >2.5 Hz (mainly 3–4.5 Hz) similar to those observed in the dog of our study.19, 30 In contrast, atypical absence seizures are observed in human patients in the context of cognitive‐related conditions and symptomatic epilepsies (eg, Lennox‐Gastaut syndrome) and are characterized by lower frequencies.19, 30 Furthermore, the frequency of spike‐wave discharges in absence seizures varies with different types of epilepsy syndromes in humans.29 In JME, absence seizures with 3–4 Hz spike‐wave discharges are described similar to the 4 Hz SWC in the dog described here and the Chihuahua with absence seizures with myoclonic features.20, 29
Short elimination half‐life time (90–120 minutes) prevents the use of valproic acid in dogs, which is the first choice AED in humans with JME, reaching effectiveness of 85–90%.9 Therefore, other AEDs with an appropriate pharmacokinetic profile are used in dogs. Levetiracetam is another effective drug to treat JME in humans and is superior to phenobarbital for the treatment of myoclonic seizures in cats with feline audiogenic reflex seizures.31, 32 For the RR in this report, treatment with imepitoin resulted only in mild reduction in intensity of myoclonic seizures, but frequency of myoclonic and absence seizures did not change. In contrast, much improvement of intensity and frequency of myoclonic seizures and complete cessation of absence seizures were achieved by levetiracetam treatment. Therefore, levetiracetam may be considered for the treatment of myoclonic seizures as well as absence seizures in veterinary medicine. However, more studies are warranted to confirm our observations.
Our study had several limitations. Because there is currently only 1 case with proven absence seizures in a RR with JME, occurrence of this seizure type could just be a coincidental finding. However, humans with idiopathic generalized epilepsies can present with various generalized seizure types such as generalized tonic‐clonic, tonic, clonic, atonic, myoclonic, or absence seizures.33 Therefore, absence seizures indeed can be a feature of JME in RRs. Moreover, several owners of RRs with JME that were presented to our clinics reported that their dogs were experiencing staring episodes. Although confirmation by video‐EEG was not attempted in those dogs, these observations suggest that absence seizures might be a more common seizure type in RRs with JME than expected. In the present case, absence seizures were confirmed by EEG at the age of 8 months, whereas for the controls (1 affected and 4 healthy littermates), EEG was performed at the age of 10 months. Therefore, absence seizures could have been an age‐related phenomenon that vanished over time. However, in contrast to the current case, owners of controls never observed staring episodes in their dogs. Brain imaging was not performed in the RR of this case report. However, the neurologic examination was normal and the dog tested positive for the DIRAS1 variant. In the previous study, RRs with JME had normal MRI findings.1 Furthermore, the RR of this report had an EEG pattern of generalized epileptic discharges and no focal discharges. Taking these points together, we considered structural brain disease very unlikely.
The current case report describes the occurrence of absence seizures in a RR diagnosed with JME. Our findings expand the spectrum of JME to include a third seizure type and highlight the potential to use this dog breed as a large animal translational model for the investigation of pathophysiologic, therapeutic, and genetic aspects of JME in humans. Furthermore, the usefulness of unsedated wireless video‐EEG for the diagnosis of seizure types and electroclinical syndromes with staring episodes is emphasized.
Supporting information
Video S1. Absence seizure with generalized 4 Hz SWC at 14:12:34 hours. The RR is nonresponsive to stimuli, slightly swaying and holds the neck in a flexed position. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.
Video S2. Dog of video S1 showing normal behavior between a myoclonic seizure and an absence seizure at 13:54:58 hours. EEG is superimposed by muscle and movement artifact. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.
Video S3. Dog of video S1 showing normal behavior after an absence seizure at 14:13:10 hours. EEG is superimposed by marked muscle and movement artifact. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.
Video S4. Dog of video S1 at 14:13:53 hours. Myoclonic seizure with generalized 4–5 Hz SWC in association with some muscle artifact during myoclonic twitches. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.
Acknowledgments
We thank PD Dr. K. Weber for assistance.
Grant support
Research grant from the Canine Health Foundation (CHF Grant No. 02248).
Conflict of Interest Declaration
FW received a travel and accommodation grant from Shire Pharmaceuticals. AF received speaker honoraria and a research grant from Boehringer Ingelheim.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Data were collected at the Centre for Clinical Veterinary Medicine, LMU Munich, Munich, Germany, and the Department of Small Animal Medicine and Surgery, University of Veterinary Medicine, Hannover, Germany.
Part of this study was presented as an oral presentation, “Absence Seizures in a Rhodesian Ridgeback with Juvenile Myoclonic Epilepsy,” at the 30th Annual Symposium of ESVN‐ECVN in Helsinki, Finland.
Footnote
Micromed BQ Home LTM 2, Micromed s.p.A. Via Giotto 2, 31201 Mogliano Veneto (Treviso), Italy
References
- 1. Wielaender F, Sarviaho R, James F, et al. Generalized myoclonic epilepsy with photosensitivity in juvenile dogs caused by a defective DIRAS family GTPase 1. Proc Natl Acad Sci USA 2017;114:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tada M, Gengyo‐Ando K, Kobayashi T, et al. Neuronally expressed Ras‐family GTPase Di‐Ras modulates synaptic activity in Caenorhabditis elegans . Genes Cells 2012;17:778–789. [DOI] [PubMed] [Google Scholar]
- 3. Genton P, Thomas P, Kasteleijn‐Nolst Trenité DGA, et al. Clinical aspects of juvenile myoclonic epilepsy. Epilepsy Behav 2013;28(S1):S8–S14. [DOI] [PubMed] [Google Scholar]
- 4. Serafini A, Rubboli G, Gigli GL, et al. Neurophysiology of juvenile myoclonic epilepsy. Epilepsy Behav 2013;28(S1):S30–S39. [DOI] [PubMed] [Google Scholar]
- 5. Baykan B, Wolf P. Juvenile myoclonic epilepsy as a spectrum disorder: A focused review. Seizure 2017;49:36–41. [DOI] [PubMed] [Google Scholar]
- 6. Wolf P, Yacubian EMT, Avanzini G, et al. Juvenile myoclonic epilepsy: A system disorder of the brain. Epilepsy Res 2015;114:2–12. [DOI] [PubMed] [Google Scholar]
- 7. Nicolson A, Marson AG. When the first antiepileptic drug fails in a patient with juvenile myoclonic epilepsy. Pract Neurol 2010;10:208–218. [DOI] [PubMed] [Google Scholar]
- 8. Yacubian EM. Juvenile myoclonic epilepsy: Challenges on its 60th anniversary. Seizure 2017;44:48–52. [DOI] [PubMed] [Google Scholar]
- 9. Welty TE. Juvenile myoclonic epilepsy. Pediatr Drugs 2006;8:303–310. [DOI] [PubMed] [Google Scholar]
- 10. Poleon S, Szaflarski JP. Photosensitivity in generalized epilepsies. Epilepsy Behav 2017;68:225–233. [DOI] [PubMed] [Google Scholar]
- 11. Guerrini R, Genton P. Epileptic syndromes and visually induced seizures. Epilepsia 2004;45(S1):14–18. [DOI] [PubMed] [Google Scholar]
- 12. Senf P, Schmitz B, Holtkamp M, Janz D. Prognosis of juvenile myoclonic epilepsy 45 years after onset: Seizure outcome and predictors. Neurology 2013;81:2128–2133. [DOI] [PubMed] [Google Scholar]
- 13. Höfler J, Unterberger I, Dobesberger J, et al. Seizure outcome in 175 patients with juvenile myoclonic epilepsy—A long‐term observational study. Epilepsy Res 2014;108:1817–1824. [DOI] [PubMed] [Google Scholar]
- 14. Rossi MA. Juvenile myoclonic epilepsy: When will it end. Epilepsy Curr 2013;13:148–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher RS. An overview of the 2017 ILAE operational classification of seizure types. Epilepsy Behav 2017;70:271–273. [DOI] [PubMed] [Google Scholar]
- 16. Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: The cortical focus theory. Arch Neurol 2005;62:371–376. [DOI] [PubMed] [Google Scholar]
- 17. Seneviratne U, Cook M, D'Souza W. The electroencephalogram of idiopathic generalized epilepsy. Epilepsia 2012;53:234–248. [DOI] [PubMed] [Google Scholar]
- 18. Leresche N, Lambert RC, Errington AC, Crunelli V. From sleep spindles of natural sleep to spike and wave discharges of typical absence seizures: Is the hypothesis still valid? Pflugers Arch 2012;463:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cortez MA, Kostopoulos GK, Snead OC. Acute and chronic pharmacological models of generalized absence seizures. J Neurosci Methods 2016;260:175–184. [DOI] [PubMed] [Google Scholar]
- 20. Poma R, Ochi A, Cortez MA. Absence seizures with myoclonic features in a juvenile Chihuahua dog. Epileptic Disord 2010;12:138–141. [DOI] [PubMed] [Google Scholar]
- 21. Packer RMA, Lucas R, Volk HA. Owner perception of focal seizures in canine epilepsy. Vet Rec 2017;180:150. [DOI] [PubMed] [Google Scholar]
- 22. Wrzosek M, Ives JR, Karczewski M, et al. The relationship between epileptiform discharges and background activity in the visual analysis of electroencephalographic examinations in dogs with seizures of different etiologies. Vet J 2017;222:41–51. [DOI] [PubMed] [Google Scholar]
- 23. Brauer C, Kästner SBR, Rohn K, et al. Electroencephalographic recordings in dogs suffering from idiopathic and symptomatic epilepsy: Diagnostic value of interictal short time EEG protocols supplemented by two activation techniques. Vet J 2012;193:185–192. [DOI] [PubMed] [Google Scholar]
- 24. Berendt M, Høgenhaven H, Flagstad A, Dam M. Electroencephalography in dogs with epilepsy: Similarities between human and canine findings. Acta Neurol Scand 1999;99:276–283. [DOI] [PubMed] [Google Scholar]
- 25. Jaggy A, Bernardini M. Idiopathic epilepsy in 125 dogs: A long‐term study. Clinical and electroencephalographic findings. J Small Anim Pract 1998;39:23–29. [DOI] [PubMed] [Google Scholar]
- 26. Jeserevics J, Viitmaa R, Cizinauskas S, et al. Electroencephalography findings in healthy and Finnish Spitz dogs with epilepsy: Visual and background quantitative analysis. J Vet Intern Med 2007;21:1299–1306. [DOI] [PubMed] [Google Scholar]
- 27. Jokinen TS, Metsahonkala L, Bergamasco L, et al. Benign familial juvenile epilepsy in Lagotto Romagnolo dogs. J Vet Intern Med 2007;21:464–471. [DOI] [PubMed] [Google Scholar]
- 28. James FMK, Cortez MA, Monteith G, et al. Diagnostic utility of wireless video‐electroencephalography in unsedated dogs. J Vet Intern Med 2017;31:1469–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panayiotopoulos CP, Obeid T, Waheed G. Differentiation of typical absence seizures in epileptic syndromes. Brain 1989;112:1039–1056. [DOI] [PubMed] [Google Scholar]
- 30. Panayiotopoulos CP. Typical absence seizures and related epileptic syndromes: Assessment of current state and directions for future research for. Epilepsia 2008;49:2131–2147. [DOI] [PubMed] [Google Scholar]
- 31. Noachtar S, Andermann E, Meyvisch P, et al. Levetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizures. Neurology 2008;70:607–616. [DOI] [PubMed] [Google Scholar]
- 32. Lowrie M, Thomson S, Bessant C, et al. Levetiracetam in the management of feline audiogenic reflex seizures: A randomised, controlled, open‐label study. J Feline Med Surg 2017;19:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522–530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Absence seizure with generalized 4 Hz SWC at 14:12:34 hours. The RR is nonresponsive to stimuli, slightly swaying and holds the neck in a flexed position. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.
Video S2. Dog of video S1 showing normal behavior between a myoclonic seizure and an absence seizure at 13:54:58 hours. EEG is superimposed by muscle and movement artifact. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.
Video S3. Dog of video S1 showing normal behavior after an absence seizure at 14:13:10 hours. EEG is superimposed by marked muscle and movement artifact. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.
Video S4. Dog of video S1 at 14:13:53 hours. Myoclonic seizure with generalized 4–5 Hz SWC in association with some muscle artifact during myoclonic twitches. Referential montage (G2 = Ref). Low pass filter: 70 Hz; high pass filter: 0.53 Hz; gain: 150 μV/cm.


