Abstract
Background
KIT inhibitors, such as toceranib (TOC), and vinblastine (VBL) have not been prospectively compared in the treatment of macroscopic mast cell tumors (MCTs). Also, it is unknown whether VBL or TOC is superior for treating MCT without c‐kit mutations.
Hypothesis/Objectives
To determine the value of KIT genotyping and localization in treatment decisions for dogs with macroscopic MCT. We hypothesized that c‐kit mutated MCT would have a better response to TOC than VBL.
Animals
Eighty‐eight client‐owned dogs with macroscopic MCT.
Methods
Prospective, randomized trial. Dogs were randomized to TOC (2.75 mg/kg EOD) or VBL (2.5 mg/m2 weekly × 4 then EOW) by KIT localization and c‐kit mutation status using an adaptive randomization scheme.
Results
Sixty dogs were allocated to TOC and 28 to VBL. Of the dogs receiving TOC, 20% had c‐kit mutations, compared to 30% receiving VBL (P = 0.74). Overall response rates were 46% (TOC) and 30% (VBL) (odds ratio = 1.56 [0.62–3.92]; P = 0.28). Median progression‐free survival (PFS) for dogs receiving VBL was 78 days (7–1,521) and for TOC 95.5 (14–990); hazard ratio (HR) = 1.34 [0.72–2.50]; P = 0.36. Median overall survival (OS) was 241.5 days (10–1,521) for the VBL group and 159 (20–990) for the TOC group; HR = 0.80 ([0.45–1.41]; P = 0.44).
Conclusions and Clinical Importance
Neither PFS nor OS was significantly different between treatment groups. As the proportion of dogs with c‐kit mutations was not different between treatment groups in this population of dogs, c‐kit mutation status did not predict treatment response.
Keywords: Cancer, Chemotherapy, Dog, Tyrosine kinase inhibitor
Abbreviations
- AE
adverse effect
- BCARA
Bayesian covariate‐adjusted response adaptive
- CR
complete response
- CSU
Colorado State University
- ECOG
Eastern Cooperative Oncology Group
- HR
hazard ratio
- IHC
immunohistochemistry
- MCT
mast cell tumor
- OR
odds ratio
- ORR
objective response rate
- OS
overall survival
- OSU‐VMC
The Ohio State University Veterinary Medical Center
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- RBVH
Red Bank Veterinary Hospital
- RTK
receptor tyrosine kinase
- SD
stable disease
- TOC
toceranib
- UWVC
University of Wisconsin Veterinary Care
- VBL
vinblastine
- VRCC
Veterinary Referral Center of Colorado
Mast cell tumors (MCTs) are the most common malignant cutaneous tumor in dogs, representing 16–21% of tumors.1 Aggressive surgery remains the mainstay of treatment for most MCTs in dogs; however, many dogs present with disease unsuitable for resection owing to size, location, or dissemination. Improvements in medical treatment for these dogs are needed. “Individualized” treatment for these dogs, based on predictors of treatment response, would be useful in maximizing treatment efficacy while minimizing cost and adverse effects (AEs).
Most MCTs in dogs express the receptor tyrosine kinase (RTK) KIT, and 13–50% of MCTs in dogs harbor internal tandem duplications in the juxtamembrane region, resulting in constitutive activation.2, 3, 4 Mutations are associated with higher grade, more aggressive behavior, and inferior outcome.3, 5, 6 Activated RTKs have the potential to enhance tumor aggressiveness via multiple mechanisms, including increased cell proliferation, apoptosis avoidance, migration, invasion, and angiogenic growth factor production.7 Multiple cancer models in humans have shown diminished activity of traditional cytotoxic therapies like chemotherapy and radiation therapy due to protection against apoptosis,8, 9, 10 and activated KIT could likewise help protect MCT in dogs from drugs such as vinblastine (VBL).
Clinical trials of 2 KIT inhibitors, toceranib phosphatea (TOC) and masitinib, have been reported in dogs with measurable MCT.11, 12 In both studies, objective response rates (ORR) were approximately 40%, and tumors with c‐kit activating mutations had an increased ORR. A more recent retrospective study of masitinib in dogs with macroscopic MCT reported an ORR of 82%; neither KIT localization nor c‐kit mutation status was assessed in these tumors.13
KIT subcellular localization has been evaluated by immunohistochemistry (IHC), and a correlation between aberrant KIT localization and activating mutations was found.3 This finding is presumably due to activated KIT molecules being removed from the cell membrane and internalized more rapidly than inactivated KIT.14 Aberrant KIT localization can also occur without a detectable c‐kit mutation, implying alternate means of constitutive activation such as gene duplication or autocrine/paracrine production of KIT's ligand, stem cell factor. It is thus possible that KIT localization could provide more accurate information regarding activation status, and thus sensitivity to KIT inhibitors, than sequencing information alone.
Previous studies suggest that cytotoxic chemotherapy used in macroscopic MCT, typically employing the drugs prednisone, VBL, and/or lomustine, has similar ORR to KIT inhibitors.15, 16, 17, 18, 19 In dogs treated with lomustine alone, ORR was 42% in a retrospective study but only 1% in a prospective, randomized trial.15, 16 The response with combination prednisone/VBL was 47%, and with lomustine/VBL 57%.17, 19 Furthermore, an inferior outcome was recently reported in dogs whose MCTs harbor c‐kit mutations or aberrant KIT localization versus wild‐type dogs when treated postsurgically with prednisone/VBL6; however, this study evaluated outcomes after combined surgery and chemotherapy, and thus, ORR was not assessed.
The primary objective of this study was to determine the predictive value of rapid PCR‐based c‐kit genotyping and immunohistochemical KIT localization in dogs with macroscopic MCT treated with prednisone and TOC or VBL. Our hypothesis was that MCT with a c‐kit mutation would have a superior response to TOC compared to VBL.
Materials and Methods
Study Design
This study was designed as a 2‐arm, multicenter, open‐label, phase III clinical trial. Dogs were enrolled from February 2011 through May 2015 at the Colorado State University (CSU) Veterinary Teaching Hospital, University of Wisconsin‐Madison Veterinary Care (UWVC), The Ohio State University Veterinary Medical Center (OSU‐VMC), Veterinary Referral Center of Colorado (VRCC; Englewood, CO), and Red Bank Veterinary Hospital (RBVH; Tinton Falls, NJ). The clinical trial was approved by each participating site's Institutional Animal Care and Use Committee (IACUC) and/or Clinical Review Board. In order to be eligible for enrollment, dogs were required to have at least 1 measurable (>1.0 cm diameter) MCT lesion with a diagnosis confirmed by either histopathology or cytology, age ≥1 year, adequate organ function as indicated by standard laboratory tests (specifically, serum transaminases ≤3 times upper normal limit, normal serum bilirubin, serum creatinine ≤1.5 times upper normal limit, neutrophils >2,000/μL, platelets > 75,000/μL, and hematocrit >25%), and performance status of 0 or 1 (according to the modified ECOG performance scheme).20 The owner provided written, informed consent before enrollment. Dogs were excluded from the study if they had received prior medical treatment for MCT other than corticosteroids, if pregnant or likely to become pregnant, if participating in another clinical trial, if scheduled for any elective procedure or medical treatment during the study period, if they had concurrent malignancy (other than MCT) or another serious systemic disorder incompatible with the study, if not going to be available for the duration of the trial or were felt to be unsuitable by the principal investigator for any other reason, or if there was anticipated poor owner compliance.
All dogs were required to have a complete blood count, serum chemistry profile, regional lymph node aspirates, thoracic radiographs, and abdominal ultrasound within 7 days of study enrollment. Before randomization, incisional biopsy and needle aspiration of one accessible MCT were performed. Biopsy and aspirate samples were shipped to CSU for analysis.
Tumor Biopsies
The biopsy sample was obtained via an incisional biopsy using a ≥6‐mm Keyes‐type punch biopsy for standard histologic grading as well as immunohistochemistry for KIT localization (patterns I–III) determination. Immunohistochemical staining was performed by standard techniques on an automated stainer.b Briefly, 4‐μm sections were cut and mounted on positively charged slides. The sections were deparaffinized and then rehydrated with descending alcohol concentrations to buffer. Heat‐induced epitope retrieval with EDTA buffer (pH 8.0) for 30 minutes was followed by endogenous peroxidase blocking with 3% hydrogen peroxide and incubation with the primary antibody at room temperature for 10 hours. The primary antibody used was a polyclonal rabbit anti‐human c‐kit (CD117) antibody at a dilution of 1:500.c A prediluted, universal biotinylated secondary antibody and a DAB MAP detection kitd were utilized to detect the immunoreactive complexes. The slides were then counterstained with Mayer's hematoxylin and evaluated by light microscopy. The predominant KIT protein staining pattern, as described in Kiupel et al.,21 was assigned by a single pathologist (EJE). Histologic grade was determined based on the Patnaik grading scheme.22 Owners and investigators were blinded to the KIT localization results.
Tumor Fine Needle Aspirates
The needle aspirate samples were subjected to PCR for c‐kit mutation detection. Internal tandem duplications in exon 8 and exon 11 were detected using primers designed to amplify the areas of reported mutation (Table 1). Together, these 2 primer pairs detect 80% of the activating mutations reported in the c‐kit gene.23 DNA was obtained by scraping cells from cytology preparations and with a commercially available kit.e Purified DNA was amplified on a thermal cyclerf by the following protocol: 94° for 5 minutes, 95° for 15 minutes, 60° for 1 minute, 40 cycles. The PCR products were analyzed on a capillary electrophoresis machineg through the Proteomics and Metabolomics Facility at CSU. During development of this assay, it was determined that at least 10% of the cells in the preparation must be mast cells for detection of c‐kit mutation. Therefore, samples were inspected for mast cell composition before use. Owners and investigators were blinded to the results of the c‐kit mutation analysis.
Table 1.
PCR primers used to detect mutations in c‐kit in mast cell tumors in dogs
| Exon | Primer Name | Location | Sequence |
|---|---|---|---|
| 8 | Ci7fa | Forward | GGT GAG GTG TTC CAG CAG TC |
| 8 | Ci8r | Reverse | CCT TCC CTC GTG CAC ATT A |
| 11 | Ce11f | Forward | CAG TGG AAG GTT GTT GAG GAG |
| 11 | Ci11r | Reverse | CAT GGA AAG CCC CTA TTT CA |
Randomization
The results of the KIT localization and c‐kit mutation status were submitted to a single individual (JCE) for treatment randomization. Randomization was determined using a Bayesian covariate‐adjusted response adaptive (BCARA) design.24 With this type of randomization scheme, dogs are allocated to one of the study arms based on the “play‐the‐winner” rule. Specifically, as more information regarding efficacy and individual dogs’ KIT localization and c‐kit mutation status profile become available during the course of the trial, a newly accrued dog is randomized with a probability to 1 of the 2 study arms which is proportional to the predicted response probability. The prediction model was based on a Bayesian generalized linear model with a logit link function. Treatment group, KIT localization, and c‐kit mutation were included as predictor variables in this model. Noninformative prior distribution were used for the intercept and slope parameters of this model. The predicted response probabilities for each arm were calculated and then used to calculate the randomization probability. There was a run‐in phase for the first 14 dogs where dogs were randomized to 1 of the 2 study arms using an equal probability. Afterward, dogs were randomized using the BCARA design. Response rates were continuously monitored over the course of the trial for efficacy and futility. A maximum sample size of 80 dogs was proposed for the trial which would provide >80% probability in identifying efficacious treatment‐marker (KIT genotype/c‐kit mutation) combinations based on the results of extensive Monte‐Carlo simulation studies where various assumptions regarding response rates, mutation status rates, accrual patterns, and stopping parameters were evaluated. Specifically, it was assumed that the odds ratio (OR) for response rates between study arms ranges between 1.0 and 4.3 depending on the KIT localization and c‐kit mutation profile.
Treatment
Dogs were randomized to receive either oral TOC (2.75 mg/kg every other day) or VBL (2.5 mg/m2 IV once weekly × 4 then every other week × 4 treatments). If considerable AEs occurred from TOC treatment, treatment was discontinued until clinical signs resolved and then was resumed on a Monday/Wednesday/Friday schedule. If considerable AEs occurred secondary to VBL, a 20% dose reduction was applied to all subsequent treatments. A dose delay of >14 days for either drug would result in removal from the study. All dogs were treated with prednisone (1 mg/kg PO every other day), diphenhydramine (2–4 mg/kg PO BID), and omeprazole (0.7 mg/kg PO q24 hours) while in the study.
Study Schedule
Once randomization was determined, dogs returned to the hospital to start treatment; this visit was considered Day 0 of the study. Physical examination and body weight was performed, and target lesions (up to 5) were identified and measured with the longest diameter recorded. Toceranib was dispensed, or the first VBL dose was administered. Rechecks were required weekly for the next 3 weeks (weeks 1, 2, and 3) and then every other week for 4 visits (weeks 5, 7, 9, and 11) for physical examination, target lesion measurement, and complete blood count ± VBL treatment. Chemistry profile was also performed at the week 5 visit. Owners completed quality of life assessment forms at each visit. Concomitant medications and AEs were recorded at each visit, and AEs were prospectively graded according to the Veterinary Comparative Oncology Group Common Terminology Criteria for Adverse Events v1.0.20
Response Assessment
Primary response assessment was performed at the week 5 visit after 4 doses of VBL or 5 weeks of TOC treatment had been given. The change in the measurements of the dog's disease was assessed as compared to the baseline measurements obtained at Day 0. Responses were determined according to a variant of the veterinary RECIST criteria25 and were classified as complete response (CR; disappearance of all target lesions), partial response (PR; ≥30% decrease in sum of longest diameter of target lesions compared to baseline), progressive disease (PD; ≥20% increase in sum of longest diameter of target lesions compared to baseline or appearance of 1 or more new lesions), or stable disease (SD; <30% decrease or <20% increase in sum of longest diameter of target lesions). Dogs were considered to have experienced clinical benefit if their disease did not meet the criteria for progressive disease (PD) at week 5. Responses were reported to the person responsible for randomization (JCE) to be used in the randomization scheme, as the responses of previously enrolled dogs influenced the treatment allocation of future dogs.
Dogs were withdrawn from the study if they developed disease progression, if unacceptable AEs occurred, at the judgment of the clinician, or at the owner's request. If dogs had stable disease (SD) or better at the week 11 visit, they were eligible to continue on the study. Recheck examinations were performed every 2 weeks, with complete blood counts performed every 2 weeks in dogs receiving VBL and every 4 weeks in dogs receiving TOC. Chemistry profiles were performed every 8 weeks in both study arms. Dogs on the VBL arm continued treatment until disease progression or 2 treatments beyond complete response (CR). Dogs on the TOC arm continued treatment until disease progression.
Statistical Analysis
Continuous data were expressed as median and range, and categorical data as frequencies and percentages. The objective response rate (ORR) at week 5 was the primary efficacy endpoint, and progression‐free survival (PFS) was a secondary endpoint. The ORR was defined as the percentage of evaluable dogs experiencing CR or partial response (PR) as their best response. The PFS was calculated from the date of treatment initiation to the date of PD or death from any cause. Dogs were censored if they had not developed PD at the time of data analysis, or if they were withdrawn or lost to follow‐up before PD development. Continuous variables were compared between groups of dogs using a two‐sample t‐test or Mann‐Whitney test depending on data normality. Categorical variables were compared between cohorts by a chi‐square or Fisher's exact test. Comparison of the frequencies of adverse events between study arms was performed by a Poisson model. The Kaplan‐Meier method was used to estimate and display the distribution of PFS and overall survival (OS). Differences between potential prognostic subsets and between study arms were compared by the log‐rank test. Multivariate analysis was performed using forward and reverse stepwise Cox regression, incorporating variables reaching significance on univariate analysis, and forcing treatment allocation into the model given the a priori hypothesis being tested. All reported P‐values are 2‐sided, and P < 0.05 was used to define statistical significance. All statistical analysis was performed with commercial software packages.h,, i
Results
A total of 94 dogs were enrolled among the 5 institutions. 2 dogs were excluded before randomization: 1 due to complications from the initial biopsy that required surgical resection of the MCT, and 1 whose initial biopsy sample did not contain any evidence of MCT and the owner declined to pursue a second biopsy. Four dogs were randomized but never started treatment at the owner's discretion: 2 dogs never returned to start treatment after randomization, 1 owner declined to treat because of the randomization result, and 1 owner had personal issues that precluded the pet's treatment. Therefore, a total of 88 dogs were randomized and started treatment as part of the clinical trial. Forty‐four dogs were treated at CSU, 20 at VRCC, 13 at UWVC, 10 at OSU‐VMC, and 1 at RBVH. Participant demographics are described in Table 2. All dogs in both treatment groups had a complete blood count and serum chemistry profile before enrollment. Staging with thoracic radiographs was performed in 52 (87%) of the dogs treated with TOC and all dogs treated with VBL (P = 0.05), and all dogs had an abdominal ultrasound. Regional lymph node aspirates were evaluated in 41 (68%) dogs in the TOC group and 18 (64%) of the dogs treated with VBL (P = 0.81). The median VBL dose given throughout the study was 2.5 mg/m2 (range 1.89–2.56 mg/m2). The median dose of TOC during the treatment period was 2.61 mg/kg (range 1.61–3.02 mg/kg).
Table 2.
Demographics of population of dogs enrolled into study comparing vinblastine to toceranib in dogs with mast cell tumors
| Toceranib (N = 60) | VBL (N = 28) | P‐Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Breed | |||
| Lab | 10 (17) | 2 (7) | 0.94 |
| Boxer | 7 (12) | 4 (14) | |
| Mixed | 8 (13) | 2 (7) | |
| Golden Retriever | 4 (7) | 3 (11) | |
| Boston Terrier | 5 (8) | 0 (0) | |
| Pug | 4 (7) | 1 (4) | |
| Staffordshire Terrier | 1 (2) | 4 (14) | |
| Other | 2 (21) | 12 (43) | |
| Sex | |||
| Female spayed | 36 (60) | 18 (64) | 0.94 |
| Male castrated | 21 (35) | 9 (32) | |
| Female intact | 1 (2) | 0 (0) | |
| Male intact | 2 (3) | 1 (4) | |
| Age (years), mean (SD) | 8.7 (3.1) | 8.8 (2.4) | 0.87 |
| Weight (kg), mean (SD) | 26.4 (12.0) | 26.7 (11.3) | 0.91 |
| Location | |||
| Limb | 21 (35) | 11 (39) | 0.48 |
| Trunk | 9 (15) | 5 (18) | |
| Head/neck | 8 (13) | 1 (4) | |
| Multiple cutaneous | 18 (30) | 7 (25) | |
| Lymph node only | 4 (7) | 3 (11) | |
| Other | 0 (0) | 1 (4) | |
| Previous treatment | |||
| Surgery | 15 (15) | 9 (32) | 0.73 |
| Chemotherapy | 0 (0) | 0 (0) | 0.99 |
| Steroids | 7 (12) | 2 (7) | 0.71 |
| First DX | 33 (55) | 11 (39) | 0.27 |
| Type of recurrence | |||
| De novo | 9 (33) | 8 (47) | 0.43 |
| Local | 12 (44) | 5 (29) | |
| Local + de novo | 1 (4) | 2 (12) | |
| Local + met | 0 (0) | 1 (6) | |
| LN met | 4 (15) | 1 (6) | |
| Possible local | 1 (4) | 0 (0) | |
| Method of diagnosis | |||
| Aspirate | 46 (77) | 28 (100) | 0.004 |
| Biopsy | 14 (23) | 0 (0) | |
| Day 0 target lesion sum | |||
| Measurements (cm) | |||
| Median | 4.59 | 4.455 | 0.53 |
| Mean (SD) | 6.95 (5.5) | 6.17 (5.37) | |
| Range | 1.12‐26.8 | 1.6‐25.8 | |
SD, standard deviation; DX, diagnosis; LN, lymph node.
Up to 5 target lesions were identified at the time of enrollment for response assessment. All target lesions were evaluable on physical examination and were measured at each study visit. Mast cell disease characteristics present in both treatment arms is displayed in Table 3, including tumor grade, metastasis present at the time of enrollment, and histopathologic/immunohistochemical findings. There was no significant difference in the number of dogs allocated to TOC or VBL treatment when comparing KIT pattern localization (P = 0.81) or c‐kit mutation status (P = 0.74).
Table 3.
Mast cell disease characteristics in dogs enrolled into current clinical trial
| Toceranib (N = 60) | Vinblastine (N = 28) | P‐Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Grade | |||
| I | 0 (0) | 0 (0) | 0.99 |
| II | 47 (78) | 23 (82) | |
| III | 10 (17) | 4 (14) | |
| Unknown | 3 (5) | 1 (4) | |
| Metastasis | 26 (43) | 12 (43) | 0.99 |
| Metastasis to LN | 24 (92) | 12 (100) | 0.97 |
| Metastasis beyond LN | 6 (23) | 4 (33) | 0.50 |
| KIT localization | |||
| Pattern I | 21 (35) | 9 (32) | 0.82 |
| Patterns II/III | 36 (60) | 18 (64) | |
| c‐kit mutation present | |||
| Yes | 12 (20) | 8 (29) | 0.42 |
| No | 47 (78) | 20 (71) | |
| Location of mutation | |||
| Exon 8 | 0 (0) | 2 (25) | 0.15 |
| Exon 11 | 12 (100) | 6 (75) | |
LN, lymph node.
Randomization of the first 14 dogs enrolled in the study was predetermined to train the randomization algorithm, with 7 dogs allocated to VBL and 7 to TOC. After the first 14 dogs, subsequent dogs were allocated to treatment arms using the BCARA randomization scheme, with an additional 24 dogs (total 31) to VBL and 54 dogs (total 61) to TOC. Three dogs assigned to VBL and 1 to TOC did not start treatment after randomization. At the time of primary response assessment at week 5, of the VBL‐treated group, 1 dog was in a CR, 7 dogs had a PR, 8 dogs had SD, and no dogs had PD. Twelve dogs were withdrawn before week 5: Ten were removed due to disease progression before week 5, 1 per the owner's request, and 1 due to a combination of AEs and PD. In the TOC arm, 7 dogs had a CR, 18 dogs had a PR, 15 dogs had SD, and 6 dogs had PD at the time of primary response assessment. Fourteen dogs were withdrawn before week 5: 8 because of disease progression and 6 at the owner's request. Dogs withdrawn from the study per the owner's request before week 5 were removed due to a lack of response to treatment (n = 3), because the owner elected to pursue surgical treatment (n = 2), or because the owner stopped administering the drug (TOC) for unknown reasons (n = 2). Therefore, there were 54 TOC dogs and 27 VBL dogs evaluable for assessment of ORR, CR rate, and clinical benefit rate at the time of primary response assessment after exclusion of the dogs that were withdrawn per the owner's request. There were no significant differences found in dogs treated with TOC compared to those treated with VBL in ORR (TOC 46%, VBL 30%; OR = 1.56 [95% confidence interval 0.62–3.92]; P = 0.15), CR rate (TOC 13%, VBL 4%; OR = 3.5 [0.41–29.9]; P = 0.19), or clinical benefit rate (TOC 74%, VBL 59%; OR = 1.25 [0.6–2.62]; P = 0.56) at week 5. A graphical representation of response at week 5 by KIT staining pattern and c‐kit mutation status in each treatment arm is shown in Figure 1.
Figure 1.
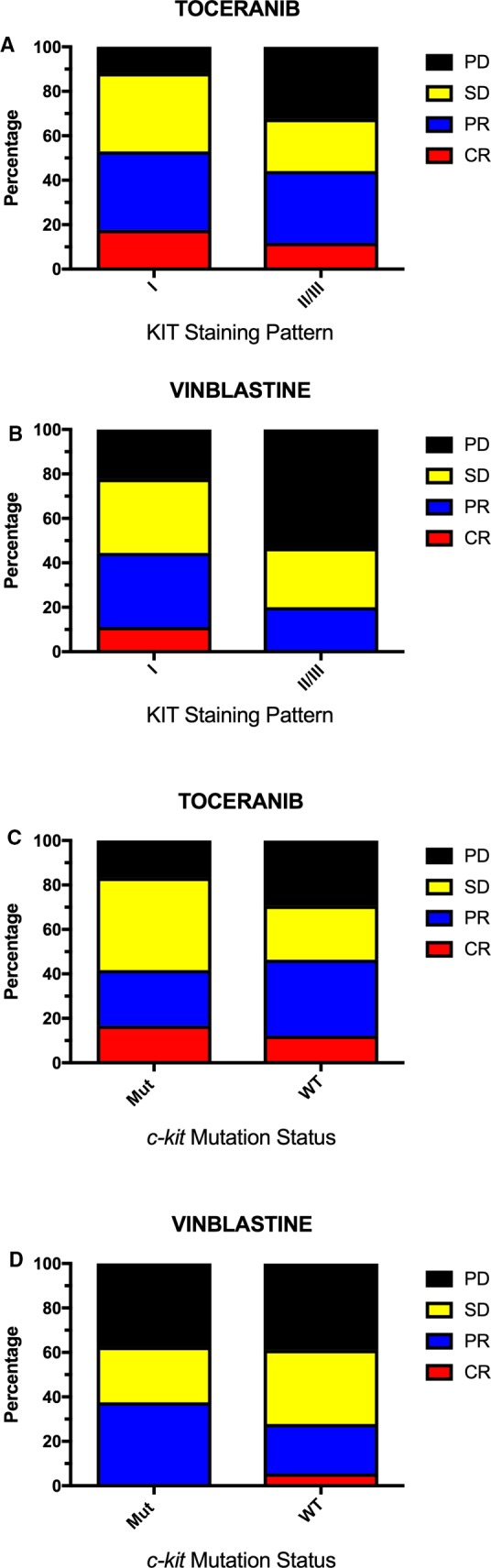
Comparison of response to treatment at week 5 by KIT staining pattern (A and B) and c‐kit mutation status (C and D) in dogs receiving toceranib or vinblastine for the treatment of macroscopic mast cell tumors.
Best response while on study treatment was also assessed in all dogs that started treatment. Of dogs receiving VBL, 1 had a CR, 11 with PR, 14 with SD, and 2 with PD. Of dogs receiving TOC, 10 achieved a CR, 28 had PR, 20 had SD, and 2 had PD. No differences in ORR (63% for TOC [50–74%]; 43% for VBL [27–61%]; P = 0.12), CR rate (17% for TOC [9–28%]; 4% for VBL [0.2–18%]; P = 0.16), or clinical benefit rate (97% for TOC [89–99%]; 93% for VBL [77–98%]; P = 0.59) were observed.
Adverse events were documented in 25 of 28 (89%) dogs receiving VBL and 56 of 60 (93%) dogs receiving TOC. Categories and grades of AEs for the TOC group compared to the VBL group are displayed in Table 4. In both treatment arms, >90% of AEs were grade 1 or 2. The overall number of AEs observed in dogs receiving TOC was significantly higher compared to those receiving VBL (P < 0.0001). The use of concomitant medications in both treatment arms is described in Table S1.
Table 4.
Adverse events experienced in dogs enrolled into current clinical trial
| Grade | Toceranib | Vinblastine | Rate Ratioa | P‐Valueb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |||
| Hematologic | 10.4% of all AEs | 13.7% of all AEs | ||||||||||
| Anemia | 21 | 3 | 7 | |||||||||
| Neutropenia | 17 | 2 | 2 | 4 | 1 | |||||||
| Thrombocytopenia | 2 | 4 | ||||||||||
| Neutrophilia | 3 | |||||||||||
| Other leukopenia | 5 | |||||||||||
| Other | 2 | |||||||||||
| Total | 47 | 5 | 16 | 4 | 1 | 1.2 | 0.57 | |||||
| GI | 38.3% of all AEs | 35.3% of all AEs | ||||||||||
| Anorexia | 32 | 14 | 1 | 5 | ||||||||
| Vomiting | 24 | 9 | 1 | 11 | ||||||||
| Diarrhea | 67 | 17 | 1 | 29 | 1 | |||||||
| Flatulence | 4 | 3 | 1 | 1 | ||||||||
| Nausea | 3 | 2 | 1 | |||||||||
| Hematochezia | 2 | 3 | 1 | |||||||||
| Other | 8 | 4 | ||||||||||
| Total | 140 | 48 | 3 | 52 | 2 | 1.7 | 0.0007 | |||||
| Constitutional | 9.8% of all AEs | 5.2% of all AEs | ||||||||||
| Lethargy | 24 | 7 | 2 | 8 | ||||||||
| Fever | 2 | 1 | 1 | |||||||||
| Weight loss | 8 | 3 | 1 | |||||||||
| Other | 1 | |||||||||||
| Total | 35 | 11 | 3 | 1 | 8 | 2.9 | 0.0015 | |||||
| Metabolic | 19% of all AEs | 18.3% of all AEs | ||||||||||
| Elevated ALP | 9 | 13 | 8 | 3 | 5 | 3 | 3 | |||||
| Elevated ALT | 9 | 10 | 5 | 2 | 1 | 2 | ||||||
| Elevated AST | 7 | 3 | 1 | |||||||||
| Elevated GGT | 2 | 2 | 1 | |||||||||
| Elevated TBili | 2 | 1 | ||||||||||
| Elevated BUN | 4 | 2 | 1 | |||||||||
| Hyperglycemia | 4 | 1 | 1 | |||||||||
| Elevated CK | 2 | 1 | 1 | |||||||||
| Elevated globulin | 3 | |||||||||||
| Other | 4 | 1 | 4 | |||||||||
| Total | 44 | 29 | 17 | 4 | 14 | 6 | 7 | 1.6 | 0.021 | |||
| Miscellaneous | 24.2% of all AEs | 27.4% of all AEs | ||||||||||
| Urinary | 12 | 7 | 1 | 7 | 1 | |||||||
| Orthopedic | 12 | 9 | 2 | 2 | 4 | 2 | ||||||
| Cardiac | 2 | 1 | 1 | 2 | 1 | |||||||
| MCT‐related | 1 | 1 | 1 | 2 | 2 | 1 | 1 | |||||
| Cutaneous | 27 | 8 | 2 | 7 | 5 | |||||||
| Bleeding | 3 | 1 | ||||||||||
| Panting | 3 | |||||||||||
| Polydipsia | 4 | |||||||||||
| Other | 12 | 5 | 5 | 4 | ||||||||
| Total | 76 | 32 | 7 | 2 | 2 | 27 | 14 | 1 | 1.3 | 0.11 | ||
| Grand total | 342 | 125 | 30 | 7 | 2 | 117 | 26 | 9 | 0 | 0 | 1.6 | <0.0001 |
GI, gastrointestinal; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; TBili, total bilirubin; BUN, blood urea nitrogen; CK, creatine kinase.
Rate ratio for total number of AEs (grade 1 to grade 5) between toceranib and vinblastine.
P‐value for comparing total number of AEs (grade 1 to grade 5) between study arms by a Poisson model.
Four dose reductions were required in 4 dogs (14%) receiving VBL, and 33 dogs (55%) receiving TOC needed a total of 46 dose adjustments. The number of dogs requiring dose reductions was significantly lower in the VBL group compared to the TOC group (P = 0.0004). The dose reductions in the dogs on VBL were all due to neutropenia. In the dogs on TOC, the numerical dose was decreased 17 times whereas the dose frequency was reduced 26 times, and both dose and frequency were adjusted 3 times. The causes for dose adjustments in the TOC dogs were variable, including gastrointestinal signs, hematologic changes, constitutional signs, and a combination of signs, among others.
Dose delays were required in 8 dogs (29%) receiving VBL, with each dog having 1 dose delay. Seven of the delays were due to neutropenia, and 1 for gastrointestinal upset. The median duration of the delay was 7 days (range 6–13). Forty‐seven dose delays occurred in 31 dogs (52%) treated with TOC, with a variety, and often a combination, of signs necessitating the delays. The median duration of delay was 7 days (range 1–16). There was no difference in number of dogs requiring dose delays between the 2 treatment groups (P = 0.064).
The median time to withdrawal from the study for dogs receiving VBL was 59.5 days (range 7–1,000) and for dogs receiving TOC was 56.5 days (range 10–796). Of the 28 dogs that received VBL, 14 (50%) were withdrawn due to PD, 10 (36%) at the owner's request, 3 (11%) due to an unrelated condition, and 1 (4%) due to AEs. Of the 60 dogs treated with TOC, 23 (38%) were withdrawn due to PD, 16 (27%) at the owner's request, 5 (8%) due to AEs, 4 (7%) due to death (3 secondary to complications of their MCT, 1 due to an unrelated condition), 3 (5%) due to an unrelated condition, 2 (3%) per the investigator's judgment, and 1 each (2%) for owner noncompliance and a combination of the owner's request and AEs. Four dogs (7%) were forced to stop treatment due to a hospital shortage of TOC. One dog was withdrawn due to the completion of study data collection, but continued on TOC treatment off study. Details regarding the characteristics of disease progression for both treatment arms are provided in Table S2.
In the 28 dogs that received VBL, the median PFS time was 78 days (range 7–1,251), compared to 95.5 days (range 14–990) in the 60 dogs in the TOC‐treated group. The hazard ratio (HR) for PFS for VBL versus TOC was 1.34 (0.72–2.50; P = 0.36) (Fig 2A). A total of 43 dogs were censored from PFS analysis, 30 that received TOC and 13 VBL. The reasons for censoring included the following: no documented PD at the time of data analysis (n = 10), euthanized without documented PD (n = 15), lost to follow‐up without PD (n = 5), and surgical excision of MCT before PD (n = 13). Median follow‐up time for censored dogs was 198 days (range 10–1,521). Median OS time in dogs receiving VBL was 241.5 days (range 10–1,521) and 159 days (range 20–990) in those receiving TOC. The HR for OS for VBL compared to TOC was 0.80 (0.45–1.41; P = 0.44) (Fig 2B). Dogs were censored from OS analysis if they were alive at the time of data analysis (n = 21), if they had died due to causes other than MCT (n = 6), or if they were lost to follow‐up (n = 12). Twenty‐five dogs in the TOC arm and 14 in the VBL arm were censored from survival analysis. Differences in PFS and OS were compared between treatment groups for dogs whose tumors were assigned KIT pattern I versus patterns II/III (Fig 3). A significant improvement in PFS was found for dogs with KIT pattern I localization that received TOC compared to those receiving VBL (HR = 4.71 [1.32–47]; P = 0.02). All other comparisons were not significantly different. PFS and OS were also compared for dogs with pattern I localization versus patterns II/II and mutant versus wild‐type c‐kit in each treatment group (Figs 4, 5). In TOC‐treated dogs, there was a significant increase in PFS for dogs with pattern I localization compared to patterns II/II (HR = 6.95 [1.99–8.83]; P = 0.0002) and wild‐type c‐kit genes compared to mutant (HR = 2.34 [1.19–7.13]; P = 0.02), as well as a prolonged OS with pattern I localization versus patterns II/III (HR = 4.37 [1.91–6.6]; P < 0.0001). There was no difference found in OS for mutant versus wild‐type c‐kit. In the VBL‐treated group, no statistically significant differences were found for any of these comparisons.
Figure 2.
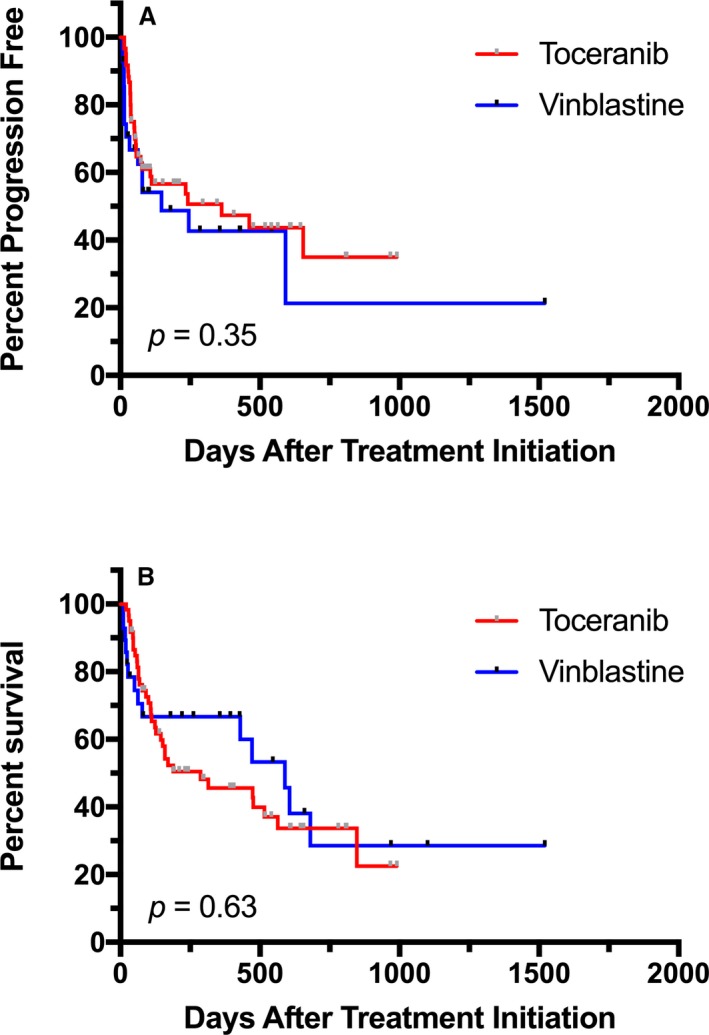
Comparison of progression‐free survival (PFS) (A) and overall survival (OS) (B) for dogs with mast cell tumors receiving vinblastine (VBL) compared to toceranib (TOC). The hazard ratio (HR) for PFS for VBL versus TOC was 1.34 (95% confidence interval 0.72–2.50) and for OS was 0.80 (95% confidence interval 0.45–1.41).
Figure 3.
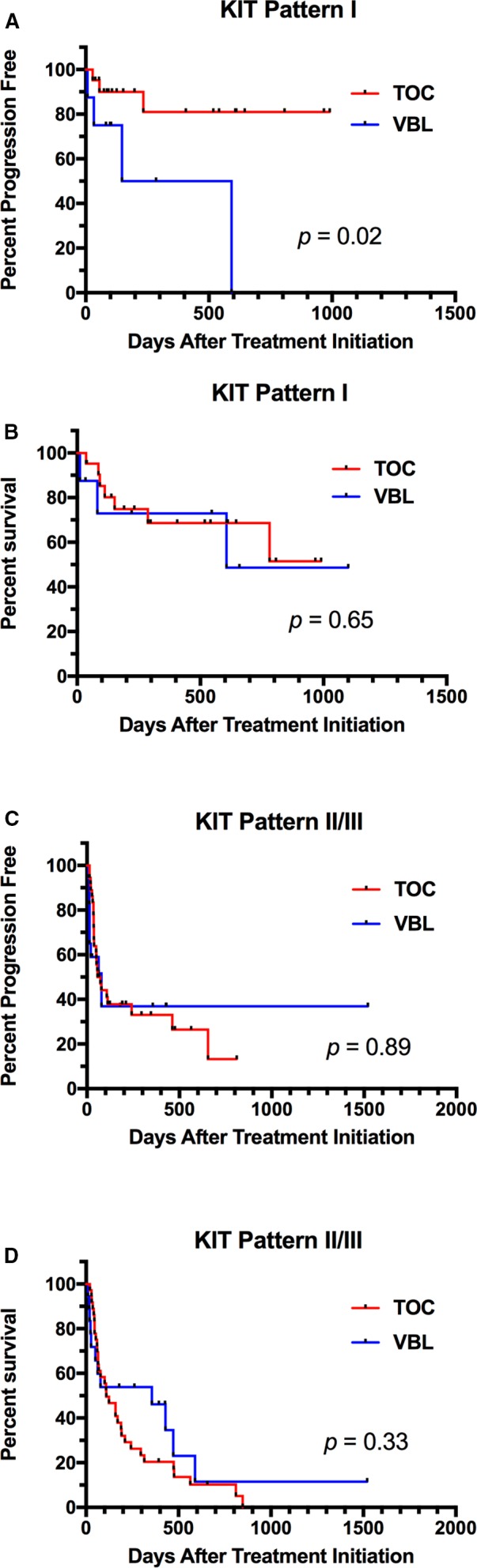
Comparison of progression‐free survival (PFS) and overall survival (OS) in dogs whose tumors expressed KIT pattern I localization (A and B) versus patterns II/III (C and D) in dogs receiving toceranib (TOC) or vinblastine (VBL). A statistically significant difference between treatments was found in PFS for tumors with KIT pattern I localization. No other comparisons were found to be statistically significant.
Figure 4.
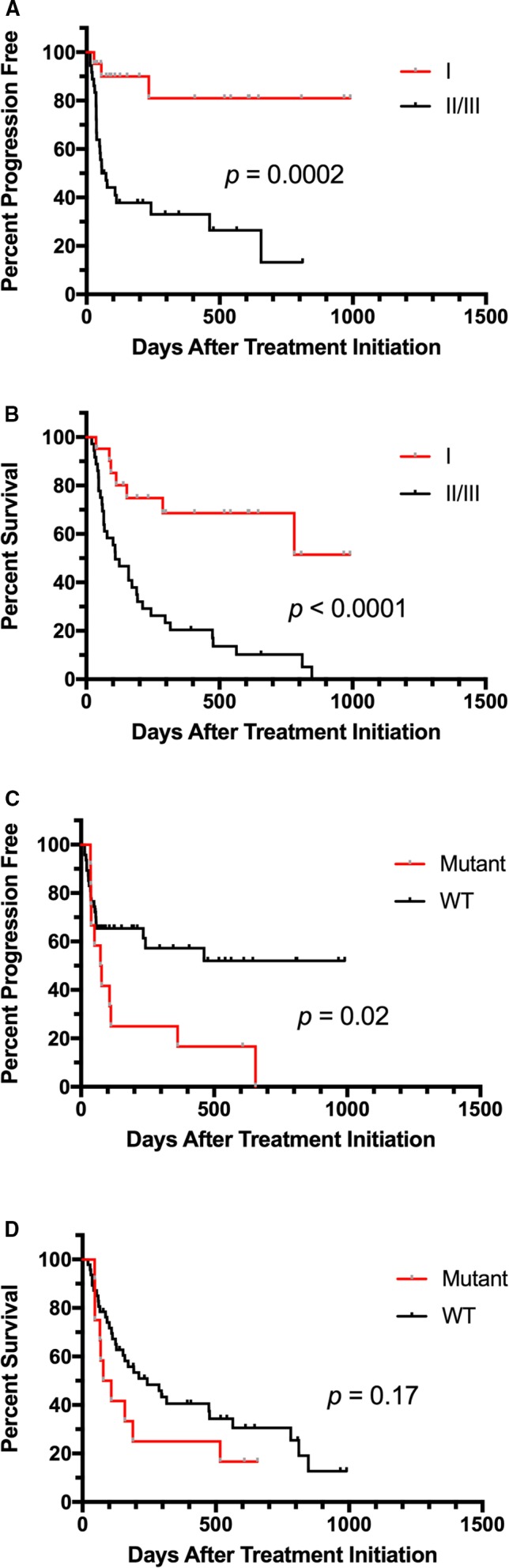
Comparison of progression‐free survival (PFS) and overall survival (OS) for pattern I versus patterns II/III (A and B) and mutant versus wild‐type c‐kit (C and D) in dogs with mast cell tumors receiving toceranib (TOC). Dogs whose tumors demonstrated pattern I localization had significant improvements in PFS and OS. Dogs with wild‐type c‐kit had a significant benefit with regard to PFS but not OS compared to dogs with mutations present.
Figure 5.
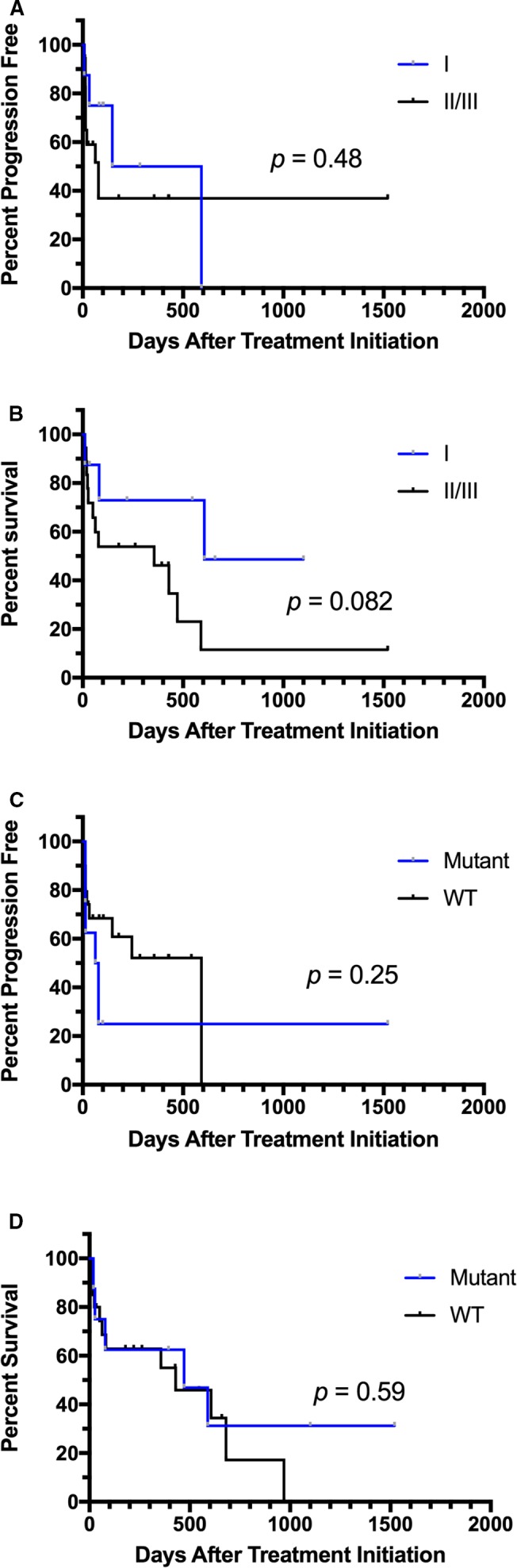
Comparison of progression‐free survival (PFS) and overall survival (OS) for pattern I versus patterns II/III (A and B) and mutant versus wild‐type c‐kit (C and D) in dogs with mast cell tumors receiving vinblastine (VBL). There were no statistically significant differences in PFS or OS in these groups.
Upon multivariate analysis incorporating histologic grade, c‐kit mutation status, KIT localization and treatment allocation, histologic grade (HR = 2.21 [1.11–4.42]) and KIT localization (HR = 2.87 [1.27–6.48]) retained significant prognostic value for PFS, and KIT localization (HR = 3.86 [1.91–7.80]) was the sole prognostic factor for OS.
When evaluating all cases enrolled into the study, tumor grade was found to be a significant predictor of outcome, with dogs with grade 2 tumors having significantly improved PFS (HR = 3.24 [1.28–8.19]; P = 0.0002) and OS (HR = 2.67 [1.16–6.10]; P = 0.02) when compared to dogs with grade 3 tumors (Fig 6). Median PFS for dogs with grade 2 tumors was 592 days compared to 37 days in dogs with grade 3 tumors. The median OS was 356 days in dogs with grade 2 tumors and 60 days for grade 3.
Figure 6.
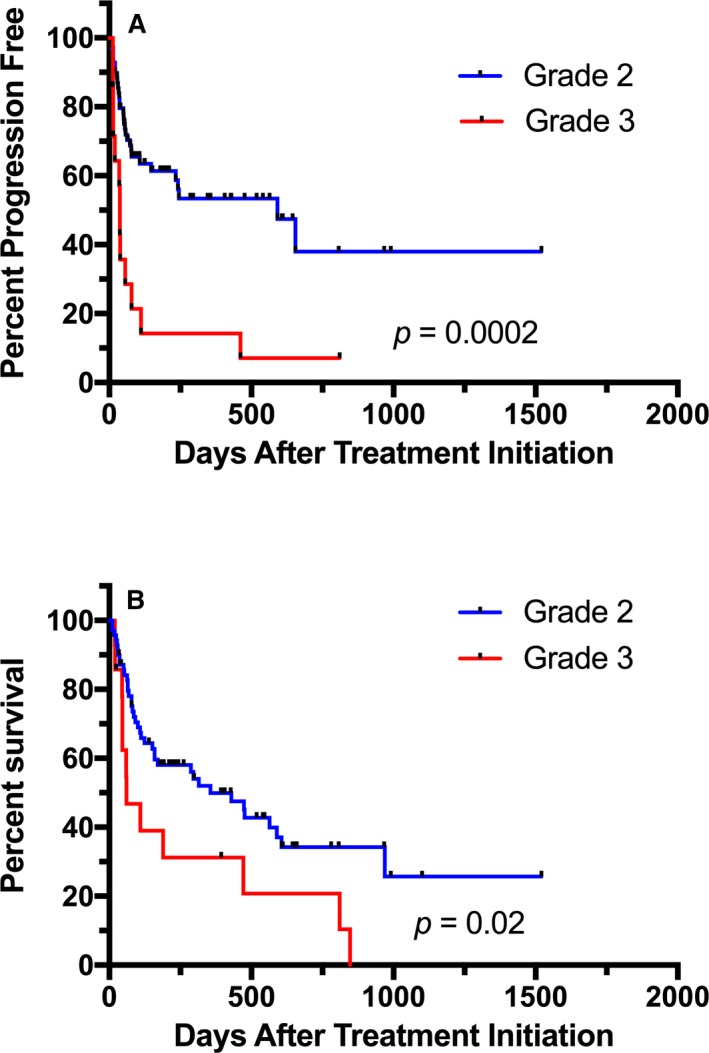
Comparison of progression‐free survival (PFS) (A) and overall survival (OS) (B) for dogs with grade 2 and grade 3 mast cell tumors (MCTs). There was a significant improvement in both PFS and OS in dogs with grade 2 tumors compared to grade 3.
Progression‐free survival was significantly shorter in dogs with grade 3 tumors compared to grade 2 in both the TOC arm (37.5 versus 655 days; HR = 5.21 [1.74–15.6]; P = 0.0042) and the VBL arm (13.5 versus 245 days; HR = 18.15 [5.78–57.0]; P = 0.0065). However, when comparing PFS for grade by treatment, no differences were seen for dogs treated with TOC versus VBL (grade 2: HR = 1.48 [0.67–3.28]; P = 0.33; grade 3: HR = 3.87 [0.77–19.24]; P = 0.10). OS for dogs treated with TOC was also significantly shorter for dogs with grade 3 tumors than grade 2 (59.5 versus 315 days; HR = 5.29 [2.54–11.0]; P = 0.011); this was not different in dogs treated with VBL (472 versus 432 days; HR = 1.1 [0.25–4.84]; P = 0.082). No difference in OS was found for dogs with grade 2 tumors (HR = 1.16 [0.59–2.29]; P = 0.67) or grade 3 tumors (HR = 1.45 [0.35–6.03]; P = 0.61) when comparing the 2 treatment groups.
Discussion
Toceranib and VBL are effective in treating some macroscopic MCTs in dogs12, 17, 19; however, the efficacies of these 2 treatments in this setting have not previously been prospectively compared, especially with specific tumor characteristics being taken into consideration. Our study compared the benefit of TOC and VBL in the treatment of dogs with macroscopic MCT, with treatment allocation performed using an adaptive randomization scheme guided by the results of rapid PCR‐based c‐kit genotyping and immunohistochemical KIT localization.
The population of dogs included in this clinical trial was similar to those of previous studies of MCT in dogs, with usual breeds and tumor locations represented. While a portion of dogs had had previous surgery for MCT removal, none had prior chemotherapy and only a small number had received prednisone. There were no significant differences in any demographic characteristics between the 2 treatment groups, nor in tumor grade or stage at diagnosis. These data help to support the validity and applicability of the study results.
Treatment allocation in this study was made based on the tumor characteristics of KIT localization and c‐kit mutation status. Because of the adaptive randomization scheme that was used,21 dogs were assigned to treatment based on the outcomes of previously enrolled dogs with similar tumor characteristics. Therefore, if one treatment was more successful for dogs with a certain KIT localization pattern or c‐kit mutation status, there would be significantly more dogs with that tumor characteristic assigned to that treatment arm. However, as there was no significant difference in the number of dogs allocated to TOC or VBL treatment when comparing KIT pattern localization or c‐kit mutation status, it is implied that there was no benefit for dogs to be assigned to one treatment over the other based on individual tumor characteristics.
While the number of dogs assigned to each treatment arm was not statistically different, the number of dogs allocated to the TOC arm (n = 60) was over twice that assigned to VBL (n = 28). This is a likely due to the study design being based on the “play‐the‐winner” rule, which allocates dogs to the treatment arm with the higher response rate at that time in the course of the study, even if the response rates are only marginally different between the treatment arms. This implies that the response rates in dogs receiving TOC tended to be higher than in those receiving VBL, although the difference was not statistically significant.
Besides a lack of difference in tumor characteristics between treatment arms, dog outcomes in the 2 arms were statistically similar. There was no significant difference found in PFS time or OS time between the dogs treated with TOC or VBL. In addition, no significant differences were found in CR rate, ORR, or clinical benefit rate between the 2 treatment groups at the time of primary response assessment. Tumor grade was found to be a significant predictor of outcome, with significant improvements in both PFS and OS found in dogs with grade 2 tumors compared to those with grade 3 tumors in both treatment groups. However, there was no difference in PFS or OS for dogs treated with TOC compared to VBL when looking at grade 2 and grade 3 tumors separately.
Despite outcomes in the 2 treatment arms being similar, there was a significant difference in toxicosis, with the dogs receiving TOC having an increased number of adverse events overall, as well as significantly more events in the categories of gastrointestinal, constitutional, and metabolic AEs. The difference in toxicosis between the treatments alone could be a reason to advocate for the use of VBL in these dogs, as AEs can affect dog's quality of life and impact the owner both emotionally and financially.
A significant prolongation in PFS time was found in dogs whose tumors had KIT pattern I localization that were treated with TOC in comparison with those treated with VBL; however, no difference in OS time was found in this group of dogs, and there was no difference in PFS time or OS time in dogs whose tumors were classified as patterns II/III between the treatment groups. In dogs treated with TOC, those with tumors that were classified as pattern I had significantly longer PFS time and OS time than those with tumors with patterns II/III localization. Additionally, dogs whose tumors expressed wild‐type c‐kit had a significantly longer PFS time than those with mutations, although there was no difference in OS time. There were no differences in PFS time or OS time in dogs treated with VBL with regard to pattern localization or mutation status. These data suggest that the use of either KIT pattern localization or c‐kit mutation status alone is not sufficient to make treatment decisions between VBL and TOC.
The results of this study indicate that KIT pattern localization and c‐kit mutation status are predictive of response to treatment in dogs with macroscopic MCT treated with TOC. An interesting finding in this study is that dogs with c‐kit mutations treated with TOC actually had worse outcomes than dogs with wild‐type c‐kit, which is contradictory to what has been reported in previous studies of KIT inhibitors in MCT in dogs.11, 12 However, these previous studies evaluated response to treatment rather than PFS, and PFS could be a more clinically relevant endpoint. Results similar to those of this study were found in a study of dogs with macroscopic MCT treated with a combination of hypofractionated radiation and TOC. In this study, dogs with tumors that had c‐kit mutations had significantly worse PFS and disease‐free rate at 1 year than those without mutations.26 These findings challenge the previous reports of decreased efficacy of TOC in dogs with wild‐type c‐kit. KIT pattern localization and c‐kit mutation status did not appear to predict response to treatment in dogs treated with VBL. In addition, these tumor characteristics are not helpful in making a treatment decision between TOC and VBL, as outcomes with the 2 treatments were not significantly different. While PFS time was improved in dogs with KIT pattern I localization receiving TOC, this did not carry over to a benefit in OS time. This could be because many of the dogs that failed TOC were rescued with VBL, and vice versa.
In conclusion, this is the first prospective study to compare the efficacy of treatment with TOC to VBL in macroscopic MCT in dogs. In addition, to the authors’ knowledge, it is the first study in veterinary oncology to use an adaptive randomization scheme. The results of this study suggest that c‐kit mutation status and KIT localization are not predictive of response to treatment with TOC or VBL in dogs with macroscopic MCT. Therefore, these diagnostics might not be useful in making a treatment decision between TOC and VBL for these dogs.
Supporting information
Table S1. Categories of concomitant medications administered to dogs with mast cell tumors receiving vinblastine compared to toceranib.
Table S2. Characteristics of disease progression in dogs being treated with toceranib or vinblastine.
Acknowledgments
This study was supported by grant (Grant Number 01426) from the American Kennel Club Canine Health Foundation, and by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Conflict of Interest Declaration
Zoetis provided the Palladia used in this study. Drs. Thamm, Clifford, Vail, and London are all paid consultants for Zoetis. Dr. London has received payment for development of educational materials and speaker honoraria from Zoetis. Dr. Clifford is an advisory board member with Zoetis.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The work in this study was performed at Colorado State University Veterinary Teaching Hospital, University of Wisconsin‐Madison Veterinary Care, The Ohio State University Veterinary Medical Center, Veterinary Referral Center of Colorado (Englewood, CO), and Red Bank Veterinary Hospital (Tinton Falls, NJ).
This was presented in abstract form at the Veterinary Cancer Society meeting, October 2016, Orlando, FL.
Footnotes
Palladia, Zoetis, Florham Park, NJ
Discovery, Ventana Medical Systems, Tucson, AZ
Catalog #A4502; DakoCytomation; Carpinteria, CA
Discovery DAB Map Detection Kit (Catalog #760‐124), Ventana Medical Systems, Tucson, AZ
QIAamp DNA Blood Mini‐kit, Qiagen, Valencia, CA
Bio‐Rad iCycler, Bio‐Rad Laboratories, Hercules, CA
ABI 3130xl Genetic Analyzer, Applied Biosystems Inc., Foster City, CA
Prism v. 6.0b, GraphPad Software, La Jolla, CA
SPSS Statistics. v. 22, IBM, Armonk, NY
References
- 1. London CA, Thamm DH. Mast cell tumors In: Withrow SJ, Vail DM, Page RL, eds. Small Animal Clinical Oncology, 5th ed St. Louis, MO: Saunders; 2013:335–355. [Google Scholar]
- 2. Zemke D, Yamini B, Yuzbasiyan‐Gurkan V. Mutations in the juxtamembrane domain of c‐KIT are associated with higher grade mast cell tumors in dogs. Vet Pathol 2002;39:529–535. [DOI] [PubMed] [Google Scholar]
- 3. Webster JD, Yuzbasiyan‐Gurkan V, Kaneene JB, et al. The role of c‐KIT in tumorigenesis: evaluation in canine cutaneous mast cell tumors. Neoplasia 2006;8:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Downing S, Chien MB, Kaas PH, et al. Prevalence and importance of internal tandem duplications in exons 11 and 12 of c‐KIT in mast cell tumors of dogs. Am J Vet Res 2002;63:1718–1723. [DOI] [PubMed] [Google Scholar]
- 5. Webster JD, Yuzbasiyan‐Gurkan V, Miller RA, et al. Cellular proliferation in canine cutaneous mast cell tumors: associations with c‐KIT and its role in prognostication. Vet Pathol 2007;44:298–308. [DOI] [PubMed] [Google Scholar]
- 6. Webster JD, Yuzbasiyan‐Gurkan V, Thamm DH, et al. Evaluation of prognostic markers for canine mast cell tumors treated with vinblastine and prednisone. BMC Vet Res 2008;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paul MK, Mukhopadhyay AK. Tyrosine kinase – role and significance in cancer. Int J Med Sci 2004;1:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ischenko I, Camaj P, Seeliger H, et al. Inhibition of Src tyrosine kinase reverts chemoresistance toward 5‐fluorouracil in human pancreatic carcinoma cells: an involvement of epidermal growth factor receptor signaling. Oncogene 2008;27:7212–7222. [DOI] [PubMed] [Google Scholar]
- 9. Liu D, Zhang Y, Dang C, et al. siRNA directed against TrkA sensitizes human pancreatic cancer cells to apoptosis induced by gemcitabine through an inactivation of PI3K/Akt‐dependent pathway. Oncol Rep 2007;18:673–677. [PubMed] [Google Scholar]
- 10. Tang MK, Zhou HY, Yam JW, Wong AS. c‐Met overexpression contributes to the acquired apoptotic resistance of nonadherent ovarian cancer cells through a cross talk mediated by phosphatidylinositol 3‐kinase and extracellular signal‐regulated kinase 1/2. Neoplasia 2010;12:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hahn KA, Ogilvie G, Rusk T, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. J Vet Intern Med 2008;22:1301–1309. [DOI] [PubMed] [Google Scholar]
- 12. London CA, Malpas PB, Wood‐Follis SL, et al. Multi‐center, placebo‐controlled, doubleblind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res 2009;15:3856–3865. [DOI] [PubMed] [Google Scholar]
- 13. Grant J, North S, Lanore D. Clinical response of masitinib mesylate in the treatment of canine macroscopic mast cell tumors. J Small Anim Pract 2016;57:283–290. [DOI] [PubMed] [Google Scholar]
- 14. Joffre C, Barrow R, Menard L, et al. A direct role for met endocytosis in tumorigenesis. Nat Cell Biol 2011;13:827–837. [DOI] [PubMed] [Google Scholar]
- 15. Rassnick KM, Moore AS, Williams LE, et al. Treatment of canine mast cell tumors with CCNU (lomustine). J Vet Intern Med 1999;13:601–605. [DOI] [PubMed] [Google Scholar]
- 16. Vail DM, von Euler H, Rusk AW, et al. A randomized trial investigating the efficacy and safety of water soluble micellar paclitaxel (Paccal Vet) for treatment of nonresectable grade 2 or 3 mast cell tumors in dogs. J Vet Intern Med 2012;26:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thamm DH, Mauldin EA, Vail DM. Prednisone and vinblastine chemotherapy for canine mast cell tumor – 41 cases (1992–1997). J Vet Intern Med 1999;13:491–497. [DOI] [PubMed] [Google Scholar]
- 18. Camps‐Palau MA, Leibman NF, Elmslie R, et al. Treatment of canine mast cell tumours with vinblastine, cyclophosphamide and prednisone: 35 cases (1997–2004). Vet Comp Oncol 2007;5:156–167. [DOI] [PubMed] [Google Scholar]
- 19. Cooper M, Tsai X, Bennett P. Combination CCNU and vinblastine chemotherapy for canine mast cell tumours: 57 cases. Vet Comp Oncol 2009;7:196–206. [DOI] [PubMed] [Google Scholar]
- 20. Vale D. Veterinary cooperative oncology group ‐ common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol 2004;2:195–213. [DOI] [PubMed] [Google Scholar]
- 21. Kiupel M, Webster JD, Kaneene JB, et al. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol 2004;41:371–377. [DOI] [PubMed] [Google Scholar]
- 22. Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol 1984;21:469–474. [DOI] [PubMed] [Google Scholar]
- 23. Letard S, Yang Y, Hanssens K, et al. Gain‐of‐function mutations in the extracellular domain of KIT are common in canine mast cell tumors. Mol Cancer Res 2008;6:1137–1145. [DOI] [PubMed] [Google Scholar]
- 24. Eickhoff JC, Kim K, Beach J, et al. A Bayesian adaptive design with biomarkers for targeted therapies. Clin Trials 2010;7:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol 2015;13:176–183. [DOI] [PubMed] [Google Scholar]
- 26. Carlsten KS, London CA, Haney S, et al. Multicenter prospective trial of hypofractionated radiation treatment, toceranib, and prednisone for measurable canine mast cell tumors. J Vet Intern Med 2012;26:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Categories of concomitant medications administered to dogs with mast cell tumors receiving vinblastine compared to toceranib.
Table S2. Characteristics of disease progression in dogs being treated with toceranib or vinblastine.


