Abstract
Hepatic fibrosis is commonly diagnosed in dogs, often as a sequela to chronic hepatitis (CH). The development of fibrosis is a crucial event in the progression of hepatic disease that is of prognostic value. The pathophysiology of hepatic fibrosis in human patients and rodent models has been studied extensively. Although less is known about this process in dogs, evidence suggests that fibrogenic mechanisms are similar between species and that activation of hepatic stellate cells is a key step. Diagnosis and staging of hepatic fibrosis in dogs requires histopathological examination of a liver biopsy specimen. However, performing a liver biopsy is invasive and assessment of fibrotic stage is complicated by the absence of a universally accepted staging scheme in veterinary medicine. Serum biomarkers that can discriminate among different fibrosis stages are used in human patients, but such markers must be more completely evaluated in dogs before clinical use. When successful treatment of its underlying cause is feasible, reversal of hepatic fibrosis has been shown to be possible in rodent models and human patients. Reversal of fibrosis has not been well documented in dogs, but successful treatment of CH is possible. In human medicine, better understanding of the pathomechanisms of hepatic fibrosis is leading to the development of novel treatment strategies. In time, these may be applied to dogs. This article comparatively reviews the pathogenesis of hepatic fibrosis, its diagnosis, and its treatment in dogs.
Keywords: Antifibrotic, Chronic hepatitis, Fibrogenesis, Hepatic stellate cell
Abbreviations
- ACE
angiotensin‐converting enzyme
- ALT
alanine transaminase
- CH
chronic hepatitis
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- ET
endothelin
- HSC(s)
hepatic stellate cell(s)
- IL‐10
interleukin‐10
- MMP
matrix metalloproteinase
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- PDGF
platelet‐derived growth factor
- RAS
renin angiotensin system
- ROS
reactive oxygen species
- TGFβ‐1
transforming growth factor beta‐1
- TIMP
tissue inhibitor of metalloproteinase
- αSMA
alpha‐smooth muscle actin
Hepatic fibrosis is characterized by progressive accumulation of fibrillary extracellular matrix (ECM) components in the liver. With persisting inflammation, the collagen profile of the liver changes, with increasing relative amounts of collagen types I and III accompanied by modification and cross‐linking of ECM components.1, 2, 3 In a fibrotic liver, the total collagen content is 3‐ to 10‐fold higher than normal.3 Development of hepatic fibrosis is an important step in the progression of many liver diseases, and it has been shown to have prognostic implications in human patients.4, 5 As is the case with human patients, hepatic fibrosis can contribute to the development of portal hypertension and acquired portosystemic collateral blood vessels in dogs.6 The true prevalence of hepatic fibrosis in dogs is not known. However, in a study of 200 dogs undergoing necropsy for any reason, 12% were found to have histological changes consistent with chronic hepatitis (CH), a disease for which hepatic fibrosis is a defining feature.7 Therefore, hepatic fibrosis is likely to be a common finding in dogs. Although the etiology of CH in dogs is different from the disease in humans, the histological appearance and progression of hepatic fibrosis is similar in both species.8, 9 Therefore, hepatic fibrosis is of importance to small animal veterinarians.
A considerable amount of research into the pathogenesis of hepatic fibrosis has been performed by studying models of induced liver disease in rodents and naturally occurring liver disease in human patients. As this process is better understood, novel strategies for its treatment are being developed for use in human patients. Hepatic fibrosis in dogs with naturally occurring CH may prove to be a valuable nonrodent model to study the efficacy of these agents.
The aims of this article are to comparatively review the pathogenesis of hepatic fibrosis, its diagnosis, as well as existing and novel strategies for its treatment in dogs. The Medline database was searched for articles relating to hepatic fibrosis. Priority was given to articles published within the last 5 years and those addressing this process in dogs. The reference lists of the articles identified in this search were used to find other pertinent articles.
Pathogenesis
Hepatic Fibrosis and Myofibroblasts
Hepatic fibrosis is a wound healing response to chronic injury and inflammation in which there is an imbalance between ECM deposition and removal, leading to excess ECM accumulation.10 In the normal liver, fibril‐forming collagens (type I, type III, type V, and type XI collagens) can be found in the capsule, in large vessels, and the portal regions.3 Only small amounts of type I and type III collagens are present in the subendothelial space. Additional components of the normal ECM include glycosaminoglycans and proteoglycans (hyaluronan, fibronectin, tenascin, or laminin) and other collagens (types VI, XIV, and XVIII).3 In human patients and rodent models of liver disease, early deposition of ECM components takes place along the subendothelial space.2, 3 In humans, von Willebrand's factor expression is used as a marker of this process and expression of von Willebrand's factor, varying in distribution from diffuse to periportal, also was found in 69% of dogs with chronic liver disease.11 The main mechanism of fibrogenesis is believed to be the activation of myofibroblast precursor cells, which results in the progressive deposition of ECM. The fibrotic liver contains increased amounts of fibrillary collagens (types I, III, and V), nonfibrillary collagens (types IV and VI), and glycosaminoglycans and proteoglycans (eg, fibronectin, tenascin and laminin, perlecan, decorin, aggrecan, and fibromodulin).1, 3 During ECM accumulation, cross‐linking of matrix proteins occurs. In advanced fibrosis, this feature has been proposed to render the ECM more resistant to degradation.12, 13
Several cells have been reported to be sources of ECM production during hepatic fibrosis: hepatic stellate cells (HSCs, Ito cells), liver resident fibroblasts (portal or centrilobular), epithelial cells that undergo epithelial‐to‐mesenchymal transition, bone marrow‐derived fibrocytes, and smooth muscle cells that surround blood vessels.1, 14 In human patients, HSCs generally are believed to be the main source for myofibroblasts in CH, whereas portal fibroblasts are considered to play an important fibrogenic role in cholestatic liver disease.15, 16 Evidence exists that perisinusoidal HSCs also are involved in the pathogenesis of hepatic fibrosis in dogs.17
The transdifferentiation of quiescent HSCs to myofibroblasts is a multi‐step process that involves cytokines, chemokines, growth factors, reactive oxygen species (ROS), and apoptotic bodies derived from hepatocytes (Fig 1).3, 18, 19, 20 In the early phase of activation, the HSC acquires responsiveness to cytokine stimuli by exposure to fibronectin or apoptotic bodies derived from damaged hepatocytes.18 In the next phase, cytokines and growth factors, produced by neighboring cells such as liver resident macrophages (Kupffer cells), hepatocytes, endothelial cells, lymphocytes, and platelets, bind to specific receptors on the HSC membrane. Stimulation of intracellular signaling pathways results in altered gene expression and a phenotypic change in the HSC.3 The last phase is the maintenance of activation, which involves paracrine and autocrine mechanisms.18
Figure 1.
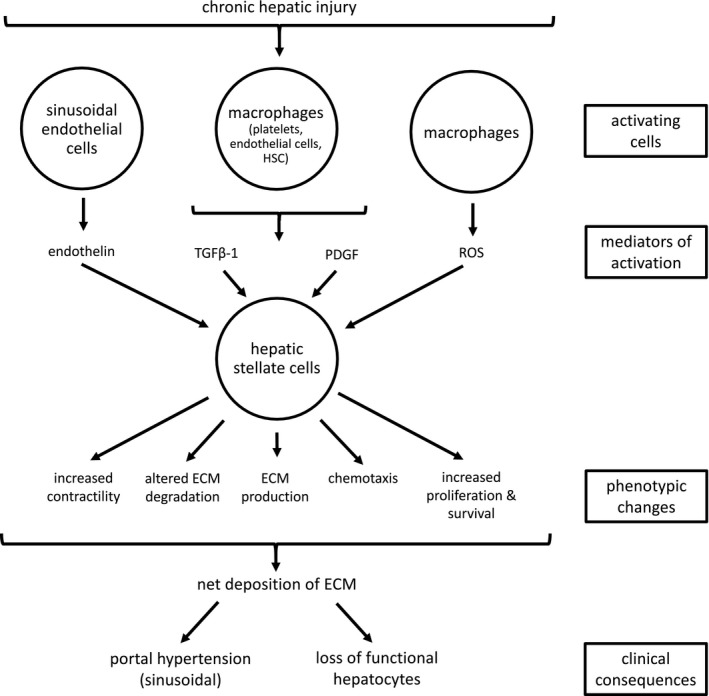
Role of hepatic stellate cells in hepatic fibrosis. The figure shows a simplified representation of the main factors involved in the activation of a hepatic stellate cell and the phenotypic changes after activation. TGFβ‐1, transforming growth factor beta 1; PDGF, platelet‐derived growth factor; ROS, reactive oxygen species; ECM, extracellular matrix.
Myofibroblast Precursor Cells
Hepatic stellate cells (Fig 2) have a dendritic morphology and are located in the perisinusoidal space in close contact with hepatocytes and sinusoidal endothelial cells. They are the main location for vitamin A storage in the healthy liver. Upon activation, HSCs change from their quiescent vitamin A‐rich state to a highly fibrogenic (myofibroblastic) phenotype. This is characterized by diminution of vitamin A droplets, enlargement of the rough endoplasmic reticulum, a ruffled nuclear membrane, and the appearance of contractile filaments.1, 3, 18, 21 They acquire increased ability for proliferation, chemotaxis, contractility, and ECM production. Activated HSCs can further promote their myofibroblastic phenotype and survival by paracrine and autocrine cytokine cross talk with surrounding cells (eg, the secretion of monocyte chemoattractant protein‐1 and chemokine [C‐C motif] ligand 5 or the release of tissue inhibitor of metalloproteinase‐1 [TIMP‐1]).18
Figure 2.
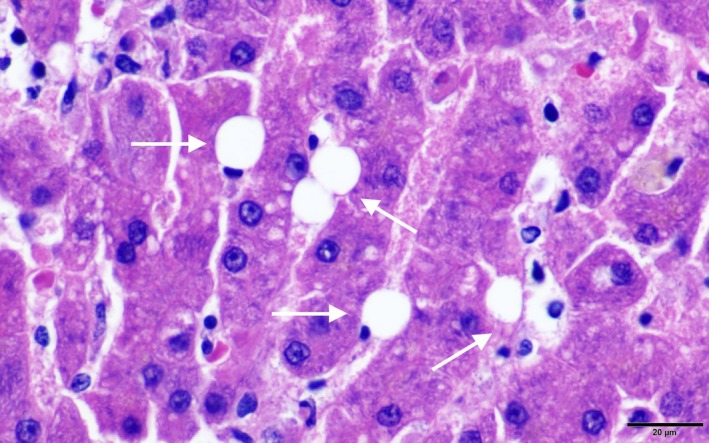
Hepatic stellate cells (Ito cell; hematoxylin and eosin). The stellate cells reside in the sinusoidal space and contain clear nonstaining vacuoles (arrows). Courtesy of Randi Gold (Texas A&M University).
The increased contractility of activated HSCs is due to the expression of the cytoskeletal protein and alpha‐smooth muscle actin (αSMA).3 Regulators of HSC contractility include endothelin‐1 (ET‐1), nitric oxide, and angiotensin II.3, 22 In human patients and rodent models of liver disease, the expression of αSMA is used as a marker of HSC activation. However, in the healthy canine liver, αSMA expression also can be found in perisinusoidal HSCs as well as in myofibroblasts and vascular smooth muscle cells within the portal tracts. In dogs with chronic liver disease, an increase in αSMA expression, in perisinusoidal spaces as well as in fibrotic septa, has been demonstrated. A positive correlation between αSMA expression and fibrosis stage was found in some studies, but not in others.11, 17, 23, 24 Figure 3 shows αSMA immunostaining in liver sections of a healthy dog without fibrosis (A) and a dog with CH and very marked fibrosis (B).
Figure 3.
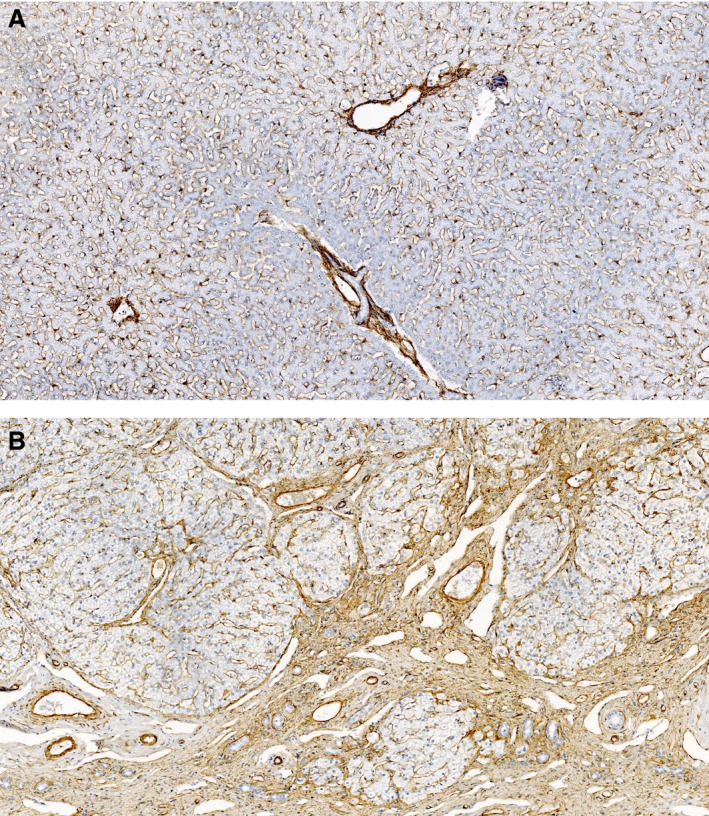
Alpha‐smooth muscle actin expression in the canine liver. A: healthy dog (absent fibrosis) with mild staining around the portal tracts and perisinusoidal spaces (hepatic stellate cells). B: dog with chronic hepatitis (very marked fibrosis) with increased staining around the portal tracts, fibrotic septa, and perisinusoidal spaces.
Portal fibroblasts are located in the mesenchyme of the portal tracts. They surround the hepatic bile ducts and are important for the integrity of the portal triads.25 In biliary fibrosis of humans, portal fibroblasts seem to be the source of myofibroblasts in the portal area.26 In studies of rodents with bile duct ligated‐induced fibrosis, they contribute to >70% of matrix deposition during early injury.16 However, their contribution during more advanced disease is still controversial, and newer studies suggest that HSCs are the major collagen producing cells in both biliary and nonbiliary fibrosis.27 Nevertheless, portal fibroblasts seem to have a role in vascular remodeling during advanced fibrosis and cirrhosis.28, 29
Epithelial‐to‐mesenchymal transition is a process whereby epithelial cells acquire mesenchymal features.30 Epithelial‐to‐mesenchymal transition can occur through hedgehog or transforming growth factor beta‐1 (TGFβ‐1) signaling pathways, and both cholangiocytes and hepatocytes can undergo this process.31, 32, 33, 34 However, newer studies show that there is no evidence of cholangiocyte or hepatocyte epithelial‐to‐mesenchymal transition in mouse models of hepatic fibrosis.35, 36 Therefore, epithelial‐to‐mesenchymal transition is not thought to play a major role in the pathogenesis of hepatic fibrosis.
Table 1 gives a summary of important liver cell types and their roles in hepatic fibrosis.
Table 1.
Hepatic cell types and their roles in fibrosis
| Cell Type | Role in Fibrosis |
|---|---|
| Hepatic stellate cells3, 18 |
|
| Portal fibroblasts26, 193 |
|
| Bone marrow‐derived mesenchymal cells194, 195, 196 |
|
| Hepatocytes18, 197, 198 |
|
| Cholangiocytes18, 199 |
|
| Macrophages18, 200, 201 |
|
| Sinusoidal endothelial cells18, 202 |
|
| Natural killer cells18, 203 |
|
HSC, hepatic stellate cell; ECM, extracellular matrix; TIMP‐1, tissue inhibitor of metalloproteinase 1; TGFβ‐1, transforming growth factor beta 1; TNF, tumor necrosis factor.
Mediators of Myofibroblast Precursor Cell Activation
Platelet‐derived Growth Factor
Early ECM changes (eg, production of fibronectin by endothelial cells) and apoptotic bodies from damaged hepatocytes are initiators of HSC activation.3, 18 Hepatic stellate cells acquire responsiveness to further paracrine activation by neighboring cell types by the expression of certain cell surface receptors.3, 18 Platelet‐derived growth factor (PDGF) is the most potent factor that induces proliferation of HSCs (Fig 1).3, 37, 38 It is released by platelets, but also by sinusoidal endothelial cells, activated liver resident macrophages, and myofibroblasts during ongoing disease (Table 1).1, 3, 39 Downstream signaling involves the renin angiotensin system (RAS)/extracellular signal‐regulated kinase and phosphoinositol 3‐kinase pathways, which enhance proliferation and migration and promote survival of the HSC.39, 40 Additionally, PDGF is a chemoattractant and guides HSCs to the site of injury.3, 41 Increased expression of PDGF mRNA has been demonstrated in liver from dogs with CH.42
Transforming Growth Factor Beta‐1
Transforming growth factor beta‐1 is considered the major factor accelerating hepatic fibrosis.3, 18, 43 Hepatocytes, liver resident macrophages, sinusoidal endothelial cells, platelets, and activated HSCs produce this cytokine (Table 1).1 Downstream signaling involves phosphorylation and thus activation of the Smad2 and Smad3 proteins.43 After forming complexes with Smad4 proteins, they are translocated into the nucleus where they interact directly on Smad‐binding elements and alter gene expression, for example, by causing upregulation of collagen types I and III, and TIMP‐1, and downregulation of MMPs.14, 39, 43 The result is increased capability of HSCs to produce ECM components and inhibition of ECM removal (Fig 1). TGFβ‐1 also seems to be an important mediator of lysyl oxidase expression. Lysyl oxidases are copper‐dependent amine oxidases that are important for cross‐linking of ECM proteins and further activation of myofibroblast precursor cells.44 TGFβ‐1 and phosphorylated Smad2/3 expression were shown to be upregulated in the liver of dogs with CH, lobular dissecting hepatitis, and cirrhosis.45 Serum concentrations of TGFβ‐1 were increased in dogs with moderate‐to‐severe hepatic fibrosis.46 Increased TIMP‐1 mRNA expression also has been demonstrated in liver from dogs with CH.42, 47
Connective Tissue Growth Factor
Connective tissue growth factor (CTGF) is another fibrogenic signal for HSCs. In addition, CTGF is involved in promoting the adhesion of HSCs to the ECM.3, 48 Expression of CTGF is increased in the fibrotic human liver and in animal models of hepatic fibrosis.49 CTGF production is considered to be TGFβ‐1/Smad2/3‐dependent, but other induction ways have been reported (eg, ET‐1, angiotensin II).50, 51, 52 Because CTGF is recognized as a profibrogenic mediator, its inhibition is a potential option for new antifibrotic therapies.51, 53 Additionally, CTGF has been evaluated as a noninvasive biomarker in human patients with CH.49, 54 Patients with advanced disease showed higher serum concentrations of CTGF, and these were linked to stage of fibrosis.49, 54 CTGF mRNA expression was shown to be upregulated in dogs with CH.1
Endothelin‐1
Endothelin‐1 is a vasoactive peptide produced by endothelial cells and by activated HSCs in cirrhotic livers of humans.18, 55 ET‐1 acts through 2 receptors: ET‐1 receptor type A and ET‐1 receptor type B, which can be found on quiescent and activated HSCs.55 This promoted proliferation, contraction, and the maintenance of the activated state.18, 56 In a recent study of 20 CH dogs, hepatic mRNA expression and plasma concentration of ET‐1 were shown to be increased in dogs with CH, and a weak correlation between plasma concentration of ET‐1 and splenic pulp pressure has been demonstrated, suggesting a possible role in the development of portal hypertension.57
Reactive Oxygen Species
Reactive oxygen species such as superoxide anion, hydrogen peroxide, or hydroxyl radicals are generated by the “respiratory burst” of phagocytic cells of the innate immune system as a first defense mechanism against invading pathogens.58, 59 Excessive production of ROS, however, leads to necrosis of surrounding cells and inflammation. The healthy liver contains several enzymatic and nonenzymatic antioxidant systems to detoxify excessive ROS. During liver disease, these antioxidant systems can become depleted, intensifying inflammation. ROS formation by phagocytic cells (eg, liver resident macrophages) is mainly the result of the enzyme nicotinamide adenine dinucleotide phosphate oxidase (NOX) 2. Besides NOX2, 6 other members of the NOX family have been identified so far: NOX1, NOX3, NOX4, NOX5, dual oxidase 1, and dual oxidase 2. Some of these have been shown to play a role in liver fibrosis: HSCs and hepatocytes express NOX2, NOX1, NOX4, dual oxidase 1, and dual oxidase 2, which may play a role in the maintenance of the activated HSC state.15, 34, 59, 60 In human patients with cirrhosis, NOX1 and NOX4 proteins are increased.61 Moreover, NOX4 expression was correlated with stage of fibrosis.34, 62 Knocking down NOX4 in mice has been shown to attenuate HSC activation and reverse the myofibrotic phenotype.34, 61 Although oxidative stress plays an important role in a variety of hepatobiliary diseases of dogs,63 its role in fibrogenesis has not yet been studied.
Renin Angiotensin System
The local RAS may play a role in the pathogenesis of hepatic fibrosis.64 A simplified view of the RAS is to describe it in 2 axes: the angiotensin‐converting enzyme (ACE)/angiotensin II/angiotensin II receptor type I axis (pathway A), and the ACE/angiotensin (1‐7)/angiotensin (1‐7) receptor axis (pathway B).65 The first step in both axes is the cleavage of angiotensin I to angiotensin II by ACE. In pathway A, angiotensin II binds to angiotensin II receptor type I, activating profibrotic mechanisms (eg, the induction of TGFβ‐1).66 In pathway B, a second enzyme (ACE2) cleaves angiotensin II, which results in the formation of angiotensin (1‐7). The G protein‐coupled Mas receptor has been recognized as the main receptor for angiotensin (1‐7). Binding of angiotensin (1‐7) to Mas activates a counter‐regulatory pathway with antifibrotic, anti‐inflammatory, and vasodilatory effects.65 Cultured activated human HSCs have been shown to express RAS components and synthesize angiotensin II,67 which has been proposed to be a trigger for the profibrogenic mediator CTGF.52 In rodent models, treatment with angiotensin (1‐7), and thus enhancement of the antifibrotic RAS pathway, has been shown to decrease hepatic fibrosis.68, 69 Furthermore, administration of ACE inhibitors or angiotensin receptor blockers, which leads to inhibition of the profibrotic RAS pathway, has been shown to attenuate hepatic fibrosis in animal models.64, 70, 71 A RNA sequencing study did not identify upregulation of the RAS in dogs with CH compared to healthy dogs, but further studies are needed to evaluate whether it has a role or not.1
Reversibility of Hepatic Fibrosis
In the past, hepatic fibrosis was thought to be an irreversible process. However, recent studies in humans and rodent models have shown that resolution of fibrosis, even in more advanced disease, is possible.3, 10, 12 In contrast, dense cirrhosis with intense ECM cross‐linking, nodule formation, and low cell density in “fibrotic scars” still is considered irreversible.3, 12 Several studies have shown that fibrosis regression takes place after specific treatment and removal of the causal agent in human patients.72 Because fibrosis now is recognized to be a continuous remodeling process, in which either net collagen deposition or resolution takes place,73 inhibiting mediators of collagen deposition or enhancing mediators of ECM degradation may result in regression of fibrosis. The balance between MMPs and TIMPs seems to play an important role in this regulation.10 Tissue inhibitor of matrix metalloproteinase‐1 overexpression has been shown to accelerate fibrosis by inhibition of metalloproteinases, but also by inhibition of HSC apoptosis. Tissue inhibitor of matrix metalloproteinase‐1 activity decreases quickly during fibrosis resolution.74, 75, 76, 77, 78 Monocyte‐derived macrophages with a proresolution phenotype seem to be important in the reversal of fibrosis, because they produce MMPs, which degrade the ECM or mediate apoptosis of myofibroblasts.10, 79, 80 Additionally, natural killer cells can induce apoptosis of HSCs and thus contribute to the inhibition of fibrosis (Table 1). The main mechanism for the resolution of fibrosis seems to be the apoptosis or senescence of activated myofibroblasts (HSCs), which removes the source of TIMP‐1, resulting in increased matrix metalloproteinase activity and the degradation of ECM.
Causes of Hepatic Fibrosis in Dogs
In our experience, the most common cause of hepatic fibrosis in dogs is CH, which is histologically characterized by hepatocyte apoptosis or necrosis, inflammation, a mononuclear cell infiltrate, and fibrosis (Fig 4).81 The fibrosis often co‐localizes with necrosis and, especially for idiopathic CH, is initially present in the periportal zones of the liver.81 With more advanced fibrosis, portal‐portal or portal‐central bridging fibrosis may develop with eventual formation of discrete nodules (Fig 4).82 In a retrospective study, copper accumulation was the underlying cause in 36% of dogs with CH.83 When copper accumulation is the primary cause of liver disease, it usually initially accumulates in the centrilobular zones. Centrilobular to bridging fibrosis was reported in Labrador retrievers with copper‐associated CH.84 In >60% of dogs with CH, no underlying cause can be found, and these patients are referred to as having idiopathic CH. Copper‐associated85 and idiopathic CH86 are reviewed elsewhere. Granulomatous hepatitis is an uncommon form of CH in dogs83 and may be the result of infectious diseases such as schistosomiasis,87 histoplasmosis,88 Angiostrongylus vasorum infection,89 leishmaniasis,90 or with lymphoma and histiocytosis.88 Regardless of the underlying cause of CH, chronic inflammation leads to fibrosis.
Figure 4.
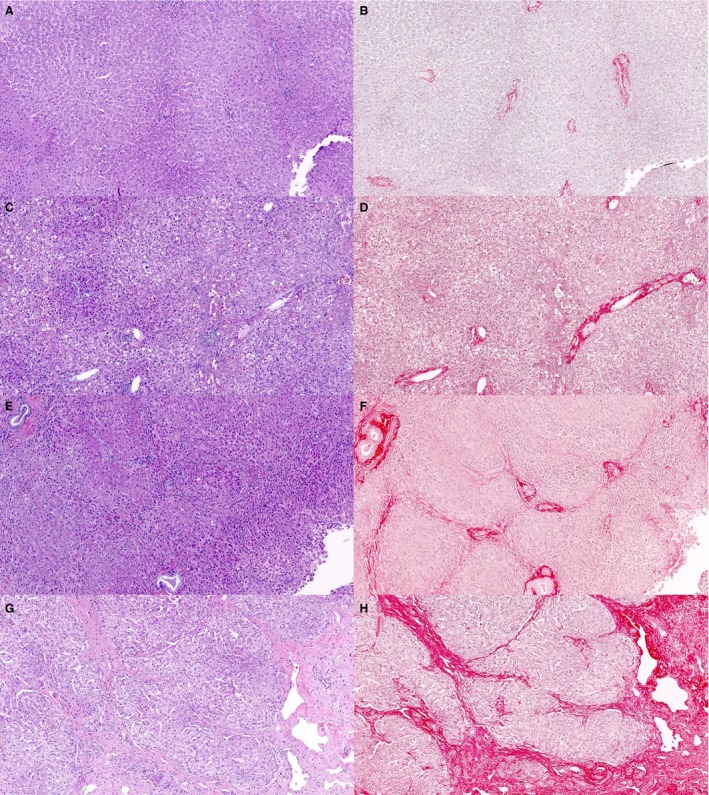
Hepatic fibrosis in dogs (hematoxylin and eosin: A, C, E, G and picrosirius red: B, D, F, H). Liver sections from dogs with various stages of fibrosis. Note the collagen fibers are more distinct when serial sections are stained with picrosirius red. A, B: absent/minimal fibrosis; C, D: moderate fibrosis with fibrous expansion of the portal tracts; E, F: marked fibrosis with portal‐portal bridging; G, H: very marked fibrosis with discrete nodule formation.
Lobular dissecting hepatitis is a distinct type of CH that typically (but not always) occurs in young dogs at an average age of 2 years. It is not clear whether lobular dissecting hepatitis is a pattern of liver injury or a distinct disease process. It has been reported in a number of breeds, including the Standard Poodle, Rottweiler, German Shepherd, Golden Retriever, and American Cocker Spaniel. This disease has a rapid clinical course and a poor prognosis with a short survival time.91 Lobular dissecting hepatitis is histologically characterized by a diffuse infiltrate of inflammatory cells and dissection of the lobular parenchyma with reticulin fibers (type III collagen) surrounding single or small groups of hepatocytes.92 The cause of lobular dissecting hepatitis is not known. However, the abnormal ECM is mainly composed of laminin and fibronectin.93
Extrahepatic bile duct obstruction can result in fibrosis around biliary ducts, presumably because of proliferation of portal myofibroblasts. Causes for extrahepatic bile duct obstruction in dogs include pancreatic or biliary tumors, inflammation, or cholelithiasis.94
Cholangitis is less well described in dogs than in cats (but may be underdiagnosed)95 and with chronicity can lead to biliary fibrosis. Biliary fibrosis can progress to portal‐portal bridging fibrosis and biliary cirrhosis (when there is concurrent nodular regeneration).8 Destructive cholangitis, characterized by loss of bile ducts with accompanying inflammation, also can lead to biliary fibrosis.8, 96 Idiosyncratic drug reactions have been implicated in causing this uncommon disease.97
Right‐sided heart failure or obstruction of the cranial vena cava leads to increased central venous pressure and passive venous hepatic congestion. Liver perfusion is impaired, and ischemia and necrosis occur.98 Chronically, this can lead to centrilobular fibrosis. A similar pattern can develop after toxin ingestion.8
Ductal plate abnormalities are a diverse group of developmental disorders of the biliary system that can be associated with increased hepatic ECM, portal hypertension, abdominal effusion, and hepatic encephalopathy. The most severe form is called congenital hepatic fibrosis and is characterized by portal‐portal bridging fibrosis, multiple small bile ducts, and discontinuous biliary profiles. Ductal plate abnormalities, including congenital hepatic fibrosis, were reported in a series of 30 boxer dogs.99 Six cases of congenital hepatic fibrosis (in a mixed breed dog and several other breeds) were reported in a separate series.100, 101 These conditions may be misdiagnosed as CH with secondary fibrosis.
Consequences of Hepatic Fibrosis
In humans, hepatic fibrosis is an important event in the progression of liver disease that can proceed to cirrhosis. Although there is no consistently used definition of cirrhosis in small animal medicine, it is generally considered the end stage of liver disease, where the deposited and remodeled ECM is connecting (bridging) and disrupting the functional architecture of the liver.8, 13 In CH, hepatocyte swelling, increased HSC contractility, fibrosis, and the formation of regenerative nodules impede portal blood flow, leading to hepatic (sinusoidal) portal hypertension.102
Portal hypertension in dogs is defined as a portal vein pressure >10 mmHg (normal values in anesthetized dog are 6–9 mmHg)103, 104 and is reviewed extensively elsewhere.104 Direct measurement of portal vein pressure is an invasive technique, which requires direct puncture of the portal vein, and therefore is rarely performed in dogs. An indirect method that has been performed in veterinary patients is catheterization of the splenic pulp. However, values obtained from this measurement seem to be 0.5–1.5 mmHg higher than for the direct measurement of the portal vein pressure.103, 104 In cirrhotic human patients, a hepatic venous pressure gradient >5 mmHg is defined as portal hypertension and a value above 10 mmHg is correlated with development of clinical consequences, including life‐threatening gastroesophageal varices.104, 105, 106, 107 In dogs, gastroesophageal varices have been described, but their clinical importance is unclear.104, 108 In addition, portal hypertension can contribute to the development of ascites and can lead to the opening of vestigial blood vessels that bypass the portal circulation (acquired portosystemic collaterals).104 Ascites is the consequence of a combination of splanchnic arterial vasodilation, decreased cardiac output, and activation of the RAS, which leads to sodium and water retention.105 In addition, high sinusoidal pressure drives fluid into the interstitial space.104 Ascites has been shown to be a negative prognostic indicator in dogs with CH.83, 109 Portosystemic shunting often results in hepatic encephalopathy, where abnormal ammonia metabolism acts synergistically with a variety of other factors, such as neurosteroids and inflammatory mediators, to cause astrocyte swelling and neurological dysfunction.110 Type C hepatic encephalopathy (as a complication of CH) was the second most common category of hepatic encephalopathy in dogs. Portal hypertensive gastropathy is common in humans and is characterized by mucosal and submucosal vascular ectasia without inflammation.104, 105 To our knowledge, the histological characteristics of the gastrointestinal tract of dogs with portal hypertension have not been well described. However, gastroduodenal ulceration has been reported to be a complication of various hepatic diseases in dogs.111, 112 Hypergastrinemia does not appear to be common in dogs with CH,113 and thus, mechanisms other than gastric hyperacidity are likely to be important. Hepatorenal syndrome and spontaneous bacterial peritonitis are other complications of portal hypertension in humans.105 Hepatorenal syndrome has not been reported to occur secondary to spontaneous liver disease in dogs, and although dogs can develop spontaneous bacterial peritonitis,114 an association with portal hypertension has not been found.104 Treatment of portal hypertension in dogs is focused on managing its complications, for example, diuretic therapy and fluid therapy for ascites, administration of lactulose and antimicrobials PO for hepatic encephalopathy, and a sodium‐restricted diet to decrease water retention. However, the optimal treatment would be to remove the underlying cause by resolving or decreasing hepatic fibrosis.
Progressive replacement of hepatocytes with fibrous tissue is another consequence of chronic hepatic disease, which can result in hepatic synthetic failure. If this develops, coagulopathies may occur.115 Dogs with liver disease traditionally were thought to be hypocoagulable because they can have prolonged clotting times (prothrombin and activated partial thromboplastin times), hypofibrinogenemia, and mild‐to‐moderate thrombocytopenia.83, 116, 117 Despite this, spontaneous bleeding is rare.118 In a recent study, dogs with CH were found to have variable thromboelastography results.118 In this study of 21 dogs, 5 were hypocoagulable, 9 were normocoagulable, and 7 were hypercoagulable. In a retrospective study of portal vein thrombosis in dogs, hepatic disease was a common concurrent condition, suggesting that hypercoagulability may have clinical consequences in these patients.119 Interestingly, in humans with CH, thrombin stimulates fibrosis by protease‐activated receptor signaling and by leading to microthrombi formation with subsequent local hypoxia.120, 121, 122
In humans with CH, advanced stages of hepatic fibrosis are associated with decreased survival times.4, 5 The prognostic implications of various stages of hepatic fibrosis (assigned by a histological scoring scheme) have not been well characterized in dogs with CH. However, those with ascites83, 109 or cirrhosis123 have decreased survival times. In human patients and rodent models, even when hepatic fibrosis is advanced, it potentially can resolve if the underlying cause is successfully treated.12
Diagnosis of Hepatic Fibrosis
Histopathology
Although the presence of increased numbers of spindle cells and mast cells on cytological evaluation of the liver was reported to diagnose hepatic fibrosis with reasonable accuracy,124 histopathologic examination of liver biopsy specimens is required for definitive diagnosis in dogs. However, liver biopsy is expensive and associated with a risk of hemorrhage and other complications (eg, postbiopsy pain, peritonitis, shock, or complications related to general anesthesia).125, 126 In small animal medicine, the following liver biopsy techniques are used: ultrasound‐guided percutaneous needle biopsy, laparoscopic biopsy, and surgical biopsy during laparotomy. No matter which technique is used, only a small portion of the organ is sampled. Because many lesions (including fibrosis) are heterogeneously distributed throughout the hepatic parenchyma, liver biopsy is susceptible to sampling error.127, 128 Substantial variation can occur in the distribution of lesions among liver lobes, and therefore, it is important to collect samples from several lobes.128 In dogs undergoing necropsy, histological diagnoses were in agreement with those from wedge samples in 66% of needle samples, 60% of cup samples, and 69% of punch samples, but these proportions were not significantly different from each other. The authors of this study concluded that the histopathologic interpretation of a liver biopsy specimen in the dog is unlikely to vary whether it contains at least 3–12 portal triads.129 However, it is recommended that pathologists be presented with specimens containing at least 11 portal triads.130, 131 Evaluation of samples with fewer portal triads results in underestimation of fibrosis stage in human patients.127, 132
In human patients, histological scoring schemes are widely used to provide a more objective assessment in patients with CH. They assess hepatic necrosis and inflammation (grade), which gives an indication of disease activity, and fibrosis (stage), which indicates the chronicity of the disease.133 These schemes include the Ishak scheme9 and the simpler METAVIR scheme.134 In general, schemes with fewer levels are more clinically applicable because there usually is better interobserver agreement when using them.133 Several studies have used a scoring scheme adapted from the Ishak scheme82 to stage hepatic fibrosis in dogs with CH.135, 136 When 6 board‐certified veterinary pathologists used this scheme to stage hepatic fibrosis from picrosirius red‐stained sections in 50 dogs, their agreement was interpreted as only being fair.137 However, it is our hope that the scheme can be refined to improve interobserver agreement.
Although fibrosis may be apparent on hematoxylin and eosin (H&E)‐stained sections (Fig 4), other histological stains differentially stain collagen fibers and allow subjectively more accurate assessment of fibrosis. These include Masson's trichrome that stains type I collagen fibers, picrosirius red (Fig 4) that stains type I and III collagen fibers, and reticulin that stains reticulin fibers (type III collagen).126 Interestingly, there was no difference in fibrosis scores assigned to serial sections of liver stained with H&E and picrosirius red.137
Computerized image analysis has been used to provide quantification of hepatic fibrosis in humans.138, 139 The histological section then is digitized, and image analysis software is used to calculate the fibrotic proportion of the section. This technique may allow a more objective quantification of hepatic fibrosis. In dogs, there was a positive correlation between the median fibrosis score assigned to each section and the fibrotic proportion.140 This technique does not detect key features in the progression of fibrosis, such as the development of bridging fibrosis. Therefore, it should not be considered to be a direct replacement for the histologic assessment of fibrosis.138
Another innovative approach is to perform gene expression analysis on hepatic fine needle aspirates. Investigators showed upregulation of collagen and other fibrosis‐related genes in livers of dogs with CH. The upregulation in gene expression for PDGF, TGFβ‐1, TIMP‐1, MMP2, and collagen type I and III, for example, showed a significant positive correlation with the severity of fibrosis.42
Serum Biomarkers
Because of the disadvantages of liver biopsy described above, serum markers of hepatic fibrosis have been developed for use in humans. In general, biomarkers of hepatic fibrosis can be divided into direct and indirect markers.141 Direct markers are proteins and other molecules involved in the pathogenesis pathways of fibrosis (eg, TGFβ‐1, tumor necrosis factor‐α, angiotensin II) or those involved in the degradation or remodeling of the ECM (eg, hyaluronic acid, procollagen peptides, MMPs, TIMPs, chitinase‐3‐like protein 1). Using such markers, the diagnosis of advanced fibrosis stages is possible.141, 142 Hyaluronic acid appears to be the most promising,141 and in a meta‐analysis of hepatitis C patients, the sensitivity and specificity for diagnosing cirrhosis were 82 and 89%, respectively.143 In human patients, these direct markers of fibrosis are not specific for hepatic fibrosis and may be increased when fibrosis of other organs is present.144 Some of these serum markers have been evaluated in dogs. Serum hyaluronic acid concentration is increased in dogs with hepatic disease, especially cirrhosis, and therefore holds some promise as a biomarker.145, 146 Serum concentrations of TGFβ‐1,46 the 7S fragment of type IV collagen,147 and procollagen type III N‐terminal peptide148 also have been found to be increased in dogs with hepatic fibrosis. Another study did not find a positive correlation between hepatic fibrosis and serum concentrations of hyaluronic acid, procollagen type III N‐terminal peptide, or TIMP‐1.149 Even in the studies that did detect a difference between groups of dogs, concentrations from the advanced liver fibrosis groups either overlapped with those from dogs with milder fibrosis or there was only a separation of concentrations for dogs with the most advanced stage of hepatic fibrosis.
Indirect markers are measurement of variables that indicate liver damage, liver function impairment, and portal hypertension, such as liver enzyme activities, albumin and bilirubin concentration, and platelet counts or a combination of these. Two commonly used combinations in human medicine are the aspartate transaminase‐to‐platelet ratio index and FibroTest2 (FibroSURE3 in the United States). The latter combines age, sex, and results for serum haptoglobin, alpha 2‐macroglobulin, apolipoprotein A1, gamma‐glutamyltransferase, and bilirubin analyses into a single index.150, 151 Recently, an index for the assessment of hepatic fibrosis was developed for use in dogs.4 This combines patient age, sex, and several biochemical variables in a proprietary algorithm to create a fibrosis score. In 1 study, this index had a negative predictive value for the diagnosis of moderate fibrosis of 90–100% and distinguished dogs with clinically relevant fibrosis with a positive predictive value of 90–100%.5
MicroRNAs are small noncoding RNAs that have a distinct expression profile depending on the liver disease.152, 153 Liver concentrations of hepatocyte‐derived microRNAs seem to correlate with serum concentrations.153, 154 In human patients with hepatic fibrosis, the expression of miR‐29 and miR‐652 is decreased, whereas the expression of miR‐571 is increased.155, 156 A recent study evaluated whether serum miRNA biomarkers hold promise for distinguishing among several hepatobiliary diseases in dogs. Two miRNAs were found to be increased in hepatobiliary disease: miR‐200c in the hepatocellular carcinoma group (6 dogs) and miR‐126 in the CH group (6 dogs).157 Measurement of microRNAs in serum potentially could be used to assess hepatic fibrosis in dogs. However, further studies with greater sample sizes are needed to evaluate the sensitivity and specificity of these markers.
Elastography
Elastography is a medical imaging method to measure soft tissue elasticity (stiffness). Liver stiffness reflects the accumulation of ECM and has been shown to correlate with fibrosis stage. Transient elastography, real‐time shear wave elastography, and acoustic radiation force impulse are new ultrasound‐based, reliable, and reproducible methods to assess liver fibrosis in humans.158, 159 These techniques are noninvasive and allow a large area of the hepatic parenchyma to be sampled, thus decreasing sampling error. For example, transient elastography has been shown to measure a volume that is 100 times larger than a typical needle biopsy specimen.160 Methods such as shear wave elastography and acoustic radiation force impulse also have been shown to be useful in patients with ascites or in obese patients.160, 161 Magnetic resonance elastography quantitatively measures acoustic shear waves in liver tissue. This method also detects early fibrosis stages with a much higher sensitivity and specificity (98 and 99%) than does transient elastography. A disadvantage of this method is higher cost compared to ultrasound‐based techniques.144 To our knowledge, the utility of elastography for the diagnosis of hepatic fibrosis in dogs has not been evaluated.
Current Treatment Options for Hepatic Fibrosis
The optimal way to stop the progression of or resolve hepatic fibrosis is to identify and treat its underlying cause. This approach is applicable in human medicine, where the underlying cause for the chronic hepatic disease usually is known.3 For example, in human patients that were treated with direct‐acting antiviral agents against hepatitis C, fibrosis resolved.162
Treatment of the Underlying Cause
In dogs with copper‐associated CH, it often is possible to address the underlying cause of hepatic fibrosis by a combination of chelation with D‐penicillamine and feeding a low copper diet. In a study of 43 Labrador retrievers, despite improved copper scores, histologic fibrosis scores were not significantly different before and after treatment with D‐penicillamine. However, the majority of dogs in this study did not have hepatic fibrosis at the time of diagnosis (median fibrosis score: 0 of 4 [absent fibrosis]), presumably because of early diagnosis.136 This decreased the likelihood of detecting a treatment effect. In our experience, hepatic fibrosis can improve after chelation with D‐penicillamine. Even if fibrosis does not resolve, chelation therapy is thought to be beneficial in these patients, although the criteria for which patients to chelate are somewhat controversial.85
Immunomodulatory Therapy
For dogs with idiopathic CH, treatment with prednisolone or another immunomodulatory drug often is initiated, especially if there is histologic evidence of active inflammation. Glucocorticoids bind to glucocorticoid receptors in the cytoplasm. These complexes are translocated to the nucleus, where they act on glucocorticoid response elements and initiate the transcription of anti‐inflammatory and immunomodulatory protein coding genes (eg, IL‐10).163 Inflammatory genes are under transcriptional control of nuclear factor‐kappa B and activator protein‐1. Glucocorticoids inhibit the effects of these transcription factors.164, 165 The response of dogs with idiopathic CH to glucocorticoids seems to be quite variable. In a retrospective study of 20 dogs with idiopathic CH that were treated with prednisolone at a dosage of 1 mg/kg PO q24h for at least 6 weeks, fibrosis resolved in 5 dogs, improved in 4 dogs, and worsened in 5 dogs, but a statistically significant difference in histological fibrosis scores before and after treatment was not found.166 However, in an older retrospective study that did not separate dogs with copper‐associated CH from those with idiopathic CH, prednisolone treatment was associated with longer median survival times.167 The effect of prednisolone on fibrosis was not evaluated in this study. In an uncontrolled study, 35 of 46 dogs (76%) with idiopathic CH achieved remission (normalization of serum ALT activity) after treatment with cyclosporine.6 The efficacy of prednisolone and other immunomodulatory medications for the treatment of idiopathic CH in dogs needs to be further evaluated, ideally with randomized controlled clinical trials.
Antioxidant Treatment
Antioxidant drugs have a cytoprotective effect by scavenging ROS or increasing tissue concentrations of antioxidant enzymes or proteins such as superoxide dismutase, catalase, glutathione peroxidase, glutathione, or metallothionein.168 Oxidative stress occurs in a variety of liver diseases and contributes to the development of hepatic fibrosis in rodent models and humans. Therefore, although there is no direct evidence that antioxidants decrease hepatic fibrosis or improve clinical outcome for most hepatobiliary diseases in dogs, there is a rationale for using them.169 Antioxidants commonly used to treat hepatobiliary disease in dogs include S‐adenosylmethionine, vitamin E, and silymarin (milk thistle extract). Silymarin also may inhibit hepatic fibrosis by decreasing HSC DNA synthesis, proliferation and migration, and decreasing hepatic collagen expression as well having anti‐inflammatory effects.170, 171, 172 Ursodeoxycholic acid is a nontoxic bile acid that has choleretic effects. It displaces hydrophobic bile acids from the circulating pool and therefore is used to treat cholestatic liver disease in dogs.173 There is evidence in other species that ursodeoxycholic acid also may have antiapoptotic properties.174 The cytoprotective and antiapoptotic effects of ursodeoxycholic acid on hepatocytes have been proposed to be a result of unspecific binding to steroid receptors and the upregulation of cellular antioxidant systems, such as glutathione and superoxide dismutase.175, 176 Because hepatocyte‐derived apoptotic bodies are a mediator of HSC activation, ursodeoxycholic acid seems to be a reasonable treatment to inhibit fibrogenesis. Ursodeoxycholic acid is used to treat primary biliary cirrhosis in humans, but resolution of fibrosis does not consistently occur and other treatments may be needed for advanced disease.177 Case reports describe the use of ursodeoxycholic acid in dogs with hepatobiliary disease, but to our knowledge, there are no studies that critically evaluate its effectiveness in this species.96, 173, 178, 179
Colchicine
Colchicine, a plant extract from Colchicum autumnale that acts as a microtubule assembly inhibitor, has been shown to decrease hepatic fibrosis in rodent models180 and also in some human patients with hepatic fibrosis.181 The suggested mechanism is the inhibition of microtubule‐associated transport of procollagen and the enhancement of collagenase activity.182, 183 However, there is insufficient evidence to support its use in humans with liver fibrosis or cirrhosis and it is commonly associated with adverse effects.182 A few case reports describe its use in dogs,184, 185 but because of the lack of proven efficacy and relatively common occurrence of adverse effects (gastrointestinal tract, central nervous system), we do not recommend its use in this species.186
Novel Treatment Strategies for Hepatic Fibrosis
There is considerable interest in developing novel treatments specifically aimed to manage hepatic fibrosis in humans. Extensive research performed to elucidate the pathogenesis of hepatic fibrosis supports the achievement of this goal. These therapeutic strategies can be divided into those that decrease myofibroblast activation, those that induce apoptosis of activated myofibroblasts, and those that induce ECM degradation and are reviewed elsewhere.21
Appealing drugs to evaluate for antifibrotic activity in dogs are those that block the RAS: ACE inhibitors (eg, enalapril, benazepril) and angiotensin receptor blockers (eg, losartan, telmisartan). Targeting the RAS with ACE inhibitors, angiotensin receptor blockers, and angiotensin (1‐7) receptor agonists has been shown to attenuate liver fibrosis in rodent models64, 187 and to downregulate fibrogenic and NADPH oxidase genes in human patients with chronic hepatitis C and fibrosis.188 However, in a cohort of human patients with hepatitis C, ACE inhibitors and angiotensin receptor blockers were not shown to have a beneficial effect.189 These drugs are used to treat proteinuria190 and generally are well tolerated in dogs. However, involvement of the RAS in hepatic fibrosis has not yet been demonstrated in dogs and so clinical trials assessing efficacy of these drugs for this purpose are premature.
Pirfenidone is an antifibrotic drug that is licensed in Europe and Japan to treat idiopathic pulmonary fibrosis in humans. It acts by inhibiting nuclear factor‐kappa B and its downstream profibrogenic mediators, including PDGF, TGFβ‐1, and interferon alpha, resulting in decreased HSC activation and ECM deposition. It has been shown to decrease hepatic fibrosis and inflammation in humans with chronic hepatitis C when given for 2 years.191 This drug has been reported to cause hepatoxicity, which may limit its use in patients with preexisting liver disease. Nevertheless, in the studies described above, adverse effects were minor. We are not aware of any reports of this drug being used in dogs with CH, although its pharmacokinetics were studied in healthy beagles.192
Conclusion
Hepatic fibrosis has been extensively studied in rodent models and in human patients. Its pathogenesis appears to be similar in dogs, but further research is needed to fully confirm or refute this supposition. Histologic assessment of a liver biopsy specimen is required for the diagnosis of hepatic fibrosis in dogs. The development and widespread institution of a practical, well‐validated, clinically relevant scheme for the histologic scoring of hepatic fibrosis (and necroinflammatory activity) in dogs with CH would be useful in both clinical and research settings and should be a priority for the veterinary community. Investigators are attempting to develop serum markers of hepatic fibrosis for use in dogs, and some of these have been shown to have some limited discriminating ability. Elastography is a useful technique for the diagnosis of hepatic fibrosis in humans and is worthy of evaluation in dogs. Even if such noninvasive tests of hepatic fibrosis are successfully developed for use in dogs, in our opinion, they are unlikely to replace biopsy, because histologic evaluation and copper quantification play a large role in the diagnosis and subcategorization of liver disease in dogs. They could, however, prove useful for monitoring response to treatment in both clinical and research settings. In small animal medicine, fully evaluating the efficacy and optimal use of existing treatments for CH, such as glucocorticoids or cyclosporine, should be a priority. A deeper understanding of the pathogenesis of hepatic fibrosis in dogs eventually may lead to the development of new medications that specifically target this process.
Acknowledgments
Grant support: None.
Parts of this manuscript will be reproduced in Dr. Eulenberg's doctoral thesis.
Conflict of Interest Declaration
The authors are affiliated with the Gastrointestinal Laboratory, Texas A&M University, which offers liver function testing and histological evaluation of liver biopsy specimens on a fee‐for‐service basis.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Where the work was done: Texas A&M University.
Footnotes
Eulenberg VM, Lawrence YA, Suchodolski JS, et al. High‐throughput RNA sequencing and differential gene expression analysis in dogs with chronic hepatitis. J Vet Intern Med;31:1307. (abstract)
FibroTest, BioPredictive, Paris, France
FibroSure, LabCorp, Burlington, NC
FibroVet, Echosens, Paris, France
Lecoindre A, Lecoindre P, Chevallier M, et al. A new combination of blood parameters for accurate non‐invasive diagnosis of liver fibrosis in dogs. J Vet Intern Med 2015;29:1197. (abstract)
Cyclosporine in the treatment of canine chronic hepatitis. Ullal T, Twedt DC, Webster CL et al. Proceedings of the 2017 European College of Veterinary Internal Medicine Conference, St. Julian's, Malta, Sept 2017
References
- 1. Gressner OA, Rizk MS, Kovalenko E, et al. Changing the pathogenetic roadmap of liver fibrosis? Where did it start; where will it go? J Gastroenterol Hepatol 2008;23:1024–1035. [DOI] [PubMed] [Google Scholar]
- 2. Bircher J. Oxford Textbook of Clinical Hepatology, 2nd ed Oxford; NY: Oxford University Press; 1999. [Google Scholar]
- 3. Friedman SL. Hepatic fibrosis In: Schiff ER, Sorrell MF, Maddrey WC, eds. Schiff's Diseases of the Liver, 10th ed Philadelphia: Lippincott Williams & Wilkins; 2007:297–315. [Google Scholar]
- 4. Everhart JE, Wright EC, Goodman ZD, et al. Prognostic value of Ishak fibrosis stage: Findings from the hepatitis C antiviral long‐term treatment against cirrhosis trial. Hepatology 2010;51:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Namisaki T, Moriya K, Noguchi R, et al. Liver fibrosis progression predicts survival in patients with primary biliary cirrhosis. Hepatol Res 2017;43:E178–E186. [DOI] [PubMed] [Google Scholar]
- 6. Rothuizen J. Important clinical syndromes associated with liver disease. Vet Clin North Am Small Anim Pract 2009;39:419–437. [DOI] [PubMed] [Google Scholar]
- 7. Watson PJ, Roulois AJ, Scase TJ, et al. Prevalence of hepatic lesions at post‐mortem examination in dogs and association with pancreatitis. J Small Anim Pract 2010;51:566–572. [DOI] [PubMed] [Google Scholar]
- 8. Cullen J. Liver and Biliary System In: Maxie G, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals, 6th ed Philadelphia, PA: Saunders Elsevier; 2015:258–352. [Google Scholar]
- 9. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–699. [DOI] [PubMed] [Google Scholar]
- 10. Ramachandran P, Iredale JP. Liver fibrosis: A bidirectional model of fibrogenesis and resolution. QJM 2012;105:813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vince AR, Hayes MA, Jefferson BJ, et al. Sinusoidal endothelial cell and hepatic stellate cell phenotype correlates with stage of fibrosis in chronic liver disease in dogs. J Vet Diagn Invest 2016;28:498–505. [DOI] [PubMed] [Google Scholar]
- 12. Issa R, Zhou X, Constandinou CM, et al. Spontaneous recovery from micronodular cirrhosis: Evidence for incomplete resolution associated with matrix cross‐linking. Gastroenterology 2004;126:1795–1808. [DOI] [PubMed] [Google Scholar]
- 13. Willard MD. Inflammatory canine hepatic disease In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat, 7th ed St. Louis, MO: Elsevier Saunders; 2010:1637–1642. [Google Scholar]
- 14. Pinzani M, Rombouts K. Liver fibrosis: From the bench to clinical targets. Dig Liver Dis 2004;36:231–242. [DOI] [PubMed] [Google Scholar]
- 15. Crosas‐Molist E, Fabregat I. Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol 2015;6:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iwaisako K, Jiang C, Zhang M, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci USA 2014;111:E3297–E3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boisclair J, Dore M, Beauchamp G, et al. Characterization of the inflammatory infiltrate in canine chronic hepatitis. Vet Pathol 2001;38:628–635. [DOI] [PubMed] [Google Scholar]
- 18. Friedman SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mallat A, Lotersztajn S. Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am J Physiol Cell Physiol 2013;305:C789–C799. [DOI] [PubMed] [Google Scholar]
- 20. Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol 2014;20:2515–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med 1993;328:1828–1835. [DOI] [PubMed] [Google Scholar]
- 22. Oakley F, Teoh V, Ching ASG, et al. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology 2009;136:e2331. [DOI] [PubMed] [Google Scholar]
- 23. Mekonnen GA, Ijzer J, Nederbragt H. Tenascin‐C in chronic canine hepatitis: Immunohistochemical localization and correlation with necro‐inflammatory activity, fibrotic stage, and expression of alpha‐smooth muscle actin, cytokeratin 7, and CD3 + cells. Vet Pathol 2007;44:803–813. [DOI] [PubMed] [Google Scholar]
- 24. Neumann S, Kaup FJ. α‐SMA and Ki‐67 immunohistochemistry as indicators for the fibrotic remodeling process in the liver of dogs. J Adv Vet Anim Res 2012;2:42–47. [Google Scholar]
- 25. Johnson SE. Parenchymal disorders In: Washabau RJ, Day MJ, eds. Canine & Feline Gastroenterology. St. Louis, MO: Elsevier Saunders; 2013:879–904. [Google Scholar]
- 26. Lua I, Li Y, Zagory JA, et al. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J Hepatol 2016;64:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wells RG. Portal fibroblasts in biliary fibrosis. Curr Pathobiol Rep 2014;2:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemoinne S, Thabut D, Housset C. Portal myofibroblasts connect angiogenesis and fibrosis in liver. Cell Tissue Res 2016;365:583–589. [DOI] [PubMed] [Google Scholar]
- 29. Lemoinne S, Cadoret A, Rautou PE, et al. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology 2015;61:1041–1055. [DOI] [PubMed] [Google Scholar]
- 30. Kalluri R, Weinberg RA. The basics of epithelial‐mesenchymal transition. J Clin Invest 2009;119:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Omenetti A, Diehl AM. Hedgehog signaling in cholangiocytes. Curr Opin Gastroenterol 2011;27:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Omenetti A, Choi S, Michelotti G, et al. Hedgehog signaling in the liver. J Hepatol 2011;54:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi SS, Omenetti A, Syn WK, et al. The role of hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol 2011;43:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sancho P, Mainez J, Crosas‐Molist E, et al. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One 2012;7:e45285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chu AS, Diaz R, Hui JJ, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial‐to‐mesenchymal transition in murine models of hepatic fibrosis. Hepatology 2011;53:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taura K, Miura K, Iwaisako K, et al. Hepatocytes do not undergo epithelial‐mesenchymal transition in liver fibrosis in mice. Hepatology 2010;51:1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borkham‐Kamphorst E, van Roeyen CR, Ostendorf T, et al. Pro‐fibrogenic potential of PDGF‐D in liver fibrosis. J Hepatol 2007;46:1064–1074. [DOI] [PubMed] [Google Scholar]
- 38. Borkham‐Kamphorst E, Weiskirchen R. The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev 2016;28:53–61. [DOI] [PubMed] [Google Scholar]
- 39. Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis 2001;21:397–416. [DOI] [PubMed] [Google Scholar]
- 40. Lechuga CG, Hernandez‐Nazara ZH, Hernandez E, et al. PI3K is involved in PDGF‐beta receptor upregulation post‐PDGF‐BB treatment in mouse HSC. Am J Physiol Gastrointest Liver Physiol 2006;291:G1051–G1061. [DOI] [PubMed] [Google Scholar]
- 41. Seppa H, Grotendorst G, Seppa S, et al. Platelet‐derived growth factor in chemotactic for fibroblasts. J Cell Biol 1982;92:584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kanemoto H, Ohno K, Sakai M, et al. Expression of fibrosis‐related genes in canine chronic hepatitis. Vet Pathol 2011;48:839–845. [DOI] [PubMed] [Google Scholar]
- 43. Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 2007;56:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perepelyuk M, Terajima M, Wang AY, et al. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol 2013;304:G605–G614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spee B, Arends B, van den Ingh TS, et al. Transforming growth factor beta‐1 signalling in canine hepatic diseases: New models for human fibrotic liver pathologies. Liver Int 2006;26:716–725. [DOI] [PubMed] [Google Scholar]
- 46. Neumann S, Kaup FJ, Beardi B. Plasma concentration of transforming growth factor‐beta1 and hepatic fibrosis in dogs. Can J Vet Res 2008;72:428–431. [PMC free article] [PubMed] [Google Scholar]
- 47. Dirksen K, Spee B, Penning LC, et al. Gene expression patterns in the progression of canine copper‐associated chronic hepatitis. PLoS One 2017;12:e0176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C‐terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem 2004;279:8848–8855. [DOI] [PubMed] [Google Scholar]
- 49. Kovalenko E, Tacke F, Gressner OA, et al. Validation of connective tissue growth factor (CTGF/CCN2) and its gene polymorphisms as noninvasive biomarkers for the assessment of liver fibrosis. J Viral Hepat 2009;16:612–620. [DOI] [PubMed] [Google Scholar]
- 50. Kemp TJ, Aggeli IK, Sugden PH, et al. Phenylephrine and endothelin‐1 upregulate connective tissue growth factor in neonatal rat cardiac myocytes. J Mol Cell Cardiol 2004;37:603–606. [DOI] [PubMed] [Google Scholar]
- 51. Sferra R, Vetuschi A, Pompili S, et al. Expression of pro‐fibrotic and anti‐fibrotic molecules in dimethylnitrosamine‐induced hepatic fibrosis. Pathol Res Pract 2017;213:58–65. [DOI] [PubMed] [Google Scholar]
- 52. Kiryu M, Niwano S, Niwano H, et al. Angiotensin II‐mediated up‐regulation of connective tissue growth factor promotes atrial tissue fibrosis in the canine atrial fibrillation model. Europace 2012;14:1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: New targets for antifibrotic therapy? Matrix Biol 2002;21:473–482. [DOI] [PubMed] [Google Scholar]
- 54. Dendooven A, Gerritsen KG, Nguyen TQ, et al. Connective tissue growth factor (CTGF/CCN2) ELISA: A novel tool for monitoring fibrosis. Biomarkers 2011;16:289–301. [DOI] [PubMed] [Google Scholar]
- 55. Pinzani M, Milani S, De Franco R, et al. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology 1996;110:534–548. [DOI] [PubMed] [Google Scholar]
- 56. Rockey DC, Fouassier L, Chung JJ, et al. Cellular localization of endothelin‐1 and increased production in liver injury in the rat: Potential for autocrine and paracrine effects on stellate cells. Hepatology 1998;27:472–480. [DOI] [PubMed] [Google Scholar]
- 57. Sakamoto Y, Sakai M, Watari T. Hepatic and plasma endothelin‐1 in dogs with chronic hepatitis. J Vet Intern Med 2017;31:764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol 2012;2012:936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys 2007;462:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liang S, Kisseleva T, Brenner DA. The role of NADPH oxidases (NOXs) in liver fibrosis and the activation of myofibroblasts. Front Physiol 2016;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lan T, Kisseleva T, Brenner DA. Deficiency of NOX1 or NOX4 prevents liver inflammation and fibrosis in mice through inhibition of hepatic stellate cell activation. PLoS One 2015;10:e0129743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bettaieb A, Jiang JX, Sasaki Y, et al. Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology 2015;149:468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Center SA, Warner KL, Erb HN. Liver glutathione concentrations in dogs and cats with naturally occurring liver disease. Am J Vet Res 2002;63:1187–1197. [DOI] [PubMed] [Google Scholar]
- 64. Lubel JS, Herath CB, Burrell LM, et al. Liver disease and the renin‐angiotensin system: Recent discoveries and clinical implications. J Gastroenterol Hepatol 2008;23:1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Santos RA, Ferreira AJ, Verano‐Braga T, et al. Angiotensin‐converting enzyme 2, angiotensin‐(1‐7) and Mas: New players of the renin‐angiotensin system. J Endocrinol 2013;216:R1–R17. [DOI] [PubMed] [Google Scholar]
- 66. Liu J, Gong H, Zhang ZT, et al. Effect of angiotensin II and angiotensin II type 1 receptor antagonist on the proliferation, contraction and collagen synthesis in rat hepatic stellate cells. Chin Med J 2008;121:161–165. [PubMed] [Google Scholar]
- 67. Bataller R, Sancho‐Bru P, Gines P, et al. Activated human hepatic stellate cells express the renin‐angiotensin system and synthesize angiotensin II. Gastroenterology 2003;125:117–125. [DOI] [PubMed] [Google Scholar]
- 68. Pereira RM, Dos Santos RA, Teixeira MM, et al. The renin‐angiotensin system in a rat model of hepatic fibrosis: Evidence for a protective role of Angiotensin‐(1‐7). J Hepatol 2007;46:674–681. [DOI] [PubMed] [Google Scholar]
- 69. Lubel JS, Herath CB, Tchongue J, et al. Angiotensin‐(1‐7), an alternative metabolite of the renin‐angiotensin system, is up‐regulated in human liver disease and has antifibrotic activity in the bile‐duct‐ligated rat. Clin Sci 2009;117:375–386. [DOI] [PubMed] [Google Scholar]
- 70. Jonsson JR, Clouston AD, Ando Y, et al. Angiotensin‐converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology 2001;121:148–155. [DOI] [PubMed] [Google Scholar]
- 71. Kurikawa N, Suga M, Kuroda S, et al. An angiotensin II type 1 receptor antagonist, olmesartan medoxomil, improves experimental liver fibrosis by suppression of proliferation and collagen synthesis in activated hepatic stellate cells. Br J Pharmacol 2003;139:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ramachandran P, Iredale JP, Fallowfield JA. Resolution of liver fibrosis: Basic mechanisms and clinical relevance. Semin Liver Dis 2015;35:119–131. [DOI] [PubMed] [Google Scholar]
- 73. Younis N, Shaheen MA, Abdallah MH. Silymarin‐loaded Eudragit RS100 nanoparticles improved the ability of silymarin to resolve hepatic fibrosis in bile duct ligated rats. Biomed Pharmacother 2016;81:93–103. [DOI] [PubMed] [Google Scholar]
- 74. Iredale JP. Models of liver fibrosis: Exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest 2007;117:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Iredale JP, Benyon RC, Arthur MJ, et al. Tissue inhibitor of metalloproteinase‐1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology 1996;24:176–184. [DOI] [PubMed] [Google Scholar]
- 76. Iredale JP, Murphy G, Hembry RM, et al. Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases‐1. Implications for regulation of matrix degradation in liver. J Clin Invest 1992;90:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Benyon RC, Iredale JP, Goddard S, et al. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 1996;110:821–831. [DOI] [PubMed] [Google Scholar]
- 78. Pellicoro A, Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Fibrogenesis Tissue Repair 2012;5:S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Iredale J. Defining therapeutic targets for liver fibrosis: Exploiting the biology of inflammation and repair. Pharmacol Res 2008;58:129–136. [DOI] [PubMed] [Google Scholar]
- 80. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016;44:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cullen JM. Summary of the World Small Animal Veterinary Association standardization committee guide to classification of liver disease in dogs and cats. Vet Clin North Am Small Anim Pract 2009;39:395–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van den Ingh TS, Van Winkle T, Cullen JM, et al. Morphological classification of parenchymal disorders of the canine and feline liver: hepatocellular death, hepatitis, and cirrhosis‐2 (updated version). In: World Small Animal Veterinary Association Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Society of Comparative Hepatology. Available at: http://www.vetvisuals.com/lms/moodle/mod/book/view.php?id=1001&chapterid=52859. Accessed July 27, 2017.
- 83. Poldervaart JH, Favier RP, Penning LC, et al. Primary hepatitis in dogs: A retrospective review (2002–2006). J Vet Intern Med 2009;23:72–80. [DOI] [PubMed] [Google Scholar]
- 84. Smedley R, Mullaney T, Rumbeiha W. Copper‐associated hepatitis in Labrador retrievers. Vet Pathol 2009;46:484–490. [DOI] [PubMed] [Google Scholar]
- 85. Dirksen K, Fieten H. Canine copper‐associated hepatitis. Vet Clin North Am Small Anim Pract 2017;47:631–644. [DOI] [PubMed] [Google Scholar]
- 86. Bexfield N. Canine idiopathic chronic hepatitis. Vet Clin North Am Small Anim Pract 2017;47:645–663. [DOI] [PubMed] [Google Scholar]
- 87. Rodriguez JY, Lewis BC, Snowden KF. Distribution and characterization of Heterobilharzia americana in dogs in Texas. Vet Parasitol 2014;203:35–42. [DOI] [PubMed] [Google Scholar]
- 88. Chapman BL, Hendrick MJ, Washabau RJ. Granulomatous hepatitis in dogs: Nine cases (1987–1990). J Am Vet Med A 1993;203:680–684. [PubMed] [Google Scholar]
- 89. Cook S, Priestnall SL, Blake D, et al. Angiostrongylus vasorum causing severe granulomatous hepatitis with concurrent multiple acquired PSS. J Am Anim Hosp Assoc 2015;51:320–324. [DOI] [PubMed] [Google Scholar]
- 90. Rallis T, Day MJ, Saridomichelakis MN, et al. Chronic hepatitis associated with canine leishmaniosis (Leishmania infantum): A clinicopathological study of 26 cases. J Comp Pathol 2005;132:145–152. [DOI] [PubMed] [Google Scholar]
- 91. Mizooku H, Kagawa Y, Matsuda K, et al. Histological and immunohistochemical evaluations of lobular dissecting hepatitis in American cocker spaniel dogs. J Vet Med Sci 2013;75:597–603. [DOI] [PubMed] [Google Scholar]
- 92. van den Ingh TS, Rothuizen J. Lobular dissecting hepatitis in juvenile and young adult dogs. J Vet Intern Med 1994;8:217–220. [DOI] [PubMed] [Google Scholar]
- 93. Schotanus BA, Kruitwagen HS, van den Ingh TS, et al. Enhanced Wnt/beta‐catenin and Notch signalling in the activated canine hepatic progenitor cell niche. BMC Vet Res 2014;10:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rothuizen J. Liver diseases of the dog In: Steiner JM, ed. Small Animal Gastroenterology. Hannover, Germany: Schluetersche Verlagsgesellschaft; 2008:241–281. [Google Scholar]
- 95. Tamborini A, Jahns H, McAllister H, et al. Bacterial cholangitis, cholecystitis, or both in dogs. J Vet Intern Med 2016;30:1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Osumi T, Ohno K, Kanemoto H, et al. A case of recovery from canine destructive cholangitis in a Miniature Dachshund. J Vet Med Sci 2011;73:937–939. [DOI] [PubMed] [Google Scholar]
- 97. Gabriel A, van den Ingh TS, Clercx C, et al. Suspected drug‐induced destructive cholangitis in a young dog. J Small Anim Pract 2006;47:344–348. [DOI] [PubMed] [Google Scholar]
- 98. Li P, Robertson TA, Zhang Q, et al. Hepatocellular necrosis, fibrosis and microsomal activity determine the hepatic pharmacokinetics of basic drugs in right‐heart‐failure‐induced liver damage. Pharm Res 2012;29:1658–1669. [DOI] [PubMed] [Google Scholar]
- 99. Pillai S, Center SA, McDonough SP, et al. Ductal plate malformation in the liver of Boxer dogs: Clinical and histological features. Vet Path 2016;53:602–613. [DOI] [PubMed] [Google Scholar]
- 100. Kaneko Y, Torisu S, Hagio M, et al. A case report of suspected hepatopulmonary syndrome secondary to ductal plate malformation with chronic active hepatitis in a dog. J Vet Med Sci 2016;78:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Brown DL, Van Winkle T, Cecere T, et al. Congenital hepatic fibrosis in 5 dogs. Vet Path 2010;47:102–107. [DOI] [PubMed] [Google Scholar]
- 102. Watson P. Complications of liver disease In: Washabau RJ, Day MJ, eds. Canine & Feline Gastroenterology. St. Louis, MO: Elsevier Saunders; 2013:936–939. [Google Scholar]
- 103. Schmidt S, Vet M, Suter PF, et al. Indirect and direct determination of the portal vein pressure in normal and abnormal dogs and normal cats. Vet Radiol 1980;21:246–259. [Google Scholar]
- 104. Buob S, Johnston AN, Webster CR. Portal hypertension: Pathophysiology, diagnosis, and treatment. J Vet Intern Med 2011;25:169–186. [DOI] [PubMed] [Google Scholar]
- 105. Sanyal AJ, Bosch J, Blei A, et al. Portal hypertension and its complications. Gastroenterology 2008;134:1715–1728. [DOI] [PubMed] [Google Scholar]
- 106. Thalheimer U, Leandro G, Samonakis DN, et al. Assessment of the agreement between wedge hepatic vein pressure and portal vein pressure in cirrhotic patients. Dig Liver Dis 2005;37:601–608. [DOI] [PubMed] [Google Scholar]
- 107. Perello A, Escorsell A, Bru C, et al. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus‐related cirrhosis. Hepatology 1999;30:1393–1397. [DOI] [PubMed] [Google Scholar]
- 108. Bertolini G, De Lorenzi D, Ledda G, et al. Esophageal varices due to a probable arteriovenous communication in a dog. J Vet Intern Med 2007;21:1392–1395. [DOI] [PubMed] [Google Scholar]
- 109. Raffan E, McCallum A, Scase TJ, et al. Ascites is a negative prognostic indicator in chronic hepatitis in dogs. J Vet Intern Med 2009;23:63–66. [DOI] [PubMed] [Google Scholar]
- 110. Lidbury JA, Cook AK, Steiner JM. Hepatic encephalopathy in dogs and cats. J Vet Emerg Crit Car 2016a;26:471–487. [DOI] [PubMed] [Google Scholar]
- 111. Stanton ME, Bright RM. Gastroduodenal ulceration in dogs. Retrospective study of 43 cases and literature review. J Vet Intern Med 1989;3:238–244. [DOI] [PubMed] [Google Scholar]
- 112. Weisse C, Berent AC, Todd K, et al. Endovascular evaluation and treatment of intrahepatic portosystemic shunts in dogs: 100 cases (2001–2011). J Am Vet Med Assoc 2014;244:78–94. [DOI] [PubMed] [Google Scholar]
- 113. Mazaki‐Tovi M, Segev G, Yas‐Natan E, et al. Serum gastrin concentrations in dogs with liver disorders. Vet Rec 2012;171:19. [DOI] [PubMed] [Google Scholar]
- 114. Culp WT, Zeldis TE, Reese MS, et al. Primary bacterial peritonitis in dogs and cats: 24 cases (1990–2006). J Am Vet Med Assoc 2009;234:906–913. [DOI] [PubMed] [Google Scholar]
- 115. Cerquetella M, Giuliano V, Rossi G, et al. Chronic hepatitis in man and in dog: A comparative update. Rev Esp Enferm Dig 2012;104:203–209. [DOI] [PubMed] [Google Scholar]
- 116. Shih JL, Keating JH, Freeman LM, et al. Chronic hepatitis in Labrador retrievers: Clinical presentation and prognostic factors. J Vet Intern Med 2007;21:33–39. [DOI] [PubMed] [Google Scholar]
- 117. Prins M, Schellens CJ, van Leeuwen MW, et al. Coagulation disorders in dogs with hepatic disease. Vet J 2010;185:163–168. [DOI] [PubMed] [Google Scholar]
- 118. Fry W, Lester C, Etedali NM, et al. Thromboelastography in dogs with chronic hepatopathies. J Vet Intern Med 2017;31:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Respess M, O'Toole TE, Taeymans O, et al. Portal vein thrombosis in 33 dogs: 1998‐2011. J Vet Intern Med 2012;26:230–237. [DOI] [PubMed] [Google Scholar]
- 120. Duplantier JG, Dubuisson L, Senant N, et al. A role for thrombin in liver fibrosis. Gut 2004;53:1682–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mercer PF, Chambers RC. Coagulation and coagulation signalling in fibrosis. Biochim Biophys Acta 2013;1832:1018–1027. [DOI] [PubMed] [Google Scholar]
- 122. Wanless IR, Wong F, Blendis LM, et al. Hepatic and portal vein thrombosis in cirrhosis: Possible role in development of parenchymal extinction and portal hypertension. Hepatology 1995;21:1238–1247. [PubMed] [Google Scholar]
- 123. Sevelius E. Diagnosis and prognosis of chronic hepatitis and cirrhosis in dogs. J Small Anim Pract 1995;36:521–528. [DOI] [PubMed] [Google Scholar]
- 124. Masserdotti C, Bertazzolo W. Cytologic features of hepatic fibrosis in dogs: A retrospective study on 22 cases. Vet Clin Pathol 2016;45:361–367. [DOI] [PubMed] [Google Scholar]
- 125. Bigge LA, Brown DJ, Penninck DG. Correlation between coagulation profile findings and bleeding complications after ultrasound‐guided biopsies: 434 cases (1993–1996). J Am Anim Hosp Assoc 2001;37:228–233. [DOI] [PubMed] [Google Scholar]
- 126. Lidbury JA. Getting the most out of liver biopsy. Vet Clin North Am Small Anim Pract 2017a;47:569–583. [DOI] [PubMed] [Google Scholar]
- 127. Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449–1457. [DOI] [PubMed] [Google Scholar]
- 128. Kemp SD, Zimmerman KL, Panciera DL, et al. Histopathologic variation between liver lobes in dogs. J Vet Intern Med 2015a;29:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kemp SD, Zimmerman KL, Panciera DL, et al. A comparison of liver sampling techniques in dogs. J Vet Intern Med 2015b;29:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rothuizen J, Twedt DC. Liver biopsy techniques. Vet Clin North Am Small Anim Pract 2009;39:469–480. [DOI] [PubMed] [Google Scholar]
- 131. Cholongitas E, Senzolo M, Standish R, et al. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol 2006;125:710–721. [DOI] [PubMed] [Google Scholar]
- 132. Colloredo G, Guido M, Sonzogni A, et al. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: The smaller the sample, the milder the disease. J Hepatol 2003;39:239–244. [DOI] [PubMed] [Google Scholar]
- 133. Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007;47:598–607. [DOI] [PubMed] [Google Scholar]
- 134. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289–293. [DOI] [PubMed] [Google Scholar]
- 135. Fieten H, Biourge VC, Watson AL, et al. Nutritional management of inherited copper‐associated hepatitis in the Labrador retriever. Vet J 2014;199:429–433. [DOI] [PubMed] [Google Scholar]
- 136. Fieten H, Dirksen K, van den Ingh TS, et al. D‐penicillamine treatment of copper‐associated hepatitis in Labrador retrievers. Vet J 2013;196:522–527. [DOI] [PubMed] [Google Scholar]
- 137. Lidbury JA, Rodrigues Hoffmann A, Ivanek R, et al. Interobserver agreement using histological scoring of the canine liver. J Vet Intern Med 2017;31:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chevallier M, Guerret S, Chossegros P, et al. A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: Comparison with morphometric studies. Hepatology 1994;20:349–355. [PubMed] [Google Scholar]
- 139. Pilette C, Rousselet MC, Bedossa P, et al. Histopathological evaluation of liver fibrosis: Quantitative image analysis vs semi‐quantitative scores. Comparison with serum markers. J Hepatol 1998;28:439–446. [DOI] [PubMed] [Google Scholar]
- 140. Lidbury JA. Canine chronic hepatitis: diagnostic evaluation and complicating syndromes. Doctoral dissertation, Texas A & M University. Available at: http://hdl.handle.net/1969.1/156453. Accessed October 1, 2017.
- 141. Plebani M, Basso D. Non‐invasive assessment of chronic liver and gastric diseases. Clin Chim Acta 2007;381:39–49. [DOI] [PubMed] [Google Scholar]
- 142. Pereira TN, Lewindon PJ, Smith JL, et al. Serum markers of hepatic fibrogenesis in cystic fibrosis liver disease. J Hepatol 2004;41:576–583. [DOI] [PubMed] [Google Scholar]
- 143. El Serafy MA, Kassem AM, Omar H, et al. APRI test and hyaluronic acid as non‐invasive diagnostic tools for post HCV liver fibrosis: Systematic review and meta‐analysis. Arab J Gastroenterol 2017;18:51–57. [DOI] [PubMed] [Google Scholar]
- 144. Baranova A, Lal P, Birerdinc A, et al. Non‐invasive markers for hepatic fibrosis. BMC Gastroenterol 2011;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kanemoto H, Ohno K, Sakai M, et al. Blood hyaluronic acid as a marker for canine cirrhosis. J Vet Med Sci 2009;71:1251–1254. [DOI] [PubMed] [Google Scholar]
- 146. Glinska‐Suchocka K, Orlowska A, Spuzak J, et al. Suitability of using serum hialuronic acid concentrations in the diagnosis of canine liver fibrosis. Pol J Vet Sci 2015;18:873–878. [DOI] [PubMed] [Google Scholar]
- 147. Glinska‐Suchocka K, Orlowska A, Kubiak K, et al. 7S fragment of type IV collagen as a serum marker of canine liver fibrosis. Pol J Vet Sci 2016a;19:647–649. [DOI] [PubMed] [Google Scholar]
- 148. Glinska‐Suchocka K, Orlowska A, Jankowski M, et al. Serum concentrations of PIIINP aminopeptide in dogs with liver fibrosis. Pol J Vet Sci 2016b;19:365–369. [DOI] [PubMed] [Google Scholar]
- 149. Lidbury JA, Hoffmann AR, Fry JK, et al. Evaluation of hyaluronic acid, procollagen type III N‐terminal peptide, and tissue inhibitor of matrix metalloproteinase‐1 as serum markers of canine hepatic fibrosis. Can J Vet Res 2016b;80:302–308. [PMC free article] [PubMed] [Google Scholar]
- 150. Imbert‐Bismut F, Ratziu V, Pieroni L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: A prospective study. Lancet 2001;357:1069–1075. [DOI] [PubMed] [Google Scholar]
- 151. Rossi E, Adams L, Prins A, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 2003;49:450–454. [DOI] [PubMed] [Google Scholar]
- 152. Lidbury J. Potential of circulating microRNAs as biomarkers in veterinary medicine. Vet J 2016;212:78–79. [DOI] [PubMed] [Google Scholar]
- 153. Arrese M, Eguchi A, Feldstein AE. Circulating microRNAs: Emerging biomarkers of liver disease. Semin Liver Dis 2015;35:43–54. [DOI] [PubMed] [Google Scholar]
- 154. Murakami Y, Toyoda H, Tanahashi T, et al. Comprehensive miRNA expression analysis in peripheral blood can diagnose liver disease. PLoS One 2012;7:e48366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Roderburg C, Mollnow T, Bongaerts B, et al. Micro‐RNA profiling in human serum reveals compartment‐specific roles of miR‐571 and miR‐652 in liver cirrhosis. PLoS One 2012;7:e32999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Roderburg C, Urban GW, Bettermann K, et al. Micro‐RNA profiling reveals a role for miR‐29 in human and murine liver fibrosis. Hepatology 2011;53:209–218. [DOI] [PubMed] [Google Scholar]
- 157. Dirksen K, Verzijl T, Grinwis GC, et al. Use of serum microRNAs as biomarker for hepatobiliary diseases in dogs. J Vet Intern Med 2016;30:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 2010;7:425–436. [DOI] [PubMed] [Google Scholar]
- 159. Sporea I, Gilja OH, Bota S, et al. Liver elastography ‐ an update. Med Ultrason 2013;15:304–314. [DOI] [PubMed] [Google Scholar]
- 160. Ferraioli G, Parekh P, Levitov AB, et al. Shear wave elastography for evaluation of liver fibrosis. J Ultrasound Med 2014;33:197–203. [DOI] [PubMed] [Google Scholar]
- 161. Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29:1705–1713. [DOI] [PubMed] [Google Scholar]
- 162. Poelstra K. Liver fibrosis in 2015: Crucial steps towards an effective treatment. Nat Rev Gastroenterol Hepatol 2016;13:67–68. [DOI] [PubMed] [Google Scholar]
- 163. Barnes PJ. Anti‐inflammatory actions of glucocorticoids: Molecular mechanisms. Clin Sci 1998;94:557–572. [DOI] [PubMed] [Google Scholar]
- 164. Kagoshima M, Ito K, Cosio B, et al. Glucocorticoid suppression of nuclear factor‐kappa B: A role for histone modifications. Biochem Soc Trans 2003;31:60–65. [DOI] [PubMed] [Google Scholar]
- 165. Adcock IM, Ito K, Barnes PJ. Glucocorticoids: Effects on gene transcription. Proc Am Thorac Soc 2004;1:247–254. [DOI] [PubMed] [Google Scholar]
- 166. Favier RP, Poldervaart JH, van den Ingh TS, et al. A retrospective study of oral prednisolone treatment in canine chronic hepatitis. Vet Q 2013;33:113–120. [DOI] [PubMed] [Google Scholar]
- 167. Strombeck DR, Miller LM, Harrold D. Effects of corticosteroid treatment on survival time in dogs with chronic hepatitis: 151 cases (1977‐1985). J Am Vet Med Assoc 1988;193:1109–1113. [PubMed] [Google Scholar]
- 168. Webster CR, Cooper J. Therapeutic use of cytoprotective agents in canine and feline hepatobiliary disease. Vet Clin North Am Small Anim Pract 2009;39:631–652. [DOI] [PubMed] [Google Scholar]
- 169. Lidbury JA. General principles in the treatment of liver disease In: Ettinger SJFE, Cote E, eds. Textbook of Veterinary Internal Medicine, 8th ed St. Louis, MO: Elsevier; 2017b:1621–1627. [Google Scholar]
- 170. Hackett ES, Twedt DC, Gustafson DL. Milk thistle and its derivative compounds: A review of opportunities for treatment of liver disease. J Vet Intern Med 2013;27:10–16. [DOI] [PubMed] [Google Scholar]
- 171. Trappoliere M, Caligiuri A, Schmid M, et al. Silybin, a component of sylimarin, exerts anti‐inflammatory and anti‐fibrogenic effects on human hepatic stellate cells. J Hepatol 2009;50:1102–1111. [DOI] [PubMed] [Google Scholar]
- 172. Manna SK, Mukhopadhyay A, Van NT, et al. Silymarin suppresses TNF‐induced activation of NF‐kappa B, c‐Jun N‐terminal kinase, and apoptosis. J Immunol 1999;163:6800–6809. [PubMed] [Google Scholar]
- 173. Meyer DJ, Thompson MB, Senior DF. Use of ursodeoxycholic acids in a dog with chronic hepatitis: Effects on serum hepatic tests and endogenous bile acid composition. J Vet Intern Med 1997;11:195–197. [DOI] [PubMed] [Google Scholar]
- 174. Honeckman A. Current concepts in the treatment of canine chronic hepatitis. Clin Tech Small Anim Pract 2003;18:239–244. [DOI] [PubMed] [Google Scholar]
- 175. Sola S, Amaral JD, Castro RE, et al. Nuclear translocation of UDCA by the glucocorticoid receptor is required to reduce TGF‐beta1‐induced apoptosis in rat hepatocytes. Hepatology 2005;42:925–934. [DOI] [PubMed] [Google Scholar]
- 176. Sola S, Amaral JD, Aranha MM, et al. Modulation of hepatocyte apoptosis: Cross‐talk between bile acids and nuclear steroid receptors. Curr Med Chem 2006;13:3039–3051. [DOI] [PubMed] [Google Scholar]
- 177. Corpechot C. Primary biliary cirrhosis beyond ursodeoxycholic acid. Semin Liver Dis 2016;36:15–26. [DOI] [PubMed] [Google Scholar]
- 178. Bautista AC, Moore CE, Lin Y, et al. Hepatopathy following consumption of a commercially available blue‐green algae dietary supplement in a dog. BMC Vet Res 2015;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. McGrotty YL, Ramsey IK, Knottenbelt CM. Diagnosis and management of hepatic copper accumulation in a Skye terrier. J Small Anim Pract 2003;44:85–89. [DOI] [PubMed] [Google Scholar]
- 180. Rodriguez L, Cerbon‐Ambriz J, Munoz ML. Effects of colchicine and colchiceine in a biochemical model of liver injury and fibrosis. Arch Med Res 1998;29:109–116. [PubMed] [Google Scholar]
- 181. Nikolaidis N, Kountouras J, Giouleme O, et al. Colchicine treatment of liver fibrosis. Hepatogastroenterology 2006;53:281–285. [PubMed] [Google Scholar]
- 182. Rambaldi A, Gluud C. Colchicine for alcoholic and non‐alcoholic liver fibrosis and cirrhosis. Cochrane Database Syst Rev 2005:CD002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Muriel P, Moreno MG, Hernandez Mdel C, et al. Resolution of liver fibrosis in chronic CCl4 administration in the rat after discontinuation of treatment: Effect of silymarin, silibinin, colchicine and trimethylcolchicinic acid. Basic Clin Pharmacol Toxicol 2005;96:375–380. [DOI] [PubMed] [Google Scholar]
- 184. Boer HH, Nelson RW, Long GG. Colchicine therapy for hepatic fibrosis in a dog. J Am Vet Med Assoc 1984;185:303–305. [PubMed] [Google Scholar]
- 185. McAlister A, Center SA, Bender H, et al. Adverse interaction between colchicine and ketoconazole in a Chinese shar pei. J Am Anim Hosp Assoc 2014;50:417–423. [DOI] [PubMed] [Google Scholar]
- 186. Ferguson FC Jr. Colchicine. I. General pharmacology. J Pharmacol Exp Ther 1952;106:261–270. [PubMed] [Google Scholar]
- 187. Pereira RM, dos Santos RA, da Costa Dias FL, et al. Renin‐angiotensin system in the pathogenesis of liver fibrosis. World J Gastroenterol 2009;15:2579–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Colmenero J, Bataller R, Sancho‐Bru P, et al. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol 2009;297:G726–G734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Abu Dayyeh BK, Yang M, Dienstag JL, et al. The effects of angiotensin blocking agents on the progression of liver fibrosis in the HALT‐C Trial cohort. Dig Dis Sci 2011;56:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Bugbee AC, Coleman AE, Wang A, et al. Telmisartan treatment of refractory proteinuria in a dog. J Vet Intern Med 2014;28:1871–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Flores‐Contreras L, Sandoval‐Rodriguez AS, Mena‐Enriquez MG, et al. Treatment with pirfenidone for two years decreases fibrosis, cytokine levels and enhances CB2 gene expression in patients with chronic hepatitis C. BMC Gastroenterol 2014;14:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Bruss ML, Margolin SB, Giri SN. Pharmacokinetics of orally administered pirfenidone in male and female beagles. J Vet Pharmacol Ther 2004;27:361–367. [DOI] [PubMed] [Google Scholar]
- 193. Karin D, Koyama Y, Brenner D, et al. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differentiation 2016;92:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Iwaisako K, Brenner DA, Kisseleva T. What's new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol 2012;27(Suppl 2):65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow‐derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 2006;45:429–438. [DOI] [PubMed] [Google Scholar]
- 196. Takemitsu H, Zhao D, Yamamoto I, et al. Comparison of bone marrow and adipose tissue‐derived canine mesenchymal stem cells. BMC Vet Res 2012;8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Masaki T, Tokuda M, Fujimura T, et al. Involvement of annexin I and annexin II in hepatocyte proliferation: Can annexins I and II be markers for proliferative hepatocytes? Hepatology 1994;20:425–435. [PubMed] [Google Scholar]
- 198. Harashima M, Harada K, Ito Y, et al. Annexin A3 expression increases in hepatocytes and is regulated by hepatocyte growth factor in rat liver regeneration. J Biochem 2008;143:537–545. [DOI] [PubMed] [Google Scholar]
- 199. Kruglov EA, Nathanson RA, Nguyen T, et al. Secretion of MCP‐1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol 2006;290:G765–G771. [DOI] [PubMed] [Google Scholar]
- 200. Pinzani M. Pathophysiology of liver fibrosis. Dig Dis 2015;33:492–497. [DOI] [PubMed] [Google Scholar]
- 201. Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 2014;60:1090–1096. [DOI] [PubMed] [Google Scholar]
- 202. Elvevold K, Smedsrod B, Martinez I. The liver sinusoidal endothelial cell: A cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol 2008;294:G391–G400. [DOI] [PubMed] [Google Scholar]
- 203. Foltz JA, Somanchi SS, Yang Y, et al. NCR1 Expression identifies canine natural killer cell subsets with phenotypic similarity to human natural killer cells. Front Immunol 2016;7:521. [DOI] [PMC free article] [PubMed] [Google Scholar]


