Abstract
Background
Prechemotherapy absolute neutrophil count (ANC) cutoffs are arbitrary and vary across institutions and clinicians. Similarly, subjective guidelines are utilized for the administration of prophylactic antibiotics in neutropenic dogs.
Objectives
To evaluate the impact of various ANC cutoffs on chemotherapy administration in dogs with lymphoma treated with CHOP chemotherapy and to determine whether an association between prechemotherapy ANC and subsequent toxicity exists. The secondary objective was to evaluate a currently used ANC cutoff to indicate prescription of prophylactic antibiotics.
Animals
Dogs diagnosed with lymphoma treated with CHOP chemotherapy (n = 64).
Methods
Six hundred and fifteen ANCs were stratified into 6 classes. The 3 ANC cutoffs 1.5 × 103/μL, 2.0 × 103/μL, and 2.5 × 103/μL were assessed. The presence of an association between prechemotherapy ANC class and toxicity was determined. Afebrile neutropenic dogs with ANC <1.5 × 103/μL but above the criteria for prophylactic antibiotics were evaluated.
Results
Chemotherapy was not administered in 7% of visits with an ANC cutoff of 1.5 × 103/μL; chemotherapy would not have been administered in 10% and 16% of visits with an ANC cutoff of 2.0 × 103/μL or 2.5 × 103/μL, respectively. There was no association among the 3 lower prechemotherapy ANC classes and toxicity. All dogs with ANC 0.75–1.5 × 103/μL recovered spontaneously without medical intervention.
Conclusion and Clinical Importance
The number of dose delays was minimized with a prechemotherapy ANC cutoff of 1.5 × 103/μL, and the prechemotherapy ANC class 1.5–1.99 × 103/μL was not associated with an increased toxicity. Further investigation of an ANC cutoff near 0.75 × 103/μL in which to prescribe prophylactic antibiotics is indicated.
Keywords: Antimicrobials, Chemotherapeutics, Hematology, Lymphoma, Oncology
Abbreviations
- ANC
absolute neutrophil count
- CBC
complete blood count
- DI
dose intensity
- DLT
dose‐limiting toxicity
- RDI
relative dose intensity
- SHC
sterile hemorrhagic cystitis
Chemotherapy in companion animals with cancer typically is administered at maximally tolerated doses and should allow good quality of life throughout treatment.1, 2, 3 Chemotherapy drugs can be associated with a broad spectrum of toxicities, notably bone marrow suppression.4, 5 Before each chemotherapy administration, CBC is recommended to assess absolute neutrophil count (ANC) and determine whether chemotherapy administration can proceed as scheduled. The ANC criteria for chemotherapy administration are arbitrary and vary across institutions and clinicians. Published ANC cutoffs in veterinary medicine range from 1.5 × 103/μL to 2.5 × 103/μL with 2.0 × 103/μL being most commonly reported.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Management of chemotherapy‐induced neutropenia in dogs is largely based on clinical experience although some standards have been published.4, 5, 16
In non‐Hodgkin's lymphoma in humans, a decrease in chemotherapy relative dose intensity (RDI) has been associated with poorer outcome.17, 18, 19, 20, 21 In dogs with lymphoma treated with a multidrug chemotherapy protocol, several studies suggested chemotherapy‐induced neutropenia prolonged first remission duration.22, 23 It remains unclear, however, if RDI is directly associated with outcome in these dogs.7, 23 Because RDI is impacted by dose delays and dose reductions, prechemotherapy ANC cutoffs likely are clinically relevant. Determination of an optimal ANC cutoff may increase chemotherapy RDI and subsequently improve remission duration in dogs with lymphoma.
As with prechemotherapy ANC cutoffs, multiple ANC cutoffs that prompt administration of prophylactic antibiotics have been used.6, 9, 10, 11 Published cutoffs vary from 1.0 × 103/μL to 2.5 × 103/μL.6, 9, 10, 11 Given recognition of increasing antimicrobial antibiotic resistance,24 the use of prophylactic antibiotic should be considered carefully. There is little evidence to support a specific ANC that increases the risk of sepsis, however, it is possible that dogs, as is the case in humans, have a low risk of sepsis if the ANC remains ≥0.5 × 103/μL.25, 26 Guidelines in the United States recommend the use of antibacterial prophylaxis for human chemotherapy patients expected to have <0.1 × 103 neutrophils/μL for >7 days, unless other factors increase the risk for complications.27 At our institution, a prechemotherapy ANC cutoff of 1.5 × 103/μL is utilized routinely and prophylactic antibiotics are administered when postchemotherapy ANC falls below 0.75 × 103/μL.
Given the lack of literature in support of strong recommendations for establishment of a prechemotherapy ANC cutoff and guidelines for usage of prophylactic antibiotics, institutional guidelines for chemotherapy for lymphoma in dogs at our institution were assessed to determine whether guideline alterations were indicated. The primary objectives were 2‐fold: (1) to compare the proportion of dogs that would require chemotherapy treatment delay if various ANC cutoffs were implemented; and (2) to determine whether an association exists between prechemotherapy ANC and subsequent chemotherapy toxicity with a standardized chemotherapy protocol. A secondary objective was to determine whether the ANC cutoff used at our institution to indicate prescription of prophylactic antibiotics resulted in the need for hospitalization and supportive care.
Materials and Methods
Dogs
All dogs included in the study were presented to an academic veterinary specialty oncology service between January 2013 and January 2016. Dogs were eligible if they had a cytological diagnosis consistent with lymphoma or histological diagnosis consistent with intermediate to high‐grade lymphoma by a board‐certified clinical pathologist or anatomic pathologist, respectively. Treatment‐naïve or relapsed dogs with lymphoma were eligible if they were prescribed a standardized 19‐week multi‐agent chemotherapy protocol. Relapsed dogs must have received the same protocol at diagnosis as treatment‐naïve dogs. Dogs were excluded if they had an incomplete medical record, were receiving systemic antibiotics for an infectious process (eg, pyoderma), received only 1 dose of chemotherapy, or presented with neutropenia. Overt pancytopenia and neutropenia at diagnosis were excluded because prechemotherapy ANC did not determine whether or not chemotherapy administration would proceed. Investigations that were recommended for all dogs included CBC with blood smear evaluation, serum biochemistry, urinalysis, thoracic radiographs, abdominal radiographs and ultrasound examination with fine needle aspiration cytology of abnormal findings, immunophenotyping, and bone marrow evaluation. Minimal investigations to permit chemotherapy included CBC and serum biochemistry.
Chemotherapy
A standardized 19‐week multi‐agent chemotherapy protocol, consisting of 4 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP), was prescribed to all dogs.28 Vincristine was administered IV at 0.7 mg/m2 on the first and third weeks of each cycle, cyclophosphamide was administered PO at 250 mg/m2 on the second week of each cycle, and doxorubicin was administered IV at 30 mg/m2 for dogs ≥10 kg and 25 mg/m2 for dogs <10 kg on the fourth week of each cycle. Mitoxantrone was substituted for doxorubicin if dilated cardiomyopathy was present or if deemed appropriate by the attending clinician and cardiologist. CBCs were performed before chemotherapy and 7 days after chemotherapy administration. Additional CBCs were performed when clinically indicated. If prechemotherapy ANC was <1.5 × 103/μL, a treatment delay of 2–7 days followed by a 10% dose reduction in the drug previously administered was instituted to maintain dose intensity (DI) for the remaining protocol. For doxorubicin or mitoxantrone, if the nadir ANC was ≥0.75 × 103/μL, a subsequent dose reduction was not performed. Chemotherapy adverse events were graded according to the Veterinary Cooperative Oncology Group guidelines (VCOG‐CTCAE) version 1.1,29 with dose‐limiting toxicity (DLT) defined as grade 4 neutropenia and ≥ grade 3 gastrointestinal toxicity. Assessment of toxicity was performed using CBC data, a weekly quality of life questionnaire completed by the owner, and weekly physical examinations. Chemotherapy dosage reduction of 10% was instituted after the occurrence of DLT. Chemotherapy dosage could be re‐escalated by 5% at the clinician's discretion. Strict antibiotic usage guidelines were instituted. Afebrile neutropenic dogs with ANC <0.75 × 103/μL were prescribed prophylactic trimethoprim–sulfonamide (15 mg/kg PO q12h) for 3–4 days. For dogs sensitive to trimethoprim–sulfonamide, enrofloxacin (10 mg/kg PO q24h) was prescribed. The choice of these antibiotics was based on current human literature,30, 31, 32, 33, 34 previous veterinary recommendations,4, 5, 26 and guidelines later published as part of a consensus by the American College of Veterinary Internal Medicine.35 Febrile neutropenia was defined as chemotherapy‐induced neutropenia (ANC <2.5 × 103/μL) in conjunction with fever (rectal temperature >39.2°C).36 Febrile neutropenic dogs were managed with inpatient supportive care. All febrile neutropenic patients had (at minimum) 3‐view thoracic radiographs, urinalysis, and urine culture to evaluate for infection before institution of antibiotics.
Prechemotherapy Absolute Neutrophil Count Stratification
All CBCs were reviewed, while dogs were on chemotherapy. To address the primary objectives, 3 specific ANC cutoffs values for chemotherapy administration were assessed: 1.5 × 103/μL (cutoff 1), 2.0 × 103/μL (cutoff 2), and 2.5 × 103/μL (cutoff 3). For analysis, prechemotherapy ANC values then were stratified into 6 classes to evaluate the impact of the ANC. To assess the 3 previously defined ANC cutoffs for chemotherapy administration, neutropenia was stratified into class C1: 1.5–1.99 × 103/μL, C2: 2.0–2.49 × 103/μL, C3: 2.5–2.99 × 103/μL, and C4: 3.0–3.49 × 103/μL. The reference interval also was represented by the class C5: 3.5–12.0 × 103/μL and neutrophilia by the class C6: >12.0 × 103/μL. To address the secondary objective, postchemotherapy ANC values were divided as follows: 0.75–0.99 × 103/μL and 1.0–1.49 × 103/μL to stratify dogs and evaluate classes for the likelihood of requiring inpatient care.
Statistical Analysis
Kruskal–Wallis tests and multiple comparisons by the Bonferroni adjustment method were used to test the difference in chemotherapy‐induced toxicity among prechemotherapy ANC classes. Dunnett‐type multiple comparison tests for proportion were employed to compare the proportions of chemotherapy treatments associated with hospitalization, DLT, and febrile neutropenia among prechemotherapy ANC classes.37 The influence of additional factors on chemotherapy‐induced neutropenia was assessed by ordinal logistic regression. These factors included initial body weight, initial body condition score as defined by the World Small Animal Veterinary Association,38 prechemotherapy neutrophil : lymphocyte ratio, and number of CHOP protocols administered. Statistical analyses were performed by commercially available statistics software (Minitaba), under the guidance of a statistician (IH). A P‐value <0.05 was considered statistically significant for all analyses. Graphs were made by commercially available graphic software (GraphPad Prismb).
Results
Dogs
Seventy‐five dogs initially met the inclusion criteria. Eleven dogs were excluded: 2 dogs for incomplete medical record information, 5 dogs for having received only 1 dose of chemotherapy, 3 dogs for having pancytopenia with neutropenia at presentation, and 1 dog for having a concurrent prostatic abscess requiring systemic antibiotic treatment. Therefore, 64 dogs were included in the study. Clinical characteristics of dogs with lymphoma were similar to those previously reported (Table 1).6, 7, 8, 9, 10, 11, 23, 39 Eight dogs were fully staged with bone marrow aspirate, whereas all dogs were minimally assessed with CBC and serum biochemistry. Although most of the dogs had multicentric lymphoma, the anatomic form was variable (Table 2).
Table 1.
Characteristics of the 64 dogs included in the study
| Parameter | |
|---|---|
| Age (years) | |
| Median (range) | 7.4 (1.6–13.3) |
| Sex | |
| Male | 35 (54%) |
| Female | 29 (46%) |
| Body weight (kg) | |
| Median (range) | 26.7 (6.2–75.2) |
| Breeds | |
| Labrador Retriever | 12 (18%) |
| Border Collie | 5 (7%) |
| Boxer | 5 (7%) |
| Cocker Spaniel | 3 (4%) |
| West Highland White Terrier | 3 (4%) |
| Crossbreed | 8 (12%) |
| Other breeds (≤2) | 31 (48%) |
Table 2.
Anatomic form of lymphoma in 64 dogs
| Anatomic Form | Number (%) |
|---|---|
| Multicentric | 43 (67.2%) |
| Mediastinal | 8 (12.5%) |
| Gastrointestinal | 6 (9.4%) |
| Hepato‐splenic | 4 (6.3%) |
| Cutaneous nonepitheliotropic | 2 (3.1%) |
| Nasal | 1 (1.5%) |
Chemotherapy Protocol Modifications
Fourteen dogs had alterations to their prescribed chemotherapy protocols. Six dogs received l‐asparaginase at the time of initial vincristine because of clinical illness at presentation. One dog received vinblastine instead of vincristine after presumptive vincristine‐induced peripheral neuropathy that occurred after 5 doses. Three dogs received chlorambucil substituted for cyclophosphamide; 2 dogs developed sterile hemorrhagic cystitis (SHC) after 1 and 2 doses of cyclophosphamide, and 1 dog because of owner concern for the risk of developing SHC. Five dogs received mitoxantrone instead of doxorubicin; 3 dogs were switched after receiving a cumulative doxorubicin dosage of 180 mg/m2 (after relapse and institution of a second CHOP protocol after completion of the first protocol), 1 dog because of clinical concern for increased risk of doxorubicin‐induced cardiac toxicity, and 1 dog because of grade III diarrhea. Cyclophosphamide was utilized as the initial drug in 2 dogs; in 1 dog, venous access could not be obtained for the first dose, whereas the other dog was given cyclophosphamide because of clinician preference.
Chemotherapy Administration
A total of 736 CBCs were reviewed for the study to obtain ANCs. Among them, 615 were prechemotherapy CBCs (including 70 before first CHOP administration [64] or subsequent CHOP protocol [6]), and 108 were CBCs performed 7 days after doxorubicin or mitoxantrone administration. Thirteen CBCs were performed at various intervals because of clinical illness and presumed chemotherapy toxicity. Chemotherapy was administered after evaluation of CBCs in 569 instances, whereas dose delay occurred 46 times because of neutropenia below the cutoff of 1.5 × 103 neutrophils/μL (38) or because of clinical illness (8). All 38 episodes of dose delays due to neutropenia resulted in a 10% dose reduction at the subsequent administration of the drug that induced neutropenia, but in 5 dogs (3 cyclophosphamide, 2 vincristine), the dose was subsequently escalated by 5% once in 3 dogs and twice in 2 dogs, without associated DLT.
Assessment of Prechemotherapy ANC Cutoffs on Chemotherapy Administration
To evaluate the potential clinical impact of altering the ANC cutoff for chemotherapy administration, prechemotherapy ANCs were stratified as described for analysis into classes, excluding the 70 ANCs from first chemotherapy administration. Overall, 93% of the ANCs were above the standard clinical cutoff of 1.5 × 103 neutrophils/μL (cutoff 1) established for routine practice at our institution. When cutoff 1 was utilized, 7% (38/545) of chemotherapy administrations were delayed. Had cutoff 2 been applied, 10% (54/545) of chemotherapy administrations would have been delayed in this particular set of dogs. Had cutoff 3 been used, 16% (85/545) of chemotherapy administrations would have been delayed. Of the 64 dogs in the study, 22 dogs (34%) were dose‐delayed at least once using cutoff 1. If ANC cutoff 2 or cutoff 3 had been used to permit chemotherapy administration, 24 (38%) and 32 (50%) of dogs would have had at least 1 dose delay, respectively.
Assessment of Prechemotherapy ANC Cutoffs on Chemotherapy Toxicity
Of the 569 chemotherapy administrations over the duration of the study, there were 344 (60%) documented instances of toxicity (Table 3). Of the 64 dogs in the study, 53 dogs (82%) experienced toxicity and multiple episodes of toxicity were common. No significant difference was observed in the overall incidence of chemotherapy‐induced toxicity among prechemotherapy ANC classes. There were 26 (5%) documented instances of DLT (Table 3). No significant difference was observed in the incidence of DLT among prechemotherapy ANC classes.
Table 3.
The number of chemotherapy administrations and toxicities associated with each prechemotherapy ANC class (C)
| PreChemotherapy ANC Classes | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|
| ANC (×103/μL) | 1.5–1.99 | 2.0–2.49 | 2.5–2.99 | 3.0–3.49 | 3.5–12.0 | >12.0 |
| Chemotherapy administration | 16 | 31 | 41 | 41 | 384 | 56 |
| Toxicity (any grade) | 8 | 25 | 31 | 26 | 226 | 28 |
| Dose‐limiting toxicity (DLT) | 2 | 0 | 3 | 5 | 11 | 5 |
| Febrile neutropenia | 1 | 0 | 1 | 3 | 3 | 2 |
| Hospitalization | 2 | 0 | 3 | 5 | 13 | 7 |
Thirty incidences in which hospitalization was necessary secondary to chemotherapy toxicity were recorded and represented 5% of all chemotherapy administrations (Table 3). Twenty‐two dogs (34%) were hospitalized during chemotherapy; 17 dogs were hospitalized once, 3 dogs twice, and 1 dog each 3 or 4 times. Considering the 17 dogs hospitalized once, the cause of hospitalization was febrile neutropenia for 7 dogs, grade 2 and grade 3 gastrointestinal toxicity for 2 and 6 dogs, respectively, and aspiration pneumonia for 1 dog. No 2 dogs were hospitalized for the same series of adverse events. Of the 3 dogs hospitalized twice, causes included 2 episodes of grade 2 gastrointestinal toxicity, grade 3 gastrointestinal toxicity and febrile neutropenia, and grade 3 gastrointestinal toxicity and SHC. The dog that was hospitalized on 3 occasions developed febrile neutropenia twice and grade 3 gastrointestinal toxicity. The dog hospitalized 4 times developed 3 episodes of grade 3 gastrointestinal toxicity and 1 episode of grade 2 gastrointestinal toxicity. One dog died in hospital after developing aspiration pneumonia and respiratory distress. All remaining dogs were discharged after a median hospital stay of 1 day (range, 1–7 days). No significant difference was observed in the incidence of hospitalization in dogs stratified by prechemotherapy ANC class.
Of 569 chemotherapy treatments administered, 194 neutropenic episodes (34%) were documented (Fig 1). Postchemotherapy neutropenia was more likely to occur in ANC class C3 compared to C5 (P < 0.001) and C6 (P = 0.002). However, postchemotherapy neutropenia in class C3 was mostly represented by grade 1 neutropenia (18 episodes), and only 6 postchemotherapy neutropenic episodes were ≥ grade 2. When only postchemotherapy neutropenia ≥ grade 2 was evaluated, no significant difference was observed between the ANC class C3 compared to C5 (P = 0.256) and C6 (P = 0.922). No significant differences were identified when other classes were compared in post hoc analysis. In particular, no significant difference in postchemotherapy neutropenia was observed when the ANC classes C1 and C2 were compared to C4, C5, or C6 (P > 0.05). Also, no significant difference was found among the ANC classes C1, C2, and C3 (P = 0.22).
Figure 1.
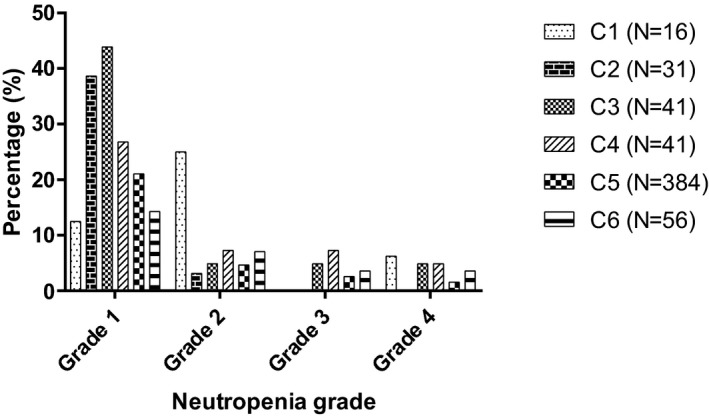
Incidence of postchemotherapy neutropenia after administration of 569 chemotherapy treatments, stratified by prechemotherapy ANC classes. Postchemotherapy neutropenia was graded according to the VCOG‐CTCAE guidelines version 1.1.29 Prechemotherapy ANC was stratified in 6 classes: class C1) 1.5–1.99 × 103/μL (N = 16), class C2) 2.0–2.49 × 103/μL (N = 31), class C3) 2.5–2.99 × 103/μL (N = 41), class C4) 3.0–3.49 × 103/μL (N = 41), class C5) 3.5–12.0 × 103/μL (N = 384), and class C6) > 12.0 × 103/μL (N = 56). There was a significant difference between the prechemotherapy ANC classes C3 and C5 (P < 0.001), and C3 and C6 (P = 0.002).
Overall, 10 episodes of febrile neutropenia occurred, representing 1.8% of all chemotherapy administrations (Table 3). Five dogs had grade IV neutropenia, 4 had grade III neutropenia, and 1 had grade I neutropenia at presentation. Drugs administered before febrile neutropenia included the following: doxorubicin (8), vincristine (1), and cyclophosphamide (1). Most (6) instances of febrile neutropenia occurred in the first cycle of CHOP, whereas 1 occurred in the second cycle and 3 occurred in the third cycle. Similarly, most (5) instances occurred at the initial administration of the drug, whereas 2 cases occurred after the second drug exposure and 3 occurred after a third exposure. No significant difference was identified in the incidence of febrile neutropenia in dogs stratified by prechemotherapy ANC class.
Of chemotherapy treatments administered, 204 (35.6%) were associated with nonhematologic toxicities. When stratified by prechemotherapy ANC class, no significant difference was found in the occurrence of nonhematologic toxicity among the ANC classes (P = 0.603; Fig 2).
Figure 2.
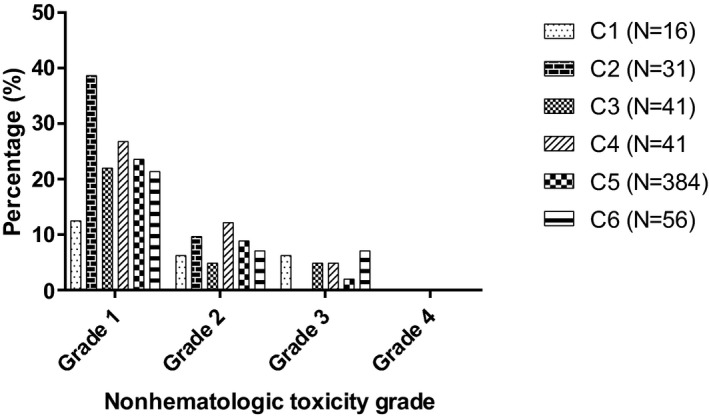
Incidence of postchemotherapy nonhematologic toxicity after administration of 569 chemotherapy treatments, stratified by prechemotherapy ANC classes. Postchemotherapy nonhematologic toxicity (constitutional or gastrointestinal) was graded according to the VCOG‐CTCAE guidelines version 1.1.29 Prechemotherapy ANC was stratified in 6 classes: class C1) 1.5–1.99 × 103/μL (N = 16), class C2) 2.0–2.49 × 103/μL (N = 31), class C3) 2.5–2.99 × 103/μL (N = 41), class C4) 3.0–3.49 × 103/μL (N = 41), class C5) 3.5–12.0 × 103/μL (N = 384), and class C6) > 12.0 × 103/μL (N = 56). There was no significant difference between the prechemotherapy ANC classes (P = 0.603).
Assessment of ANC Cutoff for Prophylactic Antibiotics Prescription
Thirty‐eight chemotherapy dose delays (7%) occurred because of prechemotherapy neutropenia, whereas dogs were afebrile and clinically well. In 6 of the dose delays (16%), dogs had prechemotherapy ANC between 0.75 and 0.99 × 103/μL, and in 22 instances (58%), dogs had prechemotherapy ANC between 1.0 and 1.49 × 103/μL. None of the dogs received prophylactic antibiotics, and all recovered well without supportive care. In 10 of the 38 dose delays (26%), dogs had prechemotherapy ANC <0.75 × 103/μL and received prophylactic antibiotics PO. None received additional supportive care.
Assessment of Additional Factors Affecting Chemotherapy‐Induced Neutropenia
The prechemotherapy neutrophil : lymphocyte count ratio varied from 0.17 to 98.5, with a median of 5.43. Ordinal regression showed no evidence of an association between prechemotherapy neutrophil : lymphocyte count ratio and the occurrence of neutropenia (P = 0.822).
Fifty‐eight dogs were treated with 1 CHOP chemotherapy protocol, 4 dogs were prescribed a second CHOP chemotherapy protocol, and 1 dog underwent 4 CHOP chemotherapy protocols. Ordinal regression showed no evidence of an association between the number of CHOP protocols and the occurrence of neutropenia (P = 0.492).
The initial body weight varied from 6.2 to 75.2 kg, with a median of 26.7 kg. Ordinal regression showed no evidence of an association between initial body weight and occurrence of neutropenia (P = 0.063). Initial body condition score varied from 3/9 to 8/9, with a median of 5/9. No significant association was observed between the initial body condition score and development of neutropenia (P = 0.822).
Discussion
Maintaining good quality of life during chemotherapy is a goal for the majority of owners of cancer‐bearing companion animals. Different strategies to alleviate adverse effects of chemotherapy may be employed and typically are dependent on the clinician, the individual pet owner's expectations. Supportive care including prophylactic medication, dose modifications (dose delay, dose reduction, or both), drug substitution, and ultimately cessation of chemotherapy are options considered when treating pets and are similar to strategies employed in humans.4, 25, 33, 40 In human patients, chemotherapy dose delays and dose reductions may negatively affect outcome, and a concerted effort is made to maintain DI.41 General guidelines for chemotherapy dose modifications have been suggested in an attempt to standardize chemotherapy administration and minimize potential impact on patient outcome.25, 33, 40, 42 For example, the South East London Cancer Network recommends a chemotherapy dose delay after a prechemotherapy ANC <1.0 × 103/μL with a 25% dose reduction in subsequent treatments if the delay was >1 week.40 Likewise, in veterinary oncology, chemotherapy dose delay followed by a 20% dose reduction has been recommended when the prechemotherapy ANC <1.5 × 103/μL,5 but there are little data to support this reduction. Nonetheless, ANC cutoff values of 2.5 × 103/μL and 2.0 × 103/μL continue to be used.6, 7, 8, 12
Given the lack of evidence supporting specific recommendations for prechemotherapy ANC cutoff values in clinical practice, the potential impact of the most commonly used ANC cutoffs (1.5 × 103/μL, 2.0 × 103/μL and 2.5 × 103/μL) was assessed using a subset of dogs subjected to the lowest cutoff. As expected, the higher the ANC cutoff, the higher the number of dose delays and potential dose reductions. Importantly, no significant difference was observed in the development of toxicity in dogs when their ANC decreased between 1.5 and 2.0 × 103/μL compared to higher cutoff values. A significant difference in the development of neutropenia was noted when ANC class C3 was compared to either C5 or C6, but no significant increased risk of neutropenia was found between C1 and C2 when compared to C5 and C6. As it is difficult to identify a reasonable biological explanation for ANC class C3 to be the only class associated with a higher likelihood of neutropenia than for classes with normal neutrophil count or neutrophilia, and this finding likely represents type I error. Also, most of the postchemotherapy neutropenic episodes in ANC class C3 were grade 1 neutropenia, and no significant difference in the development of ≥ grade 2 neutropenia was found between ANC class C3 when compared to C5 or C6. Because grade 1 neutropenia does not result in chemotherapy dose delay or dose reduction, the statistical significance in the development of neutropenia between the ANC class C3 compared to C5 and C6 is of questionable clinical relevance.
No significant association was identified between the likelihood of neutropenia and additional factors investigated. Physiologic, corticosteroid‐induced, and inflammatory leukocytoses all can affect the neutrophil : lymphocyte ratio, and all can be present in cancer‐bearing dogs.43 The neutrophil : lymphocyte ratio previously has been shown to be of clinical value in patients with cancer,44 but it was not associated with the likelihood of neutropenia in our study. The impact of the number of CHOP protocols on chemotherapy‐induced neutropenia was assessed to evaluate for possible cumulative bone marrow toxicity. Although we did not find a significant change in the likelihood of neutropenia with previous CHOP protocols in our study, it is possible that inclusion of other drugs such as lomustine in an induction protocol may alter the risk of cumulative myelosuppression. The impact of body weight on chemotherapy‐induced neutropenia also was assessed, because lower body weight previously has been associated with development of sepsis, but no significant association between weight at diagnosis and neutropenia was identified.45 However, dosage reductions are integral to the CHOP protocol in that dogs <10 kg already receive decreased chemotherapy dosage for some drugs (ie, doxorubicin is dosed at 25 mg/m2 instead of 30 mg/m2 for dogs < 10 kg). Vincristine and cyclophosphamide were dosed similarly across body weights and body condition scores. Because body condition score previously was associated with chemotherapy toxicity and the effect of cachexia and obesity on chemotherapy dosing is controversial in humans, we also evaluated if body condition score was associated with neutropenia.46 Body condition score, however, was not assigned by the same individual, a factor that may have affected the findings.
Using the standard ANC cutoff of 1.5 × 103/μL, 34% of dogs experienced at least 1 dose delay because of prechemotherapy neutropenia. However, had the cutoff been 2.5 × 103/μL (and all CBC data remained the same), approximately 50% of dogs would have experienced a delay and potentially dose reduction, thus effectively decreasing the RDI. Lowering the ANC cutoff to the lowest tolerable level may avoid unnecessary dose reductions and maintain DI throughout treatment. This concept is difficult to evaluate in clinical practice, especially because clinician preference often guides practice and standards can be challenging to implement. Our study presents hypothetical results because it assumes that all dogs would have identical CBC data regardless of the timing of chemotherapy administration, dosage administered, or both (which likely would have been altered with higher ANC cutoff values for intervention). Although it would be ideal to randomize dogs to variable ANC class levels so as to permit treatment and subsequently assess changes in the rate of toxicity, remission status, and remission duration, this approach is impractical if clinicians firmly believe the cutoff being used in their practice is correct. Multi‐institutional data could be combined whereby clinicians and practices use different criteria for treatment, but other protocols such as percentage of dose reduction, antibiotic selection, and duration of treatment may be more difficult to institute.
The term DI is used to define the drug dose delivered per time unit and is expressed as mg/m2 per week. Treatment delays and dose reductions therefore decrease DI. To characterize modifications of chemotherapy schemes, the concept of RDI, which reflects delivered DI divided by standard DI, has been introduced.47 A decrease in RDI in human patients with diffuse large B‐cell lymphoma who are treated with rituximab‐CHOP (R‐CHOP) is associated with poorer outcome.18, 20, 21 In 1 study in which dogs with lymphoma were treated with multidrug chemotherapy and half‐body irradiation, development of grade III or IV neutropenia was positively associated with remission duration, but RDI was not associated with outcome.23 In another study, dogs experiencing neutropenia during the first 9 weeks of CHOP‐based treatment had prolonged first remission duration.22 Notably, 76.9% of the neutropenia episodes in this study were grade I.22
Treatment delays also have been associated with improved outcome.7, 39 In both of these studies, treatments were delayed if the prechemotherapy ANC was <2.0 × 103/μL and the authors postulated that improved survival was attributed to neutropenic episodes. These studies indirectly support the notion that prechemotherapy ANC <2.0 × 103/μL may be associated with improved outcome measures.5, 23, 31 Comparatively, in human oncology, it has been noted since the 1970s that neutropenia is associated with improved outcome measures in several tumor types and chemotherapy protocols.48 Taken together, these data emphasize the need to investigate prechemotherapy ANC in dogs and whether a cutoff such as 1.5 × 103/μL improves remission duration. However, the exact role of RDI in veterinary medicine is unclear. Even in human oncology, RDI may have different impacts across tumor types,49 and possible artifacts might complicate its interpretation.50
Most chemotherapy dosages are based on the patient's body surface area, which does not correlate well with drug pharmacokinetics.51, 52, 53, 54 Because DI is related to chemotherapy dosage, RDI may not be clinically important in dogs with lymphoma.52, 53, 55, 56 However, a higher proportion of dogs than necessary may receive a suboptimal chemotherapy dosage after a dose delay if the neutrophil cutoff is high, making it difficult to assess the effects of RDI. Bone marrow suppression, and neutropenia in particular, may be indicative of more effective dosing. This hypothesis has, in part, led to individualized pharmacokinetic dosing of common chemotherapeutic drugs in human oncology, and early studies have suggested a potential to decrease toxicity and improve disease‐free intervals.57, 58, 59, 60 Monitoring of neutrophil count as a barometer for chemotherapy dosage and efficacy is appealing because of its simplicity and cost‐effectiveness in veterinary medicine. One dosing strategy that could be evaluated would be dose escalation in each dog until achieving the lowest safe prechemotherapy ANC. Determination of a safe prechemotherapy ANC and its timing is challenging, but 1.5 × 103/μL, could be a starting point based on our study. An alternative strategy might be to use the neutrophil nadir as a target and to aim to achieve an ANC of 0.75–1.0 × 103/μL at the time of expected nadir and maintain an ANC of at least 1.5 × 103/μL for subsequent chemotherapy administration. It is prudent before undertaking extensive study to fully determine whether RDI, neutropenia, or both impact remission duration. Although individualized dosing is attractive and may provide a benefit long‐term, it may be costly and time‐consuming for some clients, particularly if more aggressive monitoring and chemotherapy administration are undertaken.
Relative dose intensity may also be more heavily dependent on some drugs in the CHOP protocol. Indeed, a relative dose reduction in vincristine has been associated with poorer outcome in human patients diagnosed with diffuse large B‐cell lymphoma treated with R‐CHOP.19 In a recent study investigating the use of 25‐week CHOP in dogs, the authors suggested a temporal relationship existed between relapse of lymphoma and cyclophosphamide.61 Although further investigation is necessary to confirm this observation, efforts could be focused on improving DI of drugs considered most useful for the disease. Results provided here suggest that the severity of prechemotherapy neutropenia is not necessarily associated with an increase in postchemotherapy nonhematologic toxicity. Therefore, individualization of chemotherapy dosage along with optimization of RDI may improve remission status without compromising the dog's quality of life.
A secondary objective of our study was to provide an audit of a current protocol for the management of neutropenic dogs. Antibiotic resistance has become a substantial concern in both veterinary and human medicine,24 and prophylactic antibiotic treatment should be used thoughtfully, and only when deemed necessary. In human medicine, the routine use of prophylactic antibiotics typically is reserved for patients expected to develop profound neutropenia, which is variably defined (≤0.1 × 103/μL to ≤0.5 × 103/μL). Most trials investigating prophylactic antibiotics have been used in patients receiving high‐dose chemotherapy for hematologic malignancies and have demonstrated a decrease in mortality.39, 40, 41 Antibacterial prophylaxis now is considered in the current National Institute for Health and Care Excellence (NICE) guidelines for the prevention of febrile neutropenia in people with acute leukemias, stem cell transplants, or solid tumors when neutropenia ≤0.5 × 103/μL is expected.42 When considering the risk of neutropenic sepsis, the severity and duration of neutropenia are important, but other factors such as tumor type, underlying immunologic function, and potentially environmental exposure also influence the risk of neutropenic sepsis.40, 43, 44 In veterinary medicine, the neutrophil nadir is not known for each animal although estimates are made to determine the timing of each CBC. An ANC cutoff of 1.0 × 103/μL has been suggested to trigger the prescription of prophylactic antibiotics in afebrile neutropenic dogs but, as with prechemotherapy ANC values, there is little evidence to support this guideline.4, 5, 16 Clinicians may be reluctant to use a lower neutrophil cutoff to allow a margin of safety if the nadir neutrophil count was not truly captured, or because they are less tolerant of adverse chemotherapy events in pets than are their counterparts in human medicine.
Ours is the first study to suggest that an ANC cutoff as low as 0.75 × 103/μL to trigger prophylactic antibiotic prescription may be reasonable for clinical use. Six dogs presented with afebrile grade 3 neutropenia at an ANC nadir between 0.75 and 1.0 × 103/μL, and all recovered spontaneously without need for intervention. Further studies are necessary to determine whether this cutoff is reasonable as a standardized recommendation in clinical practice.
Although the percentage of dogs that experienced hospitalization (34%) in our study was higher than in previous reports (9.8–11.5%),7, 39 the overall incidence of hospitalization was 5%, as previously reported.4, 5 Upon evaluation of VCOG toxicity scores, actual scores were similar to those previously reported.62, 63 The relatively higher rate of hospitalization may have resulted from the fact that there is subjectivity about the need for hospitalization, which may relate to the clinician who evaluated the case, comfort level of the client, or both. Notably, a similar proportion of dogs that developed ≥ grade 3 gastrointestinal toxicity was similar to previous reports, and fewer dogs (38%) in our study required dose reduction compared to previous reports (54–62%).7, 39 Because our neutrophil cutoff was lower than that of other reports, we may have had fewer dose delays and fewer dose reductions may have occurred.
Similarly, the incidence of presumed SHC (3.1%) was higher than expected despite the concurrent use of furosemide at the time of cyclophosphamide administration. Although only 2 dogs developed presumptive SHC, diagnosis was based on clinical signs, abdominal ultrasound examination, urinalysis, and negative bacterial culture. In both dogs, presumed SHC resolved within 48 hours, similar to previous reports.64 Although the assumption was made that both dogs developed cyclophosphamide‐induced SHC to avoid underestimating the risk of urinary toxicity, rapid resolution also may have been consistent with non‐chemotherapy‐associated cystitis.
Our study had several limitations. Despite the 615 prechemotherapy CBCs collected for the study, there were relatively low numbers of prechemotherapy CBCs with an ANC within the lower classes (C1, C2, and C3). This situation might have led to type II error in the determination of an association between prechemotherapy ANC classes and toxicity. However, this finding further supports the contention that a prechemotherapy ANC of 1.5 × 103/μL is not associated with a higher likelihood of neutropenia. Complete blood counts were assessed at predetermined time points in the protocol, unless clinical illness supported an additional CBC. Because the timing of the neutrophil nadir likely is variable across dogs, the severity of neutropenia may have been different in some dogs. It is difficult to justify collecting serial (daily) CBC data in dogs with lymphoma during a weekly chemotherapy protocol given concerns about quality of life and potential occupational exposure, but that approach would be necessary to more accurately capture neutropenic episodes. Not all dogs were fully staged with bone marrow assessment, and missing bone marrow involvement could have affected the neutrophil counts. The lowest ANC cutoff evaluated for chemotherapy administration (ie, 1.5 × 103/μL) was applied to all dogs in our study, but the results obtained might have been different if the 3 ANC cutoffs evaluated would have been applied in 3 different groups of dogs. However, this was not performed for ethical reasons, and our approach seemed reasonable given that dogs received chemotherapy at the lowest ANC cutoff. The ANC classes were selected arbitrarily based on other published reports in the veterinary literature, and other stratification schemes may have altered results. Despite efforts to standardize a toxicity grading scale, grading of nonhematologic toxicities was partially influenced by the observations of owners. Owners also had an influence on the decision for hospitalization because some owners were more averse than others to managing toxicity at home. These limitations are inherent to the VCOG‐CTCAE criteria, which do not define when medications or hospitalization are indicated.29 Also, our study only assessed the use of CHOP in dogs with intermediate to high‐grade lymphoma and the results do not necessarily translate to other protocols and tumor types.
Conclusions
In this population of dogs with lymphoma receiving 19‐week CHOP chemotherapy, an ANC cutoff of 1.5 × 103/μL for chemotherapy administration allowed a higher number of dogs to receive scheduled treatment when compared to other reported ANC cutoffs for administration. Prechemotherapy ANC was not associated with the incidence of chemotherapy‐induced toxicity. It is necessary to determine whether alterations in ANC cutoff and improvements in RDI improve remission durations. Because there is concern for indiscriminate antibiotic use, it is also necessary to critically evaluate current prescription policies. An ANC cutoff near 0.75 × 103/μL for prophylactic antibiotic usage was well tolerated in this population of dogs, but future studies will be necessary to determine whether this same experience can be documented in a larger number of dogs.
Acknowledgments
The authors thank all members of the Oncology Service of the R(D)SVS who contributed to the management of the dogs included in this study.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
The authors acknowledge the off‐label use of trimethoprim–sulfonamide and enrofloxacin, as prescribed prophylactically in neutropenic dogs. However, the aim of this study was to provide information to reduce the indiscriminate use of prophylactic antibiotics.
Data presented in part at the European College of Veterinary Internal Medicine‐Companion Animal Annual Congress, Goteborg, Sweden, 2016.
Footnotes
aMinitab 17 Statistical Software (2010). [Computer software]. State College, PA: Minitab, Inc. (www.minitab.com)
bGraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla, CA, www.graphpad.com
References
- 1. Mellanby RJ, Herrtage ME, Dobson JM. Owners’ assessments of their dog's quality of life during palliative chemotherapy for lymphoma. J Small Anim Pract 2003;44:100–103. [DOI] [PubMed] [Google Scholar]
- 2. Vols KK, Heden MA, Kristensen AT, et al. Quality of life assessment in dogs and cats receiving chemotherapy—A review of current methods. Vet Comp Oncol 2016;15:684–691. [DOI] [PubMed] [Google Scholar]
- 3. Vail DM. Cancer clinical trials: Development and implementation. Vet Clin North Am Small Anim Pract 2007;37:1033. [DOI] [PubMed] [Google Scholar]
- 4. Vail DM. Supporting the veterinary cancer patient on chemotherapy: Neutropenia and gastrointestinal toxicity. Top Companion Anim Med 2009;24:122–129. [DOI] [PubMed] [Google Scholar]
- 5. Thamm DH, Vail DM. Aftershocks of cancer chemotherapy: Managing adverse effects. J Am Anim Hosp Assoc 2007;43:1–7. [DOI] [PubMed] [Google Scholar]
- 6. Daters AT, Mauldin GE, Mauldin GN, et al. Evaluation of a multidrug chemotherapy protocol with mitoxantrone based maintenance (CHOP‐MA) for the treatment of canine lymphoma. Vet Comp Oncol 2010;8:11–22. [DOI] [PubMed] [Google Scholar]
- 7. Sorenmo K, Overley B, Krick E, et al. Outcome and toxicity associated with a dose‐intensified, maintenance‐free CHOP‐based chemotherapy protocol in canine lymphoma: 130 cases. Vet Comp Oncol 2010;8:196–208. [DOI] [PubMed] [Google Scholar]
- 8. Curran K, Thamm DH. Retrospective analysis for treatment of naive canine multicentric lymphoma with a 15‐week, maintenance‐free CHOP protocol. Vet Comp Oncol 2016;14(Suppl 1):147–155. [DOI] [PubMed] [Google Scholar]
- 9. Price GS, Page RL, Fischer BM, et al. Efficacy and toxicity of doxorubicin/cyclophosphamide maintenance therapy in dogs with multicentric lymphosarcoma. J Vet Intern Med 1991;5:259–262. [DOI] [PubMed] [Google Scholar]
- 10. Keller ET, Macewen EG, Rosenthal RC, et al. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. J Vet Intern Med 1993;7:289–295. [DOI] [PubMed] [Google Scholar]
- 11. Siedlecki CT, Kass PH, Jakubiak MJ, et al. Evaluation of an actinomycin‐D‐containing combination chemotherapy protocol with extended maintenance therapy for canine lymphoma. Can Vet J 2006;47:52–59. [PMC free article] [PubMed] [Google Scholar]
- 12. Parsons‐Doherty M, Poirier VJ, Monteith G. The efficacy and adverse event profile of dexamethasone, melphalan, actinomycin D, and cytosine arabinoside (DMAC) chemotherapy in relapsed canine lymphoma. Can Vet J 2014;55:175. [PMC free article] [PubMed] [Google Scholar]
- 13. LeBlanc AK, Mauldin GE, Milner RJ, et al. Efficacy and toxicity of BOPP and LOPP chemotherapy for the treatment of relapsed canine lymphoma. Vet Comp Oncol 2006;4:21–32. [DOI] [PubMed] [Google Scholar]
- 14. Ponce F, Magnol JP, Ledieu D, et al. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet J 2004;167:158–166. [DOI] [PubMed] [Google Scholar]
- 15. Rassnick KM, Bailey DB, Malone EK, et al. Comparison between L‐CHOP and an L‐CHOP protocol with interposed treatments of CCNU and MOPP (L‐CHOP‐CCNU‐MOPP) for lymphoma in dogs. Vet Comp Oncol 2010;8:243–253. [DOI] [PubMed] [Google Scholar]
- 16. Boudreaux B. Antimicrobial use in the veterinary cancer patient. Vet Clin North Am Small Anim Pract 2014;44:883–891. [DOI] [PubMed] [Google Scholar]
- 17. Meyer RM, Hryniuk WM, Goodyear MD. The role of dose intensity in determining outcome in intermediate‐grade non‐Hodgkin's lymphoma. J Clin Oncol 1991;9:339–347. [DOI] [PubMed] [Google Scholar]
- 18. Gutierrez A, Bento L, Bautista‐Gili AM, et al. Differential impact of relative dose‐intensity reductions in diffuse large B‐cell lymphoma treated with R‐CHOP21 or R‐CHOP14. PLoS One 2015;10:e0123978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Utsu Y, Takaishi K, Inagaki S, et al. Influence of dose reduction of vincristine in R‐CHOP on outcomes of diffuse large B cell lymphoma. Ann Hematol 2016;95:41–47. [DOI] [PubMed] [Google Scholar]
- 20. Terada Y, Nakamae H, Aimoto R, et al. Impact of relative dose intensity (RDI) in CHOP combined with rituximab (R‐CHOP) on survival in diffuse large B‐cell lymphoma. J Exp Clin Cancer Res 2009;28:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirakawa T, Yamaguchi H, Yokose N, et al. Importance of maintaining the relative dose intensity of CHOP‐like regimens combined with rituximab in patients with diffuse large B‐cell lymphoma. Ann Hematol 2010;89:897–904. [DOI] [PubMed] [Google Scholar]
- 22. Wang SL, Lee JJ, Liao AT. Chemotherapy‐induced neutropenia is associated with prolonged remission duration and survival time in canine lymphoma. Vet J 2015;205:69–73. [DOI] [PubMed] [Google Scholar]
- 23. Vaughan A, Johnson JL, Williams LE. Impact of chemotherapeutic dose intensity and hematologic toxicity on first remission duration in dogs with lymphoma treated with a chemoradiotherapy protocol. J Vet Intern Med 2007;21:1332–1339. [DOI] [PubMed] [Google Scholar]
- 24. Paphitou NI. Antimicrobial resistance: Action to combat the rising microbial challenges. Int J Antimicrob Agents 2013;42(Suppl):S25–S28. [DOI] [PubMed] [Google Scholar]
- 25. Phillips R, Hancock B, Graham J, et al. Guidelines: Prevention and management of neutropenic sepsis in patients with cancer: Summary of NICE guidance. BMJ 2012;345:47–49. [DOI] [PubMed] [Google Scholar]
- 26. Abrams‐Ogg ACG, Kruth SA. Antimicrobial therapy for the neutropenic dog and cat In: Warren Kirk R, ed. Kirk's Current Veterinary Therapy XIII. Philadelphia, PA: Saunders; 2000:267–272. [Google Scholar]
- 27. Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013;31:794. [DOI] [PubMed] [Google Scholar]
- 28. MacDonald VS, Thamm DH, Kurzman ID, et al. Does L‐asparaginase influence efficacy or toxicity when added to a standard CHOP protocol for dogs with lymphoma? J Vet Intern Med 2005;19:732–736. [DOI] [PubMed] [Google Scholar]
- 29. Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol 2016;14:417–446. [DOI] [PubMed] [Google Scholar]
- 30. Lo N, Cullen M. Antibiotic prophylaxis in chemotherapy‐induced neutropenia: Time to reconsider. Hematol Oncol 2006;24:120–125. [DOI] [PubMed] [Google Scholar]
- 31. Wingard JR, Elmongy M. Strategies for minimizing complications of neutropenia: Prophylactic myeloid growth factors or antibiotics. Crit Rev Oncol Hematol 2009;72:144–154. [DOI] [PubMed] [Google Scholar]
- 32. Cullen M, Baijal S. Prevention of febrile neutropenia: Use of prophylactic antibiotics. Br J Cancer 2009;101(Suppl 1):S11–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gafter‐Gvili A, Fraser A, Paul M, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev 2012;1:CD004386. [DOI] [PubMed] [Google Scholar]
- 34. Aksoy DY, Tanriover MD, Uzun O, et al. Diarrhea in neutropenic patients: A prospective cohort study with emphasis on neutropenic enterocolitis. Ann Oncol 2007;18:183–189. [DOI] [PubMed] [Google Scholar]
- 35. Weese JS, Giguere S, Guardabassi L, et al. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J Vet Intern Med 2015;29:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Britton BM, Kelleher ME, Gregor TP, et al. Evaluation of factors associated with prolonged hospital stay and outcome of febrile neutropenic patients receiving chemotherapy: 70 cases (1997–2010). Vet Comp Oncol 2014;12:266–276. [DOI] [PubMed] [Google Scholar]
- 37. Zar J. Multiple Comparisons In: Biostatistical analysis, ed. Englewood Cliff, 4th ed. Upper Saddle River, NJ: Prentice Hall; 1999:564. [Google Scholar]
- 38. Freeman L, Becvarova I, Cave N, et al. WSAVA nutritional assessment guidelines. J Small Anim Pract 2011;52:385–396. [DOI] [PubMed] [Google Scholar]
- 39. Burton JH, Garrett‐Mayer E, Thamm DH. Evaluation of a 15‐week CHOP protocol for the treatment of canine multicentric lymphoma. Vet Comp Oncol 2013;11:306–315. [DOI] [PubMed] [Google Scholar]
- 40. London Cancer Alliance [Internet] . Generic Chemotherapy Protocol Guidelines; 2013. [about 8 screens]. Available from: http://www.londoncanceralliance.nhs.uk/media/56148/SELCN_Generic%20Chemo_Guidelines%20April%202013.pdf. Accessed June 3, 2017. [Google Scholar]
- 41. Chang J. Chemotherapy dose reduction and delay in clinical practice: Evaluating the risk to patient outcome in adjuvant chemotherapy for breast cancer. Eur J Cancer 2000;36:11–14. [DOI] [PubMed] [Google Scholar]
- 42. Bate J, Gibson F, Johnson E, et al. Neutropenic sepsis: Prevention and management of neutropenic sepsis in cancer patients (NICE Clinical Guideline CG151). Arch Dis Child Educ Pract Ed 2013;98:73–75. [DOI] [PubMed] [Google Scholar]
- 43. Schultze AE. Interpretation of canine leukocyte responses In: Weiss DJ, Wardrop KJ, eds. Schalm's Veterinary Hematology, 6th ed. Danvers, MA: Wiley‐Blackwell; 2010:321–334. [Google Scholar]
- 44. Faria SS, Fernandes PC Jr, Silva MJ, et al. The neutrophil‐to‐lymphocyte ratio: A narrative review. Ecancermedicalscience 2016;10:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sorenmo KU, Harwood LP, King LG, et al. Case‐control study to evaluate risk factors for the development of sepsis (neutropenia and fever) in dogs receiving chemotherapy. J Am Vet Med Assoc 2010;236:650–656. [DOI] [PubMed] [Google Scholar]
- 46. Lyman GH, Sparreboom A. Chemotherapy dosing in overweight and obese patients with cancer. Nat Rev Clin Oncol 2013;10:451–459. [DOI] [PubMed] [Google Scholar]
- 47. Tjan‐Heijnen VCG, Wagener DJT, Postmus PE. An analysis of chemotherapy dose and dose‐intensity in small‐cell lung cancer: Lessons to be drawn. Ann Oncol 2002;13:1519–1530. [DOI] [PubMed] [Google Scholar]
- 48. Gurney H. How to calculate the dose of chemotherapy. Br J Cancer 2002;86:1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liutkauskiene S, Janciauskiene R, Jureniene K, et al. Retrospective analysis of the impact of platinum dose reduction and chemotherapy delays on the outcomes of stage III ovarian cancer patients. BMC Cancer 2015;15:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loibl S, Nekljudova V, von Minckwitz G, et al. Evaluating the impact of Relative Total Dose Intensity (RTDI) on patients’ short and long‐term outcome in taxane‐ and anthracycline‐based chemotherapy of metastatic breast cancer—A pooled analysis. BMC Cancer 2011;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walko CM, Ikediobi O, Saltiel M. Pharmacogenomic applications in oncology. J Pharm Pract 2012;25:439–446. [DOI] [PubMed] [Google Scholar]
- 52. Gurney H. Dose calculation of anticancer drugs: A review of the current practice and introduction of an alternative. J Clin Oncol 1996;14:2590–2611. [DOI] [PubMed] [Google Scholar]
- 53. Gao B, Klumpen HJ, Gurney H. Dose calculation of anticancer drugs. Expert Opin Drug Metab Toxicol 2008;4:1307–1319. [DOI] [PubMed] [Google Scholar]
- 54. Walko CM, McLeod H. Pharmacogenomic progress in individualized dosing of key drugs for cancer patients. Nat Clin Pract Oncol 2009;6:153–162. [DOI] [PubMed] [Google Scholar]
- 55. Freireich EJ, Gehan EA, Rall DP, et al. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep 1966;50:219–244. [PubMed] [Google Scholar]
- 56. Pinkel D. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Can Res 1958;18:853. [PubMed] [Google Scholar]
- 57. Engels FK, Loos WJ, Van Der Bol JM, et al. Therapeutic drug monitoring for the individualization of docetaxel dosing: A randomized pharmacokinetic study. Clin Cancer Res 2011;17:353–362. [DOI] [PubMed] [Google Scholar]
- 58. Rousseau A, Marquet P. Application of pharmacokinetic modelling to the routine therapeutic drug monitoring of anticancer drugs. Fundam Clin Pharmacol 2002;16:253–262. [DOI] [PubMed] [Google Scholar]
- 59. Salas S, Mercier C, Baciuchka‐Palmaro M, et al. Therapeutic drug monitoring for dose individualization of cisplatin in testicular cancer patients based upon total platinum measurement in plasma. Ther Drug Monit 2006;28:532–539. [DOI] [PubMed] [Google Scholar]
- 60. Gamelin E, Delva R, Jacob J, et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow‐up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2099–2105. [DOI] [PubMed] [Google Scholar]
- 61. Wang S‐L, Lee J‐J, Liao AT. Assessment of temporal association of relapse of canine multicentric lymphoma with components of the CHOP protocol: Is cyclophosphamide the weakest link? Vet J 2016;213:87–89. [DOI] [PubMed] [Google Scholar]
- 62. Hosoya K, Kisseberth WC, Lord LK, et al. Comparison of COAP and UW‐19 protocols for dogs with multicentric lymphoma. J Vet Intern Med 2007;21:1355–1363. [DOI] [PubMed] [Google Scholar]
- 63. Tomiyasu H, Takahashi M, Fujino Y, et al. Gastrointestinal and hematologic adverse events after administration of vincristine, cyclophosphamide, and doxorubicin in dogs with lymphoma that underwent a combination multidrug chemotherapy protocol. J Vet Med Sci 2010;72:1391–1397. [DOI] [PubMed] [Google Scholar]
- 64. Charney SC, Bergman PJ, Hohenhaus AE, et al. Risk factors for sterile hemorrhagic cystitis in dogs with lymphoma receiving cyclophosphamide with or without concurrent administration of furosemide: 216 cases (1990–1996). J Am Vet Med Assoc 2003;222:1388–1393. [DOI] [PubMed] [Google Scholar]


