Abstract
Background
Atrial fibrillation (AF) usually is associated with a rapid ventricular rate. The optimal heart rate (HR) during AF is unknown.
Hypothesis/Objectives
Heart rate affects survival in dogs with chronic AF.
Animals
Forty‐six dogs with AF and 24‐hour ambulatory recordings were evaluated.
Methods
Retrospective study. Holter‐derived HR variables were analyzed as follows: mean HR (meanHR, 24‐hour average), minimum HR (minHR, 1‐minute average), maximum HR (maxHR, 1‐minute average). Survival times were recorded from the time of presumed adequate rate control. The primary endpoint was all‐cause mortality. Cox proportional hazards analysis identified variables independently associated with survival; Kaplan‐Meier survival analysis estimated the median survival time of dogs with meanHR <125 bpm versus ≥125 bpm.
Results
All 46 dogs had structural heart disease; 31 of 46 had congestive heart failure (CHF), 44 of 46 received antiarrhythmic drugs. Of 15 dogs with cardiac death, 14 had CHF. Median time to all‐cause death was 524 days (Interquartile range (IQR), 76–1,037 days). MeanHR was 125 bpm (range, 62–203 bpm), minHR was 82 bpm (range, 37–163 bpm), maxHR was 217 bpm (range, 126–307 bpm). These were significantly correlated with all‐cause and cardiac‐related mortality. For every 10 bpm increase in meanHR, the risk of all‐cause mortality increased by 35% (hazard ratio, 1.35; 95% CI, 1.17–1.55; P < 0.001). Median survival time of dogs with meanHR<125 bpm (n = 23) was significantly longer (1,037 days; range, 524‐open) than meanHR ≥125 bpm (n = 23; 105 days; range, 67–267 days; P = 0.0012). Mean HR was independently associated with all‐cause and cardiovascular mortality (P < 0.003).
Conclusions and Clinical Importance
Holter‐derived meanHR affects survival in dogs with AF. Dogs with meanHR <125 bpm lived longer than those with meanHR ≥ 125 bpm.
Keywords: Electrocardiography, Heart failure, Holter, Rate control, Ventricular rate
Abbreviations
- AF
atrial fibrillation
- CHF
congestive heart failure
- DCM
dilated cardiomyopathy
- DMR
diltiazem modified release
- DMVD
degenerative mitral valve disease
- DXR
diltiazem extended release
- ECG
electrocardiography
- Holter
24‐hour ambulatory ECG
- HR
heart rate
- maxHR
maximum heart rate (1‐minute average)
- meanHR
mean heart rate (24‐hour average)
- minHR
minimum heart rate (1‐minute average)
- UPenn
University of Pennsylvania
Atrial fibrillation (AF) is the most important nonphysiological arrhythmia in dogs.1 Most commonly, it is associated with structural or functional cardiac disease and atrial remodeling.2 Concurrent congestive heart failure (CHF) is frequently present, and the prognosis for patients with CHF is worsened by the development of AF.3, 4
Two different treatment strategies are used to treat AF: slowing of the ventricular response (rate control) without correction of the AF or termination of the arrhythmia (rhythm control), achieved either by electrical or medical cardioversion. Furthermore, studies in people have established that the rates of complications and death were similar in patients with AF receiving rate control treatment and in those receiving rhythm control treatment.5 Recurrence of AF shortly after cardioversion is common,6, 7 thus rate control has been the primary therapy used in the management of AF in dogs.
Guidelines for people with AF recommend a target heart rate (HR) for patients with AF similar to a “situation‐appropriate” sinus rate (<80 bpm at rest and <110 bpm during moderate exercise).8, 9, 10 Veterinary recommendations, although not evidence‐based, suggest that dogs with AF and cardiac disease should have a somewhat faster HR than normal to maintain cardiac output, but this has not been validated. A recent study in dogs proposed that an electrocardiography (ECG)‐derived HR of <160 bpm is considered adequate.3 An earlier study suggested that a 24‐hour ambulatory ECG (Holter) mean HR of ≤140 bpm indicated adequate rate control in dogs,11 but clinical trials to support these statements are lacking. Although ECG tracings are acquired quickly and at relatively low cost when recorded in a hospital setting, ECG recordings yield HR influenced by stress‐related stimulation.12 In contrast, ambulatory monitoring allows HR assessment outside the hospital setting, perhaps more accurately representing the dog's HR fluctuations during a normal daily routine in the familiar home environment. In‐clinic ECG recordings have been shown to correlate with, but overestimate, the Holter recording‐derived mean HR in dogs with AF.13
The goal of ideal rate control is to decrease clinical signs and improve survival, but drug‐related adverse effects including bradycardia and syncope can occur. The balance between benefit and risk in terms of cardiovascular morbidity and mortality in dogs is unknown. In people, stricter rate control (resting HR < 80 bpm and HR during moderate exercise <110 bpm) was not superior to a more lenient approach (resting HR < 110 bpm14, 15, 16), but these target HR overall are markedly lower than targets historically recommended in dogs with AF.3, 11
Whether more aggressive rate control is associated with an improved prognosis as compared with a more moderate treatment strategy remains unproven in dogs.
The objective of our study was to retrospectively assess the effect of HR as determined by Holter recording on survival in dogs with chronic AF associated with underlying structural heart disease with and without CHF.
Material and Methods
Data Acquisition and Analysis
For this retrospective study, medical records of all dogs having a Holter recording in their home environment as part of their diagnostic evaluation at the Small Animal Teaching Hospital, University of Liverpool, and at the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania between 2008 and 2016 were reviewed. Dogs with a diagnosis of AF and at least 1 Holter recording with a minimum of 20 hours of valid data were included in the study. Patients were excluded if AF changed to sinus rhythm or atrial flutter after the initial diagnosis of AF, if dogs developed clinically relevant bradyarrhythmia requiring a pacemaker, had prior treatment with doxorubicin or were known to have a serious systemic disease (eg, lymphoma) at time of diagnosis of AF that was expected to limit the dog's life span.
The following information was recorded from the medical records: signalment, cardiovascular diagnosis(es), rhythm diagnosis by ECG, medications administered at the time of Holter recording, Holter recording, and treatment instituted after the Holter recording. Patient outcome was obtained from medical records as well as follow‐up phone calls to referring veterinarians, owners, or both.
One or several Holter recordings were obtained from all dogs, depending on whether or not the patients required rate control treatment and how they responded to treatment.
We analyzed Holter recording data and outcome from the point of time when the clinician presumed adequate rate control was achieved, thus no further adjustments of rate control treatment were made. Some dogs had only a single Holter recording for assessment. In cases in which dogs had multiple Holter recordings, only the last Holter recording was used for the study.
For 3‐channel ambulatory Holter recording, a small area of hair was clipped over the left and right cardiac apex for each electrode pair, and ECG electrodes were connected to the Holter recorder, which was fitted into a custom‐made vest. Routine activities for the dogs were encouraged, and an activity diary was kept by the owners.
Holter recording data were acquired using the Lifecard CompactFlash (CF) Digital Holter Monitors at a sampling frequency of 1,024 Hz, stored on 90‐MB removable CF card, and transferred to a hard drive for uploading to a commercial Holter analysis company.a Commercially available Holter software designed for people was used to perform standardized semiautomatic arrhythmia analyses.b The system has been modified to accommodate differences in the ECG characteristics of dogs compared to humans, and all recordings are reviewed by a technician specifically trained in analyzing Holter recordings in dogs. Holter recording reports provided by the commercial Holter analysis company included representative ECG strips of the dog's rhythm (AF), episodes with the maximum and minimum HR and longest pause, and examples of ventricular arrhythmias, if present. Each Holter recording report was reviewed by the attending veterinary cardiologist.
Holter recording data were evaluated for the presence of chronic AF throughout the 24‐hour recording period. From the 24‐hour Holter recording report, the mean heart rate (meanHR, 24‐hour average), minimum HR (minHR, 1‐minute average), and maximum HR (maxHR, 1‐minute average) were assessed. We also assessed pauses (longest RR interval) and ventricular tachyarrhythmias (singles, couplets, and runs of ventricular tachycardia).
Outcome Measures and Statistical Analysis
The study endpoint was all‐cause mortality. A secondary endpoint was death because of cardiac disease, defined as sudden death or euthanasia related to worsening of refractory CHF. The survival time interval from the date of the last Holter recording to death was recorded. For those cases in which survival time and cause of death were not recorded in the medical records, follow‐up information was obtained by a phone interview with the referring veterinarian or owner. Demographic and descriptive characteristics of the study cohort were tabulated. Normality was assessed by visual inspection of histograms. Linear regression and the Pearson correlation coefficient were used to evaluate collinearity among minHR, meanHR, and maxHR. Overall survival and cardiovascular survival were calculated using the Kaplan‐Meier method. Differences in median survival between groups were determined using the log rank test. Dogs in the study that were alive at the end of the study or lost to follow‐up were right censored. In addition, dogs dying from noncardiovascular causes were right censored for purposes of cardiovascular survival analysis. The effect of patient demographics, diagnosis, study site, and HR on survival was analyzed using univariate Cox proportional hazards regression. Variables with P value <0.25 on univariate analysis were considered for exploratory multivariable Cox regression.c Goodness‐of‐fit of the multivariate models was determined by calculation of Harrell's C.17 The assumption of proportional hazards was tested by postestimation score test of the Schoenfeld residuals. Hazard ratios between variously constructed exploratory patient subgroups on the basis of HR were compared using 1‐way ANOVA. P < 0.05 was considered significant.
Results
Study Population
Of 844 Holter recordings acquired in dogs between January 2008 and June 2016 at the University of Liverpool, 56 (6%) were performed in dogs with AF. Of these, 17 were excluded and 39 dogs were included in the study. Of 285 Holter recordings acquired in dogs during the same period at the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania, 16 (5%) were performed in dogs with AF. Of the 16 dogs, 9 were excluded and 7 were included in this study. Dogs were excluded because of incomplete data (Holter recording < 20 hours), clinically relevant VT, spontaneous conversion to sinus rhythm, electrical cardioversion, or concurrent neoplasia.
Seventeen of the 46 dogs included in the study had repeat Holter recordings performed to optimize rate control: 13 dogs had 2 Holter recordings, 3 dogs had 3 recordings, and 1 dog had 4 recordings.
All Holter recordings were performed in the dog's home environment. The breeds represented by >2 cases were Dogue de Bordeaux (n = 6), Labrador retriever (n = 4), German shepherd (n = 4), Great Dane, Newfoundland, Mastiff, and Irish Wolfhound (n = 3 each).
There were 11 females (3 intact, 8 neutered) and 35 males (17 intact, 18 neutered) in the study. The median weight was 41.3 kg (range, 14.1–78.5; IQR, 27.9–60.7); the median age was 7 years (range, 1–14; IQR, 4–10).
Structural heart disease was identified in all dogs. The primary structural abnormalities present were as follows: dilated cardiomyopathy (DCM; n = 27), degenerative mitral valve disease (DMVD; n = 8), mitral valve dysplasia (n = 7), patent ductus arteriosus (closed; n = 2), tricuspid valve dysplasia (n = 2), subaortic stenosis (n = 2), atrial cardiomyopathy (n = 2), and arrhythmogenic right ventricular cardiomyopathy (n = 1).
Thirty‐one of the 46 dogs (67%) were in CHF at the time of diagnosis of AF (18 of 46, left‐sided; 4 of 46, right‐sided; 9 of 46, biventricular CHF) before the Holter recording examination and were receiving cardiac medications, including furosemide, enalapril, benazepril, and pimobendan. Some dogs also received spironolactone and hydrochlorothiazide.
Forty‐four of the 46 dogs (96%) were treated for rate control of AF: 1 of 46 was receiving only digoxin, 8 of 46 were receiving only diltiazem (7 diltiazem modified release [DMR] and 1 diltiazem extended release [DXR]), 29 of 46 were receiving digoxin and diltiazem (25 DMR and 4 DXR), 2 of 46 were receiving DMR and amiodarone, 2 of 46 were receiving amiodarone and digoxin and DMR, 1 of 46 was receiving only sotalol, and 1 of 46 was receiving digoxin and DXR and sotalol.
Dosages for rate control treatment were as follows: digoxin at 0.003–0.005 mg/kg PO q12h, DMR at 1.5–2.5 mg/kg PO q8h, DXR at 2–3 mg/kg PO q12h, sotalol at 1–2 mg/kg PO q12h, and amiodarone 5–7.5 mg/kg PO q24h after the initial loading dose, singly or in combination. The dogs received rate control treatment for 1–2 weeks (to ensure drugs reached steady‐state) before reexamination and repeat Holter recording.
The duration of the Holter recordings ranged from 20–26 hours. The median meanHR was 125 bpm (range, 62–203 bpm) with an IQR of 100–141 bpm. The median minHR was 82 bpm (range, 37–163 bpm) with an IQR of 61–96 bpm. The median maxHR was 217 bpm (range, 126–307 bpm) with an IQR of 159–276 bpm.
Outcome
Twenty‐five of 46 dogs (54%) were alive or lost to follow‐up at the end of the study, whereas 21 of 46 (46%) dogs were dead. Of the 21 dogs that were dead at the end of the study, 4 of 21 suffered sudden death, 11 of 21 had been euthanized because of refractory CHF, 5 of 21 had been euthanized for noncardiac reasons, and 1 of 21 had been euthanized but the reason was unclear from the clinical records. The median follow‐up time (from the last Holter recording to death or end of the study) was 98.5 days (range, 3–1,140 days) with an IQR of 28–267 days. Median time to all‐cause death was 524 days (IQR, 76–1,037 days).
Univariate Cox proportional hazard regression indicated that sex, neutering status, age, weight, cardiac diagnosis (DCM versus non‐DCM), and study site were not significantly associated with all‐cause mortality (Table 1) or cardiac‐related mortality (Table 2). The presence or absence of CHF at time of diagnosis of AF was not associated with all‐cause mortality. Of the 15 dogs that died from cardiac disease, 14 had CHF at the time of AF diagnosis. Median time to all‐cause death was 524 days (IQR, 76–1,037 days).
Table 1.
Univariate Cox proportional hazards regression for all‐cause mortality (n = 21)
| Variable | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Sex (female) | 0.84 (0.32–2.22) | 0.725 |
| Neuter status (Yes) | 1.22 (0.51–2.94) | 0.649 |
| Age | 1.03 (0.90–1.16) | 0.699 |
| Weight | 1.00 (0.98–1.02) | 0.943 |
| Diagnosis (DCM) | 0.78 (0.34–1.84) | 0.569 |
| Study site (UPenn) | 2.35 (0.64–8.61) | 0.196 |
| CHF at diagnosis (Yes) | 2.44 (0.81–7.41) | 0.113 |
| HR variables | ||
| Min HRa | 1.30 (1.14–1.49) | <0.001 |
| Max HRa | 1.25 (1.08–1.44) | 0.003 |
| Mean HRa | 1.35 (1.17–1.55) | <0.001 |
meanHR, mean heart rate (24‐hour average); minHR, minimum HR (1‐minute average); maxHR, maximum HR (1‐minute average); DCM, dilated cardiomyopathy; CHF, congestive heart failure; UPenn, University of Pennsylvania.
Hazard ratio for every 10 bpm difference.
Table 2.
Univariate Cox proportional hazards regression for cardiovascular mortality (n = 15) (Cases lost to follow‐up, alive, or dead due to noncardiac disease are censored)
| Variable | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Sex (female) | 0.79 (0.25–2.53) | 0.694 |
| Neuter status (Yes) | 1.35 (0.47–3.82) | 0.572 |
| Age | 1.01 (0.87–1.18) | 0.871 |
| Weight | 0.97 (0.94–1.00) | 0.071 |
| Diagnosis (DCM) | 3.05 (0.97–9.60) | 0.569 |
| Study site (UPenn) | 2.53 (0.51–12.45) | 0.254 |
| HR variables | ||
| Min HRa | 1.34 (1.14–1.56) | <0.001 |
| Max HRa | 1.33 (1.11–1.58) | 0.002 |
| Mean HRa | 1.42 (1.20–1.69) | <0.001 |
meanHR, mean heart rate (24‐hour average); minHR, minimum HR (1‐minute average); maxHR, maximum HR (1‐minute average); DCM, dilated cardiomyopathy; UPenn, University of Pennsylvania.
Hazard ratio for every 10 bpm difference.
All 3 indices of HR derived from the Holter recordings, including minHR, maxHR, and meanHR were significantly correlated with both all‐cause and cardiac‐related mortality (Tables 1 and 2). The 3 HR indices were significantly correlated with each other. Minimum heart rate and maxHR were significantly but modestly correlated (r = 0.353; P = 0.016). Maximum heart rate and mean HR were modestly but significantly correlated (r = 0.573, P < 0.0001); min HR and meanHR were strongly correlated (r = 0.929, P < 0.00001). Thus, there was a high degree of collinearity among indices of HR. As such, multivariable models exploring all‐cause mortality that either contained all 3 indices or the combination of minHR and meanHR were unstable. Following standard recommendations regarding entrance of 1 variable into the multivariable model for approximately every 10 events,18 we limited the number of variables analyzed in any given model to 2. Regarding all‐cause mortality, neither study site nor the presence or absence of CHF was significantly associated with mortality when analyzed along with each of the HR indices. Moreover, neither study site nor the presence of absence of CHF changed the hazard ratio of any of the HR indices by >5%. Accordingly, 2 potential multivariable models describing all‐cause mortality were further explored. Model 1 contained minHR and maxHR, and model 2 contained meanHR and maxHR. Multivariable Cox regression of model 1 indicated that both minHR and maxHR were independently associated with survival (Table 3). Model 2 indicated that after adjusting for maxHR, only meanHR was independently associated with survival (Table 3). The goodness‐of‐fit between models was very similar (C statistic: model 1, 0.803; model 2, 0.785), suggesting that the most parsimonious model was the univariate model involving meanHR as the sole predictive index. Thus, for every 10 bpm increase in meanHR, the risk of all‐cause mortality increased by 35% (95% CI, 17–55%; Table 1).
Table 3.
Multivariate Cox proportional hazards regression for all‐cause mortality
| Variable | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Model 1 | ||
| Min HRa | 1.24 (1.08–1.42) | 0.002 |
| Max HRa | 1.20 (1.00–1.42) | 0.044 |
| Model 2 | ||
| Max HRa | 1.11 (0.93–1.35) | 0.277 |
| Mean HRa | 1.29 (1.09–1.52) | 0.003 |
meanHR, mean heart rate (24‐hour average); minHR, minimum HR (1‐minute average); maxHR, maximum HR (1‐minute average).
Hazard ratio for every 10 bpm.
Several different multivariable Cox models for cardiovascular mortality were explored using body weight and each index of HR or different combinations of 2 of the 3 HR indices. Body weight was not significantly correlated with cardiovascular survival after adjustment for HR and its inclusion did not change the hazard ratio of any of the HR indices by >5%. Results from the 3 different pairings of HR indices (Table 4) indicated that, after adjustment for maxHR, minHR was correlated with cardiovascular mortality and, after adjustment for either maxHR or minHR, meanHR was correlated with cardiovascular mortality. The model involving minHR and meanHR (model 3, Table 4) returned hazard ratios with much wider CI as compared to the univariable values (Table 2), likely caused by high correlation between the 2 variables and resulting model instability. These results suggested that the most parsimonious model was the univariate model involving meanHR as the sole predictive index. Thus, for every 10 bpm increase in meanHR, the risk of cardiovascular mortality increased by 42% (95% CI, 20–69%; Table 2).
Table 4.
Multivariate Cox proportional hazards regression for cardiovascular mortality
| Variable | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Model 1 | ||
| Min HRa | 1.29 (1.09–1.52) | 0.003 |
| Max HRa | 1.11 (0.92–1.34) | 0.277 |
| Model 2 | ||
| Max HRa | 1.19 (0.94–1.50) | 0.144 |
| Mean HRa | 1.34 (1.10–1.62) | 0.003 |
| Model 3 | ||
| Min HRa | 0.71 (0.44–1.14) | 0.152 |
| Mean HRa | 2.05 (1.19–3.51) | 0.009 |
meanHR, mean heart rate (24‐hour average); minHR, minimum HR (1‐minute average); maxHR, maximum HR (1‐minute average).
Hazard ratio for every 10 bpm.
To further explore the effect of meanHR on all‐cause mortality, we compared the Kaplan‐Meier survival curves of dogs in 2 additional ways. Firstly, we divided the study population into 2 comparative cohorts based on the group's median value for meanHR (125 bpm). Dogs alive or lost to follow‐up were right‐censored observations. Median survival time of the dogs with a meanHR of <125 bpm (n = 23; 1,037 days; 95% CI, 524‐ n/a) was significantly longer as compared to dogs with a meanHR of ≥125 bpm (n = 23; 105 days; 95% CI, 67–267 days; P = 0.0012; Fig 1). The assumption of proportional hazards was met (score test, P = 0.78), meaning that the survival benefit of lower meanHR was consistent throughout the entire follow‐up time. Secondly, realizing that at some value, the beneficial effect of lowered meanHR or minHR must cease to exist, we explored the hazard ratio of dogs in the lowest tercile of either meanHR or minHR compared to the higher stratum. Thus, we stratified the dogs into 3 groups by meanHR (<112, 112–138, and >138 bpm) and compared the hazard ratios among them. One‐way ANOVA indicated that the hazard ratios in dogs with meanHR <113, between 112 and 138 and >138 were not statistically different from each other (P = 0.39). We performed similar analysis using 3 groups of minHR (<69, 69–93, and >93 bpm). One‐way ANOVA indicated that the hazard ratios in dogs with meanHR <69, between 69 and 93 and >93 were not statistically different from each other (P = 0.47).
Figure 1.
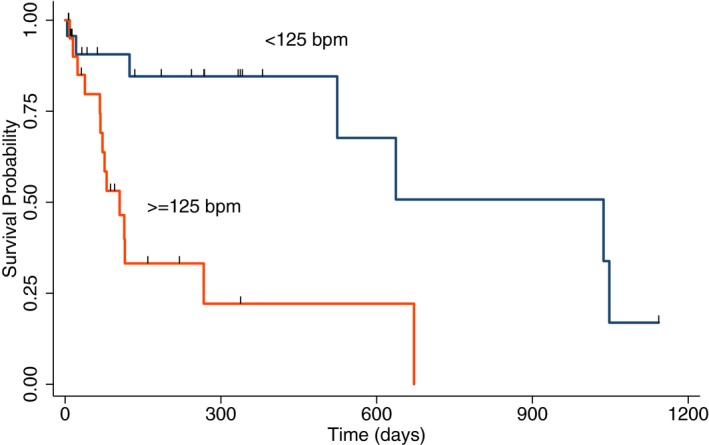
Kaplan‐Meier estimates of survival based on all‐cause mortality in two groups of dogs with AF divided equally based on the median meanHR of <125 bpm (black line, n = 23) or ≥125 bpm (orange line, n = 23). The group with meanHR < 125 bpm (1,037 days, 95% CI, 524‐ n/a) had significantly longer survival time than group with meanHR ≥125 bpm (105 days, 95% CI, 67–267 days).
Discussion
Atrial fibrillation is a very important clinical condition in dogs, resulting in substantial morbidity and mortality.1, 4, 5 The same has been reported in people, where the development of AF is associated with a 3‐fold increased risk of CHF and a worse prognosis.19 However, interdependence exists between CHF and AF where the structural and neurohormonal remodeling that occur in CHF also make the development of AF more likely.20
In people, current guidelines recommend a lenient rate control approach (HR target <110 bpm) as initial strategy in asymptomatic patients with preserved left ventricular function,10, 21, 22 but a more strict rate control approach (<80 bpm at rest and <110 bpm during moderate exercise) in symptomatic patients with impaired left ventricular function.10 Many dogs affected by AF are also in CHF and have some degree of left (or right) ventricular dysfunction, which may explain why the dogs included in our study showed significant survival benefit from a stricter rate control approach.
Rate control, as opposed to rhythm control, is the first‐choice treatment in most dogs with chronic AF and structural or functional primary cardiac disease, but there is insufficient evidence to guide HR targets in dogs. The results of our retrospective study provide insight into the role of HR in the survival of dogs with AF. Our results indicate that several different indices of Holter recording‐derived HR are significantly associated with risk for all‐cause or cardiovascular mortality. This effect was present in dogs over a broad range of HRs, including those far below what is currently recommended as a “target” or “ideal” rate. Specifically, exploratory analysis showed benefit of rate reduction in meanHRs as low as 62 bpm, and dogs with mean HR < 125 bpm lived significantly longer than those with faster rates. Historically, veterinarians have tended to be fairly lenient with rate control, considering the argument that during AF and concurrent CHF, a relatively higher HR is needed to maintain adequate cardiac output as compared to dogs in sinus rhythm with similar underlying disease. The rationale behind this supposition is that sympathetic activation may be an appropriate physiological response to maintain hemodynamic stability, and a lower HR may decrease an already impaired cardiac output, which then could have a negative impact on survival.3, 4 In contrast, our findings suggest that lower HRs are associated with decreased mortality even in dogs with CHF. In fact, within the range of min HR and meanHR represented in our study population, we did not identify a low minHR or meanHR at which the HR reduction was no longer beneficial (and may be harmful). Despite this, a lower limit of HR reduction and the “ideal” target HR likely differs among the different etiologies of AF, and additional studies are needed.
Previous studies have examined the effect of HR on survival.3, 23, 24 It is well established that HR plays an important role for cardiac function, and it has been shown in dogs that tachycardia‐induced myocardial failure occurs with pacing > 180 bpm.23, 24 A recent study in dogs with AF caused by DMVD showed that dogs with an ECG‐derived maximum HR >160 bpm had a shorter survival time than dogs with a HR < 160 bpm.3 Those HRs were acquired by short ECG recording during a hospital visit, an environment that typically increases the HR temporarily in dogs with AF.3 In our study, when maxHR and meanHR were simultaneously analyzed, only meanHR was independently associated with either all‐cause or cardiovascular mortality (Table 3, model 2; Table 4, model 2).
Males (both intact and neutered) were overrepresented (69%) in our patient population with AF, consistent with previous reports of male people and dogs having a significantly higher prevalence of AF.25, 26, 27 One possible explanation for the sex difference in AF prevalence is the overall larger size of male dogs, and thus a larger atrial mass, a factor known to predispose to AF.1, 28 A previous study in dogs found that spayed females with AF lived longer than males or intact females with AF,26 and women with AF have a lower fatality rate in all age groups as compared to men.27 Unlike in people, where male sex and older age are important risk factors for AF,29, 30 in our study, sex, neutering status, and age did not impact survival, suggesting that once AF is present, sex and age do not influence the survival time.
In our study, the underlying cardiac disease (DCM versus non‐DCM) was not associated with survival time. It is conceivable that once a dog develops AF, the underlying disease or cause of the arrhythmia is no longer relevant for outcome.
When people with CHF have severe left ventricular dysfunction, mortality from the primary disease process itself is high and addition of AF as a comorbidity seems to make little difference in terms of prognosis.31, 32, 33, 34 Previous reports in dogs with CHF indicate that development of AF is commonly associated with negative impact on survival and worse prognosis.1, 3, 4, 35 In our study, however, the presence or absence of CHF did not independently affect all‐cause mortality once HR was factored in. The effect of CHF or HR on cardiovascular mortality could not be evaluated independently because, of the 15 dogs that died from cardiac‐related cause, 14 had CHF at the time of AF diagnosis. Although the presence of CHF was not included in the univariate or multivariate regression analysis, it appears to promote cardiac‐related death in dogs with AF.
Our study had several limitations, many of which are inherent to the retrospective design of the study and selection bias. The antiarrhythmic therapies and treatment of underlying heart disease or CHF prescribed for these dogs were not standardized. These were selected according to the attending clinician's preference, and the dosages were adjusted as needed according to what the clinician deemed satisfactory rate control and could therefore have influenced the outcome. Once a meanHR considered “acceptable” by the clinician was achieved, no additional Holter recordings were performed. Therefore, a loss of effect of these drugs or the development of other clinically relevant arrhythmias (eg, ventricular arrhythmias) caused by progression of the underlying disease could have impacted survival. The effect of the different antiarrhythmic therapies on survival time and on risk of sudden death could not be assessed because of the small number of dogs and nonstandardized management. Heart rate during exercise, which appears important in human patients, could not be determined from the Holter recording data. Furthermore, the Holter recording full disclosure reports were not reviewed in every case, unless deemed necessary.
Another limitation of our study is that most of the cardiac‐related deaths occurred in dogs with CHF at the time of diagnosis of AF. Dogs in CHF with sinus rhythm have higher intrinsic HR,36 and thus CHF could have contributed to a higher meanHR in our patients with AF and therefore could have influenced our results. The number of dogs that died of cardiovascular causes was small, and further prospective studies are needed to provide definitive evidence of a link between HR or CHF and cardiovascular mortality and to determine optimal HR targets in that population.
Conclusion
Indices of HR derived by Holter recording monitoring, and in particular meanHR, were significantly associated with survival time in dogs with AF across a wide range of HR. Holter recording‐derived HR data indicate that dogs with meanHR <125 bpm lived longer than those with faster rates. These findings suggest that current rate control efforts in dogs with AF should use a therapeutic target of a Holter recording‐derived meanHR < 125 bpm. The association between HR and adverse outcome warrants prospective studies investigating strict rate control strategies.
Acknowledgments
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
This study took place at the Small Animal Teaching Hospital of the University of Liverpool (UK) and at the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania (USA).
The results of this study were presented at the 2017 ACVIM Forum, National Harbor, MD.
Footnotes
aLabCorp Ambulatory Monitoring Services, Burlington, NC
bPathfinder version 9.019, Spacelabs Healthcare
cSTATA 14.2, STATA Corp., College Station, TX
References
- 1. Bonagura JD, Ware WA. Atrial fibrillation in the dog: Clinical findings in 81 cases. J Am Anim Hosp Assoc 1986;22:111–120. [Google Scholar]
- 2. Hunter RJ, Liu Y, Lu Y, et al. Left atrial wall stress distribution and its relationship to electrophysiologic remodeling in persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2012;5:351–360. [DOI] [PubMed] [Google Scholar]
- 3. Jung SW, Sun W, Griffiths LG, et al. Atrial fibrillation as a prognostic indicator in medium to large‐sized dogs with myxomatous mitral valvular degeneration and congestive heart failure. J Vet Intern Med 2016;30:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calvert CA, Pickus CW, Jacobs GJ, et al. Signalment, survival, and prognostic factors in Doberman pinschers with end‐stage cardiomyopathy. J Vet Intern Med 1997;11:323–326. [DOI] [PubMed] [Google Scholar]
- 5. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 6. Bright JM, Martin JM, Mama K. A retrospective evaluation of transthoracic biphasic electrical cardioversion for atrial fibrillation in dogs. J Vet Cardiol 2005;7:85–96. [DOI] [PubMed] [Google Scholar]
- 7. Bright JM, zumBrunnen J. Chronicity of atrial fibrillation affects duration of sinus rhythm after transthoracic cardioversion of dogs with naturally occurring atrial fibrillation. J Vet Intern Med 2008;22:114–119. [DOI] [PubMed] [Google Scholar]
- 8. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 9. Saxonhouse SJ, Curtis AB. Risks and benefits of rate control versus maintenance of sinus rhythm. Am J Cardiol 2003;91:27D–32D. [DOI] [PubMed] [Google Scholar]
- 10. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 11. Gelzer AR, Kraus MS, Rishniw M, et al. Combination therapy with digoxin and diltiazem controls ventricular rate in chronic atrial fibrillation in dogs better than digoxin or diltiazem monotherapy: A randomized crossover study in 18 dogs. J Vet Intern Med 2009;23:499–508. [DOI] [PubMed] [Google Scholar]
- 12. Uechi M, Shimizu A, Mizuno M. Heart rate modulation by sympathetic nerves in dogs with heart failure. J Vet Med Sci 2002;64:1023–1029. [DOI] [PubMed] [Google Scholar]
- 13. Gelzer AR, Kraus MS, Rishniw M. Evaluation of in‐hospital electrocardiography versus 24‐hour Holter for rate control in dogs with atrial fibrillation. J Small Anim Pract 2015;56:456–462. [DOI] [PubMed] [Google Scholar]
- 14. Van Gelder IC, Wyse DG, Chandler ML, et al. Does intensity of rate‐control influence outcome in atrial fibrillation? An analysis of pooled data from the RACE and AFFIRM studies Europace 2006;8:935–942. [DOI] [PubMed] [Google Scholar]
- 15. Van Gelder IC, Van Veldhuisen DJ, Crijns HJ, et al. RAte Control Efficacy in permanent atrial fibrillation: A comparison between lenient versus strict rate control in patients with and without heart failure. Background, aims, and design of RACE II. Am Heart J 2006;152:420–426. [DOI] [PubMed] [Google Scholar]
- 16. Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 17. Harrell FE Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA 1982;247:2543–2546. [PubMed] [Google Scholar]
- 18. Katz MH. Multivariable analysis: A practical guide for clinicians and public health researchers. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- 19. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the longterm risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 20. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: What should we do? Eur Heart J 2015;36:3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Groenveld HF, Tijssen JG, Crijns HJ, et al. Rate control efficacy in permanent atrial fibrillation: Successful and failed strict rate control against a background of lenient rate control: Data from RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation). J Am Coll Cardiol 2013;61:741–748. [DOI] [PubMed] [Google Scholar]
- 22. Groenveld HF, Crijns HJ, Van den Berg MP, et al. The effect of rate control on quality of life in patients with permanent atrial fibrillation: Data from the RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation II) study. J Am Coll Cardiol 2011;58:1795–1803. [DOI] [PubMed] [Google Scholar]
- 23. Armstrong PW, Stopps TP, Ford SE, et al. Rapid ventricular pacing in the dog: Pathophysiologic studies of heart failure. Circulation 1986;74:1075–1084. [DOI] [PubMed] [Google Scholar]
- 24. Wilson JR, Douglas P, Hickey WF, et al. Experimental congestive heart failure produced by rapid ventricular pacing in the dog: Cardiac effects. Circulation 1987;75:857–867. [DOI] [PubMed] [Google Scholar]
- 25. Renoux C, Patenaude V, Suissa S. Incidence, mortality, and sex differences of non‐valvular atrial fibrillation: A population‐based study. J Am Heart Assoc 2014;3:e001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Westling J, Westling W, Pyle RL. Epidemiology of atrial fibrillation in the dog. Int J Appl Res Vet Med 2008;6:151–154. [Google Scholar]
- 27. Vollmar AC, Fox PR. Long‐term outcome of Irish Wolfhound dogs with preclinical cardiomyopathy, atrial fibrillation, or both treated with pimobendan, benazepril hydrochloride, or methyldigoxin monotherapy. J Vet Intern Med 2016;30:553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bohn FK, Patterson DF, Pyle RL. Atrial fibrillation in dogs. Br Vet J 1971;127:485–496. [PubMed] [Google Scholar]
- 29. van Walraven C, Hart RG, Connolly S, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: The atrial fibrillation investigators. Stroke 2009;40:1410–1416. [DOI] [PubMed] [Google Scholar]
- 30. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population‐based estimates. Am J Cardiol 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 31. Pai RG, Varadarajan P. Prognostic significance of atrial fibrillation is a function of left ventricular ejection fraction. Clin Cardiol 2007;30:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swedberg K, Olsson LG, Charlesworth A, et al. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long‐term treatment with beta‐blockers: Results from COMET. Eur Heart J 2005;26:1303–1308. [DOI] [PubMed] [Google Scholar]
- 33. Singh SN, Poole J, Anderson J, et al. Role of amiodarone or implantable cardioverter/defibrillator in patients with atrial fibrillation and heart failure. Am Heart J 2006;152:974.e977–911. [DOI] [PubMed] [Google Scholar]
- 34. Marijon E, Le Heuzey JY, Connolly S, et al. Causes of death and influencing factors in patients with atrial fibrillation: A competing‐risk analysis from the randomized evaluation of long‐term anticoagulant therapy study. Circulation 2013;128:2192–2201. [DOI] [PubMed] [Google Scholar]
- 35. Vollmar AC. The prevalence of cardiomyopathy in the Irish wolfhound: A clinical study of 500 dogs. J Am Anim Hosp Assoc 2000;36:125–132. [DOI] [PubMed] [Google Scholar]
- 36. Lopez‐Alvarez J, Boswood A, Moonarmart W, et al. Longitudinal electrocardiographic evaluation of dogs with degenerative mitral valve disease. J Vet Intern Med 2014;28:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]


